Abstract
Aim
The clinical impact of abdominal aortic calcification (AAC) in patients who undergo hepatectomy for hepatocellular carcinoma (HCC) is unknown.
Methods
To evaluate the impact of AAC on clinical outcomes, we analyzed 203 patients who underwent hepatectomy for HCC between 2010 and 2013.
Results
Kaplan–Meier survival curve analysis showed significantly worse overall survival (OS) in the high AAC group than in the low AAC group. The recurrence‐free survival (RFS) was also significantly worse in the high AAC group. In the multivariate analysis, high AAC (hazard ratio [HR], 2.51; 95% confidence interval [CI], 1.24–5.09; P = .01) was an independent risk factor for poor OS after hepatectomy for HCC. High AAC was also an independent risk factor for poor RFS (HR, 1.69; 95% CI, 1.04–2.76; P = .04).
Conclusions
Abdominal aortic calcification had a strong relationship with poor OS and RFS after hepatectomy for HCC. We suggest that AAC had a relationship with smoking and diabetes; therefore, AAC could reflect a surrogate for older age, worse health status, and inflammatory score.
Keywords: calcification, HCC, prognosis
Kaplan–Meier survival curve analysis showed significantly worse overall survival in the high abdominal aortic calcification (AAC) group than in the low AAC group.

1. INTRODUCTION
Hepatocellular carcinoma (HCC) is associated with high rates of recurrence even after curative resection. The systemic inflammation status could be useful to predict the prognosis of HCC patients after hepatectomy. 1 Inflammatory cells such as macrophages and T‐lymphocytes present in arteriosclerotic lesions are known to be involved in the progression of vascular calcification. 2 Abdominal aortic calcification (AAC) is easily evaluated on abdominal computed tomography (CT) as a marker to reflect arteriosclerosis. 3 , 4 , 5 , 6 We have previously reported that high AAC was significantly correlated with poor prognosis and systemic inflammation status among liver transplantation (LT) patients. 7 Further, the presence of systemic inflammation is associated with worse malnutrition and lower preoperative immunocompetence, influencing the risk of infections and antitumor activity. 8 , 9
This study aimed to analyze the associations between AAC and HCC recurrence after initial hepatectomy. We hypothesized that AAC related to arteriosclerosis and systemic inflammation status would reduce immunocompetence and accelerate the recurrence of HCC after hepatectomy.
2. PATIENTS AND METHODS
2.1. Patients
We evaluated HCC patients who underwent initial hepatectomy in our institute between 2010 and 2013. Of them, those with pathological coagulative necrosis (n = 19) were excluded. Thus, 203 patients were included in the analysis. Follow‐up for patients was 5 years or until time of death, with a median follow‐up of 6.08 years. Death was treated like a competing event. The indication and procedure for hepatectomy were as described previously. 10 , 11 , 12 Clinicodemographic data at the time of hepatectomy, including age, gender, Child–Pugh classification, hepatitis B surface antigen, hepatitis C virus antibody, statin use, smoking, hypertension, diabetes, blood examination data, surgical data (operation time, bleeding volume, etc), pathological findings, and tumor markers (eg, des‐gamma‐carboxy protein [DCP] and serum a‐fetoprotein [AFP]) were obtained from electronic records. The rates of HCC recurrence (recurrence of the primary tumor and multicentric carcinogenesis), extrahepatic recurrence, and long‐term survival after operation were also obtained from clinical records. The recurrence of the primary tumor was defined as intrahepatic recurrence in nearby residual liver within 2 years after the initial hepatectomy. After hepatectomy, patients were followed up using ultrasonography, contrast‐enhanced CT, or magnetic resonance imaging, combined with an evaluation of serum AFP and DCP levels at 3‐mo intervals for up to 3 years and at 6‐mo intervals for up to 5 years thereafter. The diagnosis was histologically confirmed when necessary.
2.2. Aortic abdominal calcification
CT angiographies were performed on a 320‐detector row CT scanner (Aquilion ONE ViSION, Toshiba Medical Systems, Tokyo, Japan) using a standardized examination protocol. The AAC score was calculated using AZE VirtualPlace Lexus64 Anatomia software (AZE, Schaumberg, IL). Using the Agatston method, 13 the AAC volume was automatically calculated for calcifications located in the abdominal aorta (from the origin of the renal artery to the iliac bifurcation) with attenuation greater than the predefined 130 Hounsfield units. Patients were divided into two groups according to the AAC level at a cutoff of 250 mm3, low ACC (<250 mm3; N = 53) and high ACC (≥250 mm3; N = 150) groups, using the receiver operating characteristic (ROC) curves of the overall survival (OS).
2.3. Statistical analysis
The OS and the recurrence‐free survival (RFS) were plotted using Kaplan–Meier analysis and compared using log‐rank statistics. The multivariate analyses for the variables independently related to the OS and the RFS using the Cox proportional hazard model were carried out. Univariate and multivariate Cox regression analyses were performed to assess the association of the OS and the RFS with all the variables: age, gender, hepatitis virus, AAC, total bilirubin levels, alanine aminotransferase (ALT), prothrombin time (PT), indocyanine green clearance test (ICG‐R15), operation time, blood loss, DCP, AFP, Child–Pugh, anatomical hepatectomy, the type of hepatectomy, number of tumor, tumor size, poorly differentiated, vascular invasion, and serosal invasion. All variables were included in the multivariate models and the backward elimination method with removal criterion P = .05 was used to select covariates. All statistical analyses were performed using JMP statistical software (JMP 14; SAS Institute, Cary, NC). P values <.05 were considered statistically significant.
3. RESULTS
3.1. Patient characteristics
Compared to the low AAC group, the high AAC group had a significantly lower proportion of patients with virus hepatitis, lower AFP levels, larger bleeding volume, and older age. The high AAC group has a high proportion of smokers, and patients with diabetes and high HbA1c levels. However, there were no significant differences in the oncological factors, including tumor markers and pathological findings. The clinical characteristics of the patients in each group are summarized in Table 1.
TABLE 1.
Patient characteristics and surgical procedures
|
Low AAC group N = 53 |
High AAC group N = 150 |
P‐value | |
|---|---|---|---|
| Male/female | 37/16 | 120/30 | .13 |
| Age (y), median (range) | 64 (31–84) | 73 (47–91) | <.01 |
| Child–Pugh classification A/B/C | 50/3/0 | 140/10/0 | 1.00 |
| HCC etiology Nonvirus/HCV/HBV | 9/22/22 | 57/77/16 | <.01 |
| Total bilirubin (mg/dL), median (range) | 0.7 (0.2–2.9) | 0.7 (0.3–2.2) | .87 |
| Smoking Y/N | 24/29 | 96/54 | .02 |
| Hypertension Y/N | 15/38 | 60/90 | .13 |
| Diabetes Y/N | 9/44 | 56/94 | <.01 |
| ALT (IU/L),,median (range) | 31 (10–148) | 32 (10–204) | .97 |
| Albumin (mg/dL),,median (range) | 4.1 (2.5–5.2) | 4.0 (2.3–5.4) | .38 |
| ICGR‐15 (%),,median (range) | 10 (3–66) | 14.3 (3.5–79.1) | <.01 |
| PT (%), median (range) | 87 (13.1–119) | 87 (24–116) | .44 |
| DCP (ng/mL), median (range) | 39 (5–137 910) | 81 (0–223 940) | .14 |
| AFP (mAU/mL), median (range) | 20.5 (0.5–26 170) | 6.7 (0.5–25 230) | .01 |
| Major hepatectomy Y/N | 5/48 | 23/127 | .28 |
| Total cholesterol (mg/dL), median (range) | 170 (110–312) | 166 (101–346) | .70 |
| Triglyceride (mg/dL), median (range) | 88 (42–196) | 96 (28–370) | .09 |
| HbA1c (%), median (range) | 5.3 (4.1–8.9) | 5.7 (4.3–11.2) | <.01 |
| Anatomical hepatectomy Y/N | 20/33 | 37/113 | .07 |
| Operation time (min), median (range) | 288 (161–695) | 299 (116–760) | .42 |
| Blood loss (mL), median (range) | 210 (30–3000) | 350 (20–2750) | .03 |
| Differentiation Poor/moderate/high | 9/36/8 | 11/121/18 | .09 |
| Vascular invasion Y/N | 8/45 | 31/119 | .37 |
| Serosal invasion Y/N | 8/45 | 20/130 | .75 |
| Number of tumor, median (range) | 1 (1–20) | 1 (1–11) | .97 |
| Tumor size (mm), median (range) | 24 (10–130) | 28 (7–335) | .31 |
Abbreviations: ACC, abdominal aortic calcification; AFP, a‐fetoprotein; ALT, alanine aminotransferase; DCP, des‐gamma‐carboxy protein; HCC, hepatocellular carcinoma; HCV, hepatitis virus type C; ICGR‐15, indocyanine green clearance test; PT, prothrombin time.
3.2. Overall outcomes of all patients
The observed period in the low AAC group was longer than that in high AAC group (median: 7.42 years vs 5.49 years). Kaplan–Meier survival curve analysis showed significantly worse OS in the high AAC group than in the low AAC group (P < .01, Figure 1). The RFS was also significantly worse in the high AAC group than in the low AAC group (P < .01, Figure 2). We summarize the causes of death in Table 2. In both groups, the main cause of death was HCC‐related (five in the low AAC group and 38 in the high AAC group). The rate of HCC‐related death was significantly higher in the high AAC group than that in the low AAC group (25.3% vs 9.4%, P = .02). Meanwhile, the frequency of cardiovascular disease (CVD)‐related death was similar between the two groups.
FIGURE 1.
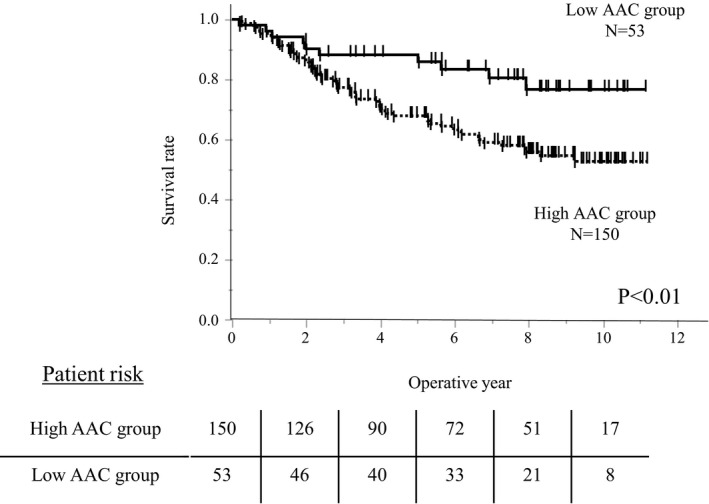
Kaplan–Meier survival curve according to the abdominal aortic calcification (AAC) levels. Kaplan–Meier survival curve analysis showed significantly worse overall survival in the high AAC group than in the low AAC group
FIGURE 2.
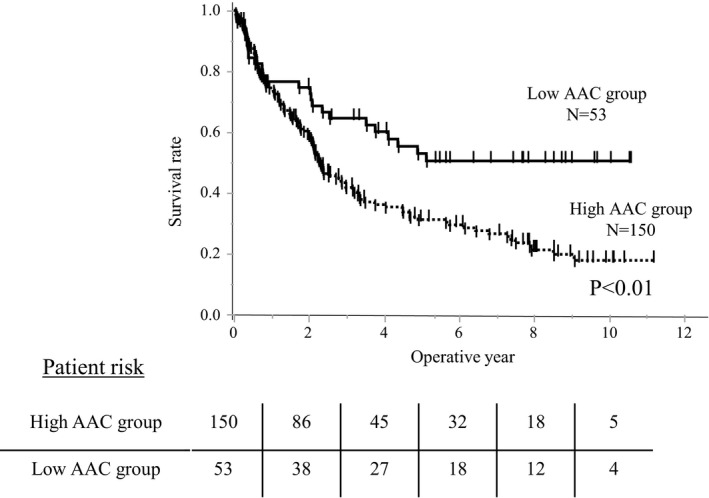
The recurrence‐free survival (RFS) curve according to the abdominal aortic calcification (AAC) levels. The RFS was significantly higher in the high AAC group than in the low AAC group
TABLE 2.
Causes of death summarized
| Cause of death |
Low AAC group N = 53 |
High AAC group N = 150 |
P‐value |
|---|---|---|---|
| Liver failure | 2 (3.8%) | 8 (5.3%) | 1.00 |
| HCC‐related death | 5 (9.4%) | 38 (25.3%) | .02 |
| CVD | 1 (1.9%) | 2 (1.3%) | 1.00 |
| Other cancer‐related death | 0 (0%) | 8 (5.3%) | .11 |
| Others | 2 (3.8%) | 4 (2.7%) | .65 |
Abbreviations: ACC, abdominal aortic calcification; CVD, cardiovascular disease; HCC, hepatocellular carcinoma.
The high AAC group had 92 intrahepatic recurrences (61.3%, N = 150), and the low AAC group had 20 intrahepatic recurrences (37.7%, N = 53). In the high AAC group, the proportion of primary tumor and multicentric carcinogenesis recurrences were 37/92 (40.2%) and 5/92 (59.8%), respectively. The proportions in the low AAC group were 9/20 (45.0%) and 11/20 (55.0%), respectively. According to these results, the intrahepatic recurrence types were similar, and a high ACC value promoted both types of recurrence.
3.3. Factors associated with overall survival and recurrence
Univariate analysis revealed that higher AAC, higher total bilirubin, lower albumin, higher ICG‐R15, longer operation time, larger blood loss, higher AFP levels, major hepatectomy, multiple tumors, tumor diameter ≥40 mm, poorly differentiated HCC, and vascular invasion were predictive factors for OS. In the multivariate analysis, high AAC (hazard ratio [HR], 2.51; 95% confidence interval [CI], 1.24–5.09; P = .01), lower albumin levels (HR, 1.98; 95% CI, 1.21–3.22; P < .01), larger blood loss (HR, 1.64; 95% CI, 1.01–2.67; P = .04), higher AFP (HR, 2.49; 95% CI, 1.48–4.17; P < .01), larger tumor size (HR, 1.92; 95% CI, 1.17–3.15; P < .01), and multiple tumors (HR, 2.15; 95% CI, 1.30–3.57; P < .01) were independent predictive factors for the OS. Table 3 shows the risk factors of poor OS.
TABLE 3.
Risk factors for overall survival
| Factors | N = 203 | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| 5Y‐survival | P value | HR | 95% CI | P value | ||
| Age (y) | ||||||
| ≥65 | 129 | 72.3% | .21 | |||
| <65 | 74 | 74.5% | ||||
| Gender | ||||||
| Male | 157 | 73.6% | .62 | |||
| Female | 46 | 72.1% | ||||
| Hepatitis virus | ||||||
| Nonvirus | 66 | 68.9% | .14 | |||
| HCV antibody | 99 | 72.6% | ||||
| HBV antigen | 38 | 81.2% | ||||
| AAC (mm3) | ||||||
| ≥250 | 150 | 67.9% | <.01 | 2.51 | 1.24‐5.09 | .01 |
| <250 | 53 | 88.2% | ||||
| Total bilirubin (mg/dL) | ||||||
| ≥1.5 | 11 | 36.4% | <.01 | |||
| <1.5 | 192 | 75.4% | ||||
| ALT (IU/L) | ||||||
| ≥50 | 45 | 79.4% | .25 | |||
| <50 | 158 | 71.3% | ||||
| Albumin (mg/dL) | ||||||
| ≥4.0 | 121 | 81.4% | <.01 | 1.98 | 1.21–3.22 | <.01 |
| <4.0 | 82 | 59.6% | ||||
| PT (%) | ||||||
| ≥80 | 154 | 72.2% | .70 | |||
| <80 | 49 | 76.3% | ||||
| ICG‐R15 (%) | ||||||
| ≥10 | 130 | 68.6% | .04 | |||
| <10 | 73 | 81.2% | ||||
| Operation time (min) | ||||||
| ≥360 | 76 | 61.7% | <.01 | |||
| <360 | 127 | 77.8% | ||||
| Blood loss (mL) | ||||||
| ≥400 | 75 | 73.2% | <.01 | 1.64 | 1.01–2.67 | .04 |
| <400 | 128 | 81.2% | ||||
| DCP (mAU/mL) | ||||||
| ≥40 | 114 | 66.3% | .06 | |||
| <40 | 89 | 81.8% | ||||
| AFP (ng/mL) | ||||||
| ≥40 | 60 | 60.0% | .03 | 2.49 | 1.48–4.17 | <.01 |
| <40 | 143 | 78.8% | ||||
| Child–Pugh | ||||||
| Class A | 190 | 73.6% | .06 | |||
| Class B | 13 | 66.6% | ||||
| Anatomical hepatectomy | ||||||
| Yes | 57 | 82.5% | .08 | |||
| No | 146 | 69.5% | ||||
| Hepatectomy | ||||||
| Minor hepatectomy | 175 | 77.8% | <.01 | |||
| Major hepatectomy | 28 | 44.6% | ||||
| Number of tumor | ||||||
| ≥2 | 64 | 53.6% | <.01 | 2.15 | 1.30–3.57 | <.01 |
| <2 | 139 | 82.3% | ||||
| Tumor size (mm) | ||||||
| ≥40 | 58 | 51.7% | <.01 | 1.92 | 1.17–3.15 | <.01 |
| <40 | 145 | 82.1% | ||||
| Poorly differentiated | ||||||
| Yes | 20 | 56.3% | .02 | |||
| No | 183 | 74.9% | ||||
| Vascular invasion | ||||||
| Yes | 39 | 50.2% | <.01 | |||
| No | 164 | 78.1% | ||||
| Serosal invasion | ||||||
| Yes | 28 | 66.5% | .30 | |||
| No | 175 | 74.2% | ||||
Abbreviations: ACC, abdominal aortic calcification; AFP, a‐fetoprotein; ALT, alanine aminotransferase; DCP, des‐gamma‐carboxy protein; HCC, hepatocellular carcinoma; HCV, hepatitis virus type C; ICGR‐15, indocyanine green clearance test; PT, prothrombin time.
With respect to risk factors for poor RFS, higher AAC, lower albumin, higher ICG‐R15, longer operation time, larger blood loss, higher DCP levels, major hepatectomy, multiple tumors, tumor diameter ≥40 mm, poorly differentiated, and vascular invasion were predictive factors in the univariate analysis. In multivariate analysis, hepatitis virus type C (HCV) antibody positivity (HR, 1.59, 95% CI, 1.06–2.40; P = .03), high AAC (HR, 1.69; 95% CI, 1.04–2.76; P = .04), larger blood loss (HR, 1.60; 95% CI, 1.11–2.32; P = .01), multiple tumors (HR, 2.56; 95% CI, 1.76–3.71; P < .01), larger tumor size (HR, 2.08; 95% CI, 1.30–3.05; P < .01), and vascular invasion (HR: 2.03; 95% CI: 1.31–3.14; P < .01) were independent predictive factors of poor RFS (Table 4).
TABLE 4.
Risk factors for the recurrence‐free survival
| Factors | N = 203 | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| 5Y‐RFS | P value | HR | 95% CI | P value | ||
| Age (y) | ||||||
| ≥65 | 129 | 34.6% | .38 | |||
| <65 | 74 | 40.5% | ||||
| Gender | ||||||
| Male | 157 | 33.3% | .09 | |||
| Female | 46 | 49.4% | ||||
| Hepatitis virus | ||||||
| Non virus | 66 | 35.2% | .25 | (1) | ||
| HCV antibody | 99 | 33.3% | 1.59 | 1.06–2.40 | .03 | |
| HBV antigen | 38 | 48.3% | 0.85 | 0.49–1.50 | .59 | |
| AAC (mm3) | ||||||
| ≥250 | 150 | 31.2% | <.01 | 1.69 | 1.04–2.76 | .04 |
| <250 | 53 | 52.9% | ||||
| Total bilirubin (mg/dL) | ||||||
| ≥1.5 | 11 | 18.2% | .10 | |||
| <1.5 | 192 | 37.9% | ||||
| ALT (IU/L) | ||||||
| ≥ 50 | 45 | 22.9% | .06 | |||
| <50 | 158 | 41.0% | ||||
| Albumin (mg/dL) | ||||||
| ≥4.0 | 121 | 42.4% | .04 | |||
| <4.0 | 82 | 28.3% | ||||
| PT (%) | ||||||
| ≥80 | 154 | 37.2% | .72 | |||
| <80 | 49 | 35.8% | ||||
| ICG‐R15 (%) | ||||||
| ≥10 | 130 | 31.0% | .04 | |||
| <10 | 73 | 47.2% | ||||
| Operation time (min) | ||||||
| ≥360 | 76 | 22.0% | <.01 | |||
| <360 | 127 | 42.9% | ||||
| Blood loss (mL) | ||||||
| ≥400 | 75 | 24.5% | <.01 | 1.60 | 1.11–2.32 | .01 |
| <400 | 128 | 44.6% | ||||
| DCP (mAU/mL) | ||||||
| ≥40 | 114 | 27.0% | <.01 | |||
| <40 | 89 | 49.3% | ||||
| AFP (ng/mL) | ||||||
| ≥40 | 60 | 38.6% | .40 | |||
| <40 | 143 | 36.0% | ||||
| Child–Pugh | ||||||
| Class A | 190 | 37.4% | .48 | |||
| Class B | 13 | 27.7% | ||||
| Anatomical hepatectomy | ||||||
| Yes | 57 | 39.0% | .51 | |||
| No | 146 | 36.1% | ||||
| Hepatectomy | ||||||
| Minor hepatectomy | 175 | 38.9% | <.01 | |||
| Major hepatectomy | 28 | 22.5% | ||||
| Number of tumor | ||||||
| ≥2 | 64 | 10.0% | <.01 | 2.56 | 1.76–3.71 | <.01 |
| <2 | 139 | 49.2% | ||||
| Tumor size (mm) | ||||||
| ≥40 | 58 | 20.1% | <.01 | 2.08 | 1.30–3.05 | <.01 |
| <40 | 145 | 43.6% | ||||
| Poorly differentiated | ||||||
| Yes | 20 | 15.0% | .02 | |||
| No | 183 | 39.0% | ||||
| Vascular invasion | ||||||
| Yes | 39 | 23.7% | <.01 | 2.03 | 1.31–3.14 | <.01 |
| No | 164 | 39.8% | ||||
| Serosal invasion | ||||||
| Yes | 28 | 48.8% | .69 | |||
| No | 175 | 34.8% | ||||
Abbreviations: ACC, abdominal aortic calcification; AFP, a‐fetoprotein; ALT, alanine aminotransferase; DCP, des‐gamma‐carboxy protein; HCC, hepatocellular carcinoma; HCV, hepatitis virus type C; ICGR‐15, indocyanine green clearance test; PT, prothrombin time.
3.4. Relationship between chronic inflammatory score and nutrition score
The high AAC group had a relationship with chronic inflammatory score 14 , 15 , 16 , 17 and nutrition score 18 (Table 5). The high AAC group had a higher C‐reactive protein (CRP) and low CRP/albumin ratio (CAR), reflecting the inflammatory score. The proportion of patients with high Glasgow prognostic score (GPS; 1/2) was 21.3% in the high AAC group and 11.3% in the low AAC group. The proportions of patients with high modified GPS (mGPS; 1/2) were 26.0% in the high AAC group and 17.0% in the low AAC group. The proportions of high CONUT score (>4) were 12.0% in the high AAC group and 9.4% in the low AAC group; however, the difference was not significant.
TABLE 5.
Relationship between chronic inflammatory score and nutrition score
| Preoperative data |
Low AAC group N = 53 |
High AAC group N = 150 |
P‐value |
|---|---|---|---|
| Albumin (mg/dL) | 4.1 (2.5–5.2) | 4.0 (2.3–5.4) | .38 |
| CRP (mg/dL) | 0.06 (0.02–0.83) | 0.13 (0.02–17.36) | <.01 |
| Platelet (×104/µL) | 13.7 (5.2–36.8) | 14.4 (4.8–33.4) | .50 |
| Lymphocyte (/μL) | 1469 (421–4490) | 1394 (325–3423) | .42 |
| PNI | 41.7 (25–52.9) | 40.9 (23.5–54.8) | .38 |
| GPS (0/1/2) | 47/6/0 | 118/26/6 | .17 |
| mGPS (0/1/2) | 44/9/0 | 111/32/7 | .19 |
| PLR | 95.7 (18.9–333.9) | 103.8 (26.8–315.5) | .21 |
| CONUT score (>4) | 5 (9.4%) | 18 (12.0%) | .61 |
| CAR | 0.015 (0.0038–0.19) | 0.032 (0.0037–6.0) | <.01 |
Abbreviations: AAC, abdominal aortic calcification; CAR, CRP/albumin ratio; CRP, C‐reactive protein; GPS, Glasgow prognostic score; mGPS, modified GPS; PLR, platelet/lymphocyte ratio; PNI, prognostic nutritional index.
4. DISCUSSION
In the present study we showed that higher AAC was significantly associated with an unfavorable prognosis, and it increased the risk of HCC recurrence in patients who underwent hepatectomy for HCC. To our best knowledge, this is the first study to demonstrate the direct relationship between AAC and the outcomes in HCC patients.
Many studies have evaluated AAC, 3 , 19 , 20 , 21 but some studies have evaluated it at other sites. 22 , 23 We used the abdominal aorta to assess aortic calcification for several reasons. First, evaluating AAC was reported as a convenient measure for evaluating vascular calcification on abdominal CT scans. 3 Since abdominal CT was always performed before hepatectomy, the AAC data could be measured in all cases. Second, calcification at any site reflects systemic inflammation and atherosclerosis 19 , 20 , 21 , 22 , 23 , 24 ; however, the liver is an abdominal organ and AAC is considered to be the most appropriate. In addition, we have previously reported that AAC level is a risk factor for prognosis and complications after LT. 25 , 26 Therefore, we used AAC in this study. One study evaluated the relationship between abdominal aortic and coronary artery calcification as detected using CT and found that the AAC can predict severe coronary artery calcification. 27 However, in our study the rate of CVD‐related death in the high AAC group was similar to that in the low AAC group. However, the rate of HCC‐related death in the AAC group was higher than that in the low AAC group (25.3% vs 9.4%). This indicates that HCC patients with high AAC have a poor prognosis. There is no accurate way to divide intrahepatic recurrence into primary tumor and multicentric carcinogenesis recurrence. According to our definition, the intrahepatic recurrence types were similar between the two groups, and the high ACC value promoted both types of recurrence. The mechanism by which high AAC values promote tumor recurrence has not yet been clarified. Several inflammatory cytokines have a negative effect on hepatic immunity 28 and carcinogenesis. 29 Further studies are needed to reveal the negative effects of high AAC on hepatic immunity and carcinogenesis.
Whether anatomical or nonanatomical hepatectomy should be performed for HCC remains controversial. At our department, we have a policy to select limited resection in cases of severe cirrhosis or tumors located on the surface of the liver. 12 Therefore, the patients who underwent anatomical hepatectomy had better liver function (high albumin levels and lower ICG‐15 levels). In this study the OS of patients who underwent anatomical hepatectomy was better than that of patients who underwent nonanatomical hepatectomy (P = .08). However, the RFS was similar between the two groups. Nonanatomical hepatectomy was not an independent risk factor for poor RFS.
Vascular calcification, which arises from chronic vascular inflammation, is a clinically significant factor of atherosclerosis. AAC is associated with chronic inflammation. 24 In gastroenterological surgery, high AAC was first reported to be a risk factor for postoperative pancreatic fistula in elderly patients undergoing pancreaticoduodenectomy. 4 Among patients with gastrointestinal cancer, those with systemic inflammation, evaluated according to the GPS, 14 mGPS, 15 platelet/lymphocyte ratio (PLR), 16 and CRP/CAR, 17 may have poor outcomes. GPS 14 and mGPS, 15 evaluated using CRP and albumin levels are well‐known chronic inflammatory markers, and PLR 16 and CAR 17 are new inflammatory markers in gastrointestinal cancer patients. CAR can reflect the systemic inflammation status and was reported as an independent prognostic marker in patients with HCC. 17 , 30 We previously reported that AAC is associated with the systematic inflammation‐based GPS and mGPS in LT patients. 25 A high GPS increased the recurrence rate of HCC after curative hepatectomy. 31 The high AAC group had a higher proportion of patients who smoked and had diabetes. These factors can cause chronic inflammation. Approximately 4% (6/150) of patients with high AAC values had a high risk of severe inflammation (≥2 GPS or mGPS), and no patients (0/53) with low AAC values had a high risk of severe inflammation. In this study preoperative CRP and CAR were also significantly higher in the high AAC group. CAR was the most valuable prognostic indicator after hepatectomy for HCC among inflammation‐based markers.
HCC remains highly refractory to therapeutic interventions. Several molecular targets of potential HCC chemoprevention therapies have been detected, and their use as antiinflammatory and immunomodulatory therapies for HCC discussed, such as metformin, cyclooxygenase 2 (COX‐2) inhibitor, statins, and aspirin. 32 Metformin, 33 COX‐2 inhibitor, 34 statins, 35 and aspirin 36 were also reported to ameliorate vascular calcification and the progression of arteriosclerosis. Future therapeutic intervention in high‐risk patients with high AAC values would potentially benefit greatly from aggressive antiinflammatory and immunomodulatory preventive treatments for HCC. Studies involving greater sample numbers of high AAC patients are needed to analyze the effect of these preventive treatments on patient outcomes after hepatectomy for HCC.
Our study has some limitations that must be taken into consideration when interpreting our findings. In particular, the retrospective cohort study design must be mentioned. The small sample size of patients between 2010 and 2013 at a single center may also weaken the conclusion. Therefore, it must not be assumed that these limitations influence the validity of the results presented here.
In conclusion, AAC has a strong relationship with poor OS and RFS after hepatectomy for HCC. We suggest that AAC had a relationship with smoking and diabetes; therefore, AAC could reflect a surrogate for older age, worse health status, and inflammatory score.
DISCLOSURE
FUNDING
: This work was supported in part by JSPS KAKENHI Grant Numbers JP19H01057, JP20K20391, JP20K09104, and AMED under Grant Number JP21fk0210051. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the article.
CONFLICT OF INTEREST
: The authors declare that they have no competing interests.
ETHICAL APPROVAL
The protocol for this research project was approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. Committee of Hiroshima University Hospital, Approval No. E‐1410. The need for written informed consent was waived owing to the retrospective nature of the study. The opt‐out method to obtain patient consent was utilized at our institution.
ACKNOWLEDGMENT
We thank Editage (www.editage.jp) for the English language review.
Imaoka Y, Ohira M, Sato K, Imaoka K, Kuroda S, Tahara H, et al. Impact of abdominal aortic calcification on clinical outcomes following initial hepatectomy for hepatocellular carcinoma: A retrospective cohort study. Ann Gastroenterol Surg.2022;6:149–158. 10.1002/ags3.12508
REFERENCES
- 1. Abe T, Tashiro H, Kobayashi T, Hattori M, Kuroda S, Ohdan H. Glasgow prognostic score and prognosis after hepatectomy for hepatocellular carcinoma. World J Surg. 2017;41(7):1860–70. [DOI] [PubMed] [Google Scholar]
- 2. Shioi A, Katagi M, Okuno Y, Mori K, Jono S, Koyama H, et al. Induction of bone‐type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor‐alpha and oncostatin M derived from macrophages. Circ Res. 2002;91(1):9–16. [DOI] [PubMed] [Google Scholar]
- 3. Yoon YE, Han WK, Lee HH, Chang MY, Huh KH, Jung DC, et al. Abdominal aortic calcification in living kidney donors. Transplant Proc. 2016;48(3):720–4. [DOI] [PubMed] [Google Scholar]
- 4. Kakizawa N, Noda H, Watanabe F, Ichida K, Suzuki K, Rikiyama T. A high abdominal aortic calcification score on CT is a risk factor for postoperative pancreatic fistula in elderly patients undergoing pancreaticoduodenectomy. World J Surg. 2018;42(4):1129–37. [DOI] [PubMed] [Google Scholar]
- 5. Okuno S, Ishimura E, Kitatani K, Fujino Y, Kohno K, Maeno Y, et al. Presence of abdominal aortic calcification is significantly associated with all‐cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2007;49(3):417–25. [DOI] [PubMed] [Google Scholar]
- 6. An C, Lee HJ, Lee HS, Ahn SS, Choi BW, Kim MJ, et al. CT‐based abdominal aortic calcification score as a surrogate marker for predicting the presence of asymptomatic coronary artery disease. Eur Radiol. 2014;24(10):2491–8. [DOI] [PubMed] [Google Scholar]
- 7. Imaoka Y, Ohira M, Nakano R, Shimizu S, Kuroda S, Tahara H, et al. Impact of abdominal aortic calcification among liver transplantation recipients. Liver Transpl. 2019;25(1):79–87. [DOI] [PubMed] [Google Scholar]
- 8. Sagawa M, Yoshimatsu K, Yokomizo H, Yano Y, Okayama S, Usui T, et al. Worse preoperative status based on inflammation and host immunity is a risk factor for surgical site infections in colorectal cancer surgery. J Nippon Med Sch. 2017;84(5):224–30. [DOI] [PubMed] [Google Scholar]
- 9. Moyes LH, Leitch EF, McKee RF, Anderson JH, Horgan PG, McMillan DC. Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2009;100(8):1236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamaoka M, Kobayashi T, Ishiyama K, Ohira M, Tahara H, Kuroda S, et al. Evaluation of the risk factors and prognostic factors of hepatectomy for hepatocellular carcinoma in patients aged 80 years or more. J Hepatobiliary Pancreat Sci. 2017;24(1):58–64. [DOI] [PubMed] [Google Scholar]
- 11. Itamoto T, Nakahara H, Amano H, Kohashi T, Ohdan H, Tashiro H, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery. 2007;141(5):589–97. [DOI] [PubMed] [Google Scholar]
- 12. Itamoto T, Nakahara H, Tashiro H, Ohdan H, Hino H, Ochi M, et al. Indications of partial hepatectomy for transplantable hepatocellular carcinoma with compensated cirrhosis. Am J Surg. 2005;189(2):167–72. [DOI] [PubMed] [Google Scholar]
- 13. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. [DOI] [PubMed] [Google Scholar]
- 14. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dagg K, Scott HR. A prospective longitudinal study of performance status, an inflammation‐based score (GPS) and survival in patients with inoperable non‐small‐cell lung cancer. Br J Cancer. 2005;92(10):1834–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Proctor MJ, Talwar D, Balmar SM, O'Reilly DS, Foulis AK, Horgan PG, et al. The relationship between the presence and site of cancer, an inflammation‐based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br J Cancer. 2010;103(6):870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DS, et al. A comparison of inflammation‐based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–41. [DOI] [PubMed] [Google Scholar]
- 17. Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C‐reactive protein/albumin ratio, a novel inflammation‐based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22(3):803–10. [DOI] [PubMed] [Google Scholar]
- 18. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–5. [PubMed] [Google Scholar]
- 19. Hollander M, Hak AE, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, et al. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke. 2003;34(10):2367–72. [DOI] [PubMed] [Google Scholar]
- 20. Wilson PW, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103(11):1529–34. [DOI] [PubMed] [Google Scholar]
- 21. van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation. 2004;109(9):1089–94. [DOI] [PubMed] [Google Scholar]
- 22. DeLoach SS, Joffe MM, Mai X, Goral S, Rosas SE. Aortic calcification predicts cardiovascular events and all‐cause mortality in renal transplantation. Nephrol Dial Transplant. 2009;24(4):1314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bos D, Leening MJ, Kavousi M, Hofman A, Franco OH, van der Lugt A, et al. Comparison of atherosclerotic calcification in major vessel beds on the risk of all‐cause and cause‐specific mortality: the Rotterdam Study. Circ Cardiovasc Imaging. 2015;8(12). [DOI] [PubMed] [Google Scholar]
- 24. Kiu Weber CI, Duchateau‐Nguyen G, Solier C, Schell‐Steven A, Hermosilla R, Nogoceke E, et al. Cardiovascular risk markers associated with arterial calcification in patients with chronic kidney disease Stages 3 and 4. Clin Kidney J. 2014;7(2):167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Imaoka Y, Ohira M, Nakano R, Shimizu S, Kuroda S, Tahara H, et al. Impact of abdominal aortic calcification among liver transplant recipients: a retrospective study. Liver Transpl. 2019.25 (1):79–87. [DOI] [PubMed] [Google Scholar]
- 26. Imaoka Y, Ohira M, Sato K, Kuroda S, Tahara H, Ide K, et al. Impact on biliary complications of donor abdominal aortic calcification among living donor liver transplantation: a retrospective study. Transpl Int. 2020;33(12):1745–53. [DOI] [PubMed] [Google Scholar]
- 27. Takayama Y, Yasuda Y, Suzuki S, Shibata Y, Tatami Y, Shibata K, et al. Relationship between abdominal aortic and coronary artery calcification as detected by computed tomography in chronic kidney disease patients. Heart Vessels. 2016;31(7):1030–7. [DOI] [PubMed] [Google Scholar]
- 28. Yang YM, Kim SY, Seki E. Inflammation and liver cancer: molecular mechanisms and therapeutic targets. Semin Liver Dis. 2019;39(1):26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chai EZ, Siveen KS, Shanmugam MK, Arfuso F, Sethi G. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem J. 2015;468(1):1–15. [DOI] [PubMed] [Google Scholar]
- 30. Yamamoto M, Kobayashi T, Kuroda S, Hamaoka M, Okimoto S, Honmyo N, et al. Verification of inflammation‐based prognostic marker as a prognostic indicator in hepatocellular carcinoma. Ann Gastroenterol Surg. 2019;3(6):667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abe T, Tashiro H, Kobayashi T, Hattori M, Kuroda S, Ohdan H. Erratum to: glasgow prognostic score and prognosis after hepatectomy for hepatocellular carcinoma. World J Surg. 2017;41(7):1860–70. [DOI] [PubMed] [Google Scholar]
- 32. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qiu X, Xu Q, Xu T, Wan P, Sheng Z, Han Y, et al. Metformin alleviates β‐glycerophosphate‐induced calcification of vascular smooth muscle cells via AMPK/mTOR‐activated autophagy. Exp Ther Med. 2021;21(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He F, Wang H, Ren WY, Ma Y, Liao YP, Zhu JH, et al. BMP9/COX‐2 axial mediates high phosphate‐induced calcification in vascular smooth muscle cells via Wnt/β‐catenin pathway. J Cell Biochem. 2018;119(3):2851–63. [DOI] [PubMed] [Google Scholar]
- 35. Shioi A, Ikari Y. Plaque calcification during atherosclerosis progression and regression. J Atheroscler Thromb. 2018;25(4):294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thobani A, Dhindsa DS, DeMoss BD, Raad M, Sandesara PB, Sperling LS, et al. Usefulness of aspirin for primary prevention of atherosclerotic cardiovascular disease. Am J Cardiol. 2019;124(11):1785–9. [DOI] [PubMed] [Google Scholar]


