Abstract
Aim
This study aimed to elucidate the effects of laparoscopic liver resection (LLR) vs open liver resection (OLR) for major complications (Clavien‐Dindo classification grade ≥ IIIa) in obese individuals with hepatocellular carcinoma (HCC).
Methods
The clinical records of 339 and 733 patients who underwent LLR and OLR, respectively, for HCC between 2000 and 2019 were retrospectively reviewed. Body mass index (BMI) groups were classified according to the definitions of the World Health Organization: underweight group, BMI ≤ 18.4 kg/m2 (LLR vs OLR: 27 vs 47); normal weight, BMI 18.5‐24.9 kg/m2 (211 vs 483); overweight, BMI 25.0‐29.9 kg/m2 (85 vs 181); and obese, BMI ≥ 30.0 kg/m2 (16 vs 22). The effects of obesity on major complications after LLR and OLR were investigated.
Results
In total, 18 (5.3%) and 127 (17.3%) patients presented with major complications after LLR and OLR, respectively. There was no significant difference in the incidence of major complications after OLR in the four BMI groups. However, a stepwise decrease in the incidence of major complications after LLR was observed from the underweight to the obese group. In addition, a multivariate analysis revealed that increased BMI was an independent preventive factor for major complications after LLR (P = .026, odds ratio: 0.84). The estimated adjusted risk of major postoperative complications decreased with increased BMI in the LLR group, while the risk did not decrease in the OLR group (P for interaction = .048).
Conclusion
Laparoscopic liver resection is beneficial for obese patients and is superior to OLR.
Keywords: body mass index, hepatectomy, laparoscopic liver resection, obesity, postoperative complication
We compared the effects of being overweight and obese on major complications after laparoscopic liver resection (LLR) and open liver resection (OLR) in patients with hepatocellular carcinoma. The estimated risk of postoperative major complications indicated downward trend in LLR according to increase in body mass index with significant interaction for OLR.

1. INTRODUCTION
The prevalence of obesity and its associated diseases is still increasing worldwide. The prevalence of obesity (body mass index [BMI] of ≥30 kg/m2) is 40% in the United States 1 and approximately 20% in Europe. 2 In Japan, obesity is defined by a BMI of ≥25 kg/m2. 3 As of 2018, 32.2% of men and 21.9% of women of ≥20 years of age were classified as obese. 4 Obesity is correlated with comorbidities and technical difficulties in surgery and is considered a risk factor for postoperative complications in several surgical fields. 5 , 6 Furthermore, several reports have shown that obese patients are at high risk of developing hepatocellular carcinoma (HCC). 7 , 8 Thus, a higher prevalence of obesity and expansion of the indications for liver resection could increase the number of liver resection procedures among obese patients with HCC in the future. Obesity is associated with an increased risk of postoperative morbidity in individuals undergoing open liver resection (OLR). 9 , 10 Recently, laparoscopic liver resection (LLR) has been widely performed and is correlated with low morbidity and mortality. 11 , 12 However, the superiority of LLR to OLR was not evaluated according to BMI. Thus, previous reports cannot support the efficacy and safety of LLR for obese individuals. 13 , 14
The current study investigated the effects of obesity on major complications (≥grade IIIa based on the Clavien‐Dindo classification system 15 ) after LLR and OLR for HCC based on BMI (from underweight to obese). Moreover, the superiority of LLR to OLR in terms of major postoperative complications based on BMI was evaluated.
2. MATERIALS AND METHODS
2.1. Study design and participants
In total, 1072 consecutive patients with HCC who underwent liver resection in our department between January 2000 and December 2019 were included in this study. Patient's height and weight were assessed preoperatively, and BMI was calculated as weight in kilograms (kg) divided by height in meters squared (m2). The patients were allocated to one of four groups based on BMI, as defined by the World Health Organization 16 : underweight group, BMI of ≤18.4 kg/m2; normal weight group, 18.5 ≤ BMI ≤ 24.9 kg/m2; overweight group, 25.0 ≤ BMI ≤ 29.9 kg/m2; and obesity group, BMI of ≥30.0 kg/m2. The local institutional review board of our institution approved this study (registration no. 1646).
2.2. Surgical procedure
In total, 923 and 149 patients who underwent their first and second hepatic procedure, respectively, were included in the analysis. LLR was performed on 339 patients (LLR group) and OLR on 733 patients (OLR group). As described in our previous study on OLR, 17 in most patients who underwent segmentectomy or more, after Glissonean sheath transection or clamping, an ultrasonic surgical aspirator was used for hepatic dissection during total or unilateral clamping of the hepatic vascular inflow. In the majority of patients who underwent partial hepatic resection, as resection of less than a segmentectomy, an ultrasonic surgical aspirator and bipolar or monopolar forceps was utilized for hepatic dissection with the Pringle maneuver. The major branches of the Glissonean sheath and the hepatic vein were sutured using non‐absorbent sutures. Patients who underwent LLR were placed in supine or left‐lateral decubitus position and on an average five trocars according to tumor location were used. Hepatic transection was performed using a laparoscopic ultrasonic surgical aspirator and a vessel sealing system with soft coagulation. 18 In general, the Pringle maneuver was applied. Hand‐assisted laparoscopy, or the so‐called hybrid procedure or laparoscopy‐assisted resection, was performed on patients with tumors that are challenging to evaluate via pure laparoscopy due to limited visualization and heavy bleeding. In this study, LLR was defined as all laparoscopic surgeries. Further, the surgical procedures were classified into partial resection, segmentectomy, sectionectomy, and resection of two or more sections according to the Brisbane 2000 Terminology of Liver Anatomy and Resections. 19
2.3. Indication for laparoscopic liver resection
We performed LLR for ≤5‐cm solitary lesions located in the peripheral liver segments 2‐6 according to the Louisville consensus. 20 Thereafter, we extended the indication for more difficult procedures including major hepatectomy. However, LLR was selected according to tumor location, types of operative procedures, tumor size, proximity to major vessels, and liver function.
2.4. Clinicopathological characteristics and surgical outcomes
The clinical data of all patients were collected prospectively, as shown in Table 1. The date of follow‐up was on March 31, 2020.
TABLE 1.
Clinicopathological characteristics and surgical outcomes of laparoscopic and open liver resection in patients with hepatocellular carcinoma (n = 1072)
| Variables | Study cohort | Laparoscopic liver resection group | Open liver resection group | P value |
|---|---|---|---|---|
| (n = 1072) | (n = 339) | (n = 733) | ||
| BMI, kg/m2; median (range) | 23.2 (12.4‐40.2) | 23.6 (12.4‐40.2) | 23.0 (15.0‐38.2) | .080 |
| BMI groups, underweight/normal/overweight/obesity, n | 74/694/266/38 | 27/211/85/16 | 47/483/181/22 | .357 |
| Age, years; median (range) | 69 (19‐87) | 70 (21‐87) | 69 (19‐87) | .288 |
| Sex, male/female, n | 832/240 | 242/97 | 590/143 | .001 |
| Comorbidities and/or previous medical history, n (%) | ||||
| Diabetes mellitus | 351 (32.7) | 121 (35.7) | 230 (31.4) | .162 |
| Hypertension | 525 (49.9) | 183 (54.0) | 342 (46.7) | .026 |
| Dyslipidemia | 196 (18.3) | 79 (23.3) | 117 (16.0) | .004 |
| Ischemic heart diseases | 51 (4.8) | 15 (4.4) | 36 (4.9) | .728 |
| Alcohol abuse (≥60 g/d), n (%) | 317 (29.6) | 109 (32.2) | 317 (43.2) | .208 |
| Underlying hepatic disease, n (%) | ||||
| HBV | 177 (16.5) | 66 (19.5) | 111 (15.1) | .248 |
| HCV | 572 (53.4) | 180 (53.1) | 392 (53.5) | |
| HBV + HCV | 11 (1.0) | 4 (1.2) | 7 (0.9) | |
| Non‐B, non‐C | 310 (28.9) | 89 (26.3) | 223 (30.4) | |
| Pathologically confirmed cirrhosis | 324 (30.2) | 91 (26.8) | 233 (31.8) | .101 |
| Laboratory tests | ||||
| Total bilirubin level, mg/dL; median (range) | 0.7 (0.7‐2.7) | 0.6 (0.2‐2.3) | 0.7 (0.1‐2.7) | <.001 |
| Albumin level, g/dL; median (range) | 4.0 (2.3‐5.3) | 4.1 (2.3‐5.3) | 3.9 (2.6‐5.0) | <.001 |
| Prothrombin time, %; median (range) | 93 (13‐147) | 93 (57‐144) | 94 (13‐147) | .816 |
| Child‐Pugh score, A/B, n | 1035/37 | 710/23 | 325/14 | .408 |
| Platelet count, ×104/µL; median (range) | 14.8 (1.3‐46.2) | 14.0 (4.0‐35.5) | 15.1 (1.3‐46.2) | <.001 |
| AST level, IU/L; median (range) | 37 (11‐201) | 32 (11‐201) | 39 (12‐187) | <.001 |
| ALT level, IU/L; median (range) | 31 (5‐270) | 26 (6‐166) | 34 (5‐270) | <.001 |
| Surgery‐related factors, n (%) | ||||
| Recurrence | 277 (25.8) | 95 (28.0) | 182 (24.8) | .267 |
| Repeat liver resection | 149 (13.9) | 43 (12.7) | 106 (14.5) | .434 |
| Types of liver resection | ||||
| Partial resection | 669 (62.4) | 278 (82.0) | 391 (53.3) | <.001 |
| Segmentectomy | 98 (9.1) | 14 (4.1) | 84 (11.5) | |
| Sectionectomy | 170 (15.9) | 34 (10.0) | 136 (18.6) | |
| Resection of two or more sections | 135 (12.6) | 13 (3.8) | 122 (16.6) | |
| Conversion, n (%) | 5 (0.5) | 5 (1.5) | ‐ | |
| Operative time, min; median (range) | 276 (75‐915) | 259 (75‐750) | 276 (75‐915) | <.001 |
| Volume of blood loss, mL; median (range) | 340 (0‐7460) | 100 (0‐3025) | 500 (0‐7460) | <.001 |
| Non‐curative surgery | 25 (2.3) | 2 (0.6) | 23 (3.1) | .008 |
| Tumor‐related factors | ||||
| AFP level, ≥ 20 ng/mL; n (%) | 412 (38.4) | 114 (33.6) | 298 (40.7) | .028 |
| Tumor size, cm; median (range) | 2.7 (0.4‐21.0) | 2.2 (0.4‐9.5) | 3.1 (0.5‐21.0) | |
| Number, solitary/multiple; n (%) | 798/274 | 286/53 | 512/221 | <.001 |
| Macrovascular invasion, n (%) | 118 (11.0) | 17 (5.0) | 101 (13.8) | <.001 |
| UICC stage, n (%) | ||||
| Ia | 274 (25.6) | 130 (38.3) | 144 (19.6) | <.001 |
| Ib | 461 (43.0) | 146 (43.1) | 315 (43.0) | |
| II | 246 (22.9) | 60 (17.7) | 186 (25.4) | |
| IIIa | 44 (4.1) | 1 (0.3) | 43 (5.9) | |
| IIIb | 42 (3.9) | 2 (0.6) | 40 (5.5) | |
| IVa | 1 (0.1) | 0 | 1 (0.1) | |
| IVb | 4 (0.4) | 0 | 4 (0.5) | |
| Pathology | ||||
| Poor HCC, n (%) | 267 (24.9) | 53 (15.6) | 214 (29.1) | <.001 |
| Number, solitary/multiple; n (%) | 765/307 | 274/65 | 491/242 | <.001 |
| Microvascular invasion, n (%) | 337 (31.4) | 76 (22.4) | 261 (35.6) | <.001 |
| Postoperative complications, n (%) | ||||
| Overall | 329 (30.7) | 59 (17.4) | 270 (36.8) | <.001 |
| Major† | 145 (13.5) | 18 (5.3) | 127 (17.3) | <.001 |
| In‐hospital death | 4 (0.4) | 0 | 4 (0.5) | .173 |
| Hospital stay, days; median (range) | 13 (4‐212) | 9 (4‐88) | 15 (5‐212) | <.001 |
Abbreviations: AFP, alpha fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; UICC, Union for International Cancer Control.
†≥Grade IIIa based on the Clavien‐Dindo classification system.
2.5. Definitions
Patients were diagnosed with diabetes mellitus (DM), hypertension, and dyslipidemia according to the guidelines of the Japan Diabetes Society, 21 Japanese Society of Hypertension, 22 and Japan Atherosclerosis Society, 23 respectively. DM was defined as a fasting plasma glucose level of ≥126 mg/dL, hemoglobin A1c level of ≥6.5%, or need for hypoglycemic drugs or insulin. Hypertension was defined as a systolic blood pressure of ≥140 mmHg, diastolic blood pressure of ≥90 mmHg, or need for antihypertensive drugs. Dyslipidemia was defined as a serum low‐density lipoprotein cholesterol level of ≥140 mg/dL, high‐density lipoprotein cholesterol level of <40 mg/dL, and/or triglyceride level of ≥150 mg/dL or a need for lipid‐lowering drugs. Regarding postoperative complications, wound infection was defined as the presence of bacteria in the wound exudate. Bile leakage was defined as a bilirubin concentration of at least three times the serum bilirubin concentration in the drainage fluid on or after postoperative day (POD) 3 or a need for radiological or surgical intervention for biliary collection or bile peritonitis. 24 Intractable ascites was defined as drainage of 1 L/day for more than 2 days or ascites in the whole abdomen. Intractable pleural effusion was diagnosed when thoracentesis was performed. 25 Pneumonia and atelectasis were considered respiratory complications. 26 Liver failure was defined as the presence of prolonged hyperbilirubinemia (total serum bilirubin concentration of ≤3.0 mg/dL) on or after POD 5 and a need for fresh frozen plasma to decrease prothrombin time (≤50%) based on the modified definitions proposed by the International Study Group of Liver Surgery 27 and Balzan et al. 28 The severity of postoperative complications was graded according to the Clavien‐Dindo classification system. 15 In this study, major complication was defined as ≥grade IIIa based on the Clavien‐Dindo classification system.
2.6. Outcomes
The primary outcome was the effect of BMI on major complications after LLR and OLR. Patients who underwent LLR and OLR initially presented with risk factors for major complications. Finally, to evaluate the superiority of LLR to OLR in patients with increased BMI, the difference in major complications between patients who underwent LLR and OLR was analyzed via a multivariate analysis.
2.7. Statistical analyses
Categorical variables were presented as numbers and percentages and were compared between groups using the Fisher's exact test or the χ 2 test, as appropriate. Continuous variables were expressed as median (range) and were compared using the Kruskal‐Wallis test. The Holm's method 29 was used to adjust P values for multiple comparisons of demographic variables among the different groups. We performed the Cochran‐Armitage trend test 30 to assess the categorical variables and the Jonckheere‐Terpstra trend test 31 to assess the continuous variables. Univariate and multivariate logistic regression analyses were performed to evaluate the relative risk for major postoperative complications. A nonlinear restricted cubic spline was contained to consider the nonlinear effect of BMI on the risk for major postoperative complications. All statistical inferences were assessed using a two‐sided significance level of 5%, except for the interaction (cross‐product term) analysis. Variables with a P value of <.05 in the univariate analysis (Cox's proportional hazard model) were included in the multivariate analysis. A P value <.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences® software version 26.0 (IBM Corp.) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphic user interface for the R software version 3.5.1 (The R Foundation for Statistical Computing). 32
3. RESULTS
3.1. Characteristics of patients who underwent laparoscopic liver resection and open liver resection
There was no significant difference in terms of median BMI among patients who underwent LLR and OLR (Table 1). Among the patients who underwent LLR/OLR, 27/47, 211/483, 85/181, and 16/22 patients were classified into the underweight, normal weight, overweight, and obese groups, respectively. In the LLR group, the proportion of female patients, patients with HT, and patients with DL was higher when compared with the OLR group. The preoperative serum concentrations of total bilirubin, albumin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were better in the LLR group than in the OLR group. However, the distribution of the Child‐Pugh scores among the two groups did not significantly differ. The proportion of patients who underwent partial liver resection was higher in the LLR group than in the OLR group, who had a shorter operative time and lower volume of blood loss. Based on tumor‐related factors or pathology, the OLR group had a more advanced HCC than the LLR group. The rate of incidence of overall and major postoperative complications was lower in the LLR group than in the OLR group, who had a shorter hospital stay.
3.2. Clinical characteristics of patients who underwent LLR and OLR according to BMI status
The clinicopathological characteristics of patients (n = 1072) after LLR or OLR according to BMI are shown in Table 2. In the LLR group, the proportion of patients with DM had a stepwise increase from the underweight to obese group. However, there was no significant difference in terms of other background characteristics, liver function test results, and tumor‐related factors among the four BMI groups. Moreover, type of liver resection, operative time, and volume of blood loss did not differ among the four groups. However, the conversion rate from laparoscopic to open surgery was high in the underweight group (P = .014). That is, the procedure was converted to open surgery in two (7.4%) patients in the underweight group because bleeding could not be controlled due to a narrow working space and in two patients in the normal weight and overweight groups because of injury in the major hepatic vein. Moreover, in one (6.3%) patient in the obesity group, the surgery was converted because of cancer invasion to the diaphragm (n = 1). By contrast, in the OLR group, the proportion of patients with DM, hypertension, dyslipidemia, and non‐B non‐C hepatitis indicated a stepwise increase from the underweight to obesity groups. There was a significant difference in the serum concentrations of albumin, AST, and ALT. However, the distribution of Child‐Pugh scores did not remarkably differ among the four groups. Although there was no difference in the type of liver resection among the four BMI groups, the proportion of patients who experienced bleeding indicated a stepwise increase from the underweight to obesity groups. In each BMI group, the proportion of patients who had intraoperative bleeding was higher in the OLR group than in the LLR group (P = .011 in the underweight group and P < .001 in the normal weight, overweight, and obesity groups).
TABLE 2.
Clinicopathological characteristics of patients with hepatocellular carcinoma (n = 1072) after laparoscopic or open liver resection according to body mass index
| Variables | Laparoscopic liver resection group | Open liver resection group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Underweight group | Normal weight group | Overweight group | Obese group | P value | P value for trend | Underweight group | Normal weight group | Overweight group | Obese group | P value | |||
| (n = 27) | (n = 211) | (n = 85) | (n = 16) | (n = 47) | (n = 483) | (n = 181) | (n = 22) | P value for trend | |||||
| BMI, kg/m2; median (range) | 17.4 (12.4‐18.4) | 22.6 (18.5‐24.9) | 26.9 (25.0‐29.7) | 33.0 (30.1‐40.2) | <.001 | <.001 | 17.4 (15.0‐18.4) | 22.1 (18.5‐24.9) | 26.5 (25.0‐29.9) | 31.2 (30.0‐38.1) | <.001 | <.001 | |
| Age, years; median (range) | 70 (46‐83) | 71 (21‐85) | 71 (47‐87) | 65 (44‐73) | .093 | 72 (19‐82) | 69 (20‐87) | 69 (37‐85) | 70 (44‐78) | 0.299 | |||
| Sex, male/female, n | 14/13 | 153/58 | 63/22 | 12/4 | .133 | 34/13 | 398/85 | 144/37 | 14/8 | 0.066 | |||
| Comorbidities and/or previous history, n (%) | |||||||||||||
| Diabetes mellitus | 7 (25.9) | 72 (34.1) | 31 (36.5) | 11 (68.8) | .029 | .019 | 9 (19.1) | 138 (28.6) | 69 (38.1) | 14 (63.6) | <.001 | <.001 | |
| Hypertension | 11 (40.7) | 111 (52.6) | 53 (62.4) | 8 (50.0) | .206 | 11 (23.4) | 213 (44.1) | 102 (56.4) | 16 (72.7) | <.001 | <.001 | ||
| Dyslipidemia | 4 (14.8) | 46 (21.8) | 24 (28.2) | 5 (31.3) | .380 | 2 (4.3) | 65 (13.5) | 45 (24.9) | 5 (22.7) | <.001 | <.001 | ||
| Ischemic heart disease | 0 | 11 (5.2) | 4 (4.7) | 0 | .509 | 1 (2.1) | 25 (5.2) | 10 (5.5) | 0 | 0.545 | |||
| Alcohol abuse (≥ 60 g/d), n (%) | 7 (25.9) | 70 (33.2) | 25 (29.4) | 7 (43.8) | .602 | 10 (21.3) | 135 (28.0) | 56 (30.9) | 7 (31.8) | 0.589 | |||
| Underlying hepatic diseases, n (%) | |||||||||||||
| HBV | 6 (22.2) | 39 (18.5) | 17 (20.0) | 4 (25.0) | .412 | 8 (17.0) | 72 (14.9) | 28 (15.5) | 3 (13.6) | <.001 | <.001 | ||
| HCV | 15 (55.6) | 116 (55.0) | 45 (52.9) | 4 (25.0) | 31 (66.0) | 278 (57.6) | 80 (44.2) | 3 (13.6) | |||||
| HBV + HCV | 0 | 4 (1.9) | 0 | 0 | 1 (2.1) | 4 (0.8) | 2 (1.1) | 0 | |||||
| Non‐B, non‐C | 6 (22.2) | 52 (24.6) | 23 (27.1) | 6 (37.5) | 7 (14.9) | 129 (26.7) | 71 (39.2) | 16 (72.7) | |||||
| Pathologically confirmed cirrhosis | 7 (25.9) | 48 (22.7) | 31 (36.5) | 5 (31.3) | .112 | 15 (31.9) | 143 (29.6) | 64 (35.4) | 11 (50.0) | .139 | |||
| Laboratory tests | |||||||||||||
| Total bilirubin level, mg/dL; median (range) | 0.6 (0.3‐1.5) | 0.6 (0.2‐2.2) | 0.6 (0.2‐2.3) | 0.8 (0.3‐2.3) | .124 | 0.6 (0.2‐1.4) | 0.7 (0.1‐2.7) | 0.7 (0.1‐2.2) | 0.6 (0.2‐2.0) | .4 | .041 | ||
| Albumin level, g/dL; median (range) | 4.1 (3.4‐5.3) | 4.1 (2.3‐5.1) | 4.2 (3.1‐4.8) | 4.0 (3.6‐4.5) | .940 | 3.9 (3.2‐4.6) | 3.9 (2.6‐5.0) | 4.0 (2.1‐5.0) | 3.7 (2.9‐4.3) | <.001 | |||
| Prothrombin time, %; median (range) | 100 (71‐122) | 93 (62‐144) | 93 (57‐138) | 89 (69‐114) | .083 | 91 (67‐123) | 93 (13‐141) | 94 (40‐130) | 95 (71‐147) | .856 | |||
| Child‐Pugh score, A/B; n (%) | 27/0 | 202/9 | 82/3 | 14/2 | .253 | 46/1 | 464/19 | 179/2 | 21/1 | .288 | |||
| Platelet count, ×104/µL; median (range) | 13.6 (6.0‐25.2) | 13.6 (4.0‐35.5) | 14.9 (4.1‐32.1) | 15.3 (5.7‐29.7) | .488 | 16.0 (7.9‐34.0) | 14.9 (2.2‐42.8) | 15.3 (1.3‐46.2) | 16.4 (1.7‐31.8) | .854 | |||
| AST level, IU/L; median (range) | 34 (23‐201) | 32 (11‐159) | 32 (15‐163) | 39 (11‐98) | .107 | 45 (14‐123) | 40 (12‐187) | 35 (13‐105) | 38 (14‐110) | .005 | <.001 | ||
| ALT level, IU/L; median (range) | 24 (13‐96) | 26 (6‐166) | 28 (8‐162) | 34 (13‐91) | .478 | 32 (6‐113) | 37 (8‐270) | 31 (5‐172) | 28 (11‐104) | .037 | .022 | ||
| Surgery‐related factors, n (%) | |||||||||||||
| Recurrence | 8 (29.6) | 60 (28.4) | 22 (25.9) | 5 (31.3) | .955 | 12 (25.5) | 115 (23.8) | 53 (29.3) | 2 (9.1) | .163 | |||
| Repeat liver resection | 3 (11.1) | 30 (14.2) | 10 (11.8) | 0 | .408 | 7 (14.9) | 65 (13.5) | 32 (17.7) | 2 (9.1) | .488 | |||
| Types of liver resection | |||||||||||||
| Partial resection | 21 (77.8) | 166 (78.7) | 77 (90.6) | 14 (87.5) | .279 | 27 (57.4) | 249 (51.6) | 103 (56.9) | 12 (54.5) | .548 | |||
| Segmentectomy | 2 (7.4) | 11 (5.2) | 0 (0.0) | 1 (6.3) | 5 (10.6) | 63 (13.0) | 15 (8.3) | 1 (4.5) | |||||
| Sectionectomy | 4 (14.8) | 24 (11.4) | 5 (5.9) | 1 (6.3) | 8 (17.0) | 84 (17.4) | 39 (21.5) | 5 (22.7) | |||||
| Resection of two or more sections | 0 | 10 (4.7) | 3 (3.5) | 0 | 7 (14.9) | 87 (18.0) | 24 (13.3) | 4 (18.2) | |||||
| Conversion, n (%) | 2 (7.4) | 1 (0.5) | 1 (1.2) | 1 (6.3) | .014 | .826 | |||||||
| Operative time, min; median (range) | 284 (135‐605) | 253 (75‐750) | 262 (87‐704) | 270 (90‐593) | .614 | 271 (93‐610) | 276 (75‐915) | 276 (84‐662) | 345 (105‐640) | .028 | .062 | ||
| Volume of blood loss, mL; median (range) | 70 (0‐1850) | 90 (0‐1625) | 100 (5‐3025) | 130 (10‐1540) | .247 | .147 | 330 (10‐5100) | 490 (0‐7460) | 550 (0‐5050) | 1230 (90‐6265) | .001 | .002 | |
| Non‐curative surgery | 0 | 2 (0.9) | 0 | 0 | .748 | 1 (2.1) | 16 (3.3) | 4 (2.2) | 2 (9.1) | .35 | |||
| Tumor‐related factors | |||||||||||||
| AFP level, ≥ 20 ng/mL, n (%) | 10 (37.0) | 75 (35.5) | 25 (29.4) | 4 (25.0) | .637 | 21 (44.7) | 197 (40.8) | 69 (38.1) | 11 (50.0) | .66 | |||
| Tumor size, cm; median (range) | 2.0 (0.7‐7.8) | 2.1 (0.4‐7.0) | 2.5 (0.7‐9.0) | 2.6 (1.7‐9.5) | .075 | 2.7 (0.6‐15.0) | 3.0 (0.7‐20.0) | 3.1 (0.5‐21.0) | 5.1 (2.2‐12.0) | .004 | .104 | ||
| Number, solitary/multiple; n (%) | 24/3 | 174/37 | 72/13 | 16/0 | .265 | 35/12 | 337/146 | 124/57 | 16/6 | .869 | |||
| Macrovascular invasion, n (%) | 1 (3.7) | 13 (6.2) | 3 (3.5) | 0 | .590 | 6 (12.8) | 67 (13.9) | 22 (12.2) | 6 (27.3) | .282 | |||
| UICC stage, n (%) | |||||||||||||
| Ia | 14 (51.9) | 86 (40.8) | 27 (27.8) | 3 (18.8) | .135 | 11 (23.4) | 100 (20.7) | 33 (18.2) | 0 | .245 | |||
| Ib | 10 (37.0) | 81 (38.4) | 42 (42.4) | 13 (81.3) | 21 (44.7) | 201 (41.6) | 80 (44.2) | 13 (59.1) | |||||
| II | 3 (11.1) | 41 (19.4) | 16 (16.8) | 0 | 9 (19.1) | 124 (25.7) | 49 (27.1) | 4 (18.2) | |||||
| IIIa | 0 | 1 (0.5) | 0 | 0 | 3 (6.4) | 26 (5.4) | 12 (6.6) | 2 (9.1) | |||||
| IIIb | 0 | 2 (0.9) | 0 | 0 | 3 (6.4) | 30 (6.2) | 5 (2.8) | 2 (9.1) | |||||
| IVa | 0 | 0 | 0 | 0 | 0 | 1 (0.2) | 0 (0.0) | 0 | |||||
| IVb | 0 | 0 | 0 | 0 | 0 | 1 (0.2) | 2 (1.1) | 1 (4.5) | |||||
| Pathology | (0.0) | ||||||||||||
| Poor HCC, n (%) | 4 (14.8) | 33 (15.6) | 13 (13.3) | 3 (18.8) | .987 | 16 (34.0) | 137 (28.4) | 52 (28.7) | 9 (40.9) | .537 | |||
| Number, solitary/multiple; n (%) | 24/3 | 164/47 | 70/15 | 16/0 | .095 | 33/14 | 325/158 | 117/64 | 16/6 | .797 | |||
| Microvascular invasion, n (%) | 4 (14.8) | 54 (25.6) | 16 (18.8) | 2 (12.5) | .301 | 17 (36.2) | 178 (36.9) | 58 (32.0) | 8 (36.4) | .719 | |||
Abbreviations: AFP, alpha fetoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; UICC, Union for International Cancer Control.
3.3. Major complications after LLR and OLR according to BMI status
The incidence of major complications after LLR indicated a stepwise decrease from the underweight to obese groups (Cochran‐Armitage trend test, P = .037, Figure 1A). A multiple analysis using Holm's test indicated that the incidence in the underweight group was higher than that in the normal weight group; however, there were no significant differences among the normal weight, overweight, and obese groups. The incidence of wound infection and intra‐abdominal infection did not differ among the four groups, and none of the patients presented with complex venous thromboembolism (Table 3). There was no significant difference in the incidence of each complication among the four BMI groups with zero in‐hospital death. In patients who underwent OLR, there was no difference in the incidence of overall or major complications among the four groups (Figure 1A). The incidence of wound infection and abdominal infection did not differ among the four groups, and only one (0.6%) patient in the overweight group had complex venous thromboembolism. One patient in the underweight group and three in the normal weight group died of liver failure. In the normal weight, overweight, and obesity groups, the incidence of major complications was higher in patients who underwent OLR than in those who underwent LLR (underweight group, P = .871; normal weight group, P < .001; overweight group, P = .006; and obesity group, P < .001).
FIGURE 1.
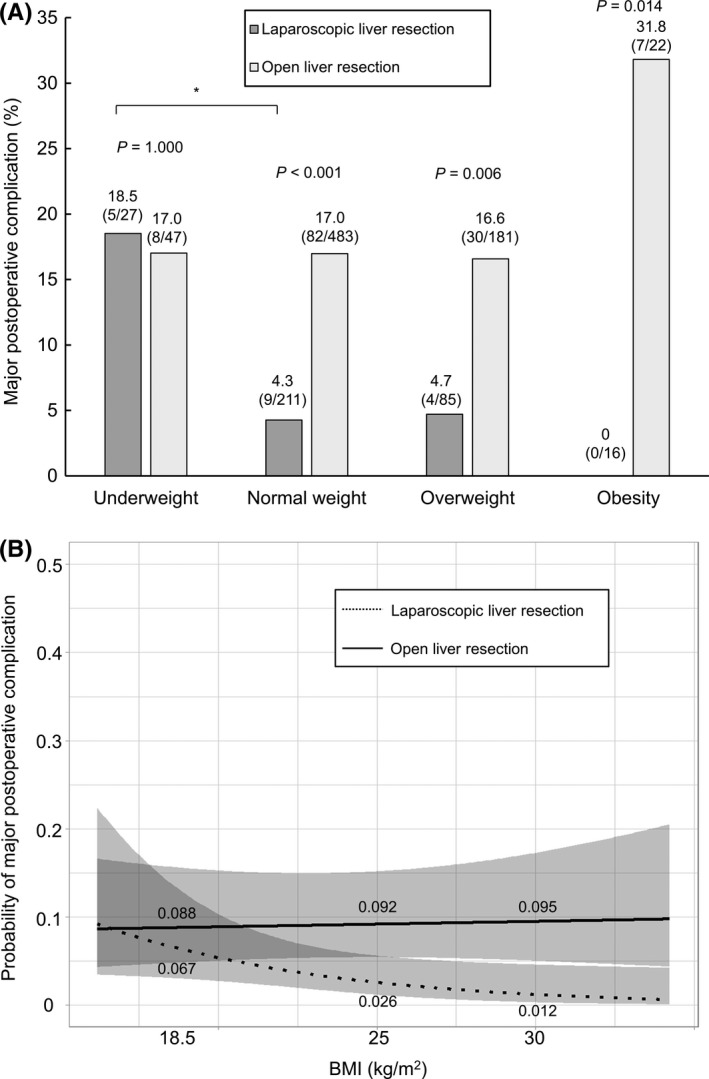
(A) Incidence of major postoperative complications according to BMI in patients who underwent LLR and OLR. The incidence of major postoperative complications had a stepwise decrease from underweight to obese patients in the LLR group (P = .013, Cochran‐Armitage trend test, P = .037), but not in the OLR group (P = .342). Underweight, BMI of ≤18.4 kg/m2; normal weight, 18.5 kg/m2 ≤ BMI ≤ 24.9 kg/m2; overweight, 25.0 kg/m2 ≤ BMI ≤ 29.9 kg/m2; and obesity, 30.0 kg/m2 ≤ BMI. BMI, body mass index; LLR, laparoscopic liver resection; OLR, open liver resection. *P < .01 using the Holm's method. (B) Risk of major postoperative complications after LLR and OLR according to BMI after adjusting for confounding variables via a multivariate analysis. A linear line with area represents the risk (mean with 95% confidence intervals). There was no difference in terms of the risk of major postoperative complications in patients with a BMI of 18.5 kg/m2. However, among patients with a BMI of 25.0 and 30.0 kg/m2, the risk was lower in the LLR group than in the OLR group. In addition, there was significant effect of interaction between patients who underwent LLR and those who underwent OLR according to increase in BMI value (P for interaction = .048). BMI, body mass index; LLR, laparoscopic liver resection; OLR, open liver resection. Major complication, ≥grade IIIa based on the Clavien‐Dindo classification system
TABLE 3.
Postoperative course in patients with hepatocellular carcinoma (n = 1072) after laparoscopic or open liver resection according to body mass index
| Variables | Laparoscopic liver resection group | Open liver resection group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Underweight group | Normal weight group | Overweight group | Obese group | P value | Underweight group | Normal weight group | Overweight group | Obese group | P value | |
| (n = 27) | (n = 211) | (n = 85) | (n = 16) | (n = 47) | (n = 483) | (n = 181) | (n = 22) | |||
| Postoperative complications | ||||||||||
| Overall | 7 (25.9) | 35 (16.6) | 16 (18.8) | 1 (6.3) | .397 | 17 (36.2) | 179 (37.1) | 64 (35.4) | 10 (45.5) | .828 |
| Wound infection | 0 | 1 (0.5) | 0 | 0 | .894 | 2 (4.3) | 22 (4.6) | 8 (4.4) | 1 (4.5) | 1.000 |
| Intra‐abdominal infection† | 2 (7.4) | 2 (0.9) | 1 (1.2) | 0 | .065 | 4 (8.5) | 38 (7.9) | 12 (6.6) | 1 (4.5) | .888 |
| Bile leakage† | 2 (7.4) | 3 (1.4) | 3 (3.5) | 0 | .197 | 3 (6.4) | 30 (6.2) | 8 (4.4) | 4 (18.2) | .091 |
| Ascites† | 0 | 1 (0.5) | 0 | 0 | .894 | 0 | 21 (4.3) | 3 (1.7) | 1 (4.5) | .193 |
| Pleural effusion† | 1 (3.7) | 1 (0.5) | 2 (2.4) | 0 | .312 | 2 (4.3) | 27 (5.6) | 8 (4.4) | 2 (9.1) | .780 |
| Bleeding† | 1 (3.7) | 1 (0.5) | 0 | 0 | .164 | 1 (2.1) | 2 (0.4) | 0 | 0 | .237 |
| Gastrointestinal bleeding† | 0 | 2 (0.9) | 0 | 0 | .748 | 0 | 2 (0.4) | 1 (0.6) | 1 (4.5) | .075 |
| Respiratory complications† | 0 | 2 (0.9) | 0 | 0 | .748 | 2 (4.3) | 8 (1.7) | 0 | 0 | .110 |
| Liver failure‡ | 0 | 1 (0.5) | 0 | 0 | .894 | 1 (2.1) | 9 (1.9) | 1 (0.6) | 0 | .574 |
| Grade (A/B/C) | 0/0/0 | 0/1/0 | 0/0/0 | 0/0/0 | 0/0/1 | 1/3/5 | 0/0/1 | 0/0/0 | ||
| Ileus† | 0 | 0 | 1 (1.2) | 0 | .392 | 0 | 1 (0.2) | 0 | 0 | .915 |
| Others† | 1 (3.7) | 1 (0.5) | 0 | 0 | .164 | 1 (2.1) | 5 (1.0) | 1 (0.6) | 0 | .744 |
| In‐hospital death | 0 | 0 0 | 0 | ‐ | 1 (2.1) | 3 (0.6) | 0 | 0 | .343 | |
| Hospital stay, days; median (range) | 10 (6‐88) | 9 (4‐79) | 9 (5‐86) | 8 (6‐17) | .366 | 16 (6‐67) | 15 (6‐212) | 15 (5‐102) | 14 (6‐134) | .901 |
†≥ Grade IIIa based on the Clavien‐Dindo classification. ‡International Study Group of Liver Surgery definition.
3.4. Risk factors for major complications after LLR and OLR
In all the cohorts including the LLR and the OLR groups, univariate and multivariate logistic regression analyses showed that age, operative time, volume of blood loss, serum albumin concentration, and LLR were independent predictive factors for major postoperative complications (Table S1). Meanwhile, BMI was not associated with major postoperative complications in all the cohorts including the LLR and OLR groups. However, in the LLR group, an increased BMI was considered an independent favorable factor and long operative time was a risk factor for postoperative complications (Table 4). On the contrary, among patients who underwent OLR, BMI was not a risk for postoperative major complication; however, long operative time was a risk factor.
TABLE 4.
Univariate and multivariate analyses of risk factors associated with major complications after laparoscopic and open liver resection
| Variables | Laparoscopic liver resection group | Open liver resection group | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| P value |
Odds ratio (95% CI) |
P value |
Odds ratio (95% CI) |
P value |
Odds ratio (95% CI) |
P value |
Odds ratio (95% CI) |
|
| BMI, per 1 kg/m2 increase | .044 | 0.87 (0.75‐0.996) | .026 | 0.84 (0.73‐0.98) | .670 | 1.01 (0.96‐1.07) | .845 | 1.01 (0.95‐1.07) |
| Age, per 1 year increase | .220 | 1.04 (0.98‐1.09) | .258 | 1.04 (0.73‐1.53) | .342 | 1.01 (0.99‐1.03) | .072 | 1.02 (0.998‐1.05) |
| Sex, male/female | .259 | 2.07 (0.59‐7.32) | .519 | 1.59 (0.39‐6.48) | .097 | 1.57 (0.92‐2.69) | .207 | 1.45 (0.82‐2.58) |
| Comorbidities and/or previous history | ||||||||
| Diabetes mellitus | .474 | 0.68 (0.24‐1.96) | .055 | 1.48 (0.99‐2.20) | ||||
| Hypertension | .728 | 0.85 (0.33‐2.18) | .464 | 1.15 (0.79‐1.69) | ||||
| Dyslipidemia | .645 | 1.28 (0.44‐3.72) | .384 | 0.78 (0.45‐1.36) | ||||
| Ischemic heart diseases | .275 | 0.32 (0.04‐2.49) | .036 | 2.20 (1.06‐4.60) | .149 | 1.81 (0.81‐4.06) | ||
| Alcohol abuse | .359 | 0.59 (0.19‐1.83) | .283 | 1.25 (0.83‐1.89) | ||||
| Viral hepatitis | .880 | 0.92 (0.32‐2.66) | .178 | 0.76 (0.51‐1.13) | ||||
| Pathologically confirmed cirrhosis | .324 | 0.53 (0.15‐1.87) | .111 | 1.38 (0.93‐2.06) | ||||
| Laboratory tests | ||||||||
| Total bilirubin per 1 g/dL increase | .437 | 0.55 (0.12‐2.51) | .638 | 0.88 (0.51‐1.52) | ||||
| Albumin per 1 g/dL increase | .269 | 0.56 (0.20‐1.56) | .021 | 0.60 (0.38‐0.92) | .151 | 0.68 (0.40‐1.15) | ||
| Prothrombin activity per 1% increase | .188 | 1.02 (0.99‐1.05) | .039 | 0.99 (0.97‐0.99) | .267 | 0.99 (0.98‐1.01) | ||
| Child‐Pugh score, B to A | .999 |
‐ ‐ |
.030 | 2.65 (1.10‐6.39) | .467 | 1.51 (0.50‐4.61) | ||
| Platelet count per 1 × 104/mL increase | .931 | 0.996 (0.92‐1.08) | .054 | 1.03 (1.00‐1.06) | ||||
| AST per 1 IU/L increase | .156 | 1.01 (0.996‐1.03) | .588 | 1.002 (0.995‐1.01) | ||||
| ALT per 1 IU/L increase | .933 | 1.001 (0.98‐1.02) | .888 | 1.00 (0.99‐1.01) | ||||
| Surgery‐related factors | ||||||||
| Recurrence | .607 | 1.30 (0.48‐3.58) | .578 | 1.13 (0.73‐1.75) | ||||
| Repeat liver resection | .837 | 0.85 (0.19‐3.85) | .705 | 0.90 (0.51‐1.57) | ||||
| Sectionectomy or more | .003 | 4.47 (1.64‐12.20) | 0.707 | 1.31 (0.32‐5.46) | <.001 | 2.16 (1.46‐3.18) | 0.549 | 1.17 (0.70‐1.95) |
| Operative time per 1 h increase | <.001 | 1.70 (1.37‐2.12) | 0.011 | 1.47 (1.09‐1.99) | <.001 | 1.34 (1.23‐1.46) | <.001 | 1.27 (1.11‐1.46) |
| Bleeding per 1 mL increase | <.001 | 4.21 (2.05‐8.63) | 0.125 | 2.22 (0.80‐6.12) | <.001 | 1.59 (1.36‐1.86) | .071 | 1.23 (0.98‐1.53) |
| Non‐curative surgery | .999 | ‐ | ‐ | .583 | 0.71 (0.21‐2.42) | |||
| Tumor‐related factors | ||||||||
| AFP level (≥ 20 ng/mL) | .978 | 0.986 (0.36‐2.70) | 0.638 | 1.10 (0.75‐1.62) | ||||
| Tumor size per 1 cm increase | .003 | 1.47 (1.14‐1.89) | .753 | 1.06 (0.73‐1.53) | .016 | 1.07 (1.01‐1.14) | .091 | 0.93 (0.86‐1.01) |
| Number, multiple to solitary | .590 | 0.66 (0.15‐2.97) | .716 | 1.08 (0.71‐1.63) | ||||
| Macrovascular invasion | .999 | ‐ | .017 | 1.82 (1.11‐2.99) | .749 | 1.12 (0.57‐2.20) | ||
| UICC stage per 1 stage increase | .179 | 1.48 (0.84‐2.62) | .011 | 1.24 (1.05‐1.46) | .834 | 0.98 (0.77‐1.32) | ||
| Pathology | ||||||||
| Poor HCC | .901 | 1.08 (0.30‐3.88) | .656 | 0.91 (0.59‐1.39) | ||||
| Number, multiple to solitary | .380 | 0.51 (0.12‐2.28) | .988 | 1.003 (0.67‐1.51) | ||||
| Microvascular invasion | .093 | 2.32 (0.87‐6.22) | .571 | 1.12 (0.76‐1.67) | ||||
Abbreviations: AFP, alpha fetoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; UICC, Union for International Cancer Control.
After adjusting for age, sex, liver function test, and surgery‐ and tumor‐related factors between the LLR and OLR groups, the risk of major postoperative complications in the LLR group decreased with increased BMI, while that in the OLR group increased with greater BMI (Figure 1B). In patients with a BMI of 25 and 30, but not 18.5, kg/m2, the risk was lower in the LLR group than in the OLR group. In addition, there was significant effect of interaction between patients who underwent LLR and those who underwent OLR according to increase in BMI value (P for interaction = .048, Figure 1B).
4. DISCUSSION
Countermeasures for the depth of surgical field and large volume of intraperitoneal fat are important in abdominal surgery, including liver resection, in overweight and obese patients. 33 , 34 In this study, a high BMI was associated with operative time and volume of blood loss in the OLR group, but not in the LLR group. Despite a large skin incision and compression of the gastrointestinal tract and greater omentum in OLR, liver parenchyma dissection and treatment of hepatic hilum are sometimes challenging, and this can be associated with long operative time and large volume of blood loss. This finding was in accordance with that of other reports. 9 , 35 , 36 , 37 , 38 In contrast, in LLR, pneumoperitoneum, head up position, and high magnification—even at deep portions in the caudal view—can provide sufficient free space to control the forceps, even in overweight and obese patients. 39 , 40 These conditions cannot affect operative time or volume of blood loss in overweight and obese patients. Even after adjusting for confounding variables, the risk of major postoperative complications was higher in overweight and obese patients (BMI of 25 and 30 kg/m2, respectively) in the OLR group than in the LLR group. Based on these data, the physical characteristics of overweight and obese patients can be risk factors for major complications after OLR compared with LLR. We showed a stepwise decrease in the incidence of major postoperative complications according to increasing BMI. Hence, LLR should be recommended for eligible overweight and obese patients to prevent major postoperative complications. Obesity is a high‐risk factor for several complications such as infections, venous thromboembolism, and respiratory complications after OLR. 10 , 41 , 42 In this study, there was no significant difference in terms of complications associated with OLR among the four BMI groups despite a greater proportion of comorbidities, including DM. This phenomenon can be attributed to controlling comorbidities before surgery and preventing surgical site infection. 43 , 44
Moreover, even after adjusting for confounding variables between the LLR and OLR groups, there was no difference in terms of the incidence of major complications among underweight patients in the LLR and OLR groups. Some studies have shown that the risk of postoperative complications after abdominal or non‐abdominal surgery according to BMI has a reverse J‐shape relationship, with underweight and morbidly obese individuals showing the highest rates and overweight and moderately obese individuals showing the lowest rates. 45 , 46 , 47 Yu et al 33 revealed that underweight patients who underwent LLR had a higher complication rate than normal body weight patients, which corresponded with our results. These phenomena are referred to as the obesity paradox, and its cause has not been fully elucidated. However, underweight‐induced hypoalbuminemia, sarcopenia, frailty, malnutrition, and a poor immune function may be correlated with poor outcomes after surgery. 48 , 49 , 50 , 51 In this study, the albumin level was basically good because the liver function reserve (e.g. the Child‐Pugh classification) was assessed before hepatectomy. In fact, there was no difference in albumin level among the BMI groups in patients who underwent LLR. In addition, we did not evaluate sarcopenia or frailty; thus, we could not elucidate the cause of the higher proportion of major complications in the underweight group after LLR in our own study. However, we could suggest that sufficient operative space could be obtained during LLR, even in overweight and obese patients, and that this was associated with surgical safety and a decreased incidence of major complications.
The current study had several limitations. That is, it has a retrospective design, and the number of patients was large. Moreover, the research was conducted at a single institution. A BMI of >40 kg/m2 was considered a risk factor for in‐hospital death. 52 However, none of the patients had a BMI of >40 kg/m2. Further, LLR was safely performed on each eligible patient. LLR and OLR have different indications according to patient and tumor characteristics, both of which may act as independent risk factors for postoperative complications. Nevertheless, the results of this study should be validated by performing multicenter and international studies.
5. CONCLUSION
Laparoscopic liver resection is superior to OLR in overweight and obese patients in regard to decrease in incidences of postoperative major complications.
DISCLOSURE
Funding
No funding was received for this study.
Conflict of Interest
Authors declare no conflict of interests for this article.
Disclosure of Ethical Statements
This research was approved by the Ethics Committee of the institution (Committee of Osaka City University Graduate School of Medicine, Approval No. 1646) and it conforms to the provisions of the Declaration of Helsinki. Informed consent was obtained from all the subjects and/or guardians.
Author Contributions
Project development: A. Ishihara, S. Tanaka, H. Shinkawa, and S. Kubo; Acquisition of data: A. Ishihara, S. Tanaka, H. Shinkawa, H. Yoshida, S. Takemura, R. Amano, K. Kimura, G. Ohira, and K. Nishio. Analysis and interpretation of data: A. Ishihara, S. Tanaka, H. Shinkawa, H. Yoshida, S. Kubo. Drafting of the manuscript: A. Ishihara and S. Tanaka. Editing of the manuscript: All authors. All authors have approved the final manuscript and agree to be accountable for all aspects of the work.
Supporting information
Table S1
ACKNOWLEDGEMENTS
This work was supported by JSPS KAKENHI (Grant Number 19K09152) and The Health, Labour and Welfare Policy Research Grants from the Ministry of Health, Labour, and Welfare of Japan (Policy Research for Hepatitis Measures [H30‐Kansei‐Shitei‐003]).
Ishihara A, Tanaka S, Shinkawa H, Yoshida H, Takemura S, Amano R, et al. Superiority of laparoscopic liver resection to open liver resection in obese individuals with hepatocellular carcinoma: A retrospective study. Ann Gastroenterol Surg.2022;6:135–148. 10.1002/ags3.12506
REFERENCES
- 1. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319(16):1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO Regional Office for Europe . Obesity ‐ Data and statistics [Internet]. Copenhagen, Denmark. c2021 [cited July 12 2021]. Available from: https://www.euro.who.int/en/health‐topics/noncommunicable‐diseases/obesity/data‐and‐statistics
- 3. McCurry J. Japan battles with obesity. Lancet. 2007;369(9560):451–2. [DOI] [PubMed] [Google Scholar]
- 4. Ministry of Health, Labour and Welfare of Japan . Report of national health and nutrition [Internet]. Tokyo, Japan; 2020. [cited 12 July 2021]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/h30‐houkoku_00001.html
- 5. Dindo D, Muller MK, Weber M, Clavien P‐A. Obesity in general elective surgery. Lancet. 2003;361(9374):2032–5. [DOI] [PubMed] [Google Scholar]
- 6. Mullen JT, Davenport DL, Hutter MM, Hosokawa PW, Henderson WG, Khuri SF, et al. Impact of body mass index on perioperative outcomes in patients undergoing major intra‐abdominal cancer surgery. Ann Surg Oncol. 2008;15(8):2164–72. [DOI] [PubMed] [Google Scholar]
- 7. Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta‐analysis of cohort studies. Br J Cancer. 2007;97(7):1005–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berentzen TL, Gamborg M, Holst C, Sørensen TIA, Baker JL. Body mass index in childhood and adult risk of primary liver cancer. J Hepatol. 2014;60(2):325–30. [DOI] [PubMed] [Google Scholar]
- 9. Gedaly R, McHugh PP, Johnston TD, Jeon H, Ranjan D, Davenport DL. Obesity, diabetes, and smoking are important determinants of resource utilization in liver resection: a multicenter analysis of 1029 patients. Ann Surg. 2009;249(3):414–9. [DOI] [PubMed] [Google Scholar]
- 10. Cucchetti A, Cescon M, Ercolani G, Di Gioia P, Peri E, Pinna AD. Safety of hepatic resection in overweight and obese patients with cirrhosis. Br J Surg. 2011;98(8):1147–54. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka S, Kawaguchi Y, Kubo S, Kanazawa A, Takeda Y, Hirokawa F, et al. Validation of index‐based IWATE criteria as an improved difficulty scoring system for laparoscopic liver resection. Surgery. 2019;165(4):731–40. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka S, Kubo S, Kanazawa A, Takeda Y, Hirokawa F, Nitta H, et al. Validation of a difficulty scoring system for laparoscopic liver resection: a multicenter analysis by the endoscopic liver surgery study group in Japan. J Am Coll Surg. 2017;225(2):249–58. [DOI] [PubMed] [Google Scholar]
- 13. Nomi T, Fuks D, Ferraz JM, Kawaguchi Y, Nakajima Y, Gayet B. Influence of body mass index on postoperative outcomes after laparoscopic liver resection. Surg Endosc. 2015;29(12):3647–54. [DOI] [PubMed] [Google Scholar]
- 14. Toriguchi K, Hatano E, Sakurai T, Seo S, Taura K, Uemoto S. Laparoscopic liver resection in obese patients. World J Surg. 2015;39(5):1210–5. [DOI] [PubMed] [Google Scholar]
- 15. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization . Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva: World Health Organization; 2000. [PubMed]
- 17. Tanaka S, Hirohashi K, Tanaka H, Shuto T, Lee SH, Kubo S, et al. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg. 2002;195(4):484–9. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka S, Takemura S, Shinkawa H, Nishioka T, Hamano G, Kinoshita M, et al. Outcomes of pure laparoscopic versus open hepatic resection for hepatocellular carcinoma in cirrhotic patients: a case‐control study with propensity score matching. Eur Surg Res. 2015;55(4):291–301. [DOI] [PubMed] [Google Scholar]
- 19. Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB (Oxford). 2000;2(3):333–9. [Google Scholar]
- 20. Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: the Louisville statement, 2008. Ann Surg. 2009;250(5):825–30. [DOI] [PubMed] [Google Scholar]
- 21. Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus: the committee of the Japan diabetes society on the diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1(5):212–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235–481. [DOI] [PubMed] [Google Scholar]
- 23. Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, et al. Japan atherosclerosis society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25(9):846–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the international study group of liver surgery. Surgery. 2011;149(5):680–8. [DOI] [PubMed] [Google Scholar]
- 25. Tanaka S, Kubo S, Tsukamoto T, Hirohashi K, Tanaka H, Shuto T, et al. Risk factors for intractable pleural effusion after liver resection. Osaka City Med J. 2004;50(1):9–18. [PubMed] [Google Scholar]
- 26. Tanaka S, Iida H, Ueno M, Hirokawa F, Nomi T, Nakai T, et al. Preoperative risk assessment for loss of independence following hepatic resection in elderly patients: a prospective multicenter study. Ann Surg. 2021;13(3):e253–61. [DOI] [PubMed] [Google Scholar]
- 27. Rahbari NN, Garden OJ, Padbury R, Brooke‐Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the international study group of liver surgery (ISGLS). Surgery. 2011;149(5):713–24. [DOI] [PubMed] [Google Scholar]
- 28. Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The "50‐50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242(6):824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 30. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–16. [DOI] [PubMed] [Google Scholar]
- 31. Mikami S, Hamano T, Fujii N, Nagasawa Y, Isaka Y, Moriyama T, et al. Serum osteoprotegerin as a screening tool for coronary artery calcification score in diabetic pre‐dialysis patients. Hypertens Res. 2008;31(6):1163–70. [DOI] [PubMed] [Google Scholar]
- 32. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu X, Yu H, Fang X. The impact of body mass index on short‐term surgical outcomes after laparoscopic hepatectomy, a retrospective study. BMC Anesthesiol. 2016;16(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 35. Tanaka S, Iimuro Y, Hirano T, Hai S, Suzumura K, Nakamura I, et al. Safety of hepatic resection for hepatocellular carcinoma in obese patients with cirrhosis. Surg Today. 2013;43(11):1290–7. [DOI] [PubMed] [Google Scholar]
- 36. Langella S, Russolillo N, Forchino F, Lo Tesoriere R, D'Eletto M, Ferrero A. Impact of obesity on postoperative outcome of hepatic resection for colorectal metastases. Surgery. 2015;158(6):1521–9. [DOI] [PubMed] [Google Scholar]
- 37. Balzan S, Nagarajan G, Farges O, Galleano CZ, Dokmak S, Paugam C, et al. Safety of liver resections in obese and overweight patients. World J Surg. 2010;34(12):2960–8. [DOI] [PubMed] [Google Scholar]
- 38. Uchida H, Iwashita Y, Saga K, Takayama H, Watanabe K, Endo Y, et al. Benefit of laparoscopic liver resection in high body mass index patients. World J Gastroenterol. 2016;22(10):3015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Soubrane O, Schwarz L, Cauchy F, Perotto LO, Brustia R, Bernard D, et al. A conceptual technique for laparoscopic right hepatectomy based on facts and oncologic principles: the caudal approach. Ann Surg. 2015;261(6):1226–31. [DOI] [PubMed] [Google Scholar]
- 40. Tomishige H, Morise Z, Kawabe N, Nagata H, Ohshima H, Kawase J, et al. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy‐specific view. World J Gastrointest Surg. 2013;5(6):173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang G, Staercke CD, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;02(04):499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang T, Tu PA, Zhang H, Lu JH, Shen YN, Yuan SX, et al. Risk factors of surgical site infection after hepatic resection. Infect Control Hosp Epidemiol. 2014;35(3):317–20. [DOI] [PubMed] [Google Scholar]
- 43. Vogt AP, Bally L. Perioperative glucose management: current status and future directions. Best Pract Res Clin Anaesthesiol. 2020;34(2):213–24. [DOI] [PubMed] [Google Scholar]
- 44. Takayama T, Aramaki O, Shibata T, Oka M, Itamoto T, Shimada M, et al. Antimicrobial prophylaxis for 1 day versus 3 days in liver cancer surgery: a randomized controlled non‐inferiority trial. Surg Today. 2019;49(10):859–69. [DOI] [PubMed] [Google Scholar]
- 45. Mullen JT, Moorman DW, Davenport DL. The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg. 2009;250(1):166–72. [DOI] [PubMed] [Google Scholar]
- 46. Tjeertes EK, Hoeks SE, Beks SB, Valentijn TM, Hoofwijk AG, Stolker RJ. Obesity–a risk factor for postoperative complications in general surgery? BMC Anesthesiol. 2015;15(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Benjamin ER, Dilektasli E, Haltmeier T, Beale E, Inaba K, Demetriades D. The effects of body mass index on complications and mortality after emergency abdominal operations: the obesity paradox. Am J Surg. 2017;214(5):899–903. [DOI] [PubMed] [Google Scholar]
- 48. Wang H, Yang J, Zhang X, Yan L, Yang J. Liver resection in hepatitis B‐related hepatocellular carcinoma: clinical outcomes and safety in overweight and obese patients. PLoS One. 2014;9(6):e99281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100(11):1523–30. [DOI] [PubMed] [Google Scholar]
- 50. Ji F, Liang Y, Fu S, Chen D, Cai X, Li S, et al. Prognostic value of combined preoperative prognostic nutritional index and body mass index in HCC after hepatectomy. HPB (Oxford). 2017;19(8):695–705. [DOI] [PubMed] [Google Scholar]
- 51. Tanaka S, Ueno M, Iida H, Kaibori M, Nomi T, Hirokawa F, et al. Preoperative assessment of frailty predicts age‐related events after hepatic resection: a prospective multicenter study. J Hepatobiliary Pancreat Sci. 2018;25(8):377–87. [DOI] [PubMed] [Google Scholar]
- 52. Saunders JK, Rosman AS, Neihaus D, Gouge TH, Melis M. Safety of hepatic resections in obese veterans. Arch Surg. 2012;147(4):331–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


