Abstract
Background
SARS-CoV-2 can cause acute pancreatitis (AP) and SARS-CoV-2 superinfection can occur in patients with AP during prolonged hospitalisation. Our objective was to characterize SARS-CoV-2 related AP and study the impact of SARS-CoV-2 superinfection on outcomes in AP.
Methods
In this multicentre prospective study, all patients with AP and SARS-CoV-2 infection between August 2020 and February 2021 were divided into two groups: SARS-CoV-2-related AP and superadded SARS-CoV-2 infection in patients with AP. The two groups were compared with each other and the whole cohort was compared with a non-COVID AP cohort.
Results
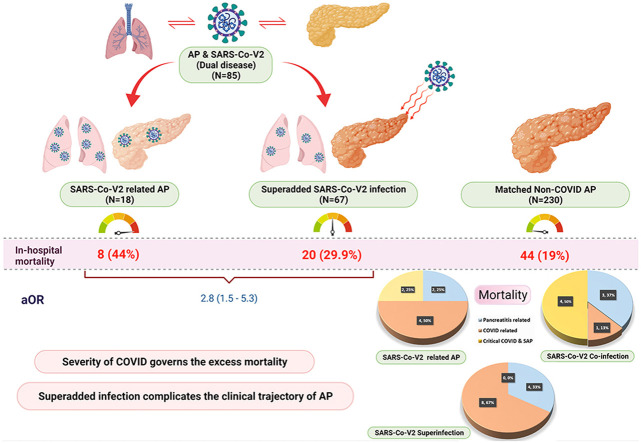
A total of 85 patients with SARS-CoV-2 and AP (SARS-CoV-2-related AP; n = 18 and AP with SARS-CoV-2 superadded infection; n = 67) were included during the study period. They had a higher mortality [28 (32.9%) vs. 44 (19.1%), aOR 2.8 (95% CI, 1.5–5.3)] than 230 propensity matched non-COVID AP patients. Mortality in SARS-CoV-2 and AP patients was due to critical COVID. SARS-CoV-2-related- AP (n = 18) had a higher but statistically insignificant mortality than SARS-CoV-2 superinfection in AP [8/18 (44.4%) vs 20/67 (29.8%), p = 0.24]. On multivariable analysis, infection with SARS-CoV-2 (aHR 2.3; 95% CI, 1.43.7) was a predictor of in-hospital mortality in addition to organ failure (OF) in patients with AP.
Conclusion
Patients with AP and SARS-CoV-2 infection had a higher mortality than matched non-COVID AP patients which was largely attributable to the severity of COVID-19. SARS-CoV-2 related AP had higher OF and in-hospital mortality.
Keywords: Acute pancreatitis, SARS-CoV-2, Coronavirus, COVID-19, Virus induced pancreatitis, Organ failure
Graphical abstract
1. Introduction
Acute pancreatitis (AP) is an acute inflammatory disease of the pancreas. It runs a mild course in a majority of patients, but severe AP (SAP) occurs in around 20% of cases with a mortality of 20–40% [[1], [2], [3]].
The current pandemic by a novel coronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the worst since the Spanish flu of 1918, has affected >364 million people and has claimed >5.6 million lives worldwide due to coronavirus disease −19 (COVID-19) as of 30th January 2022 [4]. It is projected to remain active till 2024 even after adequate control measures, particularly with the emergence of many mutants called variants of concern such as B.1.1.7 (alpha), B.1.617.2 (delta), and B.1.1.529 (omicron)[5]. Although it is primarily a respiratory pathogen with predominant tropism for lungs, an increasing number of reports have documented involvement of other organs such as liver, brain, and pancreas due to expression of ACE 2 receptors which facilitate intracellular viral entry [6,7]. Viral infections such as mumps, coxsackievirus and hepatitis E have been known to cause AP [8,9]. SARS-CoV-2 has also been shown to cause AP, the mechanism of pancreatic injury likely being multifactorial [10,11]. Although a few studies have shown isolated elevation of pancreatic enzymes which may not be due to pancreatitis, presence of ACE2 receptors on the pancreatic acinar cells, autopsy data showing presence of SARS-CoV-2 virus within the pancreas and imaging evidence of pancreatitis, lend support to the hypothesis of SARS-CoV-2 causing AP [[12], [13], [14], [15]]. In addition, nosocomial SARS-CoV-2 superinfection may also occur in patients with AP due to other etiologies during the course of hospitalisation for their primary illness [16].
Organ Failure (OF) in SAP is mediated by a cytokine storm during initial phase of illness akin to cytokine storm recently described in critical COVID [1,17,18]. Thus theoretically, concomitant COVID-19 infection in patients with AP can compound this effect leading to the development of new-onset OF and worsen the clinical course. There are a few gaps in our current knowledge regarding AP and SARS-CoV-2. SARS-CoV-2 related AP remains poorly defined and its outcome compared to AP followed by SARS-CoV-2 superinfection is not well known. Limited data exist on the comparison of AP patients with and without COVID-19 infection [[19], [20], [21]].
Therefore, the objectives of the present prospective study were to (i) to compare the severity and in-hospital mortality of patients with AP plus SARS-CoV-2 infection and those with AP but without SARS-CoV-2 infection, (ii) to define and study the outcome of SARS-CoV-2 related AP compared with that in patients with AP due to other etiologies, and (iii) to study the influence of the timing of superinfection of SARS-CoV-2 during the course of AP on its outcome.
2. Methods
Setting: It was a multicentre study carried out at 2 tertiary care academic institutions – Postgraduate Institute of Medical Education and Research, Chandigarh and All India Institute of Medical Sciences, New Delhi, India.
Study design and period: It was a prospective observational cohort study conducted from August 2020 till February 2021. The study was started after obtaining ethical approval from institute ethics committee of both the institutes. Informed consent was taken from the patient before inclusion in the study. We followed the strengthening the reporting of observational studies in epidemiology (STROBE) statement for a cohort study. All the authors had access to the study data and have reviewed and approved the final manuscript.
Study population: All consecutive patients with AP diagnosed as per Revised Atlanta Classification were screened for inclusion.
Inclusion criteria: All consecutive patients with AP and SARS-CoV-2 infection constituted the study group.
Exclusion criteria: Patients with recurrent acute pancreatitis and chronic pancreatitis were excluded.
Controls: Patients with AP hospitalized from January 2015 till December 2019 (i.e., before the start of pandemic to exclude every possible associated SARS-CoV-2 infection) were chosen as historical controls from our prospectively maintained database by propensity score matching in 1:4 ratio for age, sex and severity of pancreatitis.
2.1. Definitions
2.1.1. Acute pancreatitis and severity classification
AP and its severity were defined as per the revised Atlanta definition [22].
SARS-CoV-2 infection related AP: A patient was diagnosed to have SARS-CoV-2 related AP when the patient was hospitalized with RT-PCR confirmed SARS-CoV-2 infection and developed features of AP within 7 days of onset of symptoms of COVID-19 after excluding other causes of AP. The other causes of AP were excluded as follows: (i) gallstones: absence of gallstones on abdominal USG and <3 times elevation of ALT, (ii) no history of significant alcohol intake i.e. >40 gm/day for >5 years, (iii) normal serum calcium and triglyceride levels, (iv) no history of having undergone ERCP or trauma in the past one week and (v) no past history or family history of pancreatitis.
Superadded SARS-CoV-2 infection in acute pancreatitis: It was defined as development of superadded SARS-CoV-2 infection in patients with AP due to other etiologies. We further categorized it into two subgroups based on the incubation period of SARS-CoV-2 infection:
-
a.
Co-infection of SARS-CoV-2 with AP was defined as RT-PCR confirmed COVID-19 within 14 days of date of onset of AP, where etiology of AP was known.
-
b.
Super-infection of SARS-CoV-2 with AP was defined if a patient was hospitalized for AP and acquired SARS-CoV-2 infection at any time point after 2 weeks of onset of AP.
2.2. Assessment of severity of individual disease in patients with both AP and COVID-19
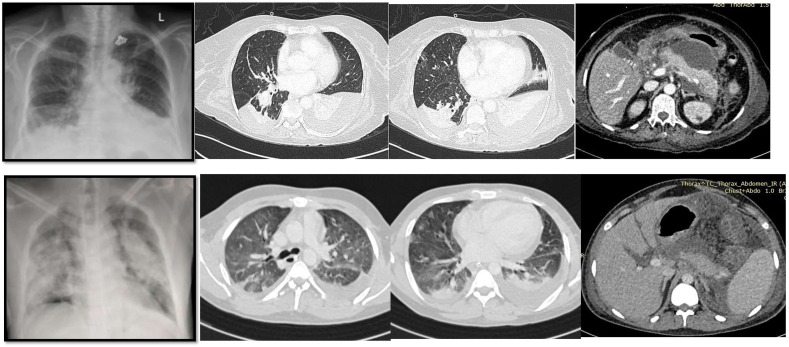
In order to assess the relative contribution of individual disease to mortality, it is important to classify the severity of AP and COVID-19 separately in patients who have both the diseases. It might be difficult because the severity of both the conditions is defined by organ failure. Respiratory failure is the first and dominant organ failure in COVID-19 due to viral pneumonia that determines its severity. A CT scan of chest was done to diagnose COVID pneumonia in every patient with respiratory failure in the present study. SARS-CoV-2 infection induced pneumonia shows characteristic findings on CT such as ground glass opacities, vascular enlargement, bilateral abnormalities, lower lobe involvement, and posterior predilection which are not seen in AP (Fig. 1 ). Critical COVID-19 infection was defined as per WHO definitions by development of acute respiratory distress syndrome (ARDS), sepsis, or septic shock requiring life supporting therapies such as mechanical ventilation (invasive or non-invasive) or vasopressor support [4]. Severity scores for AP included APACHE-II, BISAP and modified Marshall score for OF.
Fig. 1.
Upper panel showing chest x-ray and CT images of a patient with respiratory failure due to severe acute pancreatitis and mild COVID-19 infection. The images show pleural effusion and collapse consolidation of lungs. However, lung parenchyma is relatively preserved with minimal infiltrates. Lower panel shows chest x-ray and CT images of a patient with severe COVID-19 infection and moderate pancreatitis. It shows diffuse bilateral ground glass opacities with posterior predilection characteristic of COVID pneumonia.
2.3. Primary outcome
Comparison of severity and in-hospital mortality of patients with AP plus SARS-CoV-2 infection with matched AP patients without SARS-CoV-2 infection.
2.4. Secondary outcomes
-
1.
The severity and in-hospital mortality of SARS-CoV-2 related AP compared with AP patients with superadded SARS-CoV-2 infection.
-
2.
The influence of the timing of co-infection and super-infection of SARS-CoV-2 during the course of AP in terms of mortality and organ failures.
-
3.
The severity and in-hospital mortality of AP patients plus SARS-CoV-2 co-infection or super-infection as compared to matched patients with AP but no SARS-CoV-2 infection
-
4.
Differences in the outcomes (severity, organ failures, local complications and in-hospital mortality) between all three COVID positive AP groups i.e., SARS-CoV-2 related AP, co-infection and super-infection of SARS-CoV-2 with AP.
2.5. Statistical analysis
For comparison with controls, we used our prospectively collected database of 1062 COVID-19 negative AP patients and developed a propensity score model to ensure balance between the COVID-19 positive and COVID-19 negative cases. The propensity score model included the effects derived from three baseline covariates – age, sex and severity of pancreatitis. This propensity model was then used to generate a patient-specific propensity score that was used to adjust for potential confounding in the final analyses. In the current analysis, we used a matching ratio of 1:4 (COVID-19 positive: COVID-19 negative) in order to improve study power. Also, we performed the propensity score matching “with replacement”, i.e., a patient could be included in more than one matched set. We employed four iterations of the ‘Nearest available neighbour’ method to find out four matching sets of COVID-19 negative patients for each COVID-19 positive patient based on the absolute value of the difference between the propensity score of the patients in both the groups. Using this method, we matched 85 COVID-19 positive patients with 230 COVID-19 negative patients.
All the data were entered into a spreadsheet and analyzed using the SPSS (version 21.0, SPSS Inc; Chicago, USA) software. Continuous variables were expressed as mean with standard deviation or median with interquartile range. Dichotomous variables were compared using the Chi-square test or the Fischer's exact test as appropriate. We created Cox proportional hazard regression models to study the impact of COVID-19 and other variables on mortality. A p value of <0.05 was taken as statistically significant.
3. Results
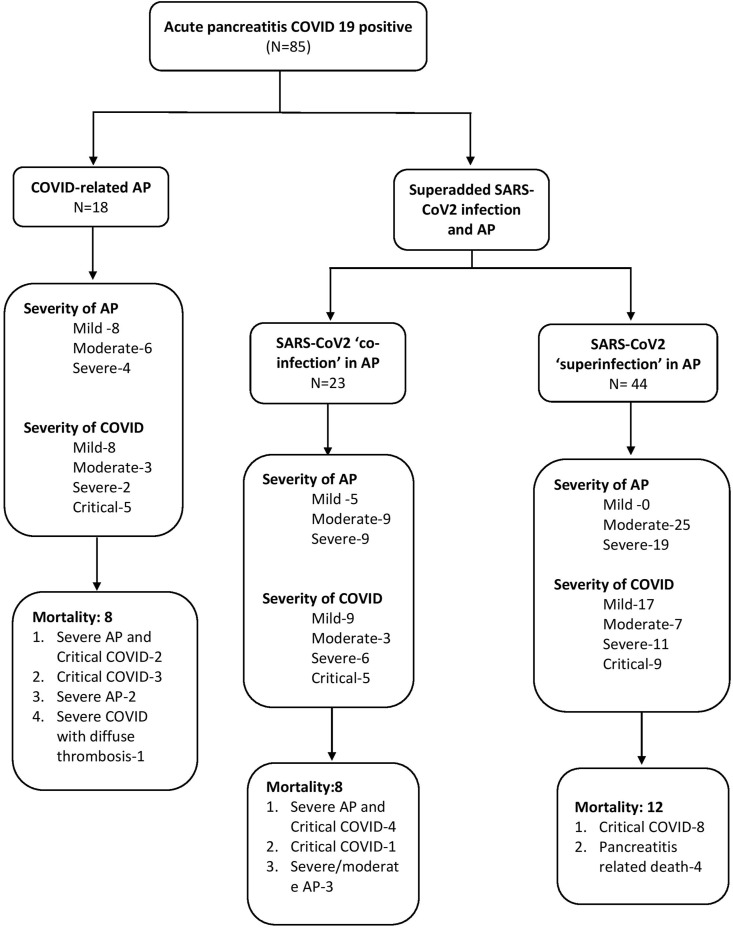
A total of 85 patients (58 males, 68.2%) with AP and COVID-19 were included during the study period. Of these, 18 had SARS-CoV-2-related AP, 23 had AP with SARS-CoV-2 ‘co-infection’ and 44 had AP with SARS-CoV-2 ‘super-infection’ (Table 1 , Fig. 2 ). The baseline parameters were similar between the 3 groups. SARS-CoV-2-related AP had a higher proportion of milder AP (44.4%) compared with SARS-CoV-2 ‘co-infection’ and ‘super-infection’ groups (21.7% and 0% respectively) (p < 0.0001).
Table 1.
Baseline characteristics of the patients in the 3 COVID positive AP subgroups.
| Parameters | SARS-CoV-2 -related AP (n = 18) | SARS-CoV-2 ‘co-infection’ in AP (n = 23) | SARS-CoV-2 ‘super-infection’ in AP (n = 44) | P value |
|---|---|---|---|---|
| Age (years) Mean ± SD |
42.17 ± 15.2 | 42.52 ± 10.5 | 37.9 ± 10.9 | 0.23 |
| Female, N (Percentage) | 4 (22.2%) | 11 (47.8%) | 12 (27.3%) | 0.14 |
| Any co-morbidity, N (Percentage) | 7 (38.9%) | 7 (30.4%) | 8 (18.2%) | 0.20 |
| Symptomatology | ||||
| Fever, N (Percentage) | 13 (72.2%) | 16 (69.6%) | 36 (81.8%) | 0.47 |
| Cough, N (Percentage) | 5 (27.8%) | 5 (21.7%) | 9 (20.5%) | 0.82 |
| Diarrhea, N (Percentage) | 3 (16.7%) | 1 (4.3%) | 5 (11.4%) | 0.43 |
| Nausea/Vomiting, N (Percentage) | 13 (72.2%) | 20 (87.0%) | 26 (59.1%) | 0.06 |
| Disease Severity | ||||
| Severity of AP, N (Percentage) | ||||
| Mild | 8 (44.4%) | 5 (21.7%) | 0 (0%) | <0.0001 |
| Moderate | 6 (33.3%) | 9 (39.1%) | 25 (56.8%) | |
| Severe | 4 (22.2%) | 9 (39.1%) | 19 (43.2%) | |
| Severity of COVID, N (Percentage) | ||||
| Mild | 8 (44.4%) | 9 (39.1%) | 17 (38.6%) | 0.98 |
| Moderate | 3 (16.7%) | 3 (13.0%) | 7 (15.9%) | |
| Severe | 2 (11.1%) | 6 (26.1%) | 11 (25%) | |
| Critical | 5 (27.8%) | 5 (21.7%) | 9 (20.4%) | |
| Number of SIRS criteria at admission, Mean ± SD |
2.89 ± 1.0 | 2.65 ± 0.83 | 2.80 ± 0.79 | 0.31 |
| BISAP score at admission, Mean ± SD |
2.1 ± 0.99 | 1.95 ± 1.2 | 1.97 ± 0.88 | 0.12 |
| Modified Marshall Score, Mean ± SD | 1.5 ± 1.65 | 1.83 ± 1.52 | 1.48 ± 1.5 | 0.69 |
| APACHE II at admission, Mean ± SD | 11.5 ± 7.0 | 11.7 ± 6.5 | 10.5 ± 5.8 | 0.45 |
| Laboratory parameters | ||||
| Hb (g/dL) Mean ± SD |
9.91 ± 1.9 | 10.20 ± 2.3 | 9.11 ± 2.2 | 0.12 |
| TLC (X 109/L) Mean ± SD |
14.75 (11.2) | 14.3 (7.9) | 15.2 (7.4) | 0.88 |
| D-Dimer# (ng/mL) Median (IQR) |
5687 (6270) | 4242 (3835) | 5597 (4945) | 0.33 |
| Fibrinogen# (g/L) Median (IQR) |
6.43 (2.2) | 4.96 (4.0) | 5.82 (4.4) | 0.41 |
| C-Reactive protein# (mg/L) Median (IQR) |
201.0 (225) | 225.0 (210) | 208.7 (218.7) | 0.71 |
| Ferritin# (ng/mL) Median (IQR) |
699.0 (436.7) | 782.9 (457.8) | 841.6 (685.9) | 0.93 |
# presented as median with interquartile range.
Abbreviations: AP acute pancreatitis; SARS-CoV-2 Severe acute respiratory syndrome coronavirus 2; COVID Coronavirus disease; SIRS Systemic inflammatory response syndrome; BISAP Bedside index of severe acute pancreatitis; APACHE Acute physiology and Chronic health evaluation.
Fig. 2.
Flow diagram showing severity of acute pancreatitis and SARS-CoV-2 infection separately and in-hospital mortality in patients with acute pancreatitis and COVID-19 infection.
i) Clinical course and outcome of COVID positive AP vs. non-COVID AP
The whole cohort of COVID positive AP (n = 85) was compared with age, sex and AP severity matched cohort of non-COVID AP (n = 230). Baseline characteristics of the two groups are given in Table 2 . COVID positive group had higher comorbidities [22 (25.9%) vs. 30 (13.2%); p = 0.007] and greater proportion of patients with SIRS at admission [79 (92.9%) vs. 110 (48.0%); p < 0.0001]. Compared to matched controls, COVID positive AP had higher rates of respiratory failure [56 (65.9% vs. 85 (37.0%), p < 0.0001] and cardiovascular failure [27 (31.8%) vs. 33 (14.4%), p = 0.001] as well as a greater need for non-invasive ventilatory support [5 (5.9%) vs. 10 (4.4%)] and mechanical ventilation [28 (32.9%) vs. 14 (6.1%), p <0.0001]. The in-hospital mortality was significantly higher in the COVID positive AP group compared to the non-COVID AP group [28 (32.9%) vs 44 (19.1%); p = 0.01].
Table 2.
Comparison between COVID positive AP with propensity matched historical cohort of AP∗.
| Parameters | COVID positive AP (n = 85) | Propensity matched Non-COVID AP cohort (n = 230) | Adjusted analysis# aOR (95% CI) |
P value@ |
|---|---|---|---|---|
| Age (years) Mean ± SD |
41.1 ± 13.0 | 40.07 ± 11.9 | 0.54 | |
| Female, N (percentage) | 27 (31.8%) | 73 (31.7%) | 0.99 | |
| Any co-morbidity, N (percentage) | 22 (25.9%) | 30 (13.2%) | 0.007 | |
| Severity of AP, N (percentage) | ||||
| Mild | 13 (15.3%) | 41 (17.8%) | ||
| Moderate | 40 (47.1%) | 96 (41.7%) | 0.68 | |
| Severe | 32 (37.6%) | 93 (40.4%) | ||
| SIRS at admission, N (percentage) | 79 (92.9%) | 110 (48.0%) | <0.0001 | |
| Outcome Parameters | ||||
| ALI, N (percentage) | 56 (65.9%) | 85 (37.0%) | 24.5 (8.6–69.9) | <0.0001 |
| AKI, N (percentage) | 25 (29.4%) | 53 (23.1%) | 2.1 (1.0–4.2) | 0.25 |
| CVSF, N (percentage) | 27 (31.8%) | 33 (14.4%) | 3.8 (1.9–7.6) | 0.001 |
| Multiple OF, N (percentage) | 34 (40.0%) | 55 (24.0%) | 3.8 (1.9–7.7) | <0.0001 |
| Need for ICU stay, N (percentage) | 48 (57.8%) | 85 (37.0%) | 2.9 (1.6–5.4) | 0.001 |
| Hospital stay (days)$ | 14.0 (16.5) | 15 (28.5) | 0.76 | |
| Need for ventilator support - NIV MV |
5 (5.9%) 28 (32.9%) |
10 (4.4%) 14 (6.1%) |
7.5 (3.7–15.3) | <0.0001 |
| Need for Dialysis, N (percentage) | 7 (8.2%) | 14 (6.1%) | 1.6 (0.6–4.5) | 0.50 |
| Interventions for local complications, N (percentage) | 34 (40.0%) | 114 (49.6%) | 0.6 (0.3–1.1) | 0.13 |
| Need for necrosectomy/Surgery, N (percentage) | 11 (12.9%) | 29 (17.3%) | 0.7 (0.3–1.6) | 0.37 |
| In-hospital mortality, N (percentage) | 28 (32.9%) | 44 (19.1%) | 2.8 (1.5–5.3) | 0.010 |
∗Propensity matched (1:4) with age, sex and severity.
@ P value represents the difference in the parameter distribution between the COVID positive and COVID negative group.
#Adjusted for age, sex, comorbidities and severity.
$ represented as median (interquartile range).
Abbreviations: AP acute pancreatitis; COVID Coronavirus disease; aOR adjusted Odds' ratio; SIRS Systemic inflammatory response syndrome; ALI Acute lung injury; AKI Acute kidney injury; CVSF Cardiovascular system failure; OF organ failure; ICU intensive care; NIV non-invasive ventilation; MV mechanical ventilation; CI confidence interval.
On Cox proportional hazard regression analysis, infection with SARS- CoV-2 was a significant predictor of mortality (aHR – 2.30; 95% CI - 1.4-3.8; p = 0.001) even after adjusting for age, sex, presence of any comorbidity and severity of pancreatitis (Supplementary Table 1). A comparative graph of the proportional mortality across time between COVID positive and non-COVID AP is shown in Supplementary Fig. 1.
ii) Clinical course and outcome of SARS-CoV-2-related AP vs. non-COVID AP
SARS-CoV-2 related AP was defined as patients with confirmed SARS-CoV-2 infection developing features of AP within 7 days of onset of symptoms of COVID-19, after excluding other causes of AP. Of these 18 patients with SARS-CoV2 related AP, 15 patients had abdominal pain at presentation, 14 had typical respiratory symptoms of COVID, all had raised pancreatic enzymes and all had imaging evidence of AP. Of the 18 patients with SARS-CoV-2 related AP, 8 (44.4%) had mild, 6 (33.3%) had moderate and 4 (22.2%) had severe AP. Severity of COVID-19 was mild in 8 (44.4%), moderate in 3 (16.7%), severe in 2 (11.1%) and critical in 5 (27.8%) patients.
The cohort of SARS-CoV-2 related AP (n = 18) was compared with a propensity matched cohort of non-COVID AP (n = 59) (Table 3 ). Organ failure rates such as respiratory (61.1% vs. 22.4%; p = 0.002), renal (38.9% vs. 12.1%; p = 0.01) and cardiovascular (44.4% vs 10.3%; p = 0.001) were significantly higher in the SARS-CoV-2 related AP group and so was the need for ICU stay (62.5% vs 20.3%; p = 0.001) and ventilatory support (p < 0.0001). While the need for interventions/surgery was similar, SARS-CoV-2 related AP had higher in-hospital mortality [8(44.4%) vs. 4 (6.8%); p < 0.0001].
Table 3.
Comparison of patients having SARS-CoV-2 -related AP and propensity matched historical non-COVID cohort∗.
| Parameters | SARS-CoV-2 -related AP (n = 18) | Propensity matched Non-COVID AP cohort (n = 59) | Adjusted analysis# aOR (95% CI) |
P value@ |
|---|---|---|---|---|
| Age in years Mean ± SD |
42.17 ± 15.2 | 39.93 ± 16.3 | 0.607 | |
| Any co-morbidity, N (percentage) | 7 (38.9%) | 11 (18.6%) | 0.076 | |
| SIRS at admission, N (Percentage) | 16 (88.9%) | 19 (32.8%) | <0.0001 | |
| ALI, N (Percentage) | 11 (61.1%) | 13 (22.4%) | – | 0.002 |
| AKI, N (Percentage) | 7 (38.9%) | 7 (12.1%) | 8.8 (1.8–43.7) | 0.010 |
| CVSF, N (Percentage) | 8 (44.4%) | 6 (10.3%) | 14.5 (2.6–80.7) | 0.001 |
| Multiple OF, N (Percentage) | 9 (50.0%) | 7 (12.1%) | 23.2 (3.5–151.8) | 0.002 |
| Need for ICU stay, N (Percentage) | 10 (62.5%) | 12 (20.3%) | 40.6 (3.9–419.5) | 0.001 |
| Hospital stay (days)$ | 11.5 (8.0) | 10 (21.5) | 0.823 | |
| Need for ventilator support NIV MV |
1 (5.6%) 7 (38.9%) |
2 (3.4%) 0 (0%) |
54.5 (5.1–576.5) | <0.0001 |
| Need for Dialysis, N (Percentage) | 2 (11.1%) | 1 (1.7%) | – | 0.138 |
| Interventions for local complications, N (Percentage) | 3 (16.7%) | 23 (39.0%) | 0.2 (0.0–1.2) | 0.095 |
| Need for necrosectomy/Surgery, N (Percentage) | 1 (5.6%) | 5 (11.4%) | 0.7 (0.0–12.2) | 0.66 |
| In- hospital mortality, N (Percentage) | 8 (44.4%) | 4 (6.8%) | 29 (4.4–192.3) | <0.0001 |
∗Propensity matched (1:4) with age, sex and severity (Atlanta).
@ P value represents the difference in the parameter distribution between the COVID positive and COVID negative group.
#Adjusted for age, sex, comorbidities and severity (Atlanta).
$ represented as median (interquartile range).
Abbreviations: AP acute pancreatitis; SARS-CoV-2 Severe acute respiratory syndrome coronavirus 2; COVID Coronavirus disease; aOR adjusted Odds' ratio; ALI Acute lung injury; AKI Acute kidney injury; CVSF Cardiovascular system failure; OF organ failure; ICU intensive care unit; NIV non-invasive ventilation; MV mechanical ventilation.
On Cox proportional hazard regression analysis, being infected with SARS-CoV-2 was the most significant predictor of mortality (aHR – 16.02; 95% CI - 3.87 – 66.28) even after adjusting for age, sex, presence of comorbidities and severity of pancreatitis (Supplementary Table 2). A comparative graph of the proportional mortality across time between SARS-CoV-2 related AP and its propensity matched non-COVID AP cohort is shown in Supplementary Fig. 2 .
iii) Clinical course and outcome of SARS-CoV-2 related AP vs. superadded SARS CoV-2 in AP
On comparing SARS-CoV-2 related AP (n = 18) with AP patients having either co- or super- SARS CoV2 infection (n = 67) (Supplementary Table 3), it was found that both the groups had similar organ failure rates and need for organ support. However, superadded SARS-CoV-2 infection group had a longer hospital stay (12.3 ± 6.1 vs. 22.2 ± 17 days; p < 0.0001) and more interventions (16.7% vs 46.3%; p = 0.03) compared to the SARS-CoV-2 related AP group. SARS-CoV-2 related AP had similar in-hospital mortality (44.4%) compared to the superadded SARS-CoV-2 infection group (29.9%) (p = 0.24). Differentiation of respiratory failure due to AP and SARS-CoV-2: Among 41 patients with AP and concomitant SARS-CoV-2 infection (18 patients with SARS-CoV-2 related AP and 23 patients with AP plus co-infection), 25 had respiratory failure. Of them, 10 had typical and 9 had suggestive features of COVID-19 pneumonia on a chest CT scan, and they were treated with steroids. Respiratory failure was considered to be due to severe AP in the remaining 6 patients.
iv) Clinical Course and outcome of AP with superadded SARS-CoV-2 infection vs. non COVID AP
On comparing patients with AP with superadded SARS-CoV-2 infection (n = 67) with a propensity matched cohort of non-COVID AP (n = 176), the former had higher rates of organ failure (respiratory: 67.2% vs. 42.6%; p = 0.001 and cardiovascular failure: 28.4% vs. 16.5%; p = 0.04) and higher proportion of patients with multi-organ failure (37.3% vs. 29.0%; p = 0.005) (Supplementary Table 4). These patients also had a greater need for ICU stay (56.7% vs. 42.6%; p = 0.049) and mechanical ventilation (31.3% vs. 8.0%; p < 0.0001) compared to non-COVID AP cohort. Superadded SARS-CoV-2 infection group had a higher in-hospital mortality (29.9%) than non-COVID controls (22.7%) but the difference was not statistically significant (p = 0.25).
v) Impact of timing of SARS-CoV-2 infection on the course of AP
‘Co-infection’ was defined as development of COVID positivity within 14 days of onset of AP, while those having SARS-CoV-2 infection beyond 14 days were termed as having ‘super-infection’. The two groups of SARS-CoV-2 ‘co-infection’ in AP (n = 23) and SARS CoV-2 ‘super-infection’ in AP (n = 44) were compared to study the impact of timing of infection on the course of AP (Supplementary Table 5). Though the occurrence of organ failure and need for ICU stay were similar between the two groups, hospital stay was longer (26.4 ± 18.9 vs. 14.4 ± 10.5; p = 0.001) and the need for interventions was higher (59.1% vs 21.7%, p = 0.005) in the ‘super-infection’ group compared to the ‘co-infection’ group. The in-hospital mortality was similar among the two groups [8 (34.8%) in co-infection group vs. 12 (27.3%) in the superinfection group, p = 0.52].
vi) Impact of SARS-CoV-2 infection on mortality in patients with different grades of severity of AP
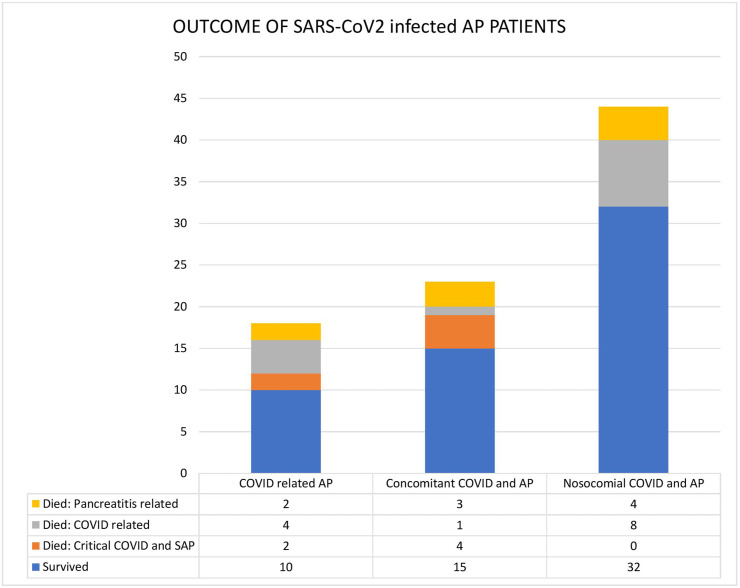
The mortality in the 4 groups of AP (SARS-CoV-2 related, SARS-CoV-2 ‘co-infection’, SARS-CoV-2 ‘super-infection’ and non-COVID AP) was compared based on the severity of AP (Table 4 , Fig. 3 ). COVID-related AP had 1 mortality even in the mild AP group secondary to critical COVID infection. In the sub-cohort of moderately severe AP, the mortality rate increased as the impact of SARS-CoV-2 infection increased. Thus, SARS-CoV-2 related AP group had the highest mortality of 66.7% (4/6) followed by SARS-CoV-2 ‘co-infection’ at 44.4% (4/9), and the super-infection group at 16%. The lowest mortality was noted in the non-COVID AP group at 8.3% (8/96) and the difference was statistically significant (p < 0.0001).
Table 4.
Mortality comparison based on the severity of pancreatitis.
| SARS-CoV-2 related AP | SARS-CoV-2 ‘co-infection’ in AP | SARS-CoV-2 ‘super-infection’ in AP | Non-COVID AP | P value | |
|---|---|---|---|---|---|
| Mild AP | 1/8 (12.5%) | 0/5 (0%) | – | 0/41 (0%) | 0.05 |
| Moderately Severe AP | 4/6 (66.7%) | 4/9 (44.4%) | 4/25 (16%) | 8/96 (8.3%) | <0.0001 |
| Severe AP | 3/4 (75%) | 4/9 (44.4%) | 8/19 (42.1%) | 36/93 (38.7%) | 0.54 |
Abbreviations: AP acute pancreatitis; COVID Coronavirus disease; SARS-CoV-2 Severe acute respiratory syndrome coronavirus 2.
Fig. 3.
Bar diagram showing in-hospital mortality in 3 subgroups of acute pancreatitis and SARS-CoV-2 infection: COVID-related AP, SARS-CoV-2 co-infection in AP (concomitant) and SARS-CoV-2 superinfection in AP (nosocomial).
4. Discussion
Patients with AP and SARS-CoV-2 infection represent a ‘dual disease’ model. In the present multicenter prospective study, we have systematically examined the interactions between SARS-CoV-2 infection and AP. We have (a) defined and clarified the clinical conundrum of SARS-CoV-2 related AP and SARS-CoV-2 superimposed on AP due to other etiologies, and (b) dissected the relative contribution of SARS-CoV-2 infection and AP to clinically relevant outcomes in patients with the ‘dual disease’.
The association of SARS-CoV-2 and AP has been described previously, but many aspects of this association are not well understood. ‘Cytokine storm’ has been thought to be the key event perpetuating organ failure both in AP and severe SARS-CoV-2 infection [1,18]. Whether the simultaneous occurrence of both diseases produces a synergistic effect is not clear. Two retrospective series have compared COVID positive AP with non-COVID AP. While one study showed significantly greater proportion of persistent organ failure (57% vs. 8%) and mortality (21% vs 2%) among COVID-positive AP [19], the other study failed to show any increased mortality, although COVID-positive patients had a higher requirement of mechanical ventilation compared with COVID-negative AP patients (28.1% vs 6.4%) [20]. These studies were however, limited by their retrospective design, small sample size and lack of adjustment for important confounding factors affecting mortality such as severity of AP. In the present study, we found COVID-positive AP patients to have significantly higher OF and in-hospital mortality rates compared with non-COVID AP patients. A multicenter prospective cohort study [21] also showed similar findings with a higher risk of persistent OF and 30-days mortality in patients with SARS-CoV-2 infection and AP. However, that study did not provide details about the timing of SARS-CoV-2 infection, severity of COVID-19, and development of organ failure.
We have defined SARS-CoV-2 related AP in the present study. Any patient, with confirmed SARS-CoV-2 infection, who developed features of AP within 7 days of onset of the COVID symptoms and without any other cause of AP was termed as having SARS-CoV-2 related AP. A few case series have highlighted an increase in the proportion of idiopathic cause of AP in SARS-CoV-2 infected AP patients, which varied from 25% to 69% suggesting SARS-CoV-2 as the likely etiology [[19], [20], [21]]. It is difficult to assign SARS-CoV-2 infection as a definite etiology of acute pancreatitis. In case of other viruses such as mumps, coxsackie and hepatitis viruses, the etiology of AP is considered to be due to the virus based on 2 important factors: (i) temporal relationship of AP during the course of the primary viral illness, and (ii) absence of a documented etiology of AP such as alcohol and gallstones. We also considered the diagnosis of SARS-CoV2 related AP based on these 2 factors. In the absence of a proven cause and effect relationship, we have used the term to ‘SARS-CoV-2 related AP’ instead of ‘SARS-CoV-2 induced AP’. An increased proportion of ‘idiopathic’ AP has been reported among SARS-CoV-2 infected patients and it has been suggested that it could be virus-induced, and hence might have been wrongly classified as “idiopathic” [20]. However, none of the previous studies has studied the temporal correlation between the onset of AP and onset of COVID symptoms to be able to define the entity of “SARS-CoV-2 related AP” or study its clinical course. Virus-induced pancreatitis such as due to mumps, coxsackie and hepatitis viruses is usually mild, and systemic features of the viral illness predominate and dictate the clinical course rather than the pancreatic injury per se. A few cases of necrotizing pancreatitis, although, have also been reported [23,24]. On the contrary, SARS-CoV-2 related AP, in our study, had worse outcomes with more OF and higher mortality than the severity matched non-COVID AP cohort. This was attributed to the severity of the COVID-19 disease as pancreatitis severity was mild in around half of these cases. Thus, involvement of organs (e.g., pancreas) other than the primary organ for which the virus has a tropism (i.e., lung for SARS-CoV-2) may be indicative of a severe disease.
The complexity of this ‘dual disease’ model lies in unraveling the relative contribution of AP and SARS-CoV-2 infection to mortality. While OF defines the severity of both AP and COVID-19, it is difficult to delineate which of the two is the chief contributor to the severity in a particular patient. We analyzed every individual patient with AP plus SARS-CoV-2 infection and distinguished the lung involvement between the two disease entities based on typical imaging findings on the chest CT scan. While SARS-CoV-2 infection exhibits ground-glass opacities, vascular enlargement, lower lobe involvement and posterior predilection, AP predominantly has vascular leak syndrome, non-cardiogenic pulmonary edema, pleural effusion and/or consolidation as the cause(s) of respiratory failure and hence lacks those typical imaging findings of COVID-19 [25,26]. Earlier studies did not make this critical distinction and thus, the increased severity of AP reported [21] among COVID-positive cases could have been due to severe COVID rather than being classified as severe AP. We specifically studied this aspect and used the clinico-radiological parameters to classify the severity of AP and COVID separately, and analyzed their relative impact on the outcome, e.g., we had a case of mild AP who expired due to critical COVID, which otherwise might have been misclassified as severe AP. This enabled us to identify the key player controlling the outcome in this “dual-disease” model, rather than using blanket severity scores. This distinction is crucial for a treating physician as specific therapy e.g., steroids can be guided by individual severity of COVID and AP. The only caveat being that in patients with COVID pneumonia, it is difficult to determine the contribution of AP to respiratory failure, if any.
Patients with moderate and severe pancreatitis are at risk of acquiring nosocomial SARS-CoV-2 infection during an ongoing epidemic due to prolonged hospital stay [16]. SARS-CoV-2 co-infection/superinfection is of concern and its effect on disease course is not well known. In our study, superadded SARS-CoV-2 infection led to a significantly higher rate of respiratory failure compared to matched AP cohort without SARS-CoV-2 infection. This reiterates the fact that SARS-CoV-2 infection, when superadded to an ongoing AP, adversely affects its outcome with a synergistic effect.
AP being a systemic illness with an initial phase of systemic inflammation (SIRS) followed by compensatory anti-inflammatory syndrome (CARS) phase, virus-specific host responses may affect its clinical course [1,16,27]. Host response to the virus is expected to be different depending on the phase of AP in which the infection occurs. To study this hypothesis, we compared the two sub-groups of the superadded SARS-CoV-2 infection cohort, i.e., ‘co-infection’ and ‘super-infection’. ‘Co-infection’ was considered when onset of AP and COVID positivity detection was within 14 days of each other, while beyond that it was termed as ‘super-infection’. This 14-day cut-off was chosen to account for the incubation period of SARS-CoV-2. A recent study has shown that concomitant COVID-19 and AP at admission had a benign course [28].
Interestingly, there was no difference in organ failure and mortality between patients who had early or late super-added SARS-CoV-2 infection. This can be explained by the pathogenetic interaction of this “dual disease” with respect to time. In the initial 14 days of AP and SARS-CoV-2 ‘co-infection’, the cytokine storm generated by the two diseases have a synergistic adverse effect on the clinical course. During the later phase of AP (beyond 14 days) in hospitalized patients, infective complications e.g., infected necrotizing pancreatitis are known to set in and a ‘super-infection’ with SARS-CoV-2 infection, at this stage, further worsens the clinical picture. Thus, any superadded infection, be it “co-infection” or “super-infection” is equally detrimental for the course of AP, as is evident from our study findings that the whole cohort of superadded SARS-CoV-2 infection with AP fared worse when compared to its non-COVID counterpart.
The strengths of our study include (a) a prospective study design, (b) systematically defined the various entities of SARS-CoV-2 related AP, SARS-CoV-2 ‘co-infection’ and ‘super-infection’ and studied their individual effects on the disease course; (c) selection of a comparative group of patients with AP before the onset of COVID-19 pandemic, thus practically excluding SARS-CoV-2 infection in them; (d) propensity matching to adjust for confounders that could affect mortality; and (e) differentiated the severity of the two components of the ‘dual disease’ to assess the relative contribution of each to the overall outcome. Our study has a few limitations: (a) we defined SARS-CoV-2 related pancreatitis based on the temporal association with COVID-19 specific symptoms and absence of other etiologies. Although this definition might suffice clinically, demonstration of the virus in the pancreatic tissue is required to provide additional evidence of causation; (b) we might have missed some patients with COVID-19 and AP as we used RT-PCR test for the diagnosis, which has a false negativity rate of 2–29% [29]; and (c) the possibility of a delayed diagnosis of SARS-CoV-2 ‘superinfection’ in some cases cannot be ruled out, which might have precluded the timely initiation of COVID specific therapy.
In summary, patients with AP and SARS-CoV-2 infection have a higher mortality and organ failures than matched controls. The excess mortality is predominantly attributable to the severity of COVID-19. A clear distinction between the severity of COVID and AP is key to decipher the dynamics of the interaction and thus predict the outcome in patients with the ‘dual disease’. Superadded SARS-CoV-2 infection complicates the clinical trajectory of AP, irrespective of the timing of infection with worse outcomes. Given the possibility of the prolonged SARS-CoV-2 pandemic in the near future due to emergence of variants of concern, patients with idiopathic AP should be investigated for SARS-CoV-2 for better informed management decisions and reduce mortality [30].
Grant support
Indian Council of Medical Research, India.
Author contributions
JS and SJM: Conception and design; data acquisition; data analysis and interpretation, drafting the work; final approval.
NK, JD, AE, AG, AD, RR, RG, SB, AVP, RB, MV, SP, JY, SKM, SA, VB, TV, VT, RS: Data acquisition, data interpretation, critical review; final approval.
VS, AB, GDP, UD: Data interpretation, critical review, final approval.
MP, AD: Data analysis; data interpretation; critical review; final approval.
RK; PKG: Conception and design; data interpretation; critical review and intellectual input; final approval.
All authors have approved the final version of the manuscript.
Declaration of competing interest
All the authors declare no potential conflicts of interest.
Acknowledgement
The Graphical abstract was made using Biorender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pan.2022.01.008.
Contributor Information
on behalf of GAIN Study group:
Aditya Vikram Pachisia, Anany Gupta, Anshuman Elhence, Anugrah Dhooria, Aritra Das, Ashish Bhalla, Goverdhan Dutt Puri, Jahnvi Dhar, Jatin Yegurla, Jayanta Samanta, Manas Vaishnav, Manya Prasad, Naveen Kumar, Pramod Kumar Garg, Rahul Sethia, Rajat Bansal, Randeep Rana, Rakesh Kochhar, Rithvik Golla, Sagnik Biswas, Sandeep Kumar Mundhra, Samagra Agarwal, Shubham Prasad, and Soumya Jagannath Mahapatra
Appendix A. Supplementary data
The following are the supplementary data to this article:
Comparison of proportional mortality across time between COVID positive and COVID negative AP patients
Comparison of proportional mortality across time between COVID-related AP and propensity matched non-COVID AP cohort
References
- 1.Garg P.K., Singh V.P. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. 2019;156:2008–2023. doi: 10.1053/j.gastro.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padhan R.K., Jain S., Agarwal S., Harikrishnan S., Vadiraja P., Behera S., et al. Primary and secondary organ failures cause mortality differentially in acute pancreatitis and should be distinguished. Pancreas. 2018;47:302–307. doi: 10.1097/MPA.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 3.Schepers N.J., Bakker O.J., Besselink M.G., Ahmed Ali U., Bollen T.L., Gooszen H.G., et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68:1044–1051. doi: 10.1136/gutjnl-2017-314657. [DOI] [PubMed] [Google Scholar]
- 4.WHO coronavirus (COVID-19) Dashboard n.d. https://covid19.who.int (accessed June 27, 2021).
- 5.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome : results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microb. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parenti D.M., Steinberg W., Kang P. Infectious causes of acute pancreatitis. Pancreas. 1996;13:356–371. doi: 10.1097/00006676-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Rawla P., Bandaru S.S., Vellipuram A.R. Review of infectious etiology of acute pancreatitis. Gastroenterol Res. 2017;10:153–158. doi: 10.14740/gr858w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samanta J., Gupta R., Singh M.P., Patnaik I., Kumar A., Kochhar R. Coronavirus disease 2019 and the pancreas. Pancreatology. 2020;20:1567–1575. doi: 10.1016/j.pan.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller J.A., Groß R., Conzelmann C., Krüger J., Merle U., Steinhart J., et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3:149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 12.Kusmartseva I., Wu W., Syed F., Van Der Heide V., Jorgensen M., Joseph P., et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metabol. 2020;32:1041–1051. doi: 10.1016/j.cmet.2020.11.005. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.F L., X L., B Z W.Z., X C Z.Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol : Off Clin Pract J Am Gastroenterol Assoc. 2020;18 doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F., Wang H., Fan J., Zhang Y., Wang H., Zhao Q. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology. 2020;159:367–370. doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNabb-Baltar J., Jin D.X., Grover A.S., Redd W.D., Zhou J.C., Hathorn K.E., et al. Lipase elevation in patients with COVID-19. Am J Gastroenterol. 2020;115:1286–1288. doi: 10.14309/ajg.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elhence A., Mahapatra S.J., Vajpai T., Garg P.K. Acute pancreatitis and nosocomial COVID-19: cause specific host responses may determine lung injury. Pancreatology. 2020;20:1258–1261. doi: 10.1016/j.pan.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samanta J., Singh S., Arora S., Muktesh G., Aggarwal A., Dhaka N., et al. Cytokine profile in prediction of acute lung injury in patients with acute pancreatitis. Pancreatology. 2018;18:878–884. doi: 10.1016/j.pan.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dirweesh A., Li Y., Trikudanathan G., Mallery J.S., Freeman M.L., Amateau S.K. Clinical outcomes of acute pancreatitis in patients with coronavirus disease 2019. Gastroenterology. 2020;159:1972–1974. doi: 10.1053/j.gastro.2020.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inamdar S., Benias P.C., Liu Y., Sejpal D.V., Satapathy S.K., Trindade A.J., et al. Prevalence, risk factors, and outcomes of hospitalized patients with coronavirus disease 2019 presenting as acute pancreatitis. Gastroenterology. 2020;159:2226–2228. doi: 10.1053/j.gastro.2020.08.044. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandanaboyana S., Moir J., Leeds J.S., Oppong K., Kanwar A., Marzouk A., et al. SARS-CoV-2 infection in acute pancreatitis increases disease severity and 30-day mortality: COVID PAN collaborative study. Gut. 2021 doi: 10.1136/gutjnl-2020-323364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banks P.A., Bollen T.L., Dervenis C., Gooszen H.G., Johnson C.D., Sarr M.G., et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 23.Feldstein J.D., Johnson F.R., Kallick C.A., Doolas A. Acute hemorrhagic pancreatitis and pseudocyst due to mumps. Ann Surg. 1974;180:85–88. doi: 10.1097/00000658-197407000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witte C.L., Schanzer B. Pancreatitis due to mumps. JAMA. 1968;203:1068–1069. [PubMed] [Google Scholar]
- 25.Adams H.J.A., Kwee T.C., Yakar D., Hope M.D., Kwee R.M. Chest CT imaging signature of coronavirus disease 2019 infection: in pursuit of the scientific evidence. Chest. 2020;158:1885–1895. doi: 10.1016/j.chest.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng R., Zhang L., Zhang Z.-M., Wang Z.-Q., Liu G.-Y., Zhang X.-M. Chest computed tomography semi-quantitative pleural effusion and pulmonary consolidation are early predictors of acute pancreatitis severity. Quant Imag Med Surg. 2020;10:451–463. doi: 10.21037/qims.2019.12.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasada R., Muktesh G., Samanta J., Sarma P., Singh S., Arora S.K., et al. Natural history and profile of selective cytokines in patients of acute pancreatitis with acute kidney injury. Cytokine. 2020;133:155177. doi: 10.1016/j.cyto.2020.155177. [DOI] [PubMed] [Google Scholar]
- 28.Kumar V., Barkoudah E., Souza D.A.T., Jin D.X., McNabb-Baltar J. Clinical course and outcome among patients with acute pancreatitis and COVID-19. Eur J Gastroenterol Hepatol. 2021;33:695–700. doi: 10.1097/MEG.0000000000002160. [DOI] [PubMed] [Google Scholar]
- 29.Prokop M., van Everdingen W., van Rees Vellinga T., Quarles van Ufford H., Stöger L., Beenen L., et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology. 2020;296:E97–E104. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal S., George J., Padhan R.K., et al. Reduction in mortality in severe acute pancreatitis: a time trend analysis over 16 years. Pancreatology. 2016;16:194–199. doi: 10.1016/j.pan.2016.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of proportional mortality across time between COVID positive and COVID negative AP patients
Comparison of proportional mortality across time between COVID-related AP and propensity matched non-COVID AP cohort