Abstract
A problem with rapid Plasmodium falciparum-specific antigen histidine-rich protein 2 (HRP2) detection tests for malaria is the persistence of antigen in blood after the disappearance of asexual-stage parasitemia and clinical symptoms, resulting in false-positive (FP) test results following treatment. The ICT P.f/P.v immunochromatographic test detects both HRP2 and a panmalarial antigen (PMA) found in both P. falciparum and Plasmodium vivax. To examine posttreatment antigen persistence with this test and whether persistent sexual-stage forms (gametocytes) are a cause of FP tests after treatment, we compared serial antigen test results with microscopy results from patients symptomatic with P. falciparum malaria in Indonesia for 28 days following treatment with chloroquine (CQ; n = 66), sulfadoxine-pyrimethamine (SP; n = 36), and artesunate plus sulfadoxine-pyrimethamine (ART + SP; n = 15). Persistent FP antigenemia following SP treatment occurred in 29% (HRP2) and 42% (PMA) of the patients on day 7 and in 10% (HRP2) and 23% (PMA) on day 14. The high rates of persistent HRP2 and PMA antigenemia following CQ and SP treatment were strongly associated with the presence of gametocytemia, with the proportion with gametocytes on day 7 posttreatment being significantly greater in those with FP results than in those with true-negative PMA and HRP2 results. Gametocyte frequency on day 14 post-SP treatment was also greater in those with FP PMA results. Following SP treatment, PMA persisted longer than HRP2, giving an FP diagnosis of P. vivax in up to 16% of patients on day 14, with all FP P. vivax diagnoses having gametocytemia. In contrast, PMA was rapidly cleared following ART + SP treatment in association with rapid clearance of gametocytemia. Gametocytes appear to be an important cause of persistent posttreatment panmalarial antigenemia in areas of endemicity and may also contribute in part to persistent HRP2 antigenemia following treatment.
Rapid dipstick antigen capture tests for the circulating Plasmodium falciparum-specific antigen histidine-rich protein 2 (HRP2) have been shown to have excellent sensitivity and specificity for the diagnosis of P. falciparum malaria, generally at least as good as those for microscopy of a thick and thin film by a skilled microscopist (25). Although currently too costly for widespread use in countries where malaria is endemic, they have become very useful as an adjunct or alternative to malaria microscopy, particularly when experienced microscopists are unavailable (24). A problem, however, when using these dipstick tests to detect asexual-stage falciparum parasitemia is the persistence of HRP2 antigen in blood after the disappearance of both asexual-stage parasitemia and clinical symptoms. This may result in false-positive (FP) diagnoses of viable asexual infection when HRP2 testing is performed after treatment of malaria (24, 26) and may reduce the usefulness of the test in predicting treatment failure (19, 21, 24).
Most published data on persistent HRP2 antigenemia after treatment relate to the Parasight-F test for HRP2 (1, 2, 4, 9, 16, 21, 24, 25), which uses an immunoglobulin G (IgG) monoclonal antibody to HRP2 in contrast to the IgM monoclonal antibody to HRP2 used in the ICT Malaria P.f and ICT P.f/P.v tests (11, 20). The ICT P.f/P.v test detects both the P. falciparum-specific antigen HRP2 and a panmalarial antigen (PMA) found in both P. falciparum and Plasmodium vivax (20) but possibly not in Plasmodium malariae (4a). An immunochromatographic diagnosis of P. falciparum is made if the HRP2 line is visible, with or without the PMA line. A diagnosis of P. vivax is made if only the PMA line is visible (20). It is not known how long the ICT P.f/P.v PMA persists after treatment in areas where malaria is endemic. Antigen persistence after treatment of P. falciparum with the ICT P.f/P.v test is important not only because of the potential for convalescent FP diagnoses and for potential inability to reliably predict treatment failure but also because persistence of the antigen after the HRP2 antigen has cleared would result in the test being falsely interpreted as P. vivax. We therefore examined the persistence of both the ICT P.f/P.v HRP2 and PMAs after three different treatment regimens for P. falciparum malaria in symptomatic Indonesians.
Because it is not known to what extent gametocytes are detected by either PMA or the IgM monoclonal antibody to HRP2, an additional aim of the study was to determine whether persistent antigenemia after treatment was associated with the presence of sexual-stage forms (gametocytes) in convalescence. We examined the persistence of HRP2 and panmalarial antigenemia following chloroquine treatment and sulfadoxine-pyrimethamine treatment, both of which are associated with high rates of posttreatment gametocytemia (6, 7, 8, 15). We then examined the persistence of each antigen following treatment with artesunate, which is associated with reduced gametocyte carriage in convalescence (12, 13).
MATERIALS AND METHODS
Study site.
The studies examining persistence of antigenemia following chloroquine and sulfadoxine-pyrimethamine were performed from February to May 1998 in Radamata Primary Health Centre, Laratama subdistrict, West Sumba, East Nusa Tenggara province, Indonesia, an area where malaria is hypoendemic, with a parasite rate in children aged 0 to 9 years of 5.1% (E. Tjitra, unpublished data) and high rates of chloroquine resistance but no demonstrable sulfadoxine-pyrimethamine resistance (18). The study examining clearance of antigenemia following combination treatment with artesunate and sulfadoxine-pyrimethamine was performed from March to April 1999 in Genyem Health Centre, Nimboran subdistrict, Irian Jaya, Indonesia. This area has moderately high malaria transmission, with parasite rates in children aged 0 to 9 years averaging 39% (Tjitra, unpublished) and high rates of resistance to chloroquine and documented sulfadoxine-pyrimethamine resistance (18). The studies were approved by the Ethics Committee of the National Institute of Health Research and Development, Indonesian Ministry of Health, Jakarta, Indonesia, and by the Joint Institutional Ethics Committee of Menzies School of Health Research and Royal Darwin Hospital, Darwin, Australia.
Patients and followup.
The studies examining antigen clearance after chloroquine and sulfadoxine-pyrimethamine treatment were performed as part of 28-day in vivo drug efficacy studies, using modified 1997 World Health Organization guidelines with standard inclusion and exclusion criteria (18, 23). Sixty-six symptomatic adults and children with a microscopic diagnosis of P. falciparum monoinfection were enrolled. All patients were treated with a standardized supervised 3-day regimen of oral chloroquine (Resochin; Bayer Pharmaceuticals, Jakarta, Indonesia) at 10 mg of base/kg of body weight on days 1 and 2 and 5 mg/kg on day 3 without primaquine and were monitored with clinical review, microscopy, and immunochromatographic testing on days 1, 2, 3, 7, 14, and 28. Those with treatment failure with chloroquine but without danger signs (23) requiring quinine treatment were then treated with sulfadoxine-pyrimethamine (Fansidar; Roche, Jakarta, Indonesia) at 1.25 mg of pyrimethamine/kg and were monitored for a further 28 days as described above.
The study examining antigen clearance after treatment with artesunate and sulfadoxine-pyrimethamine was part of a pilot study examining combination chemotherapy for malaria. Fifteen symptomatic adults and children with a microscopic diagnosis of P. falciparum monoinfection were treated with a standardized supervised 3-day regimen of oral artesunate (Artesunate; Mekophar, Ho Chi Minh City, Vietnam) at 4 mg/kg on days 1, 2, and 3 plus sulfadoxine-pyrimethamine (Fansidar; Roche, Dee Why, New South Wales, Australia) at 1.25 mg/kg pyrimethamine on day 1. They were monitored for 28 days as described above.
Microscopy and immunochromatographic testing.
Thick and thin films were examined and immunochromatographic testing was performed directly from serial finger-prick blood samples collected on days 0, 1, 2, 3, 7, 14, and 28. Thick and thin films were stained with 10% Giemsa solution and were examined at a magnification of ×1,000 by an expert microscopist with 24 years' experience (S. Suprianto) who was unaware of the clinical response to treatment or the immunochromatographic test results. Asexual- and sexual-stage parasite densities were counted per 200 leukocytes and were then expressed in trophozoites per microliter and gametocytes per microliter, assuming a leukocyte count of 8,000/μl. Only those parasitemic with P. falciparum asexual stages (with or without gametocytes) were eligible. Mixed infections with P. vivax were excluded. Thick films were considered negative if no parasites were seen in at least 100 high-power fields.
After a period of training, the ICT P.f/P.v test (AMRAD-ICT, Sydney, Australia) was performed by clinic health workers using 15-μl finger-prick capillary blood according to the manufacturer's instructions and was read by the study physician (E. Tjitra), who was blinded to microscopy results. The test was considered valid if the control line was visible and positive if the HRP2 and/or PMA lines were visible. As per manufacturer's instructions, an immunochromatographic diagnosis of P. falciparum was made if the HRP2 line was visible, with or without the PMA line. A diagnosis of P. vivax was made if only the PMA line was visible. Coinfection with both P. falciparum and P. vivax cannot be distinguished from infection with P. falciparum alone; the test interpretation of two visible lines is P. falciparum.
All discordant slides and 20% of concordant slides were cross-checked by an expert microscopist in Darwin with over 20 years' experience, who was blinded to patient diagnosis, previous microscopy, and immunochromatographic test results. A thick film was considered negative on cross-checking only if at least 200 high-power fields were negative. Results of microscopy and immunochromatographic testing were compared following each drug on each of the follow-up days.
Data analysis.
Results were analyzed using Epi-Info version 6 (3). Because gametocytes do not cause disease, immunochromatographic test results were considered FP on each day of follow-up if positive for HRP2 or PMAs but microscopically negative for asexual-stage parasites with or without gametocyte positivity (25). Antigen test results were considered true negative if antigen testing was negative and microscopy was negative for asexual-stage parasitemia, with or without gametocyte positivity. To examine whether the presence of gametocytes was associated with FP persistent antigenemia after treatment for malaria, the proportion showing gametocytemia in those with true-negative antigen test results was compared, using a two-tailed Fisher's exact test, with the proportion showing gametocytemia in those with FP antigen test results for each antigen on days 7 and 14 after each treatment. Mean gametocyte counts in each group were compared using the Mann-Whitney U test.
RESULTS
Microscopy findings following treatment.
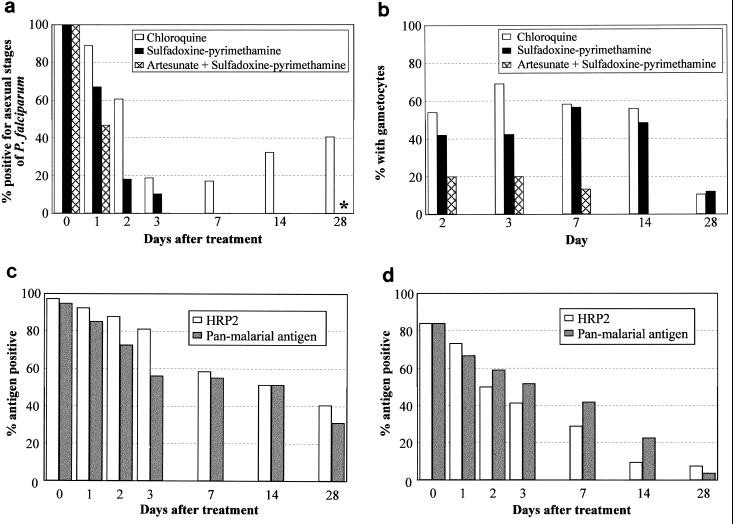
Sixty-six Sumbanese children and adults with symptomatic P. falciparum malaria treated with chloroquine, 36 of the 37 recipients of failed chloroquine treatment subsequently treated with sulfadoxine-pyrimethamine, and all 15 patients treated with artesunate plus sulfadoxine-pyrimethamine could be evaluated by both microscopy and immunochromatographic testing at least once. High frequencies of recurrent asexual-stage P. falciparum parasitemia were found following treatment with chloroquine, but there were no recurrences of asexual-stage P. falciparum parasitemia following sulfadoxine-pyrimethamine monotherapy or treatment with a combination of artesunate and sulfadoxine-pyrimethamine (Fig. 1a). Over 50% of chloroquine-treated patients who tested negative for asexual-stage parasites on microscopy had P. falciparum gametocytemia on days 2, 3, 7, and 14, with a similar proportion among patients who received sulfadoxine-pyrimethamine (Fig. 1b). In contrast, as expected, gametocytes occurred infrequently following artesunate combination therapy and were cleared rapidly (Fig. 1b).
FIG. 1.
Microscopic findings (a and b) following chloroquine treatment of P. falciparum malaria, sulfadoxine-pyrimethamine treatment of patients who had received failed chloroquine treatment, and treatment with artesunate plus sulfadoxine-pyrimethamine. (a) Recurrence of asexual P. falciparum parasitemia following chloroquine treatment is shown. ∗, P. vivax asexual parasitemia was noted on day 28 in one sulfadoxine-pyrimethamine-treated patient and in two patients following treatment with artesunate plus sulfadoxine-pyrimethamine. (b) Proportion with P. falciparum gametocytemia in those negative for asexual-stage P. falciparum parasites on each day of follow-up. (c) Antigen persistence following chloroquine treatment. The proportion of all patients having HRP2 or panmalarial antigenemia on each day of follow-up is shown. (d) Antigen persistence following sulfadoxine-pyrimethamine treatment. The proportion of all patients having HRP2 or panmalarial antigenemia on each day of follow-up is shown.
Antigen persistence following treatment.
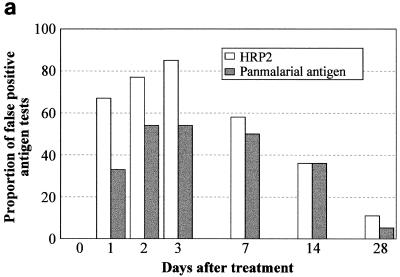
FP results with the HRP2 and PMAs were found in 48% (32 of 66) and 41% (27 of 66) of patients, respectively, on at least 1 day of follow-up following chloroquine, with each antigen found in 61% (22 of 36) of patients following sulfadoxine-pyrimethamine treatment and in 100% (15 of 15) and 47% (7 of 15), respectively, following treatment with artesunate plus sulfadoxine-pyrimethamine. Following chloroquine treatment, both total-positive (Fig. 1c) and FP (Fig. 2a) HRP2 and panmalarial antigenemia were more frequent and persisted longer than total positives (Fig. 1d) and FPs (Fig. 2b) following sulfadoxine-pyrimethamine treatment. Following sulfadoxine-pyrimethamine treatment, a higher proportion of patients was FP for PMA than for HRP2 antigen on each day of follow-up to day 14 (Fig. 2b). Because PMA positivity in the absence of HRP2 positivity results in a diagnosis of P. vivax malaria, this caused a false-convalescent immunochromatographic diagnosis of vivax malaria on days 1, 2, 3, 7, and 14 in 1 (3%), 2 (9%), 3 (10.3%), 4 (12.9%), and 5 (16.1%) evaluable patients, respectively. Significantly, in all of these 15 FP diagnoses of vivax malaria, P. falciparum gametocytes were present on microscopy. Following chloroquine treatment, a FP diagnosis of P. vivax occurred on day 7 only and was made in 2 of 24 (8%) patients negative for P. falciparum asexual-stage parasites on microscopy.
FIG. 2.
FP persistence of HRP2 and panmalarial antigenemia following treatment with chloroquine (a), sulfadoxine-pyrimethamine (b), and artesunate plus sulfadoxine-pyrimethamine (c) of symptomatic P. falciparum malaria. Results do not include true-positive results associated with asexual-stage parasitemia on microscopy and are expressed as FP antigen results, as a proportion of the total FP and true-negative results for each antigen on each day of follow-up.
In contrast to the findings following treatment with chloroquine and sulfadoxine-pyrimethamine, therapy with artesunate plus sulfadoxine-pyrimethamine was followed by high frequencies of persistent HRP2 antigenemia but rapid clearance of panmalarial antigenemia (Fig. 2c), which paralleled the rapid clearance of gametocytemia. There were no FP diagnoses of P. vivax in convalescence. There was a recrudescence of panmalarial antigenemia on day 28 in the absence of asexual-stage or sexual-stage parasites on microscopy, which may have reflected emerging subpatent reinfection in this area of moderately high transmission in Irian Jaya.
Relationship between gametocytes and antigen persistence following treatment.
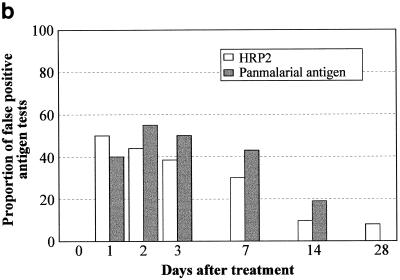
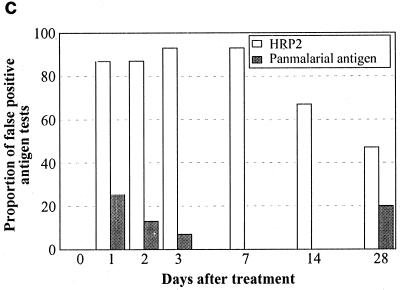
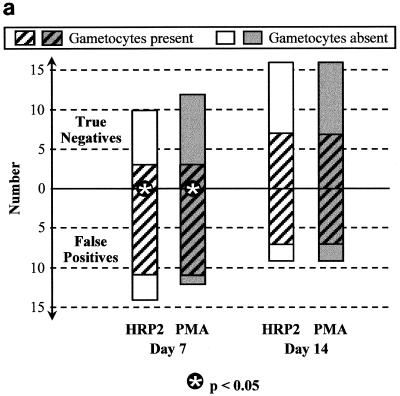
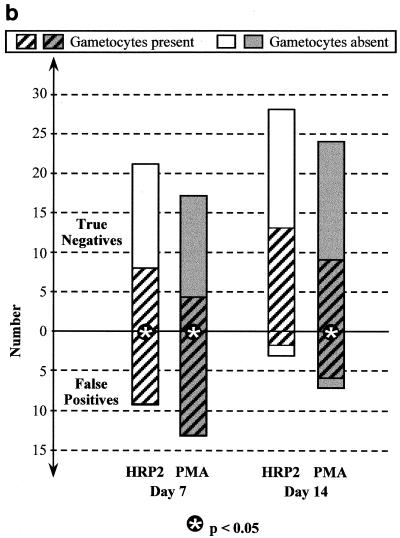
On each day of follow-up after both chloroquine (Fig. 3a) and sulfadoxine-pyrimethamine (Fig. 3b), the majority of FP results for both HRP2 and PMAs were associated with gametocytemia, whereas gametocytemia was found in only a minority of true-negative antigen results. On day 7 posttreatment, the proportion with gametocytes was significantly greater among those with FP HRP2 tests than among those with true-negative results: 11 of 14 versus 3 of 10 (P = 0.035) after chloroquine treatment and 9 of 9 versus 8 of 21 (P = 0.001) after sulfadoxine-pyrimethamine treatment. With fewer HRP2 FPs by day 14, the difference between the proportion with gametocytes in those with FP HRP2 results and that in those with true-negative results was no longer statistically significant on day 14. The association between PMA false positivity and gametocytemia was even stronger. On day 7 posttreatment, the proportion with gametocytes was significantly greater among those with FP PMA results than among those with true-negative results: 11 of 12 versus 3 of 12 (P = 0.0009) after chloroquine treatment and 13 of 13 versus 4 of 17 (P = 0.0001) after sulfadoxine-pyrimethamine treatment. On day 14, gametocyte frequency was also greater in patients with FP PMA results than in those with true-negative results following sulfadoxine-pyrimethamine treatment: 6 of 7 versus 9 of 24 (P = 0.02).
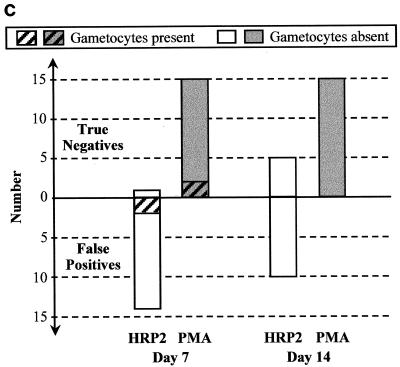
FIG. 3.
Frequency of P. falciparum gametocytemia on days 7 and 14 after treatment with chloroquine (a), sulfadoxine-pyrimethamine (b), and artesunate plus sulfadoxine-pyrimethamine (c) in those with FP immunochromatographic antigen tests (below the zero line) compared with those with true negative tests (above the zero line). ∗ indicates a significant difference (P < 0.05) using Fisher's two-tailed exact test.
In marked contrast to the findings following chloroquine and sulfadoxine-pyrimethamine treatment, there were no FP PMA results following treatment with artesunate plus sulfadoxine-pyrimethamine (Fig. 3c). Importantly, this lack of convalescent-stage FP PMA results was strongly associated with lack of gametocytemia, with gametocytes being present at very low levels (mean, 120/μl) in only 2 of 15 patients (13%) on day 7 and absent in all patients on day 14. Conversely, a high proportion of those with FP HRP2 antigen tests following therapy with artesunate plus sulfadoxine-pyrimethamine did not have gametocytemia, suggesting that other causes of persistent HRP2 antigenemia are important in this high-transmission setting.
Although a greater proportion of those with FP antigen tests following both chloroquine and sulfadoxine-pyrimethamine treatment had gametocytemia, gametocytes were also found in those with true-negative antigen tests. Comparison of mean gametocyte counts indicated that those with FP antigen tests had higher mean gametocyte counts than did those with true-negative tests (Table 1), particularly following sulfadoxine-pyrimethamine treatment. This was a consistent trend which, despite small numbers, reached statistical significance for PMA. Although the numbers were small, there was a trend for mean gametocyte counts to be higher in patients with true-negative convalescent-stage HRP2 antigen test results than in those with true-negative PMA test results, suggesting a potentially higher gametocyte detection threshold for HRP2. When day-0 asexual-stage parasite counts in those with convalescent-stage FP antigen tests were compared with counts for those with true-negative results, there were no consistent differences.
TABLE 1.
Gametocyte density in patients with FP panmalarial and HRP2 antigenemia after treatment compared to that in those with true-negative antigen resultsa
| Antigen | Drug used | Day after treatment | Results for FPs
|
Results for true negatives
|
P | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | Gametocyte densityb
|
n | Gametocyte density
|
||||||
| Mean | (SEM) | Mean | (SEM) | ||||||
| PMA | Chloroquine | 7 | 11 | 768 | (212) | 3 | 80 | (0) | 0.07 |
| 14 | 7 | 263 | (92) | 7 | 114 | (44) | 0.24 | ||
| Sulfadoxine-pyrimethamine | 7 | 13 | 712 | (176) | 4 | 130 | (41) | 0.008 | |
| 14 | 6 | 280 | (92) | 9 | 80 | (16) | 0.006 | ||
| HRP2 | Chloroquine | 7 | 11 | 652 | (212) | 3 | 508 | (428) | 0.64 |
| 14 | 7 | 263 | (92) | 7 | 114 | (44) | 0.24 | ||
| Sulfadoxine-pyrimethamine | 7 | 9 | 812 | (246) | 8 | 310 | (100) | 0.07 | |
| 14 | 2 | 120 | (80) | 13 | 168 | (48) | 0.80 | ||
Mean gametocyte counts in each group are compared using the Mann-Whitney U test.
Density is given as gametocytes per microliter.
DISCUSSION
We have demonstrated high rates of persistent positive results for both antigens with the ICT P.f/P.v test after treatment of symptomatic Indonesians with P. falciparum malaria using the two most commonly used antimalarial drugs worldwide, chloroquine and sulfadoxine-pyrimethamine. There are several lines of evidence to suggest that persistence of PMA following clearance of asexual-stage parasites in residents of areas of malaria endemicity results largely from detection of circulating sexual stages in convalescence. In this study we found a consistent association between gametocytemia and FP panmalarial antigenemia on days 7 and 14 following treatment with both chloroquine and sulfadoxine-pyrimethamine. Moreover, gametocyte density was higher in those with convalescent-stage PMA false positivity than in those with true-negative results, consistent with gametocyte density being above the antigen detection threshold in those with FP tests. Thirdly, and in marked contrast to the findings following chloroquine and sulfadoxine-pyrimethamine treatment, artesunate therapy resulted in rapid clearance of gametocytes which was associated with a rapid clearance of panmalarial antigenemia and in an absence of FP results by day 7. This last finding is consistent with results from a recent hospital series (in an area where malaria is not endemic) of eight cases with P. falciparum monitored longitudinally with the ICT P.f/P.v test after treatment with an unnamed drug(s) (5). In these patients, PMA reactivity declined in parallel with the decline in parasitemia, with no FP PMA results in convalescence. Significantly, these patients were treated early and none developed gametocytemia, further evidence that detection of gametocytes is the predominant cause of the convalescent-stage FP panmalarial antigenemia found in endemic areas.
Potential alternative causes of persistent panmalarial antigenemia early in convalescence are unlikely to have contributed to a major degree to the day-7 and -14 FP results that we observed. While it could be argued that in an area of known chloroquine resistance, persistent asexual-stage parasitemia below the threshold for microscopic detection could potentially result in persistent true-positive panmalarial antigenemia following chloroquine treatment and could also, as an epiphenomenon, result in posttreatment gametocytemia (7), this would not explain the persistent panmalarial antigenemia following sulfadoxine-pyrimethamine treatment (and its association with gametocytemia), in which there were no parasitological or clinical failures observed in 28 days of follow-up. Conversely, late recurrence of panmalarial antigenemia following initial clearance, as we found following therapy with artesunate plus sulfadoxine-pyrimethamine, is consistent with emerging reinfection in this high-transmission region (Tjitra, unpublished) at a level below the detection limit for microscopy.
Antigen persistence after treatment of P. falciparum with the ICT P.f/P.v test is important not only because of the potential for FP diagnosis of asexual-stage infection in convalescence but also because the persistence of PMA after the HRP2 antigen has cleared, as we observed following chloroquine therapy and particularly sulfadoxine-pyrimethamine therapy, results in the test being falsely interpreted as positive for P. vivax. This is likely to be a particular problem in semiimmune residents of areas of malaria endemicity, where greater chronicity of infection prior to treatment results in higher frequencies of gametocytemia on presentation (6) and where previous self-treatment is common. Moreover, treatment of chloroquine-resistant P. falciparum with chloroquine, as is widely practiced in areas of malaria endemicity, results in higher frequencies of gametocytemia in convalescence than following treatment of chloroquine-sensitive strains (7, 14). Rates of gametocytemia in areas of endemicity where chloroquine is still used are now much higher than those noted before chloroquine resistance emerged (7). However, because gametocyte carriage is markedly reduced following artesunate therapy (12), persistent FP panmalarial antigenemia is unlikely to be a major problem in areas of malaria endemicity, such as Thailand and Vietnam, which have switched to artesunate-containing combination therapies (12). Because posttreatment gametocytemia is also unusual in nonimmune returned travelers owing to the usually shorter duration of infection prior to treatment, convalescent-stage panmalarial antigenemia is also unlikely to be a problem in these patients (5). Testing for PMA after treatment may therefore prove to be potentially useful only in predicting treatment failure in those settings, as described above, where posttreatment gametocytemia is uncommon.
The cause of persistent HRP2 antigenemia after malaria treatment is not known. Potential causes include persistent viable asexual-stage parasitemia below the detection limit of microscopy, delayed clearance of circulating antigen (free or in antigen-antibody complexes), rheumatoid factor, and persistent sexual-stage forms (gametocytes) (24, 25; M. P. Grobusch, U. Alpermann, S. Schwenke, T. Jelinek, and D. C. Warhurst, Letter, Lancet 353:297). It is still not clear to what extent HRP2 is found in gametocytes. While HRP2 has been described in published reviews as being found in immature but not mature gametocytes (25), to date there are no published primary data supporting this assertion. Recently, HRP2 mRNA transcript and protein have both been demonstrated in late-stage gametocytes (R. Haywood, D. Sullivan, and K. Day, personal communication). An early study with Kenyan children found no association between posttreatment gametocytemia and Parasight-F HRP2 false positivity 6 days after treatment (2), but until now this association has not been specifically looked for in convalescence since. We have demonstrated an association between circulating gametocytemia and persistent ICT P.f/P.v. HRP2 antigen reactivity following both chloroquine and sulfadoxine-pyrimethamine treatments, suggesting that detection of gametocytes may contribute to persistent FP HRP2 reactivity in convalescence. Importantly, the lack of demonstrable sulfadoxine-pyrimethamine resistance at the Sumba study site (18) makes it unlikely that the association between FP HRP2 antigen reactivity and gametocytemia following sulfadoxine-pyrimethamine treatment is related to an epiphenomenon of persistent submicroscopic asexual-stage parasitemia causing both persistent HRP2 and gametocytemia. An Indian study also found a high frequency of persistent ICT P.f. HRP2 antigen following treatment with sulfadoxine-pyrimethamine (42% on day 7), with half of these FP HRP2 results on day 7 being gametocytemic, but rates of gametocytemia in those with true-negative results on day 7 were not reported (17). In cross-sectional evaluations of the Parasight-F (10) and ICT P.f/P.v immunochromatographic tests (20), the sensitivity of the HRP2 antigen for gametocytes in those negative for asexual stages was 22 and 73%, respectively. It is possible that there are differences between the ability of the IgG monoclonal antibody to HRP2 used in the Parasight-F test to detect gametocytes and that of the IgM monoclonal antibody to HRP2 used in the ICT Malaria P.f and ICT P.f/P.v tests, however, proving this theory will require direct comparative studies.
While we found an association between gametocytemia and HRP2 antigen reactivity following treatment, we and others have also found evidence that other causes of persistent HRP2 are important. Indeed, in contrast to PMA reactivity, other causes of persistent HRP2 reactivity are likely to be more important than posttreatment gametocytemia. We found high frequencies of persistent HRP2 reactivity following artesunate combination therapy, despite the rapid clearance of gametocytemia. Persistent ICT P.f/P.v HRP2 reactivity to day 31 has also been reported in a patient without patent gametocytemia (5). We found higher rates of FP HRP2 antigenemia following chloroquine treatment than following sulfadoxine-pyrimethamine therapy, despite similar frequencies of posttreatment gametocytemia and despite the drugs sharing similar rates of asexual-stage parasite clearance when parasites are drug sensitive (22). This may relate to chloroquine resistance but not sulfadoxine-pyrimethamine resistance being found at the study site and suggests that subclinical drug-resistant asexual-stage parasitemia below the detection limit of microscopy may contribute to the higher frequency of persistence following chloroquine (19).
The gametocyte detection threshold for HRP2 may be higher than that for PMA. All of the convalescent FP diagnoses of P. vivax (i.e., HRP2 negative and PMA positive) had gametocytes. In the presence of declining gametocyte levels following sulfadoxine-pyrimethamine treatment, HRP2 reactivity fell more quickly than PMA reactivity, with a trend of higher gametocyte counts in those with true-negative convalescent HRP2 reactivity than in those with true-negative PMA reactivity.
In conclusion, it is likely that gametocytes are the predominant cause of persistent PMA reactivity after treatment of malaria. Persistent PMA reactivity in convalescence does not appear to occur in those patients who do not develop gametocytes following treatment and is unlikely to be a diagnostic problem in these patients. In contrast, because gametocyte-associated PMA reactivity persists longer than HRP2 reactivity after treatment, a high percentage of those with posttreatment gametocytemia have FP ICT P.f/P.v diagnoses of P. vivax in convalescence. While gametocytes are associated with, and likely contribute to, persistent ICT P.f/P.v HRP2 antigen reactivity after chloroquine and sulfadoxine-pyrimethamine treatment of P. falciparum malaria, other factors are likely to be more important causes of the FP HRP2 reactivity seen in convalescence.
ACKNOWLEDGMENTS
We thank Mary Dyer for expert cross-checking of malaria microscopy; Mary Garcia of AMRAD-ICT for providing the ICT P.f/P.v tests; Ken Ilett for assistance with supply of the artesunate; Umar Fahmi Achmadi, Sumarjati Arjoso, Harijani Marwoto, Thomas Suroso, and Ferdinand Laihad of the Ministry of Health, Jakarta, Indonesia, for their support; Elizabeth Stubbs for logistic help; Bambang Purnomo, Budi Subianto, Tony Dimpudus, Ingko Gunawan, Krisman Hutadjulu, Ester Ayomi, Agus Berek, Frankie Hartanto, Sunarno, Frans Pello, Markus, Wayan, Yulius Weng, Gede Utomo, Neli, and their staff; and the Regional, Provincial, District, and Subdistrict Health Offices of East Nusa Tenggara and Irian Jaya, Indonesia, for support and technical assistance.
We are grateful for Northern Territory Government 50th Anniversary of Indonesian Independence Malaria-Tuberculosis Research Fellowships and to Mark Nicholson and Alice Hill, who assisted in meeting the costs of the artesunate studies. The expense of the ICT P.f/P.v kits and some logistical costs for the Sumba studies were underwritten by AMRAD-ICT, Sydney, New South Wales, Australia.
REFERENCES
- 1.Banchongaksorn T, Yomokgul P, Panyim S, Rooney W, Vickers P. A field trial of the ParaSight-F test for the diagnosis of Plasmodium falciparum infection. Trans R Soc Trop Med Hyg. 1996;90:244–245. doi: 10.1016/s0035-9203(96)90231-x. [DOI] [PubMed] [Google Scholar]
- 2.Beadle C, Long G, Weiss W, McElroy P, Maret S, Oloo A, Hoffman S. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet. 1994;343:564–568. doi: 10.1016/s0140-6736(94)91520-2. [DOI] [PubMed] [Google Scholar]
- 3.Dean A G, Dean J A, Coulombier D, Brendel K A, Smith D C, Burton A H, Dicker R C, Sullivan K, Fagan R F, Arner T G. Epi Info, version 6: a word-processing database and statistics program for public health on IBM-compatible microcomputers. Atlanta, Ga: Centers for Disease Control and Prevention; 1995. [Google Scholar]
- 4.Di Perri G, Olliaro P, Nardi S, Allegranzi B, Deganello R, Vento S, Lanzafame M, Cazzadori A, Bonora S, Concia E. The ParaSight-F rapid dipstick antigen capture assay for monitoring parasite clearance after drug treatment of Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:403–405. doi: 10.1016/s0035-9203(97)90257-1. [DOI] [PubMed] [Google Scholar]
- 4a.Dyer M E, Tjitra E, Currie B J, Anstey N M. Failure of the ‘pan-malarial’ antibody of the ICT Malaria P.f/P.v immunochromatographic test to detect symptomatic Plasmodium malariae infection. Trans R Soc Trop Med Hyg. 2000;94:518. doi: 10.1016/s0035-9203(00)90072-5. [DOI] [PubMed] [Google Scholar]
- 5.Eisen D, Saul A. Disappearance of pan-malarial antigen reactivity using the ICT Malaria P.f/P.v kit parallels decline of patent parasitaemia as shown by microscopy. Trans R Soc Trop Med Hyg. 2000;94:169–170. doi: 10.1016/s0035-9203(00)90262-1. [DOI] [PubMed] [Google Scholar]
- 6.Field J, Shute P. The microscopic diagnosis of human malaria. 2. A morphological study of the erythrocytic parasites. Kuala Lumpur, Malaysia: Institute for Medical Research; 1956. [Google Scholar]
- 7.Handunetti S, Gunawardena D, Pathirana P, Ekanayake K, Weerasinghe S, Mendis K. Features of recrudescent chloroquine-resistant Plasmodium falciparum infections confer a survival advantage on parasites and have implications for disease control. Trans R Soc Trop Med Hyg. 1996;90:563–567. doi: 10.1016/s0035-9203(96)90325-9. [DOI] [PubMed] [Google Scholar]
- 8.Hogh B, Gamage-Mendis A, Butcher G A, Thompson R, Begtrup K, Mendis C, Enosse S M, Dgedge M, Barreto J, Eling W, Sinden R E. The differing impact of chloroquine and pyrimethamine/sulfadoxine upon the infectivity of malaria species to the mosquito vector. Am J Trop Med Hyg. 1998;58:176–182. doi: 10.4269/ajtmh.1998.58.176. [DOI] [PubMed] [Google Scholar]
- 9.Karbwang J, Tasanor O, Kanda T, Wattanagoon Y, Ibrahim M, Na-Bangchang K, Thanavibul A, Rooney W. ParaSight-F test for the detection of treatment failure in multidrug resistant Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1996;90:513–515. doi: 10.1016/s0035-9203(96)90298-9. [DOI] [PubMed] [Google Scholar]
- 10.Kodisinghe H M, Perera K L, Premawansa S, Naotunne T, Wickramasinghe A R, Mendis K N. ParaSight-F dipstick test as a routine diagnostic tool for malaria in Sri Lanka. Trans R Soc Trop Med Hyg. 1997;91:398–402. doi: 10.1016/s0035-9203(97)90255-8. [DOI] [PubMed] [Google Scholar]
- 11.Makler M T, Palmer C J, Ager A L. A review of practical techniques for the diagnosis of malaria. Ann Trop Med Parasitol. 1998;92:419–433. doi: 10.1080/00034989859401. [DOI] [PubMed] [Google Scholar]
- 12.Price R, Nosten F, Luxemberger C, ter Kuile F, Paiphun L, Chongsuphajaisiddhi T, White N. Effects of artemisinin derivatives on malaria transmissibility. Lancet. 1996;347:1654–1658. doi: 10.1016/s0140-6736(96)91488-9. [DOI] [PubMed] [Google Scholar]
- 13.Price R, Nosten F, Simpson J A, Luxemburger C, Phaipun L, ter Kuile F, van Vugt M, Chongsuphajaisiddhi T, White N J. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am J Trop Med Hyg. 1999;60:1019–1023. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- 14.Robert V, Awono-Ambene H P, Le Hesran J Y, Trape J F. Gametocytemia and infectivity to mosquitoes of patients with uncomplicated Plasmodium falciparum malaria attacks treated with chloroquine or sulfadoxine plus pyrimethamine. Am J Trop Med Hyg. 2000;62:210–216. doi: 10.4269/ajtmh.2000.62.210. [DOI] [PubMed] [Google Scholar]
- 15.Robert V, Molez J F, Trape J F. Short report: gametocytes, chloroquine pressure, and the relative parasite survival advantage of resistant strains of falciparum malaria in west Africa. Am J Trop Med Hyg. 1996;55:350–351. doi: 10.4269/ajtmh.1996.55.350. [DOI] [PubMed] [Google Scholar]
- 16.Shiff C J, Premji Z, Minjas J N. The rapid manual ParaSight-F test. A new diagnostic tool for Plasmodium falciparum infection. Trans R Soc Trop Med Hyg. 1993;87:646–648. doi: 10.1016/0035-9203(93)90273-s. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Valecha N, Sharma V P. Malaria diagnosis by field workers using an immunochromatographic test. Trans R Soc Trop Med Hyg. 1997;91:396–397. doi: 10.1016/s0035-9203(97)90254-6. [DOI] [PubMed] [Google Scholar]
- 18.Tjitra E, Suprianto S, Dyer M, Currie B, Anstey N. Evaluation of the therapeutic efficacy of chloroquine and sulfadoxine-pyrimethamine in uncomplicated falciparum malaria in Eastern Indonesia: high rates of chloroquine treatment failure with poor hematological recovery. Am J Trop Med Hyg. 1999;61(Suppl.):S286–S287. . (Abstract.) [Google Scholar]
- 19.Tjitra E, Suprianto S, Dyer M, Currie B, Anstey N. Utility of the ICT Malaria P.f/P.v immunochromatographic test in diagnosing/predicting treatment outcome following treatment of uncomplicated falciparum malaria in Eastern Indonesia. Am J Trop Med Hyg. 1999;61(Suppl.):S472. [Google Scholar]
- 20.Tjitra E, Suprianto S, Dyer M, Currie B J, Anstey N M. Field evaluation of the ICT Malaria P.f/P.v immunochromatographic test for detection of Plasmodium falciparum and Plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in eastern Indonesia. J Clin Microbiol. 1999;37:2412–2417. doi: 10.1128/jcm.37.8.2412-2417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vakharia S, Gopinathan N, Kshirsagar N A. The ParaSight-F test for detecting treatment failure. Trans R Soc Trop Med Hyg. 1997;91:490–491. doi: 10.1016/s0035-9203(97)90298-4. [DOI] [PubMed] [Google Scholar]
- 22.Warrell D. Treatment and prevention of malaria. In: Gilles H, Warrell D, editors. Essential malariology. 3rd ed. London, United Kingdom: Edward Arnold; 1993. pp. 164–195. [Google Scholar]
- 23.World Health Organization. Assessment of therapeutic efficacy of antimalarial drugs for uncomplicated falciparum malaria. Geneva, Switzerland: Division of Control of Tropical Diseases, World Health Organization; 1997. [Google Scholar]
- 24.World Health Organization. New perspectives malaria diagnosis. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 25.World Health Organization. A rapid dipstick antigen capture assay for the diagnosis of falciparum malaria. WHO informal consultation on recent advances in diagnostic techniques and vaccines for malaria. Bull W H O. 1996;74:47–54. [PMC free article] [PubMed] [Google Scholar]