Abstract
Purpose
This study aimed to develop and validate new metrics to objectively assess the lipid layer thickness (LLT) through the analysis of grey intensity values obtained from the Placido disk pattern reflected onto the tear film.
Methods
Ocular surface parameters were measured using Oculus Keratograph 5 M in 94 healthy volunteers (43.8 ± 26.8 years). Subjects’ LLT was subjectively classified into 4 groups using an interferometry-based grading scale. New metrics based on the intensity of the Placido disk images were calculated and compared between groups. The repeatability of the new metrics and their diagnostic ability was analysed through receiver operating characteristics (ROC) curves. The level of agreement between the new objective tool and the existing subjective classification scale was analysed by means accuracy, weighted Kappa index and F-measure.
Results
Mean pixel intensity, median pixel intensity and relative energy at 5.33 s after blinking achieved the highest performance, with a correlation with LLT between r = 0.655 and 0.674 (p < 0.001), sensitivity between 0.92 and 0.94, specificity between 0.79 and 0.81, area under the ROC curve between 0.89 and 0.91, accuracy between 0.76 and 0.77, weighted Kappa index of 0.77 and F-measure between 0.86 and 0.87.
Conclusion
The analysis of grey intensity values in videokeratography can be used as an objective tool to assess LLT. These new metrics could be included in a battery of clinical tests as an easy, repeatable, objective and accessible method to improve the detection and monitoring of dry eye disease and meibomian gland dysfunction.
Keywords: Dry eye disease, High-speed videokeratoscopy, Image processing, Lipid layer thickness, Meibomian gland dysfunction, Tear film
Introduction
The lipid layer is the outermost layer of the tear film (TF) and is almost entirely derived from meibum, which is secreted by the meibomian glands. The lipid layer plays a vital role in the stabilization of the TF. It also spreads the whole TF over the ocular surface, lowers the surface tension at the air interface of the TF and prevents the aqueous layer from evaporating [1, 2].
Given the key role of the lipid layer in maintaining the properties of the TF, the assessment of the lipid layer thickness (LLT) is essential in dry eye disease (DED) and Meibomian gland dysfunction (MGD) [3, 4]. One of the most common methods for assessing the lipid layer is the evaluation of the colour and brightness of its interference patterns using an interferometer.
The Tearscope Plus™ is an interferometer developed to assess the LLT [4]. However, this is a subjective technique which requires an experienced clinician to classify the interference patterns. It has been reported that subjective diagnostic tests, such as grading scales, rely on the examiner’s ability, which might decrease inter and intra-observer repeatability [3, 5–7]. Likewise, in some cases, the grading of the interference patterns is difficult to perform, especially when dealing with thinner lipid layers [3, 8]. Currently, only the LipiView® system can provide quantitative values of the LLT. However, it has a small area of measurement and it only measures the LLT in blinking conditions [9, 10].
Lately, several studies have tried to solve the aforementioned problems by developing algorithms, based on the analysis of the texture, structure or colour of the interference patterns, which objectively assess the LLT [8, 11–19]. Likewise, other authors have used high-resolution microscopy systems to characterize the LLT [20] or have combined optical coherence tomography with interferometry to develop novel imaging systems [19, 21]. Nonetheless, none of these methods has been globally accepted and most of them are considerably time-consuming. Moreover, they require interferometers to be performed, which are too costly and sophisticated to be implemented in the clinic, being more suitable for research purposes.
During corneal topography measurement, the TF acts as a mirror and reflects the projected Placido disk ring pattern. Placido disk rings show lighter than the background. The healthy TF surface forms a well-structured and reflected pattern with good intensity of reflection, while an altered TF produces an irregular pattern with low reflectivity [22]. Accordingly, the primary aim of the present study was to develop and validate a novel method to objectively assess the lipid layer through the analysis of grey intensity values obtained from the Placido disk pattern reflected onto the TF, without the need of an interferometer, thus making the method widely accessible.
The base of the method is that a thicker lipid layer has more lipids [1], which will reflect the light of the Placido disk ring pattern with higher intensity. We hypothesized that high grey intensity values might be related to a thicker lipid layer, while low grey intensity values might be related to a thinner lipid layer. This method was developed following previous research, which shows that the analysis of grey level intensity values of videokeratoscopy images may significantly improve the diagnosis of DED in comparison to other image analysis approaches [22].
Material and methods
Ninety-four healthy volunteers ranging in age from 18 to 90 years (43.8 ± 26.8 years) were enrolled in this study. Only the right eye of participants was assessed to avoid subjects’ data duplication. Subjects had no prior history of ocular disease or injury in the last 3 months. No exclusion based on ocular surface parameters was made to evaluate different TF status. Contact lens users were instructed not to wear their contact lenses within a week before the examination. The work was performed in accordance with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the University of Valencia. Written consent of each subject was obtained after a verbal explanation of the study protocol.
Ocular surface measurements
Participants’ ocular surface was evaluated using Oculus Keratograph 5 M (K5 M; Oculus GmbH, Wetzlar, Germany). Measurements were taken by the same experienced researcher following the guidelines of the Tear Film and Ocular Surface Dry Eye Workshop II (TFOS DEWS II) Diagnostic Methodology report [3] and were performed in the following order to avoid TF destabilization: Ocular Surface Disease Index (OSDI), Dry Eye Questionnaire-5 (DEQ-5), total bulbar redness, tear meniscus height (TMH), LLT, non-invasive keratograph break-up time (NIKBUT), meibomian glands expressibility and upper eyelid meibography. The illuminance, temperature and humidity of the room were maintained constant at 200 lx, 24.1 ± 1.6 °C and 44.9 ± 5.0%, respectively.
OSDI and DEQ-5 were used for scoring the ocular surface symptoms of subjects. Bulbar redness was assessed three consecutive times, and an average value was calculated [23], while TMH was obtained by capturing the meniscus immediately post-blink [24].
The LLT was recorded using Oculus Keratograph 5 M and assessed through the lipid layer interference pattern, which was subjectively classified by a masked and experienced examiner into 4 groups using a standardised grading scale [6, 25]: 1 = open meshwork (13–15 nm); 2 = closed meshwork (30–50 nm); 3 = wave (50–80 nm); and 4 = colour fringe (90–140 nm).
The moment of the first break-up of the TF (first NIKBUT) and the average time of all break-ups (mean NIKBUT) were also obtained. A total of three measurements were carried out, one every 3 min so that the TF stabilized between assessments, and the mean and median values of these three measurements were calculated [3].
The expressibility of the central 8 meibomian glands of the upper eyelid was assessed using a subjective grading scale [6, 26, 27]. Upper eyelid meibography was captured using non-contact infrared meibography, and meibomian glands drop-out was objectively calculated using ImageJ tool (Wayne Rasband, National Institutes of Health, Bethesda, MD) as the ratio between gland loss area and eyelid area [28].
Data analysis using the proposed algorithm
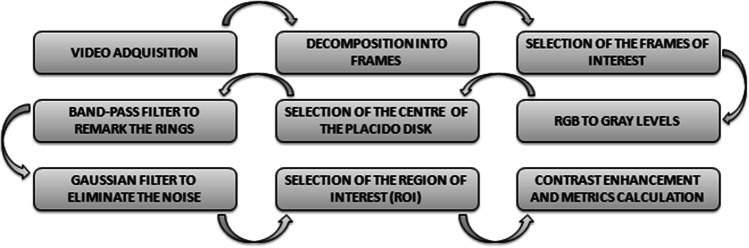
Oculus Keratograph 5 M was used to record a video of the NIKBUT measurement at 32 frames per second with a spatial resolution of 680 × 512 pixels. This video was recorded and saved to be later analysed. The proposed software was developed using Matlab R2019a® (MathWorks, Natick, MA). The software automatically decomposed the video into frames with a time interval of 0.031 s between them. The examiner manually selected the frames at 0.33, 5.33, 10.33, 15.33 and 20.33 s after blinking. The frame of 0.33 was selected since the eye was completely open after this time in all videos. Likewise, intervals of 5.00 s from this moment on were chosen to analyse whether the grey intensity values changed over time.
Once the frames of interest were selected, the software automatically processed the images. First, RGB images were transformed into grey-level images. Given that input images contained irrelevant information of external areas, the centre of the Placido disk ring pattern was isolated by the examiner through Matlab. After clicking the centre of the image, the software automatically selected a square of 241 × 241 pixels surrounding the centre of the rings (region of interest, ROI), as the area to perform the image processing.
Next, a band-pass filter was used to eliminate the background illumination and highlight the rings. Furthermore, the images were then smoothed by applying a 4-pixel sigma Gaussian filter to remove the remaining noise from the background [29]. After that, the final ROI was selected by the examiner, who manually selected the region of the image comprising solely the pupil, to avoid the influence of the iris on the results.
Finally, to increase the differences between normal and altered TFs, each pixel value of the resulting image was multiplied by 255 and divided by 85, thus enhancing the contrast between rings and non-ring spaces. These values were selected since they produced the highest possible contrast enhancement.
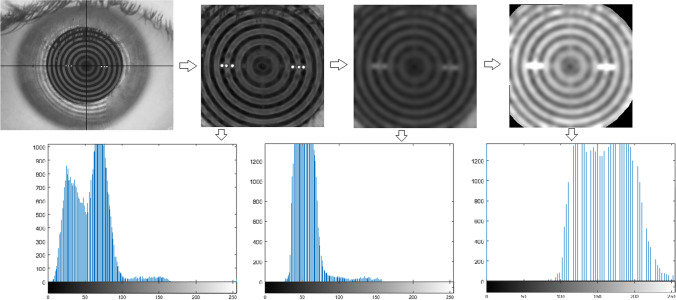
Once the images were processed, histograms were obtained from their pixel intensity values and metrics were calculated (Fig. 1). Figure 2 shows a summary of the main steps of image processing.
Fig. 1.
Images and histograms of the main steps of the image processing in a random frame. From left to right: selection of the centre of the image; band-pass filter implementation; Gaussian filter implementation; selection of the final ROI and contrast enhancement (final image). In the histograms, axis “x” represents the grey level intensities (0–255), while axis “y” shows the number of pixels
Fig. 2.
The main steps of the image processing
The base of our method is that a thicker lipid layer has more lipids [1], which will reflect the Placido disk rings with higher intensity. Thus, higher grey intensity values might be related to a thicker LLT, while lower grey intensity values could be related to a thinner LLT.
Mean, standard deviation (SD), median, mode, kurtosis and skewness of the histogram of the grey level intensity values were calculated. The minimum grey level in the image was also calculated. Besides, energy, relative energy, entropy and SD irregularity were calculated as follows [22]:
where p = pixel grey value; n = number of pixels of the ROI; pmax = maximum pixel intensity; and x = mean pixel intensity values.
Metrics were divided by the number of pixels of the ROI (n) so that all images were comparable independently of the size of the ROI. Finally, the total area under the pixel intensity three-dimensional curve of the image was calculated and divided by the number of pixels in the ROI (Fig. 3).
Fig. 3.
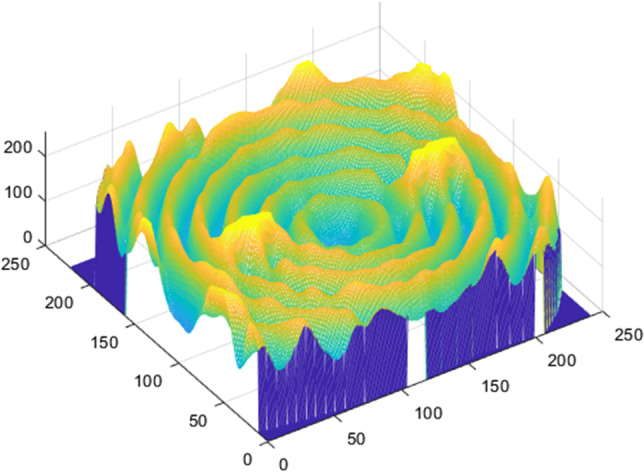
Three-dimensional graphic of the grey intensity values in the image. The “x” and “y” axes represent the size of the image and “z” the grey intensity value for each pixel
Statistical analysis
Statistical analysis was carried out using SPSS v26.0 for Windows (IBM Corp, Armonk, NY, USA). Outcomes were shown as the mean ± SD.
Differences in new metrics depending on time after blinking
Repeated mixed model ANOVA was used to evaluate the differences in pixel intensity values depending on the moment after blinking. Bonferroni was used to assess the post hoc differences between paired moments.
Repeatability of new metrics
As three NIKBUT videos were recorded, the three videos were analysed so as to calculate the repeatability of the software in the calculation Placido disk’ reflectivity metrics. Repeatability of each Placido disk’ reflectivity metric was assessed by calculating the within-subject SD (Sw), coefficient of variation (CoV) and the repeatability coefficient (CoR) [30–32].
Correlations of new metrics with DED signs and symptoms
Rho Spearman correlations were used to analyse the correlations between ocular surface signs and symptoms and new metrics, for the whole sample. Moreover, the sample was divided into different groups according to the cut-off values reported by the Diagnostic Methodology report of TFOS DEWS [3].
Differences in Placido disk’ reflectivity metrics between groups was assessed by means of Mann–Whitney U test or Kruskal–Wallis test. A p-value less than 0.05 was defined as statistically significant.
Multiple linear regressions
Multiple linear regressions were performed to assess the predictability of tear film-dynamic metrics to ocular signs that had statistically significant correlations. Multiple linear models were constructed with new metrics as dependent variables and current metrics as independent variables to assess the relative importance of each independent variable and their contribution to the change of dependent variables. The following assumptions were checked: the linear relationship between the independent and dependent variables, normal distribution of residuals, homoscedasticity of residuals and predicted values and absence of multicollinearity between independent variables.
Diagnostic ability and validation of new metrics
Each new metric was validated by means receiver operating characteristics (ROC) curves. The probability density functions for an altered (LLT = 1) or normal (LLT ≥ 2) LLT were calculated [3], and different parameters were obtained for each ROC curve: sensitivity, specificity, area under the ROC curve, the cut-off value that optimizes the diagnosis, Youden index and discriminant power [33].
Finally, each Placido disk image was objectively classified into LLT groups depending on the cut-off values obtained in the ROC curves. The level of agreement between this objective classification and the subjective ones was analysed by calculating the accuracy, Kappa index, weighted Kappa index with quadratic weights and F-measure for each metric as in previous studies [34–37]. The three indexes denote high level of agreement between tests when the values are near 1 [34–37].
Results
The described algorithm was applied to ninety-four eyes from 94 volunteers, 54 females (57.4%) and 40 males (42.6%). The mean age was 43.8 ± 26.8 years, ranging from 18 to 90 years. The algorithm was able to obtain objective metrics in all subjects.
Placido disk reflectivity metrics over time
Table 1 shows the mean values and SD for each Placido disk reflectivity metric at 0.33, 5.33, 10.33, 15.33 and 20.33 s after blinking. Repeated mixed model ANOVA showed statistical higher pixel intensity values at 10.33, 15.33 and 20.33 s than at 0.33 s. Nevertheless, CoV revealed a low variability of metrics over time. Thus, pixel intensity of the Placido disk was stable in the same subject throughout the measuring period. CoV between seconds after blinking achieved values between 4.42 and 16.92%. Total area under pixel intensity curve, mean pixel intensity, SD of pixel intensity, median pixel intensity and skewness had a CoV < 10%, which evidenced that metrics did not change after blinking.
Table 1.
Mean values for each Placido disk reflectivity metric
| Metric | Number of subjects | Mean ± SD | Significance level |
Statistically significant post hoc differences (p-value) |
CoV between seconds after blinking (%) | |
|---|---|---|---|---|---|---|
| Total area | 0.33 s | 94 | 113.63 ± 9.57 | < 0.0011 | 1–4 < 0.001 | 4.42 |
| 5.33 s | 90 | 116.79 ± 8.48 | 1–5 < 0.001 | |||
| 10.33 s | 66 | 117.15 ± 9.18 | ||||
| 15.33 s | 49 | 119.22 ± 8.30 | ||||
| 20.33 s | 37 | 120.30 ± 7.87 | ||||
| Minimum pixel intensity | 0.33 s | 94 | 67.12 ± 17.37 | 0.0821 | 13.58 | |
| 5.33 s | 90 | 69.10 ± 16.20 | ||||
| 10.33 s | 66 | 70.00 ± 18.84 | ||||
| 15.33 s | 49 | 75.12 ± 18.98 | ||||
| 20.33 s | 37 | 70.58 ± 18.16 | ||||
| Energy | 0.33 s | 94 | 240.78 ± 8.89 | < 0.0011 | 1–3 < 0.001 | 14.84 |
| 5.33 s | 90 | 244.72 ± 7.06 | 1–4 < 0.001 | |||
| 10.33 s | 66 | 249.45 ± 5.02 | 1–5 < 0.001 | |||
| 15.33 s | 49 | 251.82 ± 3.32 | ||||
| 20.33 s | 37 | 253.82 ± 2.02 | ||||
| Relative energy | 0.33 s | 94 | 0.46 ± 0.26 | < 0.0011 | 1–3 < 0.001 | 15.78 |
| 5.33 s | 90 | 0.54 ± 0.26 | 1–4 < 0.001 | |||
| 10.33 s | 66 | 0.57 ± 0.28 | 1–5 < 0.001 | |||
| 15.33 s | 49 | 0.63 ± 0.27 | ||||
| 20.33 s | 37 | 0.68 ± 0.27 | ||||
| Entropy | 0.33 s | 94 | 2.0 × 10−4 ± 1.2 × 10−4 | 0.9061 | 16.92 | |
| 5.33 s | 90 | 2.0 × 10−4 ± 1.2 × 10−4 | ||||
| 10.33 s | 66 | 2.0 × 10−4 ± 1.2 × 10−4 | ||||
| 15.33 s | 49 | 2.0 × 10−4 ± 1.2 × 10−4 | ||||
| 20.33 s | 37 | 2.0 × 10−4 ± 1.2 × 10−4 | ||||
| SD irregularity | 0.33 s | 94 | 0.08 ± 0.12 | 0.0021 | 1–4 = 0.005 | 15.26 |
| 5.33 s | 90 | 0.10 ± 0.12 | 1–5 = 0.001 | |||
| 10.33 s | 66 | 0.12 ± 0.14 | ||||
| 15.33 s | 49 | 0.13 ± 0.12 | ||||
| 20.33 s | 37 | 0.17 ± 0.17 | ||||
| Mean pixel intensity | 0.33 s | 94 | 130.38 ± 26.74 | < 0.0011 | 1–3 < 0.001 | 9.31 |
| 5.33 s | 90 | 137.24 ± 26.96 | 1–4 < 0.001 | |||
| 10.33 s | 66 | 140.58 ± 29.76 | 1–5 < 0.001 | |||
| 15.33 s | 49 | 146.55 ± 29.27 | ||||
| 20.33 s | 37 | 153.06 ± 31.80 | ||||
| SD of pixel intensity | 0.33 s | 94 | 26.89 ± 4.63 | < 0.0011 | 1–3 < 0.001 | 8.73 |
| 5.33 s | 90 | 29.62 ± 4.84 | 1–4 < 0.001 | |||
| 10.33 s | 66 | 30.43 ± 4.50 | 1–5 < 0.001 | |||
| 15.33 s | 49 | 31.19 ± 4.92 | ||||
| 20.33 s | 37 | 32.62 ± 4.83 | ||||
| Median pixel intensity | 0.33 s | 94 | 127.12 ± 27.47 | < 0.0011 | 1–4 < 0.001 | 9.73 |
| 5.33 s | 90 | 136.27 ± 27.97 | 1–5 < 0.001 | |||
| 10.33 s | 66 | 139.68 ± 31.12 | ||||
| 15.33 s | 49 | 145.59 ± 30.56 | ||||
| 20.33 s | 37 | 152.52 ± 33.03 | ||||
| Mode pixel intensity | 0.33 s | 94 | 142.40 ± 40.12 | < 0.0011 | 1–3 = 0.004 | 16.79 |
| 5.33 s | 90 | 150.03 ± 47.08 | 1–4 = 0.001 | |||
| 10.33 s | 66 | 152.90 ± 50.89 | 1–5 < 0.001 | |||
| 15.33 s | 49 | 164.27 ± 55.38 | ||||
| 20.33 s | 37 | 175.22 ± 60.98 | ||||
| Kurtosis | 0.33 s | 94 | 0.017 ± 0.001 | < 0.0011 | 1–4 < 0.001 | 11.37 |
| 5.33 s | 90 | 0.015 ± 0.003 | 1–5 < 0.001 | |||
| 10.33 s | 66 | 0.015 ± 0.003 | ||||
| 15.33 s | 49 | 0.014 ± 0.003 | ||||
| 20.33 s | 37 | 0.014 ± 0.003 | ||||
| Skewness | 0.33 s | 94 | 0.14 ± 0.02 | < 0.0011 | 1–4 < 0.001 | 6.90 |
| 5.33 s | 90 | 0.13 ± 0.01 | 1–5 < 0.001 | |||
| 10.33 s | 66 | 0.13 ± 0.02 | ||||
| 15.33 s | 49 | 0.13 ± 0.02 | ||||
| 20.33 s | 37 | 0.12 ± 0.02 |
CoV coefficient of variation, s seconds, SD standard deviation.
1Repeated mixed model ANOVA.
Repeatability of Placido disk reflectivity metrics
Table 2 shows the repeatability scores for each metric. All metrics showed acceptable repeatability since Sw, CoR and CoV values were low, and the variability between the three measurements was not high. Sw achieved values between 2 × 10−6 and 7.07, CoR between 6 × 10−6 and 19.59 and CoV between 0.09 and 5.15.
Table 2.
Repeatability of each Placido disk reflectivity metric
| Metric | Sw | CoR | CoV (%) |
|---|---|---|---|
| At 0.33 s | |||
| Total area | 0.63 | 1.74 | 0.54 |
| Minimum pixel intensity | 2.95 | 8.16 | 4.42 |
| Energy | 0.23 | 0.64 | 0.09 |
| Relative energy | 0.01 | 0.04 | 2.85 |
| Entropy | 2 × 10−6 | 6 × 10−6 | 1.95 |
| SD irregularity | 0.003 | 0.01 | 3.66 |
| Mean pixel intensity | 1.28 | 3.55 | 0.97 |
| SD pixel intensity | 0.34 | 0.96 | 1.25 |
| Median pixel intensity | 1.30 | 3.59 | 0.99 |
| Mode pixel intensity | 7.02 | 19.44 | 5.11 |
| Kurtosis | 3 × 10−4 | 9 × 10−4 | 2.39 |
| Skewness | 0.002 | 0.006 | 1.56 |
| At 5.33 s | |||
| Total area | 0.54 | 1.49 | 0.47 |
| Minimum pixel intensity | 2.82 | 7.82 | 4.23 |
| Energy | 0.23 | 0.64 | 0.09 |
| Relative energy | 0.01 | 0.04 | 2.84 |
| Entropy | 2 × 10−6 | 6 × 10−6 | 1.94 |
| SD irregularity | 0.003 | 0.01 | 3.64 |
| Mean pixel intensity | 1.06 | 2.93 | 0.80 |
| SD pixel intensity | 0.34 | 0.93 | 1.21 |
| Median pixel intensity | 1.29 | 3.56 | 0.98 |
| Mode pixel intensity | 7.01 | 19.40 | 5.10 |
| Kurtosis | 3 × 10−4 | 8 × 10−4 | 2.01 |
| Skewness | 0.001 | 0.004 | 1.11 |
| At 10.33 s | |||
| Total area | 0.59 | 1.64 | 0.51 |
| Minimum pixel intensity | 2.76 | 7.64 | 4.15 |
| Energy | 0.23 | 0.64 | 0.09 |
| Relative energy | 0.01 | 0.04 | 2.89 |
| Entropy | 2 × 10−6 | 8 × 10−6 | 2.49 |
| SD irregularity | 0.003 | 0.01 | 3.97 |
| Mean pixel intensity | 1.33 | 3.67 | 1.00 |
| SD pixel intensity | 0.35 | 0.97 | 1.26 |
| Median pixel intensity | 1.30 | 3.61 | 0.99 |
| Mode pixel intensity | 7.03 | 19.48 | 5.12 |
| Kurtosis | 3 × 10−4 | 9 × 10−4 | 2.09 |
| Skewness | 0.002 | 0.004 | 1.21 |
| At 15.33 s | |||
| Total area | 0.60 | 1.67 | 0.52 |
| Minimum pixel intensity | 2.81 | 7.78 | 4.19 |
| Energy | 0.23 | 0.64 | 0.09 |
| Relative energy | 0.01 | 0.04 | 2.90 |
| Entropy | 3 × 10−6 | 8 × 10−6 | 2.56 |
| SD irregularity | 0.003 | 0.01 | 4.05 |
| Mean pixel intensity | 1.35 | 3.75 | 1.02 |
| SD pixel intensity | 0.42 | 1.16 | 1.50 |
| Median pixel intensity | 1.32 | 3.66 | 1.01 |
| Mode pixel intensity | 7.06 | 19.57 | 5.14 |
| Kurtosis | 3 × 10−4 | 9 × 10−4 | 2.07 |
| Skewness | 0.002 | 0.005 | 1.42 |
| At 20.33 s | |||
| Total area | 0.68 | 1.87 | 0.58 |
| Minimum pixel intensity | 3.14 | 8.69 | 4.69 |
| Energy | 0.23 | 0.64 | 0.09 |
| Relative energy | 0.01 | 0.04 | 2.93 |
| Entropy | 3 × 10−6 | 8 × 10−6 | 2.62 |
| SD irregularity | 0.003 | 0.01 | 4.21 |
| Mean pixel intensity | 1.44 | 4.00 | 1.09 |
| SD pixel intensity | 0.42 | 1.16 | 1.51 |
| Median pixel intensity | 1.34 | 3.71 | 1.02 |
| Mode pixel intensity | 7.07 | 19.59 | 5.15 |
| Kurtosis | 3 × 10−4 | 9 × 10−4 | 2.37 |
| Skewness | 0.004 | 0.01 | 2.73 |
CoR repeatability coefficient, CoV coefficient of variation, s seconds, SD standard deviation; Sw within-subject standard deviation
Correlations between new metrics and DED signs and symptoms
Following the results of the previous sections, showing no variation of the metrics over time, only the metrics at 0.33, 5.33 and 10.33 s after blinking were further assessed. Metrics at 15.33 and 20.33 s were excluded from further analysis as most patients need to suppress blinking forcefully, and thus, they do not represent in most cases a real scenario.
Spearman’s significant correlations between each Placido disk reflectivity metric and DED signs and symptoms are shown in Table 3. Generally, there were moderate negative correlations between new metrics based on the grey intensity of pixels of Placido disk images and age, meibomian glands drop-out percentage, bulbar redness, TMH and OSDI. Meanwhile, Placido disk reflectivity metrics were positively correlated with LLT and NIKBUT. The correlation with LLT was the strongest. Given that LLT was statistically correlated with age (r = − 0.298, p = 0.002), glands drop-out (r = − 0.271, p = 0.004), mean first NIKBUT (r = − 0.209, p = 0.008), median first NIKBUT, mean mean NIKBUT and median mean NIKBUT, it might be possible that the correlation of new metrics with the other ocular surface metrics was as consequence of the correlation with LLT. Nevertheless, LLT was not statistically correlated with bulbar redness, TMH and OSDI.
Table 3.
Statistically significant Rho Spearman correlations between Placido disk reflectivity metrics and DED signs and symptoms
| New metrics | Current metrics | Correlation coefficient (r) | Significance level |
|---|---|---|---|
| At 0.33 s | |||
| Total area | Age | − 0.372 | < 0.001 |
| Drop-out percentage | − 0.277 | 0.007 | |
| LLT | 0.413 | < 0.001 | |
| Mean mean NIKBUT | 0.209 | 0.048 | |
| Minimum pixel intensity | Age | − 0.387 | < 0.001 |
| Drop-out percentage | − 0.236 | 0.022 | |
| LLT | 0.345 | 0.001 | |
| Energy | Age | − 0.346 | < 0.001 |
| Drop-out percentage | − 0.236 | < 0.001 | |
| LLT | 0.408 | < 0.001 | |
| Relative energy | Age | − 0.356 | < 0.001 |
| Drop-out percentage | − 0.266 | 0.010 | |
| LLT | 0.407 | < 0.001 | |
| TMH | − 0.205 | 0.048 | |
| Entropy | Age | − 0.670 | < 0.001 |
| Drop-out percentage | − 0.517 | < 0.001 | |
| Bulbar redness | − 0.615 | < 0.001 | |
| TMH | − 0.395 | < 0.001 | |
| Mean first NIKBUT | 0.223 | 0.033 | |
| Median first NIKBUT | 0.226 | 0.030 | |
| OSDI | − 0.316 | 0.002 | |
| SD irregularity | Age | − 0.448 | < 0.001 |
| Drop-out percentage | − 0.322 | 0.002 | |
| Bulbar redness | − 0.306 | 0.003 | |
| LLT | 0.454 | < 0.001 | |
| Mean pixel intensity | Age | − 0.388 | < 0.001 |
| Drop-out percentage | − 0.285 | 0.005 | |
| Bulbar redness | − 0.210 | 0.043 | |
| LLT | 0.426 | < 0.001 | |
| Mean mean NIKBUT | 0.210 | 0.046 | |
| SD pixel intensity | Age | − 0.507 | < 0.001 |
| Drop-out percentage | − 0.331 | 0.001 | |
| Bulbar redness | − 0.400 | < 0.001 | |
| LLT | 0.446 | < 0.001 | |
| Median pixel intensity | Age | − 0.383 | < 0.001 |
| Drop-out percentage | − 0.294 | 0.004 | |
| LLT | 0.426 | < 0.001 | |
| Mode pixel intensity | Age | − 0.305 | 0.003 |
| Drop-out percentage | − 0.234 | 0.023 | |
| LLT | 0.418 | < 0.001 | |
| Kurtosis | LLT | − 0.515 | < 0.001 |
| Mean mean NIKBUT | − 0.252 | 0.016 | |
| Median mean NIKBUT | − 0.251 | 0.017 | |
| Skewness | LLT | − 0.510 | < 0.001 |
| Mean mean NIKBUT | − 0.237 | 0.024 | |
| Median mean NIKBUT | − 0.230 | 0.029 | |
| At 5.33 s | |||
| Total area | LLT | 0.647 | < 0.001 |
| Mean first NIKBUT | 0.265 | 0.011 | |
| Mean mean NIKBUT | 0.233 | 0.029 | |
| Minimum pixel intensity | LLT | 0.589 | < 0.001 |
| Mean first NIKBUT | 0.229 | 0.030 | |
| Energy | LLT | 0.548 | < 0.001 |
| Mean first NIKBUT | 0.223 | 0.019 | |
| Relative energy | LLT | 0.655 | < 0.001 |
| Mean first NIKBUT | 0.237 | 0.024 | |
| Entropy | Age | − 0.642 | < 0.001 |
| Drop-out percentage | − 0.572 | < 0.001 | |
| Bulbar redness | − 0.564 | < 0.001 | |
| TMH | − 0.403 | < 0.001 | |
| Mean first NIKBUT | 0.221 | 0.036 | |
| Median first NIKBUT | 0.219 | 0.038 | |
| OSDI | − 0.260 | 0.013 | |
| SD irregularity | Age | − 0.327 | 0.002 |
| Drop-out percentage | − 0.249 | 0.018 | |
| LLT | 0.662 | < 0.001 | |
| Mean pixel intensity | LLT | 0.665 | < 0.001 |
| Mean first NIKBUT | 0.235 | 0.026 | |
| SD of pixel intensity | Age | − 0.408 | < 0.001 |
| Drop-out percentage | − 0.272 | 0.009 | |
| Bulbar redness | − 0.247 | 0.019 | |
| LLT | 0.572 | < 0.001 | |
| Median pixel intensity | LLT | 0.674 | < 0.001 |
| Mean first NIKBUT | 0.246 | 0.020 | |
| Mean mean NIKBUT | 0.220 | 0.040 | |
| Mode pixel intensity | LLT | 0.657 | < 0.001 |
| Mean first NIKBUT | 0.233 | 0.027 | |
| Kurtosis | LLT | − 0.672 | < 0.001 |
| Skewness | LLT | − 0.673 | < 0.001 |
| At 10.33 s | |||
| Total area | LLT | 0.645 | < 0.001 |
| Minimum pixel intensity | LLT | 0.523 | < 0.001 |
| Energy | LLT | 0.660 | < 0.001 |
| Relative energy | LLT | 0.654 | < 0.001 |
| Entropy | Age | − 0.668 | < 0.001 |
| Drop-out percentage | − 0.596 | < 0.001 | |
| Bulbar redness | − 0.542 | < 0.001 | |
| TMH | − 0.536 | < 0.001 | |
| Mean first NIKBUT | 0.260 | 0.034 | |
| Median first NIKBUT | 0.282 | 0.021 | |
| OSDI | − 0.300 | 0.014 | |
| SD irregularity | Age | − 0.282 | 0.021 |
| LLT | 0.689 | < 0.001 | |
| Mean pixel intensity | LLT | 0.684 | < 0.001 |
| SD of pixel intensity | Age | − 0.371 | 0.002 |
| LLT | 0.644 | < 0.001 | |
| Median pixel intensity | LLT | 0.687 | < 0.001 |
| Mode pixel intensity | LLT | 0.654 | < 0.001 |
| Kurtosis | LLT | − 0.659 | < 0.001 |
| Skewness | LLT | − 0.665 | < 0.001 |
LLT lipid layer thickness, NIKBUT non-invasive keratograph break-up time, OSDI ocular surface disease index, s seconds, TMH tear meniscus height
Entropy was the only metric which was not correlated with LLT. Likewise, new metrics were not correlated with meibomian glands expressibility or DEQ-5 score. The metrics measured at 5.33 and 10.33 can be considered the best to describe the LLT since they revealed the strongest correlations.
Differences between groups
The new metrics were analysed according to age and the different ocular surface parameters. Table 4 shows the statistically significant differences in Placido disk reflectivity metrics between classification groups. These outcomes were in accordance with correlations. Statistically higher pixel intensity values were found in young subjects, lower glands drop-out, high NIKBUT, low TMH and thick LLT. However, no statistical differences were found between grade 3 (wave) and 4 (colour fringe) interference patterns in the assessment of LLT (p > 0.005).
Table 4.
Statistically significant differences in Placido disk reflectivity metrics for each ocular surface parameter
| New metrics | n | Groups | Mean ± SD | Significance level | Statistically significant post hoc differences (p-value) |
|---|---|---|---|---|---|
| Age | |||||
| At 0.33 s | |||||
| Total area | 60 | < 49 years | 117.43 ± 11.62 | < 0.0011 | |
| 34 | > 49 years | 111.47 ± 7.46 | |||
| Minimum pixel intensity | 60 | < 49 years | 75.44 ± 20.64 | < 0.0011 | |
| 34 | > 49 years | 62.40 ± 13.20 | |||
| Energy | 60 | < 49 years | 254.18 ± 4.38 | < 0.0011 | |
| 34 | > 49 years | 245.28 ± 4.38 | |||
| Relative energy | 60 | < 49 years | 0.59 ± 0.30 | < 0.0011 | |
| 34 | > 49 years | 0.38 ± 0.19 | |||
| Entropy | 60 | < 49 years | 3.0 × 10−4 ± 1.5 × 10−4 | < 0.0011 | |
| 34 | > 49 years | 1.0 × 10−4 ± 4 × 10−5 | |||
| SD irregularity | 60 | < 49 years | 0.13 ± 0.16 | < 0.0011 | |
| 34 | > 49 years | 0.04 ± 0.03 | |||
| Mean pixel intensity | 60 | < 49 years | 143.32 ± 33.52 | < 0.0011 | |
| 34 | > 49 years | 119.92 ± 17.24 | |||
| SD of pixel intensity | 60 | < 49 years | 30.05 ± 5.21 | < 0.0011 | |
| 34 | > 49 years | 25.10 ± 3.09 | |||
| Median pixel intensity | 60 | < 49 years | 142.32 ± 34.97 | < 0.0011 | |
| 34 | > 49 years | 118.50 ± 17.22 | |||
| Mode pixel intensity | 60 | < 49 years | 155.03 ± 53.85 | 0.0061 | |
| 34 | > 49 years | 125.85 ± 24.52 | |||
| At 5.33 s | |||||
| Entropy | 59 | < 49 years | 3.0 × 10−4 ± 2.1 × 10−4 | < 0.0011 | |
| 31 | > 49 years | 1.0 × 10−4 ± 5 × 10−5 | |||
| SD irregularity | 59 | < 49 years | 0.15 ± 0.15 | 0.0021 | |
| 31 | > 49 years | 0.07 ± 0.08 | |||
| Mean pixel intensity | 59 | < 49 years | 147.34 ± 32.78 | 0.0441 | |
| 31 | > 49 years | 131.92 ± 21.83 | |||
| SD of pixel intensity | 59 | < 49 years | 32.53 ± 5.47 | < 0.0011 | |
| 31 | > 49 years | 28.09 ± 3.68 | |||
| Mode | 59 | < 49 years | 169.26 ± 58.13 | 0.0431 | |
| 31 | > 49 years | 139.93 ± 36.77 | |||
| At 10.33 s | |||||
| Entropy | 43 | < 49 years | 3.0 × 10−4 ± 1.7 × 10−4 | < 0.0011 | |
| 23 | > 49 years | 1.0 × 10−4 ± 4 × 10−5 | |||
| SD irregularity | 43 | < 49 years | 0.17 ± 0.19 | 0.0111 | |
| 23 | > 49 years | 0.08 ± 0.09 | |||
| SD of pixel intensity | 43 | < 49 years | 32.62 ± 4.10 | 0.0021 | |
| 23 | > 49 years | 29.10 ± 4.37 | |||
| Bulbar redness | |||||
| At 0.33 s | |||||
| Total area | 60 | < 1 | 116.59 ± 11.70 | 0.0081 | |
| 34 | > 1 | 111.18 ± 7.73 | |||
| Minimum pixel intensity | 60 | < 1 | 73.32 ± 20.77 | 0.0041 | |
| 34 | > 1 | 63.60 ± 14.12 | |||
| Energy | 60 | < 1 | 254.08 ± 4.40 | < 0.0011 | |
| 34 | > 1 | 245.44 ± 21.94 | |||
| Relative energy | 60 | < 1 | 0.57 ± 0.29 | 0.0021 | |
| 34 | > 1 | 0.39 ± 0.21 | |||
| Entropy | 60 | < 1 | 3.0 × 10−4 ± 1.5 × 10−4 | < 0.0011 | |
| 34 | > 1 | 1.0 × 10−4 ± 4 × 10−5 | |||
| SD irregularity | 60 | < 1 | 0.13 ± 0.16 | < 0.0011 | |
| 34 | > 1 | 0.05 ± 0.04 | |||
| Mean pixel intensity | 60 | < 1 | 140.99 ± 33.54 | 0.0011 | |
| 34 | > 1 | 121.23 ± 18.82 | |||
| SD of pixel intensity | 60 | < 1 | 29.98 ± 5.08 | < 0.0011 | |
| 34 | > 1 | 25.14 ± 3.27 | |||
| Median pixel intensity | 60 | < 1 | 139.94 ± 34.98 | 0.0011 | |
| 34 | > 1 | 119.85 ± 18.87 | |||
| Mode pixel intensity | 60 | < 1 | 152.92 ± 54.38 | 0.0111 | |
| 34 | > 1 | 127.05 ± 25.23 | |||
| Skewness | 60 | < 1 | 0.14 ± 0.03 | 0.0441 | |
| 34 | > 1 | 0.15 ± 0.01 | |||
| At 5.33 s | |||||
| Entropy | 58 | < 1 | 3.0 × 10−4 ± 2.1 × 10−4 | < 0.0011 | |
| 32 | > 1 | 1.0 × 10−4 ± 5 × 10−5 | |||
| SD irregularity | 58 | < 1 | 0.14 ± 0.15 | 0.0281 | |
| 32 | > 1 | 0.07 ± 0.08 | |||
| SD of pixel intensity | 58 | < 1 | 31.99 ± 5.58 | 0.0021 | |
| 32 | > 1 | 28.30 ± 3.84 | |||
| Entropy | 40 | < 1 | 3.0 × 10−4 ± 1.7 × 10−4 | < 0.0011 | |
| 25 | > 1 | 1.0 × 10−4 ± 4 × 10−5 | |||
| SD of pixel intensity | 40 | < 1 | 131.72 ± 4.24 | 0.0241 | |
| 25 | > 1 | 129.42 ± 5.59 | |||
| Meibomian glands drop-out | |||||
| At 0.33 s | |||||
| Total area | 50 | < 1/3 | 115.94 ± 10.62 | 0.0081 | |
| 44 | > 1/3 | 111.59 ± 8.12 | |||
| Minimum pixel intensity | 50 | < 1/3 | 71.80 ± 19.69 | 0.0081 | |
| 44 | > 1/3 | 63.00 ± 13.97 | |||
| Energy | 50 | < 1/3 | 254.12 ± 4.20 | 0.0021 | |
| 44 | > 1/3 | 245.94 ± 4.18 | |||
| Relative energy | 50 | < 1/3 | 0.54 ± 0.28 | 0.0071 | |
| 44 | > 1/3 | 0.39 ± 0.22 | |||
| Entropy | 50 | < 1/3 | 2.0 × 10−4 ± 1.4 × 10−4 | < 0.0011 | |
| 44 | > 1/3 | 2.0 × 10−4 ± 7.0 × 10−5 | |||
| SD irregularity | 50 | < 1/3 | 0.12 ± 0.15 | 0.0011 | |
| 44 | > 1/3 | 0.05 ± 0.04 | |||
| Mean pixel intensity | 50 | < 1/3 | 136.92 ± 31.60 | 0.0051 | |
| 44 | > 1/3 | 120.87 ± 18.90 | |||
| SD of pixel intensity | 50 | < 1/3 | 28.65 ± 5.29 | 0.0021 | |
| 44 | > 1/3 | 25.35 ± 3.30 | |||
| Median pixel intensity | 50 | < 1/3 | 135.95 ± 32.77 | 0.0051 | |
| 44 | > 1/3 | 119.34 ± 18.90 | |||
| Mode pixel intensity | 50 | < 1/3 | 148.50 ± 48.31 | 0.0201 | |
| 44 | > 1/3 | 125.76 ± 27.54 | |||
| At 5.33 s | |||||
| Entropy | 48 | < 1/3 | 3.0 × 10−4 ± 2.0 × 10−4 | < 0.0011 | |
| 42 | > 1/3 | 1.0 × 10−4 ± 8.0 × 10−5 | |||
| SD irregularity | 48 | < 1/3 | 0.13 ± 0.14 | 0.0311 | |
| 42 | > 1/3 | 0.07 ± 0.09 | |||
| SD of pixel intensity | 48 | < 1/3 | 31.08 ± 5.39 | 0.0131 | |
| 42 | > 1/3 | 28.34 ± 3.93 | |||
| At 10.33 s | |||||
| Entropy | 33 | < 1/3 | 3.0 × 10−4 ± 1.6 × 10−4 | < 0.0011 | |
| 33 | > 1/3 | 1.0 × 10−4 ± 4.0 × 10−5 | |||
| SD irregularity | 33 | < 1/3 | 0.14 ± 0.16 | 0.0461 | |
| 33 | > 1/3 | 0.09 ± 0.10 | |||
| SD of pixel intensity | 33 | < 1/3 | 31.48 ± 4.06 | 0.0211 | |
| 33 | > 1/3 | 29.17 ± 4.80 | |||
| TMH | |||||
| At 0.33 s | |||||
| Entropy | 60 | > 0.20 mm | 3.0 × 10−4 ± 1.6 × 10−4 | < 0.0011 | |
| 34 | < 0.20 mm | 2.0 × 10−4 ± 5.0 × 10−5 | |||
| At 5.33 s | |||||
| Entropy | 55 | > 0.20 mm | 3.0 × 10−4 ± 2.2 × 10−4 | < 0.0011 | |
| 35 | < 0.20 mm | 1.0 × 10−4 ± 5.0 × 10−5 | |||
| At 10.33 s | |||||
| Entropy | 38 | > 0.20 mm | 3.0 × 10−4 ± 1.7 × 10−4 | < 0.0011 | |
| 28 | < 0.20 mm | 1.0 × 10−4 ± 4.0 × 10−5 | |||
| Mean first NIKBUT | |||||
| At 0.33 s | |||||
| Entropy | 41 | > 10 s | 2.0 × 10−4 ± 1.3 × 10−4 | 0.0341 | |
| 53 | < 10 s | 2.0 × 10−4 ± 9.0 × 10−5 | |||
| At 5.33 s | |||||
| Entropy | 41 | > 10 s | 2.0 × 10−4 ± 1.8 × 10−4 | 0.0421 | |
| 49 | < 10 s | 2.0 × 10−4 ± 1.2 × 10−4 | |||
| At 10.33 s | |||||
| Entropy | 36 | > 10 s | 2.0 × 10−4 ± 1.6 × 10−4 | 0.0261 | |
| 30 | < 10 s | 2.0 × 10−4 ± 1.0 × 10−4 | |||
| LLT | |||||
| At 0.33 s | |||||
| Total area | 28 | Grade 1 | 109.67 ± 8.76 | < 0.0012 | 1–3 < 0.001 |
| 38 | Grade 2 | 111.96 ± 8.70 | 1–4 = 0.002 | ||
| 15 | Grade 3 | 120.70 ± 10.16 | 2–3 = 0.001 | ||
| 13 | Grade 4 | 119.26 ± 7.30 | 2–4 = 0.008 | ||
| Minimum pixel intensity | 28 | Grade 1 | 60.75 ± 11.06 | 0.0012 | 1–3 = 0.001 |
| 38 | Grade 2 | 62.60 ± 12.72 | 1–4 = 0.041 | ||
| 15 | Grade 3 | 83.79 ± 22.62 | 2–3 = 0.001 | ||
| 13 | Grade 4 | 75.92 ± 21.02 | 2–4 = 0.046 | ||
| Energy | 28 | Grade 1 | 239.86 ± 26.07 | < 0.0012 |
1–2 = 0.032 1–3 < 0.001 1–4 ≤ 0.001 2–3 = 0.002 2–4 = 0.001 |
| 38 | Grade 2 | 243.52 ± 22.66 | |||
| 15 | Grade 3 | 254.55 ± 2.08 | |||
| 13 | Grade 4 | 254.99 ± 0.01 | |||
| Relative energy | 28 | Grade 1 | 0.33 ± 0.13 | < 0.0012 | 1–3 < 0.001 |
| 38 | Grade 2 | 0.40 ± 0.20 | 1–4 = 0.002 | ||
| 15 | Grade 3 | 0.73 ± 0.29 | 2–3 = 0.001 | ||
| 13 | Grade 4 | 0.72 ± 0.29 | 2–4 = 0.016 | ||
| SD irregularity | 28 | Grade 1 | 0.03 ± 0.01 | < 0.0012 | 1–3 < 0.001 |
| 38 | Grade 2 | 0.05 ± 0.03 | 1–4 < 0.001 | ||
| 15 | Grade 3 | 0.18 ± 0.18 | 2–3 = 0.008 | ||
| 13 | Grade 4 | 0.15 ± 0.17 | 2–4 = 0.014 | ||
| Mean pixel intensity | 28 | Grade 1 | 115.09 ± 11.56 | < 0.0012 | 1–3 < 0.001 |
| 38 | Grade 2 | 121.47 ± 17.40 | 1–4 = 0.001 | ||
| 15 | Grade 3 | 156.54 ± 35.25 | 2–3 = 0.002 | ||
| 13 | Grade 4 | 155.23 ± 32.90 | 2–4 = 0.008 | ||
| SD of pixel intensity | 28 | Grade 1 | 24.50 ± 2.25 | < 0.0012 | 1–3 = 0.001 |
| 38 | Grade 2 | 26.06 ± 3.35 | 1–4 < 0.001 | ||
| 15 | Grade 3 | 30.37 ± 6.17 | 2–3 = 0.027 | ||
| 13 | Grade 4 | 30.84 ± 5.66 | 2–4 = 0.008 | ||
| Median pixel intensity | 28 | Grade 1 | 113.68 ± 11.76 | < 0.0012 | 1–3 < 0.001 |
| 38 | Grade 2 | 119.92 ± 17.47 | 1–4 = 0.001 | ||
| 15 | Grade 3 | 156.21 ± 36.16 | 2–3 = 0.002 | ||
| 13 | Grade 4 | 157.08 ± 34.84 | 2–4 = 0.009 | ||
| Mode pixel intensity | 28 | Grade 1 | 120.22 ± 15.53 | < 0.0012 | 1–3 < 0.001 |
| 38 | Grade 2 | 122.68 ± 19.12 | 1–4 = 0.002 | ||
| 15 | Grade 3 | 178.72 ± 57.03 | 2–3 < 0.001 | ||
| 13 | Grade 4 | 178.92 ± 55.12 | 2–4 = 0.002 | ||
| Kurtosis | 28 | Grade 1 | 0.019 ± 0.003 | < 0.0012 | 1–3 < 0.001 |
| 38 | Grade 2 | 0.018 ± 0.003 | 1–4 < 0.001 | ||
| 15 | Grade 3 | 0.014 ± 0.003 | 2–3 = 0.001 | ||
| 13 | Grade 4 | 0.015 ± 0.003 | 2–4 = 0.030 | ||
| Skewness | 28 | Grade 1 | 0.15 ± 0.01 | < 0.0012 | 1–3 < 0.001 |
| 38 | Grade 2 | 0.15 ± 0.01 | 1–4 = 0.001 | ||
| 15 | Grade 3 | 0.13 ± 0.02 | 2–3 = 0.001 | ||
| 13 | Grade 4 | 0.13 ± 0.02 | 2–4 = 0.023 | ||
| At 5.33 s | |||||
| Total area | 27 | Grade 1 | 110.03 ± 6.85 | < 0.0012 |
1–2 = 0.004 1–3 < 0.001 1–4 < 0.001 2–3 = 0.002 2–4 = 0.005 |
| 37 | Grade 2 | 116.70 ± 7.62 | |||
| 15 | Grade 3 | 123.84 ± 5.40 | |||
| 11 | Grade 4 | 124.25 ± 3.88 | |||
| Minimum pixel intensity | 27 | Grade 1 | 57.44 ± 8.43 | < 0.0012 | 1–2 = 0.001 |
| 37 | Grade 2 | 69.08 ± 14.20 | 1–3 < 0.001 | ||
| 15 | Grade 3 | 82.93 ± 19.00 | 1–4 < 0.001 | ||
| 11 | Grade 4 | 79.64 ± 14.03 | 2–3 = 0.023 | ||
| Energy | 27 | Grade 1 | 238.28 ± 23.74 | < 0.0012 |
1–2 = 0.007 1–3 < 0.001 1–4 < 0.001 2–3 < 0.001 2–4 < 0.001 |
| 37 | Grade 2 | 242.24 ± 20.14 | |||
| 15 | Grade 3 | 254.14 ± 2.28 | |||
| 11 | Grade 4 | 254.45 ± 0.39 | |||
| Relative energy | 27 | Grade 1 | 0.33 ± 0.13 | < 0.0012 |
1–2 = 0.005 1–3 < 0.001 1–4 < 0.001 2–3 = 0.002 2–4 = 0.003 |
| 37 | Grade 2 | 0.52 ± 0.23 | |||
| 15 | Grade 3 | 0.79 ± 0.20 | |||
| 11 | Grade 4 | 0.80 ± 0.17 | |||
| SD irregularity | 27 | Grade 1 | 0.04 ± 0.01 | 0.0012 |
1–2 = 0.023 1–3 < 0.001 1–4 < 0.001 2–3 = 0.001 2–4 = 0.001 |
| 37 | Grade 2 | 0.06 ± 0.04 | |||
| 15 | Grade 3 | 0.23 ± 0.18 | |||
| 11 | Grade 4 | 0.20 ± 0.15 | |||
| Mean pixel intensity | 27 | Grade 1 | 116.87 ± 9.94 | < 0.0012 |
1–2 = 0.005 1–3 < 0.001 1–4 < 0.001 2–3 = 0.002 2–4 = 0.002 |
| 37 | Grade 2 | 132.69 ± 19.70 | |||
| 15 | Grade 3 | 166.37 ± 27.89 | |||
| 11 | Grade 4 | 166.26 ± 23.58 | |||
| SD of pixel intensity | 27 | Grade 1 | 27.05 ± 2.86 | < 0.0012 | 1–3 < 0.001 |
| 37 | Grade 2 | 28.25 ± 3.58 | 1–4 < 0.001 | ||
| 15 | Grade 3 | 34.20 ± 5.77 | 2–3 = 0.001 | ||
| 11 | Grade 4 | 34.85 ± 3.59 | 2–4 < 0.001 | ||
| Median pixel intensity | 27 | Grade 1 | 115.00 ± 10.78 | < 0.0012 |
1–2 = 0.004 1–3 < 0.001 1–4 < 0.001 2–3 = 0.001 2–4 = 0.002 |
| 37 | Grade 2 | 131.68 ± 10.78 | |||
| 15 | Grade 3 | 166.93 ± 29.20 | |||
| 11 | Grade 4 | 166.73 ± 24.05 | |||
| Mode pixel intensity | 27 | Grade 1 | 116.33 ± 21.15 | < 0.0012 |
1–2 = 0.003 1–3 < 0.001 1–4 < 0.001 2–3 = 0.006 2–4 = 0.003 |
| 37 | Grade 2 | 140.35 ± 23.95 | |||
| 15 | Grade 3 | 198.64 ± 55.22 | |||
| 11 | Grade 4 | 202.90 ± 52.29 | |||
| Kurtosis | 27 | Grade 1 | 0.017 ± 0.001 | < 0.0012 | 1–3 < 0.001 |
| 37 | Grade 2 | 0.016 ± 0.002 | 1–4 < 0.001 | ||
| 15 | Grade 3 | 0.012 ± 0.002 | 2–3 < 0.001 | ||
| 11 | Grade 4 | 0.012 ± 0.002 | 2–4 = 0.001 | ||
| Skewness | 27 | Grade 1 | 0.14 ± 0.01 | < 0.0012 | 1–3 < 0.001 |
| 37 | Grade 2 | 0.14 ± 0.01 | 1–4 < 0.001 | ||
| 15 | Grade 3 | 0.12 ± 0.01 | 2–3 < 0.001 | ||
| 11 | Grade 4 | 0.12 ± 0.01 | 2–4 = 0.001 | ||
| At 10.33 s | |||||
| Total area | 18 | Grade 1 | 110.14 ± 8.54 | < 0.0012 | 1–3 < 0.001 |
| 28 | Grade 2 | 115.47 ± 8.34 | 1–4 < 0.001 | ||
| 11 | Grade 3 | 125.88 ± 3.45 | 2–3 < 0.001 | ||
| 9 | Grade 4 | 124.59 ± 2.56 | 2–4 = 0.012 | ||
| Minimum pixel intensity | 18 | Grade 1 | 58.00 ± 11.69 | < 0.0012 |
1–3 < 0.001 1–4 = 0.004 2–3 = 0.001 |
| 28 | Grade 2 | 66.43 ± 15.16 | |||
| 11 | Grade 3 | 91.64 ± 19.27 | |||
| 9 | Grade 4 | 88.33 ± 14.93 | |||
| Energy | 18 | Grade 1 | 238.22 ± 20.54 | < 0.0012 | 1–3 < 0.001 |
| 28 | Grade 2 | 242.01 ± 19.16 | 1–4 < 0.001 | ||
| 11 | Grade 3 | 254.01 ± 2.09 | 2–3 = 0.001 | ||
| 9 | Grade 4 | 254.04 ± 0.42 | 2–4 = 0.004 | ||
| Relative energy | 18 | Grade 1 | 0.36 ± 0.18 | < 0.0012 | 1–3 < 0.001 |
| 28 | Grade 2 | 0.50 ± 0.25 | 1–4 < 0.001 | ||
| 11 | Grade 3 | 0.89 ± 0.16 | 2–3 < 0.001 | ||
| 9 | Grade 4 | 0.80 ± 0.13 | 2–4 = 0.010 | ||
| SD irregularity | 18 | Grade 1 | 0.04 ± 0.01 | < 0.0012 | 1–3 < 0.001 |
| 28 | Grade 2 | 0.07 ± 0.05 | 1–4 < 0.001 | ||
| 11 | Grade 3 | 0.29 ± 0.21 | 2–3 < 0.001 | ||
| 9 | Grade 4 | 0.21 ± 0.13 | 2–4 = 0.004 | ||
| Mean pixel intensity | 18 | Grade 1 | 117.99 ± 13.74 | < 0.0012 | 1–3 < 0.001 |
| 28 | Grade 2 | 132.00 ± 22.31 | 1–4 < 0.001 | ||
| 11 | Grade 3 | 178.50 ± 25.59 | 2–3 < 0.001 | ||
| 9 | Grade 4 | 170.99 ± 19.92 | 2–4 = 0.003 | ||
| SD of pixel intensity | 18 | Grade 1 | 27.04 ± 2.96 | < 0.0012 | 1–3 < 0.001 |
| 28 | Grade 2 | 29.48 ± 3.57 | 1–4 < 0.001 | ||
| 11 | Grade 3 | 33.99 ± 4.04 | 2–3 = 0.006 | ||
| 9 | Grade 4 | 35.10 ± 4.34 | 2–4 = 0.004 | ||
| Median pixel intensity | 18 | Grade 1 | 116.17 ± 14.40 | < 0.0012 | 1–3 < 0.001 |
| 28 | Grade 2 | 130.82 ± 23.06 | 1–4 < 0.001 | ||
| 11 | Grade 3 | 179.45 ± 27.88 | 2–3 < 0.001 | ||
| 9 | Grade 4 | 170.67 ± 20.63 | 2–4 = 0.004 | ||
| Mode pixel intensity | 18 | Grade 1 | 117.67 ± 23.25 | < 0.0012 | 1–3 < 0.001 |
| 28 | Grade 2 | 137.79 ± 31.60 | 1–4 < 0.001 | ||
| 11 | Grade 3 | 214.36 ± 49.28 | 2–3 < 0.001 | ||
| 9 | Grade 4 | 192.67 ± 49.86 | 2–4 = 0.002 | ||
| Kurtosis | 18 | Grade 1 | 0.017 ± 0.002 | < 0.0012 | 1–3 < 0.001 |
| 28 | Grade 2 | 0.015 ± 0.003 | 1–4 = 0.001 | ||
| 11 | Grade 3 | 0.012 ± 0.002 | 2–3 = 0.001 | ||
| 9 | Grade 4 | 0.013 ± 0.003 | 2–4 = 0.015 | ||
| Skewness | 18 | Grade 1 | 0.14 ± 0.01 | < 0.0012 | 1–3 < 0.001 |
| 28 | Grade 2 | 0.13 ± 0.01 | 1–4 = 0.001 | ||
| 11 | Grade 3 | 0.11 ± 0.01 | 2–3 = 0.001 | ||
| 9 | Grade 4 | 0.11 ± 0.01 | 2–4 = 0.011 | ||
LLT lipid layer thickness, m millimetres, n number of patients, NIKBUT non-invasive keratograph break-up time, s seconds, SD standard deviation, TMH tear meniscus height
1Mann-Whitney U test
2Kruskal-Wallis test
Multiple linear regressions
Since the metrics at 5.33 s after blinking have proved to differentiate between grades 1 (open meshwork), 2 (closed meshwork) and 3 (wave) of the LLT, only the metrics at 5.33 s after blinking will be assessed in this section of the manuscript.
Multiple linear regressions (Table 5) were performed to show the current metrics that were associated with new metrics, avoiding that the interaction between current metrics mislead results. Multiple linear regressions showed that new metrics were statistically significant associated with LLT, explaining the variability between 7.1 and 47.0% depending on the metric. Kurtosis and skewness showed a weak association with gland drop-out percentage instead of with LLT. Energy also appeared to be associated with the first median NIKBUT together with LLT. No association was found with the remaining variables. Generally, these results suggest that the main predictor factor of new metrics was LLT.
Table 5.
Multiple linear regressions for new metrics at 5.33 s where the independent variables included were gland drop-out percentage, bulbar redness, lipid layer thickness, tear meniscus height, first and mean NIKBUT, gland expressibility, OSDI and DEQ-5
| New metrics | Current metrics | β | SE | Sβ | Significance level | Adjusted R square |
|---|---|---|---|---|---|---|
| Total area | Constant | 129.99 | 140.09 | < 0.001 | 0.470 | |
| LLT | 18.64 | 2.62 | 0.71 | < 0.001 | ||
| Minimum pixel intensity | Constant | 40.34 | 9.86 | < 0.001 | 0.325 | |
| LLT | 9.72 | 1.83 | 0.60 | < 0.001 | ||
| Energy | Constant | 261.47 | 8.09 | < 0.001 | 0.214 | |
| LLT | 5.27 | 1.50 | 0.42 | 0.001 | ||
| First median NIKBUT | 1.24 | 0.61 | 0.64 | 0.045 | ||
| Relative energy | Constant | 0.87 | 0.14 | < 0.001 | 0.404 | |
| LLT | 0.17 | 0.03 | 0.69 | < 0.001 | ||
| Entropy | Constant | 0.000 | 0.000 | < 0.001 | 0.050 | |
| First median NIKBUT | 0.00000063 | 0.00 | 0.73 | 0.037 | ||
| SD irregularity | Constant | 0.13 | 0.03 | < 0.001 | 0.071 | |
| LLT | 0.014 | 0.005 | 0.39 | 0.005 | ||
| Mean pixel intensity | Constant | 137.78 | 15.63 | < 0.001 | 0.457 | |
| LLT | 20.26 | 2.91 | 0.70 | < 0.001 | ||
| SD of pixel intensity | Constant | 25.15 | 1.81 | < 0.001 | 0.193 | |
| LLT | 1.22 | 0.34 | 0.45 | 0.001 | ||
| Median pixel intensity | Constant | 140.60 | 16.64 | < 0.001 | 0.468 | |
| LLT | 21.89 | 3.09 | 0.70 | < 0.001 | ||
| Mode pixel intensity | Constant | 130.92 | 22.42 | < 0.001 | 0.432 | |
| LLT | 28.37 | 4.17 | 0.70 | < 0.001 | ||
| Kurtosis | Constant | 0.012 | 0.001 | < 0.001 | 0.114 | |
| Gland drop-out percentage | 0.000031 | 0.000 | 0.33 | 0.042 | ||
| Skewness | Constant | 0.119 | 0.005 | < 0.001 | 0.099 | |
| Gland drop-out percentage | 0.000 | 0.000 | 0.36 | 0.029 |
β unstandardized coefficient, Sβ standardized coefficient, LLT lipid layer thickness, NIKBUT non-invasive break-up time, SD standard deviation, SE standard error
Diagnostic capability and validation of the new metrics
Table 6 summarizes the diagnostic power and the cut-off values for each new metric when grade 1 LLT was compared with other grades. New developed metrics were powerful indicators to detect subjects with an altered lipid layer (grade 1 — open meshwork) since the area under the curve, sensitivity and specificity obtained were high. Mean pixel intensity, median pixel intensity and relative energy were the metrics with the highest sensitivity, specificity, area under the curve, Youden index, discriminant power, accuracy, Kappa index and F-measure.
Table 6.
ROC curve parameters of newly developed metrics to differentiate grade 1 LLT from other grades at 5.33 s
| Metric | Sensitivity | Specificity | Area under the curve (CI) | Cut-off value | Youden index | Discriminant power | Accuracy | Kappa index | F-measure |
|---|---|---|---|---|---|---|---|---|---|
| Total area | 0.94 | 0.76 | 0.89 (0.83–0.96) | 117.74 | 0.70 | 2.18 | 0.83 | 0.72 | 0.81 |
| Minimum pixel intensity | 0.92 | 0.74 | 0.88 (0.82–0.95) | 67.50 | 0.65 | 1.89 | 0.82 | 0.70 | 0.80 |
| Energy | 0.87 | 0.77 | 0.82 (0.71–0.88) | 239.15 | 0.65 | 1.77 | 0.82 | 0.70 | 0.80 |
| Relative energy | 0.92 | 0.81 | 0.91 (0.83–0.96) | 0.48 | 0.73 | 2.13 | 0.86 | 0.76 | 0.84 |
| SD irregularity | 0.89 | 0.77 | 0.86 (0.79–0.94) | 0.05 | 0.66 | 1.83 | 0.82 | 0.70 | 0.79 |
| Mean pixel intensity | 0.94 | 0.79 | 0.89 (0.83–0.96) | 128.62 | 0.74 | 2.29 | 0.86 | 0.76 | 0.84 |
| SD of pixel intensity | 0.83 | 0.70 | 0.78 (0.68–0.88) | 28.08 | 0.53 | 1.35 | 0.74 | 0.57 | 0.73 |
| Median pixel intensity | 0.92 | 0.81 | 0.91 (0.84–0.97) | 124.50 | 0.73 | 2.13 | 0.86 | 0.76 | 0.84 |
| Mode pixel intensity | 0.83 | 0.77 | 0.87 (0.80–0.94) | 133.50 | 0.61 | 1.56 | 0.80 | 0.66 | 0.78 |
| Kurtosis | 0.89 | 0.76 | 0.83 (0.74–0.92) | 0.015 | 0.64 | 1.77 | 0.78 | 0.63 | 0.76 |
| Skewness | 0.92 | 0.72 | 0.84 (0.75–0.92) | 0.13 | 0.63 | 1.84 | 0.80 | 0.66 | 0.78 |
CI 95% confidence interval, SD standard deviation
Tables 7 and 8 show the diagnostic power of each new metric to differentiate between grades 1 and 2, and between grades 2 and 3, respectively. This step allowed finding the cut-off values for each new metric to objectively classify the lipid layer into different grades. The cut-off value which optimizes the diagnosis determines the best score to diagnose the disease. Thus, a subject with a higher score than the cut-off value in kurtosis and skewness was classified into the thinner LLT group, while a subject with a higher score than the cut-off value in the rest of the newly developed metrics was classified into the thicker LLT group. The SD of pixel intensity had a low specificity to distinguish between grades 1 and 2, which could lead to the lipid layer being misclassified.
Table 7.
ROC curve parameters of new developed metrics to differentiate between grades 1 and 2 LLT at 5.33 s
| Metric | Sensitivity | Specificity | Area under the curve (CI) | Cut-off value | Youden index | Discriminant power |
|---|---|---|---|---|---|---|
| Total area | 0.89 | 0.70 | 0.83 (0.73–0.94) | 116.20 | 0.59 | 1.62 |
| Minimum pixel intensity | 0.86 | 0.74 | 0.86 (0.77–0.95) | 64.50 | 0.60 | 1.58 |
| Energy | 0.86 | 0.73 | 0.81 (0.71–0.91) | 238.59 | 0.59 | 1.55 |
| Relative energy | 0.92 | 0.74 | 0.83 (0.72–0.94) | 0.48 | 0.66 | 1.90 |
| SD irregularity | 0.83 | 0.70 | 0.78 (0.66–0.90) | 0.05 | 0.54 | 1.36 |
| Mean pixel intensity | 0.92 | 0.74 | 0.84 (0.73–0.95) | 126.93 | 0.66 | 1.90 |
| SD of pixel intensity | 0.83 | 0.48 | 0.66 (0.53–0.80) | 28.22 | 0.31 | 0.84 |
| Median pixel intensity | 0.92 | 0.74 | 0.85 (0.74–0.95) | 124.50 | 0.66 | 1.90 |
| Mode pixel intensity | 0.83 | 0.70 | 0.82 (0.71–0.93) | 133.50 | 0.54 | 1.36 |
| Kurtosis | 0.89 | 0.63 | 0.74 (0.60–0.87) | 0.015 | 0.52 | 1.44 |
| Skewness | 0.83 | 0.67 | 0.74 (0.60–0.87) | 0.14 | 0.5 | 1.27 |
CI 95% confidence interval, SD standard deviation
Table 8.
ROC curve parameters of new developed metrics to differentiate between grade 2 and 3 LLT at 5.33 s
| Metric | Sensitivity | Specificity | Area under the curve (CI) | Cut-off value | Youden index | Discriminant power |
|---|---|---|---|---|---|---|
| Total area | 0.78 | 0.81 | 0.80 (0.65–0.95) | 123.97 | 0.59 | 1.50 |
| Minimum pixel intensity | 0.71 | 0.69 | 0.74 (0.57–0.91) | 79.50 | 0.40 | 0.94 |
| Energy | 0.71 | 0.84 | 0.78 (0.62–0.93) | 248.19 | 0.55 | 1.41 |
| Relative energy | 0.74 | 0.88 | 0.81 (0.67–0.96) | 0.72 | 0.62 | 1.65 |
| SD irregularity | 0.89 | 0.69 | 0.82 (0.68–0.97) | 0.12 | 0.58 | 1.58 |
| Mean pixel intensity | 0.78 | 0.88 | 0.83 (0.68–0.97) | 150.69 | 0.65 | 1.76 |
| SD of pixel intensity | 0.82 | 0.69 | 0.80 (0.65–0.95) | 32.36 | 0.50 | 1.25 |
| Median pixel intensity | 0.70 | 0.88 | 0.83 (0.69–0.97) | 160.50 | 0.58 | 1.55 |
| Mode pixels | 0.96 | 0.63 | 0.80 (0.63–0.96) | 183.00 | 0.59 | 2.08 |
| Kurtosis | 0.82 | 0.75 | 0.82 (0.68–0.96) | 0.013 | 0.57 | 1.42 |
| Skewness | 0.93 | 0.69 | 0.84 (0.70–0.97) | 0.12 | 0.61 | 1.83 |
CI 95% confidence interval, SD standard deviation
Once the cut-off values were calculated, the lipid layer was objectively classified. The level of agreement between the newly developed objective and existing subjective classifications was evaluated (Table 9). Since different LLT grades were evaluated, the weighted Kappa index was calculated [37]. Mean pixel intensity, median pixel intensity and relative energy were the metrics with the highest area under the curve, best relationship between sensitivity and specificity and higher agreement between objective and subjective methods for LLT classification.
Table 9.
Agreement between the subjective and objective classification of LLT for each parameter at 5.33 s
| Metric | Accuracy | Kappa index | F-measure |
|---|---|---|---|
| Total area | 0.72 | 0.73 | 0.84 |
| Minimum pixel intensity | 0.68 | 0.66 | 0.81 |
| Energy | 0.72 | 0.73 | 0.84 |
| Relative energy | 0.76 | 0.76 | 0.86 |
| SD irregularity | 0.71 | 0.73 | 0.83 |
| Mean pixel intensity | 0.77 | 0.77 | 0.87 |
| SD of pixel intensity | 0.63 | 0.61 | 0.78 |
| Median pixel intensity | 0.76 | 0.77 | 0.86 |
| Mode pixel intensity | 0.71 | 0.67 | 0.83 |
| Kurtosis | 0.69 | 0.69 | 0.82 |
| Skewness | 0.70 | 0.69 | 0.82 |
SD standard deviation
Discussion
The assessment of LLT plays an essential role in DED and MGD because of the relevance of the lipid layer in the TF [1, 4]. Existing tests lack objectivity, preciseness, are time-consuming or are inaccessible for most clinicians due to the need of an interferometer to be performed [8, 10, 11, 13–19]. The present article introduces a new self-developed technique for the non-invasive objective evaluation of the LLT which can be implemented in any Placido disk topograph.
The present work has tested the validity and applicability of new metrics calculated from the grey level intensity values of the Placido disk pattern reflected onto the TF. Alonso-Caneiro et al. [22] performed a similar study, in which they used texture analysis of videokeratoscopy images and denoted that the proposed technique offered clinical utility in the diagnosis of DED (area under the curve from 0.77 to 0.82, sensitivity of 0.9 and specificity of 0.6). However, the authors did not explain why this could be a predictor of DED since they did not study the correlations of the metric with ocular surface parameters. Therefore, they did not evidence which parameter of the TF they were measuring.
The present work makes three important contributions: (1) the development of a new method to assess LLT in an unbiased, objective, quick and non-invasive way; (2) the possibility of assessing the lipid layer without the need of an interferometer, making the method widely accessible; (3) the validation of the new technique through the study of its repeatability, diagnostic capability and correlations with ocular surface parameters.
Correlations between Placido disk reflectivity metrics and ocular surface parameters
Moderate positive significant correlations were found between grey level intensities of the Placido disk pattern and LLT and NIKBUT. The correlations between new developed metrics and age, meibomian glands drop-out, bulbar redness, TMH and OSDI (Table 3) might be a consequence of their correlation with LLT since LLT is also correlated with age, meibomian glands drop-out and NIKBUT [38–42].
Despite the above, in the present study, LLT revealed no correlation with bulbar redness, TMH and OSDI. Finis et al.[41] neither found a significant correlation between DED symptoms and LLT, although this was not in accordance with others [39, 40, 43, 44]. New metrics, though less strongly correlated with bulbar redness, TMH and OSDI than with LLT, could still be used to assess these ocular surface parameters.
Entropy measures the randomness of a grey level distribution [22] and as a result might change as the TF becomes thinner and the Placido disk pattern becomes more unstructured [22]. This metric was not correlated with LLT, although it revealed a significant correlation with glands drop-out, bulbar redness, TMH, NIKBUT and OSDI, and thus, it might be used to predict these parameters.
Moreover, despite that new metrics were correlated with LLT, no statistically significant correlations were found with meibomian glands expressibility, although previous research did find a correlation between these parameters [41].
Differences between groups
When the sample was subjectively divided into 4 different LLT groups, using grade scales of interference patterns, statistically significant differences in the new metrics were found between them (Table 4). The measurements at 5.33 s after blinking were the best to differentiate among the different LLT grades since metrics were able to distinguish between grades 1 and 2 and grades 2 and 3. Nonetheless, the algorithm could not differentiate between grades 3 (wave) and 4 (colour fringe pattern). This could be due to the fact that grade 4 differs from grade 3 in that 4 is the only grade, in the interference scale, to imply a coloured pattern, which cannot be detected using grey level values. Hence, as already reported by other authors [8], it would be necessary to incorporate a colour analysis to differentiate between grades 3 and 4.
Nevertheless, since the TFOS DEWS II diagnostic report reported that a subject is classified as having DED when the LLT has a grade of 1, differentiating between grade 3 and 4 has a low clinical utility. Additionally, thinner patterns are more difficult to characterize by an examiner [3, 14].
In addition to being capable of differentiating between LLT grades, the metrics at 5.33 s after blinking are performed under more realistic conditions than at later times, as subjects are not required to forcefully suppress blinking. Moreover, metrics at 0.33 s might not have achieved a similar performance than at 5.33 s in assessing LLT since at 0.33 s after blinking, the lipid layer might not have stabilized yet.
Placido disk reflectivity metrics over time
Repeated mixed model ANOVA showed statistical higher pixel intensity values at 10.33, 15.33 and 20.33 s than at 0.33 s (Table 1). This might be due to the fact that the sample size decreased as the seconds after blink increased. Thus, only subjects with larger NIKBUT values were able to maintain the eye opened for 20.33 s. This may be behind the observed differences as LLT and NIKBUT were positively correlated with pixel intensity.
Nevertheless, despite that ANOVA revealed differences in the metrics between periods, when all subjects were analysed together, CoV, which evaluated the variability in each subject individually, revealed a low variability of metrics over time.
Repeatability of each Placido disk reflectivity metric
The present method has the limitation that is semiautomatic since the centre of the Placido disk pattern and the ROI must be selected manually by the examiner. In spite of this, the repeatability was acceptable in all metrics (Table 2) and the analysis can be carried out in less than 10 s. It has been previously reported that this time is considered appropriate for a clinical test [45].
Multiple linear regressions
As correlations showed, LLT was the clinical parameter that was more strongly correlated with new metrics. Nevertheless, other parameters were also correlated. This could be a bias since different metrics can confound results, affecting the classification of LLT. Therefore, multiple linear regression analysis has been performed to show which current metrics are independently associated with new metrics. Results showed that for most metrics, LLT was the only parameter associated. This suggests that new metrics are predictors of LLT and can be used to objectively assess it. Nevertheless, kurtosis and skewness were associated with gland drop-out and energy with LLT together with NIKBUT.
Diagnostic capability and validation of the new metrics
ROC curves were calculated to analyse the diagnostic ability of the new metrics. It has been previously reported that a 70% level of sensitivity and specificity is acceptable for the diagnosis of a disease [6]. Sensitivity and specificity were higher than 0.7 for most of the developed new metrics.
According to the classification on previous reports [46], the newly developed metrics showed areas under the curve between acceptable (0.74) and outstanding (0.91) discrimination. Thus, new metrics can be considered powerful aides to objectively assess the lipid layer.
It has been reported that accuracy, F-measure and kappa index denote good agreement between tests when they are close to 1 [33–37]. Generally, the agreement between new metrics and subjective classification methods of LLT showed an accuracy between 0.63 and 0.77, an F-measure between 0.78 and 0.87 and a Kappa index between 0.61 and 0.77 (very good agreement) (Table 8).
Mean pixel intensity, median pixel intensity and relative energy at 5.33 s after blinking were the metrics with the highest diagnostic capability in terms of sensitivity, specificity, area under the curve, Youden index and discriminant power (Table 5) and the metrics with the highest agreement with the subjective grading in terms of accuracy, Kappa index and F-measure (Table 8).
In comparison with previous studies on the analysis of interference patterns [8, 10–18], the new metrics showed slightly lower diagnostic ability and agreement with the subjective classification of LLT. Nevertheless, this method adds the possibility of objectively assessing the LLT without the need of having an interferometer, which might broad the assessment of the lipid layer in clinical practice.
This study had some limitations to consider. First, statistically significant correlations between new metrics and age were found. Consequently, age might act as a possible confounding factor. As in previous studies, age could not be excluded from the analysis because of its strong association with DED and MGD [39, 47]. Furthermore, the surrounding illumination and the focussing of the Placido disk pattern should be carefully controlled. In addition, LLT has not been measured objectively. However, it has been measured subjectively with a validated grading scale, which suggests that the present method is able to objectify the subjective measurement of this grading scale. It has been reported that this subjective grading scale is correlated with LLT [3, 4, 6, 7]. Therefore, these issues are not expected to affect results significantly. Future studies could assess the predictability of LLT measured objectively with the new metrics. Finally, the method only measures the grey intensity values of the Placido disk pattern within the pupil. Nevertheless, this issue is not expected to influence the outcomes since all the metrics have been designed to be pupil-independent. Moreover, the present study has demonstrated that the analysis of the pixels within the pupil area is enough to assess LLT.
Conclusions
Overall, the analysis of grey level intensity values in videokeratography is able to assess TF behaviour. Grey level intensity can be used as an alternative biomarker to objectively grade LLT. It has been demonstrated that the method is quick, objective, non-invasive, repeatable and with acceptable sensitivity and specificity. Therefore, it could be easily included in a battery of tests to improve the detection and monitoring of DED and MGD in clinical practice.
Further research is needed to assess the performance of these metrics in subjects diagnosed with DED or MGD. Likewise, the software could be further developed to be fully automatic and to distinguish between grades 3 and 4 of LLT. Nonetheless, although these outcomes are preliminary, they are highly encouraging. This study could be the base for future works which attempt to assess LLT objectively without the need of an interferometer.
Abbreviations
- CI
Confidence interval
- CoR
Coefficient of repeatability
- CoV
Coefficient of variation
- DED
Dry eye disease
- DEQ-5
Dry Eye Questionnaire-5
- LLT
Lipid layer thickness
- MGD
Meibomian gland dysfunction
- NIKBUT
Non-invasive keratograph break-up time
- OSDI
Ocular Surface Disease Index
- ROC
Receiver operating characteristics
- ROI
Region of interest
- SD
Standard deviation
- Sw
Within-subject standard deviation
- TF
Tear film
- TFOS DEWS II
Tear Film and Ocular Surface Dry Eye Workshop II
- TMH
Tear meniscus height
Funding
Open access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by the University of Valencia (“Atracció de Talent” scholarship, UV-INV-PREDOC18F2-886420) awarded to José Vicente García-Marqués; and the Ministerio de Educación, Cultura y Deporte (“Formación de Profesorado Universitario” scholarship, FPU17/03665) awarded to Cristian Talens-Estarelles.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Valencia and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclaimer
The funding sources had no role in the design of the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Willcox MDP, Argüeso P, Georgiev GA, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15:366–403. doi: 10.1016/j.jtos.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewen King-Smith P, Reuter KS, Braun RJ, et al. Tear film breakup and structure studied by simultaneous video recording of fluorescence and tear film lipid layer images. Investig Ophthalmol Vis Sci. 2013;54:4900–4909. doi: 10.1167/iovs.13-11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15:539–574. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Guillon J-P. Non-invasive tearscope plus routine for contact lens fitting. Contact Lens Anterior Eye. 1998;21:S31–S40. doi: 10.1016/S1367-0484(98)80035-0. [DOI] [PubMed] [Google Scholar]
- 5.Arita R, Itoh K, Maeda S, et al. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology. 2009;116:2058–2063.e1. doi: 10.1016/j.ophtha.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Investig Ophthalmol Vis Sci. 2011;52:2006–2049. doi: 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols JJ, Berntsen DA, Mitchell GL, Nichols KK. An assessment of grading scales for meibography images. Cornea. 2005;24:382–388. doi: 10.1097/01.ico.0000148291.38076.59. [DOI] [PubMed] [Google Scholar]
- 8.Remeseiro B, Penas M, Barreira N, et al. Automatic classification of the interferential tear film lipid layer using colour texture analysis. Comput Methods Programs Biomed. 2013;111:93–103. doi: 10.1016/j.cmpb.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Markoulli M, Duong TB, Lin M, Papas E. Imaging the tear film: a comparison between the Subjective Keeler Tearscope-Plus™ and the Objective Oculus® Keratograph 5M and LipiView® interferometer. Curr Eye Res. 2018;43:155–162. doi: 10.1080/02713683.2017.1393092. [DOI] [PubMed] [Google Scholar]
- 10.Remeseiro B, Penedo MG, García-Resúa C et al (2014) Dry eye characterization by analyzing tear film images. In: Ng EYK, Acharya-Rajendra U, Rangayyan RM, Suri JS (eds) Ophthalmological imaging and applications, 1st edn. New York, pp 449–475
- 11.Remeseiro B, Oliver KM, Tomlinson A, et al. Automatic grading system for human tear films. Pattern Anal Appl. 2015;18:677–694. doi: 10.1007/s10044-014-0402-x. [DOI] [Google Scholar]
- 12.da Cruz LB, Souza JC, de Sousa JA, et al (2020) Interferometer eye image classification for dry eye categorization using phylogenetic diversity indexes for texture analysis. Comput Methods Programs Biomed 188. 10.1016/j.cmpb.2019.105269 [DOI] [PubMed]
- 13.Hwang H, Jeon H-J, Yow KC, et al (2017) Image-based quantitative analysis of tear film lipid layer thickness for meibomian gland evaluation. Biomed Eng Online 16. 10.1186/s12938-017-0426-8 [DOI] [PMC free article] [PubMed]
- 14.García-Resúa C, Fernández MJG, González Penedo MF, et al. New software application for clarifying tear film lipid layer patterns. Cornea. 2013;32:538–546. doi: 10.1097/ICO.0b013e31824d0d04. [DOI] [PubMed] [Google Scholar]
- 15.Peteiro-Barral D, Remeseiro B, Méndez R, Penedo MG. Evaluation of an automatic dry eye test using MCDM methods and rank correlation. Med Biol Eng Comput. 2017;55:527–536. doi: 10.1007/s11517-016-1534-5. [DOI] [PubMed] [Google Scholar]
- 16.Wu D, Boyer KL, Nichols JJ, King-Smith PE. Texture based prelens tear film segmentation in interferometry images. Mach Vis Appl. 2010;21:253–259. doi: 10.1007/s00138-008-0155-x. [DOI] [Google Scholar]
- 17.Ramos L, Penas M, Remeseiro B, et al (2011) Texture and color analysis for the automatic classification of the eye lipid layer. Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics) 6692 LNCS:66–73
- 18.Bai Y, Nichols JJ. In vivo thickness measurement of the lipid layer and the overall tear film by interferometry. Opt Lett. 2019;44:2410–2413. doi: 10.1364/OL.44.002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu H, Wang MR, Wang J, Shen M (2014) Tear film measurement by optical reflectometry technique. J Biomed Opt 19. 10.1117/1.JBO.19.2.027001 [DOI] [PMC free article] [PubMed]
- 20.Bai Y, Ngo W, Nichols JJ. Characterization of the thickness of the tear film lipid layer using high resolution microscopy. Ocul Surf. 2019;17:356–359. doi: 10.1016/j.jtos.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai Y, Nichols JJ. Advances in thickness measurements and dynamic visualization of the tear film using non-invasive optical approaches. Prog Retin Eye Res. 2017;58:28–44. doi: 10.1016/j.preteyeres.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Alonso-Caneiro D, Szczesna-Iskander DH, Iskander DR, et al. Application of texture analysis in tear film surface assessment based on videokeratoscopy. J Optom. 2013;6:185–193. doi: 10.1016/j.optom.2013.07.006. [DOI] [Google Scholar]
- 23.Wu S, Hong J, Tian L, et al. Assessment of bulbar redness with a newly developed keratograph. Optom Vis Sci. 2015;92:892–899. doi: 10.1097/OPX.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 24.Xie W, Zhang X, Xu Y, Yao Y-F. Assessment of tear film and bulbar redness by keratograph 5M in pediatric patients after orthokeratology. Eye Contact Lens. 2018;44:S382–S386. doi: 10.1097/ICL.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 25.Guillon J-P. Use of the tearscope plus and attachments in the routine examination of the marginal dry eye contact lens patient. Adv Exp Med Biol. 1998;438:859–867. doi: 10.1007/978-1-4615-5359-5_121. [DOI] [PubMed] [Google Scholar]
- 26.Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease. Classification and grading of lid changes. Eye. 1991;5:395–411. doi: 10.1038/eye.1991.65. [DOI] [PubMed] [Google Scholar]
- 27.Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–1270. doi: 10.1001/archopht.1995.01100100054027. [DOI] [PubMed] [Google Scholar]
- 28.Pult H, Nichols JJ. A review of meibography. Optom Vis Sci. 2012;89:E760–769. doi: 10.1097/OPX.0b013e3182512ac1. [DOI] [PubMed] [Google Scholar]
- 29.Esmaeili M, Dehnavi A, Rabbani H, Hajizadeh F. Three-dimensional segmentation of retinal cysts from spectral-domain optical coherence tomography images by the use of three-dimensional curvelet based K-SVD. J Med Signals Sens. 2016;6:166–171. doi: 10.4103/2228-7477.175873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martínez-Albert N, Esteve-Taboada JJ, Montés-Micó R, et al. Repeatability assessment of biometric measurements with different refractive states and age using a swept-source biometer. Expert Rev Med Devices. 2019;16:63–69. doi: 10.1080/17434440.2019.1557517. [DOI] [PubMed] [Google Scholar]
- 31.McAlinden C, Khadka J, Pesudovs K. Precision (repeatability and reproducibility) studies and sample-size calculation. J Cataract Refract Surg. 2015;41:2598–2604. doi: 10.1016/j.jcrs.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud. 2010;47:931–936. doi: 10.1016/j.ijnurstu.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Sokolova M, Japkowicz N, Szpakowicz S (2006) Beyond accuracy, F-score and ROC: a family of discriminant measures for performance evaluation. In: AAAI Workshop - technical report. pp 24–29
- 34.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–363. [PubMed] [Google Scholar]
- 35.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfield GH, Fitzpatrick-Lins K. A coefficient of agreement as a measure of thematic classification accuracy. Photogramm Eng Remote Sens. 1986;52:223–227. [Google Scholar]
- 37.Cohen J. Weighted kappa: Nominal scale agreement provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 38.Eom Y, Lee J-S, Kang S-Y, et al. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am J Ophthalmol. 2013;155:1104–1110.e2. doi: 10.1016/j.ajo.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Pult H, Riede-Pult BH, Nichols JJ. Relation between upper and lower lids’ meibomian gland morphology, tear film, and dry eye. Optom Vis Sci. 2012;89:E310–E315. doi: 10.1097/OPX.0b013e318244e487. [DOI] [PubMed] [Google Scholar]
- 40.Hosaka E, Kawamorita T, Ogasawara Y, et al. Interferometry in the evaluation of precorneal tear film thickness in dry eye. Am J Ophthalmol. 2011;151:18–23.e1. doi: 10.1016/j.ajo.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Finis D, Pischel N, Schrader S, Geerling G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea. 2013;32:1549–1553. doi: 10.1097/ICO.0b013e3182a7f3e1. [DOI] [PubMed] [Google Scholar]
- 42.Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–164. doi: 10.1016/S1542-0124(12)70150-7. [DOI] [PubMed] [Google Scholar]
- 43.Best N, Drury L, Wolffsohn JS. Clinical evaluation of the oculus keratograph. Contact Lens Anterior Eye. 2012;35:171–174. doi: 10.1016/j.clae.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Foulks GN. The correlation between the tear film lipid layer and dry eye disease. Surv Ophthalmol. 2007;52:369–374. doi: 10.1016/j.survophthal.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Remeseiro B, Bolon-Canedo V, Peteiro-Barral D, et al. A methodology for improving tear film lipid layer classification. IEEE J Biomed Heal Informatics. 2014;18:1485–1493. doi: 10.1109/JBHI.2013.2294732. [DOI] [PubMed] [Google Scholar]
- 46.Hosmer DW, Lemeshow S, Sturdivant RX (2013) Applied logistic regression. Hoboken, New Jersey
- 47.Rico-del-Viejo L, Benítez-del-Castillo JM, Gómez-Sanz FJ, et al. The influence of meibomian gland loss on ocular surface clinical parameters. Contact Lens Anterior Eye. 2019;42:562–568. doi: 10.1016/j.clae.2019.04.004. [DOI] [PubMed] [Google Scholar]