Abstract
Background
The advanced lung cancer inflammation index (ALI), which comprehensively evaluates the patient body composition and inflammation/nutritional status, is reportedly associated with the patient outcome in lung cancer. However, the clinical significance in colorectal cancer (CRC) patients after curative resection remains unclear.
Methods
A total of 813 CRC patients after curative resection between April 2005 and June 2019 in a single institution were retrospectively enrolled. The association of the preoperative ALI (calculated as follows: body mass index × albumin value/neutrophil‐to‐lymphocyte ratio) with clinicopathological factors, postoperative complications, and survival was analyzed.
Results
A low ALI was significantly associated with male gender, older age, a higher depth of tumor invasion, progressed TNM stage, and preoperative carcinoembryonic antigen (CEA) positivity. Both postoperative complications and severe complications occurred more frequently in the ALI‐low group than in the ALI‐high group (P < .001 and P < .001, respectively), especially postoperative complications in stage III patients (P < .001) and severe complications in stages II and III patients (P = .024 and P = .004, respectively). In addition, a low ALI was an independent predictor of a poor overall survival (hazard ratio: 2.30, 95% confidence interval: 1.52–3.50, P < .001) and relapse‐free survival (hazard ratio: 1.73, 95% confidence interval: 1.22–2.44, P = .002), especially in older patients, and in patients without lymph node metastasis or severe postoperative complications.
Conclusion
This study suggests that preoperative ALI may serve as a novel independent predictive index for severe postoperative complications and recurrence in CRC patients after curative resection.
Keywords: advanced lung cancer inflammation index, colorectal cancer, postoperative complication, recurrence, systemic inflammation
The preoperative ALI may serve as a novel independent predictive index for severe postoperative complications and recurrence in CRC patients after curative resection.
1. INTRODUCTION
Tumor factors, such as the TNM stage, and pathological and molecular features, are strongly associated with cancer progression and patient outcome. Similarly, in recent years the importance of host factors has become known. Weight loss, undernutrition, and systemic inflammation in cancer patients are common conditions and can reportedly predict patient outcome. 1 , 2 In colorectal cancer (CRC), a low body mass index (BMI), hypoalbuminemia, and high inflammation status have been reported to be predictive markers for postoperative complications, recurrence, and a poor prognosis. 3 , 4 , 5 , 6 In addition, recent basic research has unveiled an association between such host factors and the host immune status. 7 Therefore, it is important for cancer treatment strategies to evaluate host factors known to be related to patient outcome. However, there have been few reports on predictive indices that comprehensively indicate the body composition, nutrition, and systemic inflammation status in CRC patients.
The advanced lung cancer inflammation index (ALI) was initially reported as a screening tool for patients with non‐small‐cell lung cancer. This index is based on the patient's BMI, serum albumin level, and neutrophil‐to‐lymphocyte ratio (NLR) (ALI; BMI × albumin value/NLR). 8 Its predictive value has been reported in patients after curative resection with lung cancer, 9 head and neck cancer, 10 esophageal cancer, 11 and gastric cancer. 12 In CRC, Shibutani et al 13 reported the utility of the ALI as a prognosticator in unresectable metastatic CRC, and Xie et al 14 reported its utility as a predictor for the postoperative outcome. Recently, Kusunoki et al 15 reported that low ALI status was significantly associated with the prognosis in CRC patients after resection. However, no study has focused on the association of the ALI with postoperative severe complications and recurrence in CRC patients after curative resection.
Given the above, we hypothesized that the preoperative ALI could predict postoperative severe complications and recurrence in CRC patients who underwent potentially curative resection. To test this hypothesis, we used a dataset of 813 CRC cases from a single institution and examined the association of the preoperative ALI with the clinicopathological factors, postoperative complications, and survival outcome in CRC patients after curative resection.
2. PATIENTS AND METHODS
2.1. Patients and study design
A total of 1013 consecutive CRC patients who underwent resection at Kumamoto University Hospital (Kumamoto, Japan) between April 2005 and June 2019 were enrolled. Of them, 813 who underwent potentially curative resection were enrolled in this study. Figure 1A shows the inclusion/exclusion criteria in this study. The surgical procedures were based on the Japanese colorectal cancer treatment guidelines. 16 , 17 , 18 TNM staging was based on the 7th edition of the Union for International Cancer Control classification. 19
FIGURE 1.
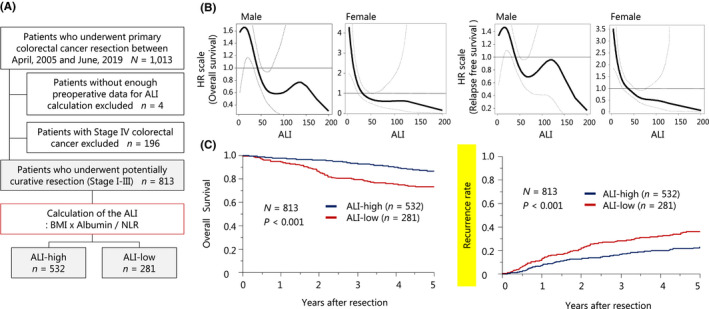
The preoperative ALI and long‐term patient outcome. (A) Flow chart for this study (inclusion/exclusion criteria). (B) Spline plots showing the HR for the overall survival and RFS using the preoperative ALI. (C) The probabilities (Kaplan–Meier plots) for the overall survival and recurrence rate. ALI, advanced lung cancer inflammation index; BMI, body mass index; HR, hazard ratio; NLR, neutrophil‐to‐lymphocyte ratio
The protocol of this study was approved by the Human Ethics Review Committee of the Graduate School of Medicine, Kumamoto University (Institutional Review Board number 1047), and carried out according to the Declaration of Helsinki and Good Clinical Practice Guidelines.
2.2. Clinicopathological factors and preoperative serum data
We collected and tested serum samples obtained within 2 wk before surgery. Laboratory measurements included albumin (g/dL), total neutrophil count (TNC; /mm3), total lymphocyte count (TLC; /mm3), carcinoembryonic antigen (CEA; ng/mL), and carbohydrate antigen 19‐9 (CA19‐9: U/mL). Positivity of CEA (>5.0 ng/mL) and CA19‐9 (>37 U/mL) was defined based on past reports. 20 , 21 The BMI (kg/m2) was calculated from the preoperative patient height (m) and weight (kg), measured by our clinical staff on the date of admission. The NLR was calculated by dividing the TNC by the TLC. The ALI was calculated as follows: BMI × albumin value/NLR. The cutoff value was calculated separately for male and female patients based on past reports. 14 In this study we used a classification and regression tree (CART) analysis for the patient overall survival (OS) to determine the optimal cutoff value of ALI. The value for males was 43.099, and we defined ≤43.099 as a low ALI and >43.099 as a high ALI. The value for females was 13.197, and we defined ≤13.197 as a low ALI and >13.197 as a high ALI (Figure S1).
2.3. Statistical analyses
All statistical analyses were performed with JMP v. 10 (SAS Institute, Cary, NC), or R V. 3.6.3 (R Development Core Team, Vienna, Austria). We performed all analyses in patients who underwent potentially curative resection and had the necessary preoperative data for calculating the ALI (n = 813, Stages I–III, Figure 1A). We analyzed categorical variables using the chi‐square test and continuous variables using Student's t‐test. Spline plots showed the hazard ratio (HR) of death or relapse as a continuous function of the ALI. The OS was defined as the interval from the date of resection to the date of death from any cause, and the relapse‐free survival (RFS) was defined as the interval from the date of resection to the first date of confirmed recurrence or death. The Kaplan–Meier method and log‐rank test were used for the survival analysis. Severe postoperative complications were defined as those of Clavien–Dindo classification ≥III. A multivariate Cox proportional hazards analysis (using the maximum likelihood model) was adjusted for the sex, age, tumor location, depth of tumor invasion, lymph node metastasis, and severe postoperative complications to calculate the HR and 95% confidence intervals (CIs) for the ALI status.
3. RESULTS
3.1. Association between the preoperative ALI and patient prognosis
We first examined the association between the preoperative ALI and patient prognosis. Spline plots showed the estimated shapes of the HR for death or relapse as a continuous function of the ALI (Figure 1B). For both death and relapse, the ALI showed a declining pattern in spline plots, regardless of gender, such that the risk gradually decreased as the ALI value increased. Therefore, we performed a CART analysis to determine the cutoff value of the ALI depending on the sex and separated all cases into two populations: ALI‐high, n = 532; and ALI‐low, n = 281 (Figure 1A, Figure S1). Patients with a high ALI had significantly longer OS and RFS than those with a low ALI (Figure 1C). In the patients with a high ALI and those with a low ALI, the 5‐year OS rates were 86.5% and 73.1% (P < .001), respectively, while the 5‐year RFS rates were 77.3% and 63.9% (P < .001), respectively.
3.2. Association of the preoperative ALI with the clinicopathological factors and postoperative complications
Next we examined the association of the preoperative ALI with clinicopathological factors and postoperative complications. Table 1 shows the association between the preoperative ALI and clinicopathological factors. A low ALI was significantly associated with male gender (P < .001), older age (P = .016), deeper tumor invasion (P < .001), a progressed TNM stage (P < .001), and CEA positivity (>5.0 ng/mL) (P < .001).
TABLE 1.
Association between ALI and clinicopathological factors
| Characteristics |
Overall n = 813 |
ALI‐high n = 532 |
ALI‐low n = 281 |
P value a |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Sex | ||||
| Male | 464 (57%) | 219 (41%) | 245 (87%) | <.001 |
| Female | 349 (43%) | 313 (59%) | 36 (13%) | |
| Age | ||||
| <65 | 291 (36%) | 206 (39%) | 85 (30%) | .016 |
| ≥65 | 522 (64%) | 326 (61%) | 196 (70%) | |
| BMI (kg/m2) | ||||
| <18.5 | 95 (12%) | 54 (10%) | 41 (15%) | <.001 |
| 18.5≤, <25 | 536 (66%) | 328 (62%) | 208 (74%) | |
| ≥25 | 182 (22%) | 150 (28%) | 32 (11%) | |
| Tumor location | ||||
| Right side | 264 (32%) | 161 (30%) | 103 (37%) | .065 |
| Left side | 549 (68%) | 371 (70%) | 178 (63%) | |
| Depth of tumor invasion | ||||
| T1 | 198 (24%) | 154 (29%) | 44 (16%) | <.001 |
| T2 | 160 (20%) | 115 (22%) | 45 (16%) | |
| T3 | 342 (42%) | 205 (38%) | 137 (49%) | |
| T4 | 113 (14%) | 58 (11%) | 55 (19%) | |
| Lymph node metastasis | ||||
| Negative | 580 (71%) | 372 (70%) | 205 (73%) | .364 |
| Positive | 233 (29%) | 160 (30%) | 76 (27%) | |
| Stage | ||||
| I | 307 (38%) | 226 (43%) | 81 (29%) | <.001 |
| II | 274 (34%) | 149 (28%) | 125 (44%) | |
| III | 232 (28%) | 157 (29%) | 75 (27%) | |
| CEA (ng/mL) | ||||
| ≤5 | 590 (73%) | 412 (77%) | 178 (63%) | <.001 |
| >5 | 223 (27%) | 120 (23%) | 103 (37%) | |
| CA19‐9 (U/mL) | ||||
| ≤37 | 685 (84%) | 451 (85%) | 234 (83%) | .578 |
| >37 | 128 (16%) | 81 (15%) | 47 (17%) | |
Abbreviations: ALI, advanced lung cancer inflammation index; CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen.
P value was based on the chi‐square test for categorical factors.
Figure 2 shows the association between the preoperative ALI and postoperative complications. Postoperative complications occurred in 275 patients (33.8%), including stage I in 86 (28.1%), stage II in 102 (37.5%), and stage III in 87 (37.2%); and severe postoperative complications (defined as Clavien–Dindo classification ≥III) occurred in 116 patients (14.3%), including stage I in 33 (10.8%), stage II in 47 (17.3%), and stage III in 36 (15.4%). Both postoperative complications and severe complications occurred more frequently in the ALI‐low group than in the ALI‐high group (P < .001; Figure 2A, and P < .001; Figure 2B). In addition, in the subgroup analysis the rate of postoperative complications in patients with stage III CRC was significantly higher in the ALI‐low group than in the ALI‐high group (P < .001; Figure 2A), while that of severe postoperative complications in patients with stage II and stage III CRC was significantly higher in the ALI‐low group than in the ALI‐high group (P = .024 and P = .004; Figure 2B).
FIGURE 2.
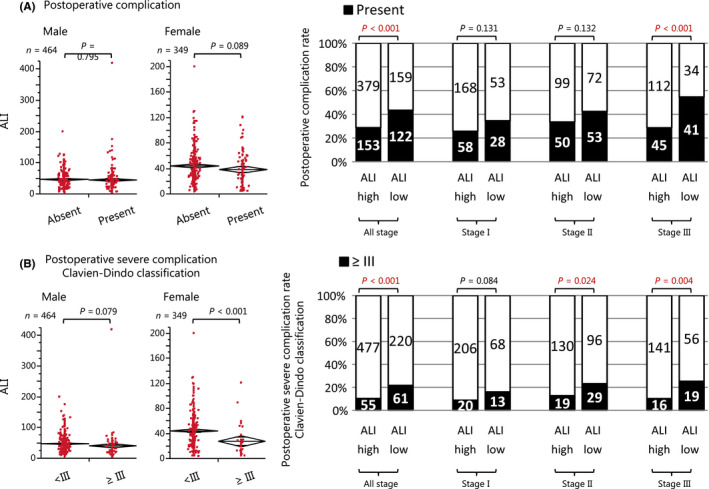
The preoperative ALI and short‐term patient outcome. (A) The association between the preoperative ALI and postoperative complications. (B) The association between the preoperative ALI and severe postoperative complications (Clavien–Dindo classification ≥III). ALI, advanced lung cancer inflammation index
3.3. Clinical impact of the preoperative ALI on the survival outcome
Finally, we examined the independent clinical impact of the preoperative ALI on the survival outcome. In multivariate analysis (Table 2), a low ALI was a significant predictor of a higher OS mortality (HR: 2.30, 95% CI: 1.52‐3.50, P < .001), as was an older age (P < .001), lymph node metastasis (P = .046), and severe postoperative complications (P = .047). Similarly, a low ALI was a significant predictor of a higher RFS mortality (HR: 1.73, 95% CI: 1.22–2.44, P = .002), as was female gender (P = .031), older age (P < .001), deeper tumor invasion (P < .001), lymph node metastasis (P < .001), and severe postoperative complications (P = .008). In the subgroup analysis, regarding the OS, all patients had a risk benefit with a high ALI, except for younger patients and patients with severe postoperative complications (Figure 3A). In addition, regarding the RFS, all patients had a risk benefit with a high ALI, except for younger patients, patients with deeper tumor invasion, positive lymph node metastasis, and severe postoperative complications (Figure 3B). A high ALI was an independent predictor with quantitative interaction for a better OS in older patients (P < .001, P for interaction = .016), and for a better OS and RFS in patients without lymph node metastasis (OS: P < .001, P for interaction = .002, and RFS: P < .001, P for interaction < .001), or severe postoperative complication (OS: P < .001, P for interaction = .002, and RFS: P = .001, Pfor interaction = .002).
TABLE 2.
Association between ALI and patient survival outcome
| Clinicopathological factors |
Univariate HR (95% CI) |
P value |
Multivariate HR (95% CI) a |
P value | |
|---|---|---|---|---|---|
| Overall survival | |||||
| Sex | Male/Female | 1.13 (0.79–1.61) | .496 | 0.75 (0.49–1.14) | .174 |
| Age | ≥65/<65 | 2.14 (1.45–3.25) | <.001 | 1.96 (1.32–3.00) | <.001 |
| Tumor location | Right side/left‐side | 1.41 (0.99–1.99) | .055 | 1.22 (0.85–1.74) | .286 |
| Depth of tumor invasion | T3‐T4/T1‐T2 | 1.59 (1.12–2.30) | .009 | 1.20 (0.82–1.77) | .346 |
| Lymph node metastasis | Positive/negative | 1.52 (1.06–2.17) | .024 | 1.47 (1.01–2.12) | .046 |
| Postoperative complication | CD classification ≥III/<III | 1.73 (1.12–2.58) | .015 | 1.57 (1.01–2.39) | .047 |
| ALI | Low/High | 2.27 (1.62–3.21) | <.001 | 2.30 (1.52–3.50) | <.001 |
| Relapse free survival | |||||
| Sex | Male/Female | 0.91 (0.69–1.20) | .496 | 0.69 (0.49–0.97) | .031 |
| Age | ≥65/<65 | 1.77 (1.30–2.45) | <.001 | 1.73 (1.27–2.41) | <.001 |
| Tumor location | Right side/left‐side | 1.09 (0.81–1.46) | .553 | 0.97 (0.71–1.31) | .846 |
| Depth of tumor invasion | T3‐T4/T1‐T2 | 2.47 (1.82–3.41) | <.001 | 1.86 (1.35–2.60) | <.001 |
| Lymph node metastasis | Positive/negative | 2.31 (1.74–3.05) | <.001 | 1.94 (1.45–2.60) | <.001 |
| Postoperative complication | CD classification ≥III/<III | 1.82 (1.28–2.52) | .001 | 1.64 (1.14–2.31) | .008 |
| ALI | Low/High | 1.70 (1.28–2.24) | <.001 | 1.73 (1.22–2.44) | .002 |
Abbreviations: ALI, advanced lung cancer inflammation index; CD classification, Clavien–Dindo classification; CI, confidence interval; HR, hazard ratio.
Multivariate Cox proportional hazards regression model was adjusted for sex, age, tumor location, depth of tumor invasion, lymph node metastasis, postoperative complication, and ALI status/PNI.
FIGURE 3.
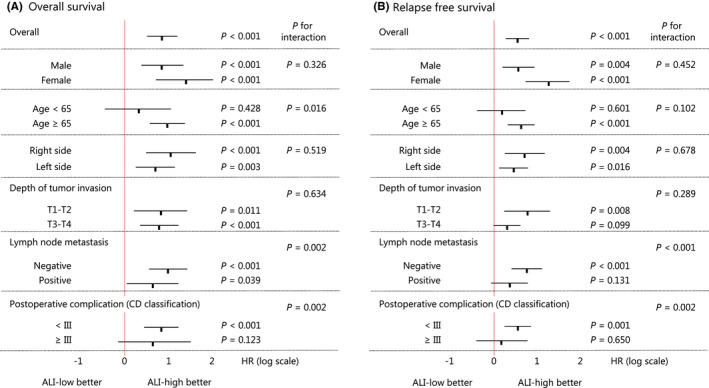
Results of a subgroup analysis according to the ALI for the long‐term patient outcome. ALI, advanced lung cancer inflammation index
4. DISCUSSION
We assessed the usability of the preoperative ALI in CRC patients after curative resection. With a retrospective review of 813 CRC cases, our study showed that a low ALI was significantly associated with postoperative complications and severe complications, especially postoperative complications in stage III CRC patients and severe complications in stages II and III CRC patients. In addition, a low ALI was an independent predictor of a poor OS and RFS, especially in older patients, and in patients without lymph node metastasis or severe postoperative complications.
In our analysis, we found that the ALI was a significant predictor of recurrence in CRC. Gastrointestinal cancer is more prone to malnutrition, hypoalbuminemia, and systemic inflammation due to tumor obstruction and consumption than other cancers, so our results are reasonable, considering previous studies that explored the usefulness of ALI for predicting the outcome in various other cancers. 9 , 10 , 11 , 12 , 15 Cancer patients are likely to be malnourished because of their high metabolism, 1 and malnutrition/cachexia is reportedly associated with an impaired immune function and poor outcome. 6 Furthermore, inflammation is known to lead to hypoalbuminemia, irrespective of the nutritional status, and hypoalbuminemia is a negative prognostic factor in cancer patients. 22 , 23 , 24 Similarly, the NLR, which measures the inflammation/immunity ratio, has been related to postoperative recurrence in various solid tumors. 25 Neutrophils develop a proinflammatory status related to tumor progression by activating inflammatory markers, such as vascular endothelial growth factor in the tumor microenvironment. 26 , 27 In contrast, the peripheral lymphocyte count reflects the immune system suppressing tumor progression and metastasis. 28 , 29 Therefore, the ALI, which is calculated based on body composition, nutrition, and systemic inflammation, may be a comprehensive indicator predicting recurrence in CRC after curative resection. Furthermore, our data showed that the ALI was particularly useful in older cases and cases without lymph node metastasis. This may aid in deciding on postoperative treatment strategies in CRC, including the introduction of adjuvant chemotherapy and attempts to improve the preoperative ALI.
Severe postoperative complications are largely associated with recurrence and are an independent prognosticator for cancer patients after curative resection. 30 Surgical invasion and severe postoperative complications induce inflammatory cytokines and contribute to cancer progression. 31 , 32 In addition, inflammatory reactions facilitate the survival of cancer cells and promote metastasis. 33 , 34 Therefore, predicting severe postoperative complications is important for developing CRC treatment strategies. Our study showed that the ALI was a novel predictor of severe postoperative complications, especially in progressed CRC patients, and additionally predicted a poor OS and RFS in patients without severe postoperative complications. This means that the usefulness of ALI is more sensitive in patients with larger surgical invasiveness and in patients without postoperative complications, a prognostic factor. Our finding may aid in the development of strategies for improving the prognosis of CRC patients. However, Xie et al 14 recently demonstrated conflicting findings, noting that the ALI was a predictor of low‐grade complications rather than severe ones. This may be due to our inclusion criteria of patients with metastatic CRC, indicating that patients with metastatic CRC are more likely to have severe complications, regardless of the ALI. In addition, the use of different cutoff values for nonmetastatic and metastatic CRC may have affected the results. To verify this, a prospective study is needed that allows for larger and more sufficient subgroup analyses.
In the present study, we found that a low ALI was significantly associated with male gender, older age, deeper tumor invasion, progressed TNM stage, and CEA positivity in CRC patients after curative resection. Among these factors, the frequency of a low ALI was significantly higher in men (52.5%; 245/464) than in women (10.3%; 36/349). This is because the optimal cutoff value, calculated using a CART analysis for the OS, is completely different between men and women (men: 43.099, women: 13.197). The optimal cutoff value of the ALI remains controversial, despite strong evidence of the practicality of the ALI through different stages of treatment in various cancers. 8 , 9 , 10 , 11 , 12 , 13 , 14 One reason for this may be the different treatment settings and tumor stages. 35 , 36 Alternatively, it may be due to the differences in the importance of the BMI by sex and the ambiguity of the BMI to assess patient body composition for cancer patients. The BMI is an established nutritional index, but some reports have shown no association between the BMI and CRC development in women. 37 , 38 In addition, the utility of the BMI is usually limited due to its inaccurate representation of body compositions. The BMI allows no distinction between fat mass and lean mass, and body weight loss is indistinct in patients with pleural effusion or body edema. Furthermore, we previously reported that the clinically meaningful body composition type differed between male and female CRC patients. 39 The ALI may be a simple and comprehensive index for assessing host factors, but further improvements are necessary, taking into account the sex as well as the fat mass and lean mass content, such as the muscle and intracellular fluid.
Colorectal cancer is the third leading cause of death from cancer worldwide, 40 and requires radical resection for cure. 41 Given our results that ALI is a predictor of postoperative severe complications and recurrence, ALI has the potential to help surgeons and oncologists in CRC treatment. Some reports have shown that preoperative interventions for the CRC patients improve the outcome. van Rooijen et al 42 reported that multimodal prehabilitation can reduce postoperative complications, and Gills et al 43 reported that nutritional prehabilitation and an exercise program significantly decreased the length of the hospital stay. Furthermore, as immune checkpoint inhibitors have resulted in breakthroughs in cancer treatment, understanding the host factors related to tumor inflammation and the immune microenvironment is important for improving cancer treatment. 44 Preoperative interventions for the improvement of ALI and evaluation of patient status using ALI might help in developing treatment strategies for CRC.
Several limitations associated with the present study warrant mention. The limited sample size in a single institution and retrospective design may cause selection bias. We were unable to compare the ALI with the various useful body composition, nutrition, and systemic inflammation markers reported in CRC. As a trial, we tested the usefulness of the prognostic nutritional index (PNI), and detected that PNI is a prognostic marker as well (cutoff value: 45, HR for OS: 2.42 and HR for RFS: 1.77). However, our aim in this study was to find out if preoperative ALI could predict postoperative severe complications and recurrence in CRC patients after curative resection, not to compare the usefulness of ALI with other markers. Furthermore, we discussed using only the preoperative ALI data and not taking the postoperative ALI into consideration. We did not consider the data, such as tumor pathological and molecular features, or basic information on the tumor microenvironment either. The ALI might be affected by other preoperative factors; for example, preoperative renal dysfunction, hepatic dysfunction, or steroid use are known to modify the ALI, as they can affect the body composition, nutrition, and systemic inflammation status. In addition, the optimal cutoff value calculated in this study is completely different between men and women. Despite the above limitations, our study revealed for the first time that ALI could predict postoperative severe complications and recurrence in CRC patients after curative resection. Further large‐scale population‐based prospective studies are required to fully validate our findings.
In conclusion, we reported that the ALI was an independent predictor of postoperative severe complications and recurrence in CRC patients after curative resection. Calculating the preoperative ALI may be useful for developing postoperative treatment strategies in CRC.
DISCLOSURE
Conflict of Interest: The authors declare no conflicts of interest for this article.
Ethical Approval: The protocol of this study was approved by the Human Ethics Review Committee of the Graduate School of Medicine, Kumamoto University (Institutional Review Board number 1047), and carried out according to the Declaration of Helsinki and Good Clinical Practice Guidelines.
Presentation: We have not presented this article elsewhere.
Supporting information
Figure S1
Horino T, Tokunaga R, Miyamoto Y, Hiyoshi Y, Akiyama T, Daitoku N, et al. The advanced lung cancer inflammation index is a novel independent prognosticator in colorectal cancer patients after curative resection. Ann Gastroenterol Surg.2022;6:83–91. 10.1002/ags3.12499
REFERENCES
- 1. Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2(11):862–71. [DOI] [PubMed] [Google Scholar]
- 2. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature. 2008;454(7203):436–44. [DOI] [PubMed] [Google Scholar]
- 3. Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil‐to‐lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol. 2017;115(4):470–9. [DOI] [PubMed] [Google Scholar]
- 4. Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: a systematic review and meta‐analysis. Tech Coloproctol. 2016;20(8):517–35. [DOI] [PubMed] [Google Scholar]
- 5. Almasaudi AS, Dolan RD, Edwards CA, McMillan DC. Hypoalbuminemia reflects nutritional risk, body composition and systemic inflammation and is independently associated with survival in patients with colorectal cancer. Cancers. 2020;12(7):1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22(8):2663–8. [DOI] [PubMed] [Google Scholar]
- 7. Alwarawrah Y, Kiernan K, MacIver NJ. Changes in nutritional status impact immune cell metabolism and function. Front Immunol. 2018;9:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non‐small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobayashi S, Karube Y, Inoue T, Araki O, Maeda S, Matsumura Y, et al. Advanced lung cancer inflammation index predicts outcomes of patients with pathological stage ia lung adenocarcinoma following surgical resection. Ann Thorac Cardiovasc Surg. 2019;25(2):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jank BJ, Kadletz L, Schnoll J, Selzer E, Perisanidis C, Heiduschka G. Prognostic value of advanced lung cancer inflammation index in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2019;276(5):1487–92. [DOI] [PubMed] [Google Scholar]
- 11. Feng JF, Huang Y, Chen QX. A new inflammation index is useful for patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2014;7:1811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yin C, Toiyama Y, Okugawa Y, Omura Y, Kusunoki Y, Kusunoki K, et al. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: a propensity score matching analysis. Clin Nutr. 2021;40(3):1130–6. [DOI] [PubMed] [Google Scholar]
- 13. Shibutani M, Maeda K, Nagahara H, Fukuoka T, Matsutani S, Kimura K, et al. The prognostic significance of the advanced lung cancer inflammation index in patients with unresectable metastatic colorectal cancer: a retrospective study. BMC Cancer. 2019;19(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xie H, Huang S, Yuan G, Kuang J, Yan L, Wei L, et al. The advanced lung cancer inflammation index predicts short and long‐term outcomes in patients with colorectal cancer following surgical resection: a retrospective study. PeerJ. 2020;8:e10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kusunoki K, Toiyama Y, Okugawa Y, Yamamoto A, Omura Y, Ohi M, et al. Advanced lung cancer inflammation index predicts outcomes of patients with colorectal cancer after surgical resection. Dis Colon Rectum. 2020;63(9):1242–50. [DOI] [PubMed] [Google Scholar]
- 16. Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17(1):1–29. [DOI] [PubMed] [Google Scholar]
- 17. Tokyo: Kanehara & Co L, The Japanese Society for Cancer of the Colon and Rectum . JSCCR Guidelines 2005 for the Treatment of Colorectal Cancer; 2005.
- 18. Tokyo: Kanehara & Co L, The Japanese Society for Cancer of the Colon and Rectum . JSCCR Guidelines 2010 for the Treatment of Colorectal Cancer; 2010. [DOI] [PubMed]
- 19. Leslie HS, Mary KG, Christian W. TNM Classification of Malignant Tumours, 7th edn. New York, NY: Wiley‐Blackwell; 2009. [Google Scholar]
- 20. Tarantino I, Warschkow R, Worni M, Merati‐Kashani K, Koberle D, Schmied BM, et al. Elevated preoperative CEA is associated with worse survival in stage I‐III rectal cancer patients. Br J Cancer. 2012;107(2):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stiksma J, Grootendorst DC, van der Linden PW. CA 19–9 as a marker in addition to CEA to monitor colorectal cancer. Clin Colorectal Cancer. 2014;13(4):239–44. [DOI] [PubMed] [Google Scholar]
- 22. Ishida S, Hashimoto I, Seike T, Abe Y, Nakaya Y, Nakanishi H. Serum albumin levels correlate with inflammation rather than nutrition supply in burns patients: a retrospective study. J Med Invest. 2014;61(3–4):361–8. [DOI] [PubMed] [Google Scholar]
- 23. Zhou QP, Li XJ. C‐reactive protein to albumin ratio in colorectal cancer: a meta‐analysis of prognostic value. Dose Response. 2019;17(4):1559325819889814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakamoto Y, Mima K, Imai K, Miyamoto Y, Tokunaga R, Akiyama T, et al. Preoperative C‐reactive protein‐to‐albumin ratio and clinical outcomes after resection of colorectal liver metastases. Surg Oncol. 2020;35:243–8. [DOI] [PubMed] [Google Scholar]
- 25. Kosumi K, Baba Y, Ishimoto T, Harada K, Nakamura K, Ohuchi M, et al. Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patients. Surg Today. 2016;46(4):405–13. [DOI] [PubMed] [Google Scholar]
- 26. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–46. [DOI] [PubMed] [Google Scholar]
- 27. Chen Y, Chen K, Xiao X, Nie Y, Qu S, Gong C, et al. Pretreatment neutrophil‐to‐lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee J, Kim DM, Lee A. Prognostic role and clinical association of tumor‐infiltrating lymphocyte, programmed death ligand‐1 expression with neutrophil‐lymphocyte ratio in locally advanced triple‐negative breast cancer. Cancer Res Treat. 2019;51(2):649–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, et al. Prognostic nutritional index, tumor‐infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271(4):693–700. [DOI] [PubMed] [Google Scholar]
- 30. Miyamoto Y, Hiyoshi Y, Tokunaga R, Akiyama T, Daitoku N, Sakamoto Y, et al. Postoperative complications are associated with poor survival outcome after curative resection for colorectal cancer: A propensity‐score analysis. J Surg Oncol. 2020;122(2):344–9. [DOI] [PubMed] [Google Scholar]
- 31. Wilmore DW. From Cuthbertson to fast‐track surgery: 70 years of progress in reducing stress in surgical patients. Ann Surg. 2002;236(5):643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsumoto Y, Tsujimoto H, Ono S, Shinomiya N, Miyazaki H, Hiraki S, et al. Abdominal infection suppresses the number and activity of intrahepatic natural killer cells and promotes tumor growth in a murine liver metastasis model. Ann Surg Oncol. 2016;23(Suppl 2):S257–65. [DOI] [PubMed] [Google Scholar]
- 33. Nojiri T, Hosoda H, Tokudome T, Miura K, Ishikane S, Otani K, et al. Atrial natriuretic peptide prevents cancer metastasis through vascular endothelial cells. Proc Natl Acad Sci USA. 2015;112(13):4086–91. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. DerHagopian RP, Sugarbaker EV, Ketcham A. Inflammatory oncotaxis. JAMA. 1978;240(4):374–5. [PubMed] [Google Scholar]
- 35. Renfro LA, Loupakis F, Adams RA, Seymour MT, Heinemann V, Schmoll HJ, et al. Body mass index is prognostic in metastatic colorectal cancer: pooled analysis of patients from first‐line clinical trials in the ARCAD database. J Clin Oncol. 2016;34(2):144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML, et al. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol. 2016;2(9):1137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta‐analysis of prospective studies. Am J Clin Nutr. 2007;86(3):556–65. [DOI] [PubMed] [Google Scholar]
- 38. Buron Pust A, Alison R, Blanks R, Pirie K, Gaitskell K, Barnes I, et al. Heterogeneity of colorectal cancer risk by tumour characteristics: large prospective study of UK women. Int J Cancer. 2017;140(5):1082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tokunaga R, Nakagawa S, Miyamoto Y, Ohuchi M, Izumi D, Kosumi K, et al. The clinical impact of preoperative body composition differs between male and female colorectal cancer patients. Colorectal Dis. 2020;22(1):62–70. [DOI] [PubMed] [Google Scholar]
- 40. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 41. Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. 2019;19(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos‐Reed N, et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta‐analysis. Gastroenterology. 2018;155(2):391–410 e4. [DOI] [PubMed] [Google Scholar]
- 44. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): an open‐label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


