Abstract
Aim
This study aimed to evaluate the indications of laparoscopic repeat liver resection (LRLR) for recurrent hepatocellular carcinoma from the viewpoint of its difficulty.
Methods
One hundred and one patients who underwent LRLR and 59 patients who underwent open repeat liver resection (ORLR) were included. The difficulty was classified according to the preoperative predictive factors for difficult LRLR, including an open approach during previous liver resection, history of two or more previous liver resections, history of previous major liver resection, tumor near the resected site of the previous liver resection, and intermediate or high difficulty with the difficulty scoring system. We compared the surgical outcomes between the LRLR and ORLR groups based on the difficulty class (low‐ or intermediate difficiulty class, 0 to 3 predictive factors; high difficiulty class, 4 or 5 factors).
Results
In the low‐ or intermediate difficiulty class, intraoperative blood loss and the proportion of patients with postoperative complications were significantly lower in LRLR than in ORLR, and the duration of the postoperative hospital stay was significantly shorter in LRLR than in ORLR. In the high difficiulty class, total operative time and operative time before starting hepatic parenchymal resection were significantly longer in LRLR than in ORLR, and there were no significant differences in other surgical outcomes between the two groups.
Conclusion
LRLR is recommended for patients in the low or intermediate difficulty class. However, LRLR does not have an advantage with longer operative time for patients in the high difficulty class compared with ORLR.
Keywords: difficulty scoring system, laparoscopic repeat liver resection, recurrent hepatocellular carcinoma
Laparoscopic repeat liver resection (LRLR) is recommended for patients in our suggested low or intermediate difficulty class. On the other hands, LRLR does not have an advantage with longer operative time for patients in the high difficulty class, compared with open repeat liver resection.

1. INTRODUCTION
Liver resection is commonly accepted as a curative treatment for hepatocellular carcinoma (HCC), which is usually featured in treatment guidelines. 1 , 2 Although repeat liver resection has also been accepted as an effective treatment for recurrent HCC (HCCR) in patients with preserved liver function, 3 , 4 , 5 , 6 it is a challenging clinical procedure because the adhesion around the previous liver resection site can often make operative procedures difficult and sometimes results in incidental complications. 7 , 8
Laparoscopic liver resection (LLR) is a minimally invasive treatment modality and has demonstrated feasible short‐ and long‐term outcomes comparable to open liver resection. 9 , 10 , 11 , 12 Recently, the difficulty scoring system (DSS) for initial LLR has been proposed based on the experience at three high‐volume centers in Japan. 13 A multicenter validation study demonstrated that the DSS could predict the difficulty of surgical procedures and short‐term outcomes in patients who underwent initial LLR. 14 Although most previous studies have demonstrated the safety of laparoscopic repeat liver resection (LRLR) for patients comparable to initial LLR or open repeat liver resection (ORLR), 15 , 16 , 17 , 18 , 19 , 20 , 21 it is difficult to compare the advantages and disadvantages of LRLR because the difficulty of LRLR is different from those of initial LLR and ORLR. Therefore, the feasible indication of LRLR remains unclear.
Some previous studies have shown that unfavorable surgical outcomes, such as a long operative time and large intraoperative blood loss, after ORLR or LRLR were associated with a history of previous open liver resection, two or more previous liver resections, the relationship between current and previous locations of liver tumor, and a high DSS score. 15 , 22 , 23 , 24 , 25 Our previous study revealed five preoperative predictive factors for difficult LRLR: a history of an open approach during previous liver resection, history of two or more previous liver resections, history of previous major liver resection, tumor near the resected site of the previous liver resection, and intermediate or high difficulty in the DSS. 26 These are similar to the risk factors for unfavorable surgical outcomes as described above.
This study aimed to evaluate the indications of LRLR by comparing intra‐ and postoperative outcomes of LRLR with those of ORLR, based on risk factors for unfavorable surgical outcomes, including the difficulty classification of LRLR reported in our previous study.
2. METHODS
2.1. Study population
A total of 244 patients underwent repeat liver resection for HCCR at the Departments of Hepato‐Biliary‐Pancreatic Surgery, Osaka City University Hospital and Osaka City General Hospital between 2010 and 2019. To eliminate operative bias, the patients who underwent repeat liver resection that involved segmentectomy or extensive surgery, noncurative resection, concomitant resection of other organs (except for the gallbladder), resection of multiple lesions, and resection of the caudate lobe (segment 1) were excluded from this study. A total of 160 patients who underwent repeat partial liver resection were included in the study (101 underwent LRLR and 59 underwent ORLR). The median age of the subjects was 70 (range, 32–87) years, and 134 subjects were male. Of the 160 patients, 76 were seropositive for anti‐hepatitis C virus antibody and 42 were seropositive for hepatitis B surface antigen. Eleven patients had alcoholic hepatitis, and five had nonalcoholic steatohepatitis.
This study conformed to the ethical guidelines of the Declaration of Helsinki and was retrospective in nature, and we obtained approval from the Ethics Committees of Osaka City University (No. 3166) and Osaka City General Hospital (No. 1910076). All participants provided written informed consent.
2.2. Preoperative risk (predictive) factors for unfavorable surgical outcomes and difficulty classification for LRLR
The preoperative risk factors for unfavorable surgical outcomes after LRLR were selected based on previous studies 15 , 22 , 23 , 24 , 25 and our own experiences, including our previous study. 26 The risk factors in the previous studies 15 , 22 , 23 , 24 , 25 included a history of previous open liver resection, two or more previous liver resections, relationship between current and previous locations of liver tumor, and a high DSS score. Our previous study 26 showed that a history of an open liver resection, history of two or more previous liver resections, history of previous major liver resection (not less than sectionectomy), tumor near the resected site of the previous liver resection (a tumor was classified if it was in the same segment as the previous liver resection site or in an adjacent segment), and intermediate or high difficulty in the DSS were preoperative predictive factors for difficult LRLR. Therefore, the suggested preoperative predictive factors for difficult LRLR 26 overlapped with the previous reported risk factors for unfavorable surgical outcomes of repeat liver resection. 15 , 22 , 23 , 24 , 25 Similarly in this study, in patients who underwent LRLR, all five preoperative predictive factors (history of previous open liver resection, history of two or more liver resections, history of previous major liver resection, tumor near the resected site of the previous liver resection, and intermediate or high difficulty in the DSS) were indicated as independent risk factors for prolonged operative time and/or severe adhesion (Table 1). Prolonged operative time had been defined as >321 min (equivalent to the 75th percentile for the study population; n = 25). Severe adhesion had been defined by the occurrence of one or more of three situations: the patient required >120 min before the start of liver dissection; injury occurred to other organs due to the dissection procedure; or the patient required conversion to open surgery because of the adhesion. Accordingly, 27 patients were classified as having severe adhesion. 26
TABLE 1.
Associations between five predictive factors for LRLR and surgical outcomes in patients who underwent LRLR
| Variables | Prolonged operative time | Severe adhesion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||
| n (%) | P‐value | OR | 95% CI | P‐value | n (%) | P‐value | OR | 95% CI | P‐value | |
| Approach of previous liver resection | ||||||||||
| Laparoscopic (n = 52) | 7 (13) | 6 (12) | ||||||||
| Open (n = 49) | 18 (37) | .0061 | 5.2 | 1.69‐18.1 | .0035 | 21 (43) | .0003 | 5.8 | 1.90‐19.9 | .0016 |
| Number of previous liver resections | ||||||||||
| One (n = 78) | 15 (19) | 18 (23) | ||||||||
| Two or more (n = 23) | 10 (43) | .018 | 4 | 1.15‐15.3 | .029 | 9 (39) | .14 | |||
| Tumor near the resected surface of previous liver resection | ||||||||||
| No (n = 42) | 4 (9.5) | 5 (12) | ||||||||
| Yes (n = 59) | 21 (36) | .0044 | 2.3 | 0.625‐9.75 | .21 | 22 (37) | .0032 | 4.1 | 1.14‐17.8 | .03 |
| Range of previous liver resection | ||||||||||
| less than sectionectomy (n = 81) | 18 (22) | 18 (22) | ||||||||
| not less than sectionectomy (n = 20) | 7 (35) | .26 | 9 (45) | .047 | 5.6 | 1.35‐27.6 | .017 | |||
| Difficulty score a | ||||||||||
| Low (n = 58) | 6 (10) | 9 (16) | ||||||||
| Intermediate or high (n = 43) | 19 (44) | <.0001 | 8.9 | 2.57‐37.8 | .0003 | 18 (42) | .0031 | 5.5 | 1.62‐22.9 | .0055 |
Abbreviations: 95% CI, 95% confidence interval; LRLR, laparoscopic repeat liver resection; OR, odds ratio.
According to the difficulty scoring system. 13
In our previous study, 26 we categorized patients who underwent LRLR into three classifications (patients who had 0 or 1 predictive factor were categorized as having low difficulty, those with 2 or 3 predictive factors as having intermediate difficulty, and those with 4 or 5 predictive factors as having high difficulty) (Figure 1). In the investigation of the feasible indication of LRLR, we compared the surgical outcomes between the LRLR and ORLR groups based on our difficulty classification (low or intermediate difficulty class, 0 to 3 preoperative predictive factors; high difficulty class, 4 or 5 preoperative predictive factors) to avoid bias of surgical difficulty (Figure 1).
FIGURE 1.
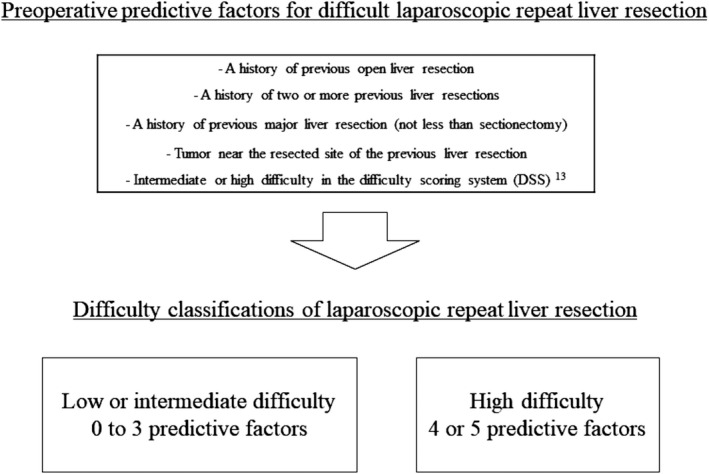
Preoperative predictive factors for difficult laparoscopic repeat liver resection and difficulty classifications suggested in our previous study 26
2.3. Statistical analysis
The Mann–Whitney U‐test was used to compare continuous variables. Categorical variables were summarized as numbers and percentages and compared between groups using Fisher's exact test or the chi‐squared test, as appropriate. P < .05 was considered statistically significant. Statistical analyses were performed using JMP v. 11 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Surgical outcomes in patients who underwent LRLR according to difficulty classification
Surgical outcomes of each difficulty class (low or intermediate and high difficulty class) after LRLR are described in Table 2. In patients who underwent LRLR, significant differences were observed for operative time and intraoperative blood loss and operative time before starting hepatic parenchymal resection between high and other difficulty groups. Rates of conversion to open surgery from LRLR tended to increase in patients with a high difficulty class without a significant difference. Moreover, although the duration of the postoperative hospital stay (in days) did not significantly differ, the incidence of postoperative complications (Clavien–Dindo classification ≥grade IIIa 27 ) was significantly higher in high difficiulty groups than in low‐ or intermediate difficiulty groups.
TABLE 2.
Surgical outcomes in patients who underwent LRLR according to difficulty classification
| The number of predictive factors | 0 to 3 | 4 or 5 | P‐value |
|---|---|---|---|
| Category of difficulty classification | Low or Intermediate (n = 92) | High (n = 9) | |
| Operative results, median (range) | |||
| Operative time, min | 214 (69–704) | 425 (153–681) | .0003 |
| Operative time before starting hepatic parenchymal dissection, min | 78 (16–410) | 223 (30–321) | .0004 |
| Blood loss, mL | 50 (2–1500) | 330 (3–2170) | .0049 |
| Conversion, n (%) | 4 (4.4) | 2 (22) | .088 |
| Postoperative outcomes | |||
| Postoperative complication a , n (%) | 1 (1.1) | 3 (33) | .0019 |
| Postoperative hospital stays, median (range), d | 8 (2–30) | 8 (5–95) | .40 |
Abbreviation: LRLR, laparoscopic repeat liver resection.
Clavien–Dindo classification ≥grade IIIa. 27
3.2. Patients’ backgrounds between patients who underwent LRLR and ORLR
Patients’ backgrounds are described in Table 3. There were no differences in age, sex, body mass index, comorbid liver diseases, or tumor diameter between the LRLR and ORLR groups. The proportion of patients with a history of previous open liver resection was significantly lower in the LRLR group than in the ORLR group. The proportion of patients with a history of previous major liver resection tended to be lower in the LRLR group than in the ORLR group. Although there was no difference in the proportion of patients in the low‐ or intermediate difficiulty class between the two groups, the proportion of patients in the high difficiulty class was significantly higher in the ORLR group than in the LRLR group. Comparisons of patient's backgrounds in each difficulty class between patients who underwent LRLR and ORLR are summarized in Tables S1 and S2. In low‐ or intermediate difficiulty class, the proportion of patients with a history of previous open liver resection was significantly lower in the LRLR group than in the ORLR group. Conversely, in the high difficiulty class, the proportion of patients with a history of two or more previous liver resections was significantly higher in the LRLR group than in the ORLR group.
TABLE 3.
Comparison of preoperative backgrounds between patients who underwent laparoscopic and open repeat liver resection
| Variables | LRLR (n = 101) | ORLR (n = 59) | P‐value |
|---|---|---|---|
| Age, median (range), years | 71 (32–86) | 68 (43–87) | .11 |
| Sex, male/female | 84/17 | 50/9 | .99 |
| Body mass index, median (range), kg/m2 | 23 (17–39) | 24 (17–31) | .69 |
| Comorbid liver disease, n (%) | |||
| Anti‐HCV positive | 46 (46) | 30 (51) | .62 |
| HBs antigen positive | 29 (29) | 13 (22) | .46 |
| Alcoholic hepatitis | 8 (7.9) | 3 (5.1) | .75 |
| Nonalcoholic steatohepatitis | 2 (2.0) | 3 (5.1) | .36 |
| Tumor diameter, median (range), cm | 1.5 (0.4–3.8) | 1.7 (0.6–8.5) | .098 |
| A history of previous open liver resection, n (%) | 49 (49) | 51 (86) | <.0001 |
| A history of two or more previous liver resections, n (%) | 23 (23) | 14 (24) | 0.99 |
| A history of previous major liver resection (not less than sectionectomy), n (%) | 20 (20) | 19 (32) | .088 |
| Tumor near the resected site of the previous liver resection, n (%) | 59 (58) | 39 (66) | .40 |
| Intermediate or high difficulty in the difficulty scoring system a , n (%) | 43 (43) | 32 (54) | 0.19 |
| Low or intermediate difficulty class b , n (%) | 92 (91) | 44 (75) | .32 |
| High difficulty class b , n (%) | 9 (8.9) | 15 (25) | .0062 |
3.3. Surgical outcomes between patients who underwent LRLR and ORLR
A significantly longer total operative time, less intraoperative blood loss, and shorter postoperative hospital stay were observed in the LRLR group than in the ORLR group. There was no difference in the operative time before starting liver parenchymal resection and the proportion of patients with postoperative complications between the groups (Table 4).
TABLE 4.
Comparisons of surgical outcomes between patients who underwent laparoscopic and open repeat liver resection
| Variables | LRLR (n = 101) | ORLR (n = 59) | P‐value |
|---|---|---|---|
| Total operative time, median (range), min | 221 (69–704) | 182 (87–559) | .025 |
| Operative time before starting hepatic parenchymal dissection, median (range), min | 80 (16–410) | 72 (30–185) | .40 |
| Intraoperative blood loss, median (range), mL | 50 (2–2170) | 190 (10–1870) | <.0001 |
| Postoperative complications, n (%) a | 4 (4.0) | 7 (12) | .10 |
| Postoperative hospital stay, median (range), d | 8 (2–95) | 10 (7–50) | .0003 |
Abbreviations: LRLR, laparoscopic repeat liver resection; ORLR, open repeat liver resection.
Clavien–Dindo classification ≥grade IIIa. 27
3.4. Comparisons of surgical outcomes between LRLR and ORLR in the low or intermediate difficulty class
In the low or intermediate difficulty class (patients with 0 to 3 preoperative predictive factors), there were no significant differences in the total operative time and operative time before starting hepatic parenchymal resection (Table 5). Intraoperative blood loss was significantly less in the LRLR group than in the ORLR group (P < .0001), and the duration of postoperative hospital stay was significantly shorter in the LRLR group than in the ORLR group (P < .0001). Moreover, the proportion of patients with postoperative complications was also significantly lower in the LRLR group than in the ORLR group (P = .014). In the LRLR group, one patient had bile leakage. In the ORLR group, two patients had bile leakage, one had intractable ascites, and two had pleural effusion.
TABLE 5.
Comparisons of surgical outcomes between laparoscopic and open repeat liver resection in patients in the low or intermediate difficulty class
| Variables | LRLR (n = 92) | ORLR (n = 44) | P‐value |
|---|---|---|---|
| Operative results, median (range) | |||
| Total operative time, min | 214 (69–704) | 185 (87–559) | .16 |
| Operative time before starting hepatic parenchymal dissection, min | 78 (16–410) | 72 (30–185) | .77 |
| Blood loss, mL | 50 (2–1500) | 155 (10–1675) | <.0001 |
| Postoperative outcomes | |||
| Postoperative complicationa, n (%) | 1 (1.1) | 5 (11) | .014 |
| Postoperative hospital stay, median (range), d | 8 (2–30) | 10 (7–37) | <.0001 |
Abbreviations: LRLR, laparoscopic repeat liver resection; ORLR, open repeat liver resection.
aClavien–Dindo classification ≥grade IIIa. 27
3.5. Comparisons of surgical outcomes between LRLR and ORLR in the high difficulty class
In the high difficulty class (patients with 4 or 5 preoperative predictive factors), total operative time and operative time before starting hepatic parenchymal resection were significantly longer in the LRLR group than in the ORLR group (P = .0009 and P = .0017, respectively; Table 6). There were no significant differences in intraoperative blood loss and duration of postoperative hospital stay between the two groups. The proportion of patients with postoperative complications was not different between the two groups. In the LRLR group, one patient had bile leakage and two had intractable ascites. In the ORLR group, one patient had bile leakage, one had intractable ascites, and one had pleural effusion.
TABLE 6.
Comparisons of surgical outcomes between laparoscopic and open repeat liver resection in patients in the high difficulty class
| Variables | LRLR (n = 9) | ORLR (n = 15) | P‐value |
|---|---|---|---|
| Operative results, median (range) | |||
| Total operative time, min | 425 (153–681) | 176 (134–295) | .0009 |
| Operative time before starting hepatic parenchymal dissection, min | 223 (30–321) | 76 (41–131) | .0017 |
| Blood loss, mL | 330 (3–2170) | 250 (10–1870) | .81 |
| Postoperative outcomes | |||
| Postoperative complicationa, n (%) | 3 (33) | 2 (13) | .33 |
| Postoperative hospital stay, median (range), d | 8 (5–95) | 11 (7–50) | .28 |
Abbreviations: LRLR, laparoscopic repeat liver resection; ORLR, open repeat liver resection.
aClavien–Dindo classification ≥grade IIIa. 27
4. DISCUSSION
Some previous studies reported that LRLR was not inferior to ORLR in terms of short‐ and/or long‐term outcomes. 15 , 16 , 17 , 18 , 19 , 20 , 21 They reported less blood loss and a shorter hospital stay in LRLR than in ORLR due to minimal damage to structures surrounding the liver, reduction of adhesion formation, and the need for adhesiolysis in LRLR. 15 , 16 , 17 , 18 Although these studies concluded that LRLR can be performed safely in selected patients, the definition of such “selected patients” remains unclear. LRLR can often be difficult, and a safe laparoscopic procedure is not always performed for all patients with HCCR. Therefore, in this study we evaluated the surgical outcomes of LRLR and ORLR based on the difficulty of repeat liver resection, based on previous studies 15 , 22 , 23 , 24 , 25 and our previous report, 26 to determine a feasible indication for LRLR.
This study showed that LRLR could be performed with less intraoperative blood loss, and a shorter postoperative hospital stay than ORLR, when all subjects are compared, as previously reported. 15 , 16 , 17 , 18 , 19 , 20 , 21 In the low or intermediate difficulty class, the intraoperative blood loss and proportion of patients with postoperative complications were significantly lower in the LRLR group than in the ORLR group, and the duration of the postoperative hospital stay was significantly shorter in the LRLR group than in the ORLR group. Therefore, LRLR for HCCR, as a minimally invasive treatment modality, was useful in patients in the low or intermediate difficulty class compared with ORLR. However, in the high difficulty class the total operative time and operative time before starting hepatic parenchymal resection were significantly longer in the LRLR group than in the ORLR group, and there was no statistical superiority in other surgical outcomes in patients who underwent LRLR compared to ORLR. In addition, a high conversion rate (22%) was observed in patients who underwent LRLR in the high difficulty class. The results suggested that LRLR is recommended for patients in the low or intermediate difficulty class (0 to 3 preoperative predictive factors, those with a history of previous open liver resection, history of two or more previous liver resections, history of previous major liver resection, tumor near the resected site of the previous liver resection, and intermediate or high difficulty in the DSS). In contrast, presently LRLR cannot be currently recommended for patients in the high difficulty class (4 or 5 preoperative predictive factors) because LRLR does not have an advantage for such patients, compared with ORLR. In patients in the high difficulty class, LRLR should be recommended to be performed only by an experienced team in high‐volume centers with consideration of conversion to open surgery.
This study included some limitations. First, this was a dual‐center retrospective study and included a small number of patients with some selection biases. Patients who underwent repeat anatomical liver resection or partial resection in the caudate lobe were excluded because there were only a few patients who underwent such procedures laparoscopically. A multicenter study with a large number of patients as a validation study must be performed to assess the more appropriate indications of LRLR for HCCR. Second, recently devices and techniques of laparoscopic surgery are advancing; therefore, our “current” difficulty classification will not be suitable in the future. However, presently the indication of a surgical approach for repeat liver resection is unclear, and our present classifications may be useful to decide the surgical approach in patients who require repeat liver resection. Third, our present study classified the difficulty simply based on the number of predictive factors, with consideration that the five predictive factors were indicated as the independent risk factors in our present and previous studies. 26 The importance and weight may be different in each factor. A scoring system should be established by a large‐number study, considering the importance and weight of each factor, based on the present results.
In conclusion, the difficulty classification evaluated by five preoperative predictive factors consisting of history of previous open liver resection, history of two or more previous liver resections, history of previous major liver resection (not less than sectionectomy), tumor near the resected site of the previous liver resection, and intermediate or high difficulty in the DSS was useful in making the decision for the indication of LRLR. LRLR is recommended for patients in the low or intermediate difficulty class (0 to 3 predictive factors). In contrast, presently LRLR cannot be recommended for patients in the high difficulty class (4 or 5 preoperative predictive factors) because LRLR has a longer operative time without any benefits in other surgical outcomes for such patients, compared with ORLR.
DISCLOSURES
Conflict of Interest: None.
Funding Information: This work was supported by the Health, Labour and Welfare Policy Research Grants from the Ministry of Health, Labour and Welfare of Japan (Policy Research for Hepatitis Measures [H30‐Kansei‐Shitei‐003]).
Author Contributions: Study design: Masahiko Kinoshita, Akishige Kanazawa, and Shoji Kubo designed the study. Acquisition of data: Masahiko Kinoshita, Akishige Kanazawa, Shogo Tanaka, Shigekazu Takemura, Ryosuke Amano, Kenjiro Kimura, Hiroji Shinkawa, Go Ohira, Kohei Nishio. Data analysis: Masahiko Kinoshita, Akishige Kanazawa, and Shoji Kubo. Manuscript drafted by Masahiko Kinoshita, Akishige Kanazawa, and Shoji Kubo. All the authors reviewed the manuscript.
Supporting information
Table S1
Table S2
ACKNOWLEDGMENTS
This work was supported by the Health, Labour and Welfare Policy Research Grants from the Ministry of Health, Labour and Welfare of Japan (Policy Research for Hepatitis Measures [H30‐Kansei‐Shitei‐003]). The authors thank Enago (www.enago.jp) for the English language review.
Kinoshita M, Kanazawa A, Tanaka S, Takemura S, Amano R, Kimura K, et al. Indications of Laparoscopic Repeat Liver Resection for Recurrent Hepatocellular Carcinoma. Ann Gastroenterol Surg.2022;6:119–126. 10.1002/ags3.12493
REFERENCES
- 1. Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence‐based comparison and review. Cancer. 2014;120(18):2824–38. [DOI] [PubMed] [Google Scholar]
- 2. Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH‐HCC guidelines) 2019 update. Hepatol Res. 2019;49(10):1109–13. [DOI] [PubMed] [Google Scholar]
- 3. Chan AC, Poon RT, Cheung TT, Chok KSH, Chan SC, Fan ST, et al. Survival analysis of re‐resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg. 2012;36(1):151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238(5):703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakajima Y, Ko S, Kanamura T, Nagao M, Kanehiro H, Hisanaga M, et al. Repeat liver resection for hepatocellular carcinoma. J Am Coll Surg. 2001;192(3):339–44. [DOI] [PubMed] [Google Scholar]
- 6. Shuto T, Kinoshita H, Hirohashi K, Kubo S, Tanaka H, Tsukamoto T, et al. Indications for, and effectiveness of, a second hepatic resection for recurrent hepatocellular carcinoma. Hepatogastroenterology. 1996;43(10):932–7. [PubMed] [Google Scholar]
- 7. Tanaka S, Hirohashi K, Tanaka H, Shuto T, Lee SH, Kubo S, et al. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg. 2002;195(4):484–9. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka S, Tanaka H, Kubo S, Shuto T, Takemura S, Yamamoto T, et al. Bowel injury associated with liver surgery for hepatocellular carcinoma. Hepatogastroenterology. 2006;53(70):571–5. [PubMed] [Google Scholar]
- 9. Chan AC, Dai JW, Chok KS, Cheung TT, Lo CM. Prognostic influence of spontaneous tumor rupture on hepatocellular carcinoma after interval hepatectomy. Surgery. 2016;159(2):409–17. [DOI] [PubMed] [Google Scholar]
- 10. Beppu T, Wakabayashi G, Hasegawa K, Gotohda N, Mizuguchi T, Takahashi Y, et al. Long‐term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi‐institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22(10):711–20. [DOI] [PubMed] [Google Scholar]
- 11. Takahara T, Wakabayashi G, Beppu T, Aihara A, Hasegawa K, Gotohda N, et al. Long‐term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi‐institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22(10):721–7. [DOI] [PubMed] [Google Scholar]
- 12. Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery. The Louisville Statement, 2008. Ann Surg. 2009;250(5):825–30. [DOI] [PubMed] [Google Scholar]
- 13. Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21(10):745–53. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka S, Kubo S, Kanazawa A, Takeda Y, Hirokawa F, Nitta H, et al. Validation of a difficulty scoring system for laparoscopic liver resection: a multicenter analysis by the endoscopic liver surgery study group in Japan. J Am Coll Surg. 2017;225(2):249–58. [DOI] [PubMed] [Google Scholar]
- 15. Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamamoto S, Yamazoe S, et al. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2013;20(5):512–7. [DOI] [PubMed] [Google Scholar]
- 16. Noda T, Eguchi H, Wada H, Iwagami Y, Yamada D, Asaoka T, et al. Short‐term surgical outcomes of minimally invasive repeat hepatectomy for recurrent liver cancer. Surg Endosc. 2018;32(1):46–52. [DOI] [PubMed] [Google Scholar]
- 17. Isetani M, Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, et al. Pure laparoscopic hepatectomy as repeat surgery and repeat hepatectomy. World J Gastroenterol. 2015;21(3):961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morise Z, Aldrighetti L, Belli G, Ratti F, Belli A, Cherqui D, et al. Laparoscopic repeat liver resection for hepatocellular carcinoma: a multicentre propensity score‐based study. Br J Surg. 2020;107(7):889–95. [DOI] [PubMed] [Google Scholar]
- 19. Ome Y, Hashida K, Yokota M, Nagahisa Y, Yamaguchi K, Okabe M, et al. The feasibility and efficacy of pure laparoscopic repeat hepatectomy. Surg Endosc. 2018;32(8):3474–9. [DOI] [PubMed] [Google Scholar]
- 20. Liu K, Chen Y, Wu X, Huang Z, Lin Z, Jiang J, et al. Laparoscopic liver re‐resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: a propensity score matching study. Surg Endosc. 2017;31(11):4790–8. [DOI] [PubMed] [Google Scholar]
- 21. Peng L, Zhou Z, Xiao W, Hu X, Cao J, Mao S. Systematic review and meta‐analysis of laparoscopic versus open repeat hepatectomy for recurrent liver cancer. Surg Oncol. 2019;28:19–30. [DOI] [PubMed] [Google Scholar]
- 22. Okamura Y, Yamamoto Y, Sugiura T, Ito T, Ashida R, Ohgi K, et al. Novel patient risk factors and validation of a difficulty scoring system in laparoscopic repeat hepatectomy. Sci Rep. 2019;9(1):17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gon H, Kido M, Tanaka M, Kuramitsu K, Komatsu S, Awazu M, et al. Laparoscopic repeat hepatectomy is a more favorable treatment than open repeat hepatectomy for contralateral recurrent hepatocellular carcinoma cases. Surg Endosc. 2021;35(6):2896–906. 10.1007/s00464-020-07728-9 [DOI] [PubMed] [Google Scholar]
- 24. Gutt CN, Oniu T, Schemmer P, Mehrabi A, Büchler MW. Fewer adhesions induced by laparoscopic surgery? Surg Endosc. 2004;18(6):898–906. [DOI] [PubMed] [Google Scholar]
- 25. Yamashita Y, Shirabe K, Tsuijita E, Takeishi K, Ikegami T, Yoshizumi T, et al. Third or more repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery. 2013;154(5):1038–45. [DOI] [PubMed] [Google Scholar]
- 26. Kinoshita M, Kanazawa A, Kodai S, Shimizu S, Murata A, Nishio K, et al. Difficulty classifications of laparoscopic repeated liver resection in patients with recurrent hepatocellular carcinoma. Asian J Endosc Surg. 2020;13(3):366–74. [DOI] [PubMed] [Google Scholar]
- 27. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


