Abstract
Objective: To study the differences in clinical characteristics, risk factors, and complications across age-groups among the inpatients with the coronavirus disease 2019 (COVID-19).
Methods: In this population-based retrospective study, we included all the positive hospitalized patients with COVID-19 at Wuhan City from December 29, 2019 to April 15, 2020, during the first pandemic wave. Multivariate logistic regression analyses were used to explore the risk factors for death from COVID-19. Canonical correlation analysis (CCA) was performed to study the associations between comorbidities and complications.
Results: There are 36,358 patients in the final cohort, of whom 2,492 (6.85%) died. Greater age (odds ration [OR] = 1.061 [95% CI 1.057–1.065], p < 0.001), male gender (OR = 1.726 [95% CI 1.582–1.885], p < 0.001), alcohol consumption (OR = 1.558 [95% CI 1.355–1.786], p < 0.001), smoking (OR = 1.326 [95% CI 1.055–1.652], p = 0.014), hypertension (OR = 1.175 [95% CI 1.067–1.293], p = 0.001), diabetes (OR = 1.258 [95% CI 1.118–1.413], p < 0.001), cancer (OR = 1.86 [95% CI 1.507–2.279], p < 0.001), chronic kidney disease (CKD) (OR = 1.745 [95% CI 1.427–2.12], p < 0.001), and intracerebral hemorrhage (ICH) (OR = 1.96 [95% CI 1.323–2.846], p = 0.001) were independent risk factors for death from COVID-19. Patients aged 40–80 years make up the majority of the whole patients, and them had similar risk factors with the whole patients. For patients aged <40 years, only cancer (OR = 17.112 [95% CI 6.264–39.73], p < 0.001) and ICH (OR = 31.538 [95% CI 5.213–158.787], p < 0.001) were significantly associated with higher odds of death. For patients aged >80 years, only age (OR = 1.033 [95% CI 1.008–1.059], p = 0.01) and male gender (OR = 1.585 [95% CI 1.301–1.933], p < 0.001) were associated with higher odds of death. The incidence of most complications increases with age, but arrhythmias, gastrointestinal bleeding, and sepsis were more common in younger deceased patients with COVID-19, with only arrhythmia reaching statistical difference (p = 0.039). We found a relatively poor correlation between preexisting risk factors and complications.
Conclusions: Coronavirus disease 2019 are disproportionally affected by age for its clinical manifestations, risk factors, complications, and outcomes. Prior complications have little effect on the incidence of extrapulmonary complications.
Keywords: COVID-19, age, risk factors, complications, death
Introduction
The coronavirus disease 2019 (COVID-19) is a novel coronavirus infection disease causing respiratory failure and a high death rate in vulnerable populations (1). Despite increasing clinical experience and a rapidly expanding of literature on diverse aspects of COVID-19, the relationship between common risk factors and mortality from COVID-19 remains incompletely understood.
Prior studies have identified greater age, male sex, and metabolic comorbidities, such as hypertension and diabetes as risk factors for poor outcomes in patients with COVID-19 (2, 3). These comorbidities are more common among older age groups, and overwhelming evidence from around the world suggests that age itself is the most significant risk factor for severe disease and death from COVID-19 (3). Emerging data suggest that the effect of comorbidities on COVID-19 may be age-dependent (4–6). However, due to the small sample size of previous studies, it is challenging to isolate the independent effects of these clustered characteristics as a function of patient age. Besides, COVID-19 can affect multiple organ systems (7, 8), and cause complications, such as acute respiratory distress syndrome (ARDS), respiratory failure, acute myocardial infarction (AMI), acute kidney injury (AKI), coagulopathy (9), sepsis, or systemic inflammation (10). Since these complications are extremely life-threatening, their prevalence can act as determining factors in the morbidity and mortality rates of the disease (11, 12). However, there is a lack of research about the effects of age on the incidence of complications and the association between complications and prior comorbidities of patients (13).
To address these deficiencies in the literature, in this study we determine clinical characteristics, risk factors for death, and complications across age groups in the whole dataset of inpatients with COVID-19 across Wuhan city, China. Wuhan has emerged as the original epicenter of COVID-19, with some 50,000 individuals infected and nearly 4,000 deaths recorded during the early phase of this pandemic. Using this unique cohort, we explored the association between complications and prior comorbidities of patients.
Methods
Study Design and Participants
This is a retrospective cohort study using data collected from all inpatients in Wuhan with positive COVID-19 test results recorded from December 29, 2019, when the patients were first admitted to hospital, and extending to April 15, 2020, this being the first day with no new cases declared in Wuhan city. The compilation of data was coordinated by the Wuhan Health Commission, which mandated the reporting of clinical information from every designated hospital (such as, 61 Wuhan hospitals and mobile cabin hospitals) in Wuhan that had admitted patients with laboratory-confirmed COVID-19. We included all the inpatients with positive COVID-19 in this administrative dataset. We excluded patient records with incomplete clinic data. The follow-up of patients continued through July 1, 2020. This study was designed by the authors and approved with the provision of a waiver of authorization and informed consent by the ethics committee of Tongji Hospital (IRB ID: TJ-C20200121). The data are anonymous, and the requirement for informed consent was therefore waived.
Data Collection and Data Extraction
Demographic, clinical, laboratory, treatment, and outcome data were extracted from electronic medical records using a standardized data collection and processing method. Detailed information about procedures is provided in Supplementary Materials. The laboratory data were collected at the time of admission of the patient.
Definitions
Age groups were set as <18, 18–40, 40–60, 60–80, and >80 years since differences in mortality were reported according to a previous study (14). The clinical outcomes were defined as hospital discharge or death. COVID-19 related deaths were defined as those occurring in patients who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) through reverse transcription-PCR (RT-PCR). The cardiac injury was defined as an elevation of at least one of the three cardiac biomarkers above the 99th percentile upper reference limit, or if new abnormalities were shown in electrocardiography and echocardiography (15). Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition (16). Respiratory failure was defined as the requirement for oxygen supplementation or mechanical ventilation (17). AKI was identified according to the Kidney Disease: Improving Global Outcomes definition (18). Cardiac arrhythmias were recorded according to the ECG measured upon initial admission and duration of the hospital stay, or were collected from the medical records of patients (19). Coagulopathy was defined as a 3-s prolongation of prothrombin time or a 5-s prolongation of activated partial thromboplastin time, or by any evidence of pulmonary embolism, lower limb venous thrombosis, arterial embolism, or disseminated intravascular coagulation (DIC) (20). Acute liver injury (ALI) was defined as a state in which the blood laboratory results of patients met at least one of three criteria: serum total bilirubin of 1.0 mg/dl or greater; aspartate aminotransferase of 41 IU/L or greater; and alanine aminotransferase of 41 IU/L or greater. AMI and acute ischemic stroke (AIS) was diagnosed as previously described (21, 22). Gastrointestinal bleeding was defined as the presence of hematemesis, melena, or hematochezia. Sepsis was defined according to The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) (23).
Statistical Analysis
Continuous variables with non-normal distributions were described as a median interquartile range (IQR). Categorical variables were described as number and percentage (%). Comparison between two groups was performed with Wilcoxon test for non-parametric variables, Fisher's exact test or the chi-square test was performed for categorical variables. Multivariate logistic regression models were developed to explore the relative contributions of risk factors. Variables with P < 0.1 in univariate regression were included in multiple regression. There were no significant interactions between variables enrolled in multivariate analyses. The value of P < 0.05 was considered statistically significant. Canonical correlation analysis (CCA) was used to analyze the correlation between preexisting risk factors and the incidence of complications. The sign of the standardized coefficient indicates the direction in which the raw variable influences the canonical variate. The magnitude of the standardized coefficient represents the magnitude of the influence of variable on the canonical variate. Data preprocessing and statistical analysis were calculated with Ubuntu 16.04.6 and an R-studio server (R-4.0.3 R Foundation for Statistical Computing). CCA was performed using the “matcor” function in the CCA package (detail in Supplementary Materials).
Data Availability
The data that support the findings of this study were available from the corresponding author upon reasonable request.
Results
Baseline Characteristics and Outcomes of Patients With COVID-19 in Different Age Groups
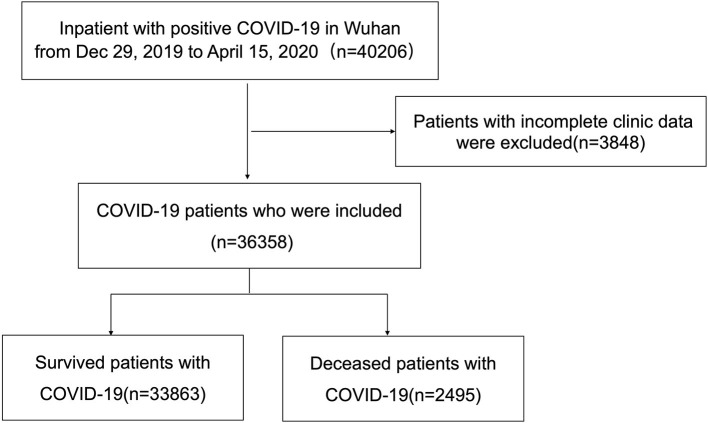
In total, 40,206 patients were found positive for COVID-19 and received in-hospital treatment in Wuhan, Hubei Province. After excluding patients with critical missing data, the final cohort consisted of 36,358 patients (Figure 1). There were 473, 6,106, 12,950, 14,805, and 2,497 patients with COVID-19 included in the age group of <18, 18–40, 40–60, 60–80, and >80 years old, respectively. The most common symptoms on admission were fever (66.1%) and cough (62.0%), followed by shortness of breath (39.7%) and fatigue (38.5%). Patients aged <18 years had a lower incidence in almost all the symptoms but the running nose. Patients aged between 40 and 80 years had a higher incidence of fever, cough, muscle soreness, and fatigue. Hemoptysis and anorexia showed increasing incidence with age. Patients aged older than 80 years showed a significantly increased incidence of disturbance of consciousness (4.5%).
Figure 1.
Flowchart of patient inclusion and exclusion.
Patients aged <18 years had less comorbidities. Patients aged >40 years had a greater prevalence of smoking and alcohol consumption. The incidence of comorbidities, such as hypertension, diabetes mellitus, hyperlipidemia, heart disease, tuberculosis, malignant tumor, kidney disease, chronic obstructive pulmonary disease (COPD), cerebral hemorrhage, and ischemic stroke increased with age groups. Patients aged >80 years had a significantly greater history of heart disease. Patients aged <60 years were more likely to have a history of liver disease.
Elderly patients showed a greater prevalence of positive laboratory tests, such as leukocytosis, lymphopenia, decreased platelet count, hemoglobinemia, lower albumin, increased D-D-dimer, and prolonged prothrombin times. ALT elevation occurred more frequently in younger patients. The mortality rate of patients with COVID-19 increased with age, with patients aged over 80 years having a particularly high mortality rate (21.6% vs. 0.8%, 1.0%, 3.4%, 9.8%) (Table 1).
Table 1.
Baseline characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) in different age groups.
| characteristic | All | ≤18 | >18, ≤40 | >40, ≤60 | >60, ≤80 | >80 | P |
|---|---|---|---|---|---|---|---|
| n | 36,358 | 473 | 5,633 | 12,950 | 14,805 | 2,497 | |
| Age | 59[47.0, 69.0] | 9.00 [4.00, 14.00] | 34.00[30.00, 37.00] | 52[48.0, 57.0] | 68[64.0, 72.0] | 85[83.0,88.0] | |
| Sex(female/male) | 18,696/1766 (51.4/48.6) | 208/265 (44.0/56.0) | 2,760/2,873 (49.0/51.0) | 6,855/6,095 (52.9/47.1) | 7,604/7,201 (51.4/48.6) | 1,269/1,228 (50.8/49.2) | <0.001 |
| Systolic pressure | 126.0 [114.0, 138.0] | 114.50 [100.00, 122.75] | 120.00 [110.00, 130.00] | 125.0 [113.0, 135.0] | 130.0 [116.0, 141.0] | 130.0 [118.0, 144.0] | <0.001 |
| Diastolic pressure | 80.0 [72.0, 90.0] | 71.50 [61.00, 80.00] | 80.00 [71.00, 88.00] | 80.0 [74.0, 92.0] | 80.0 [73.0, 90.0] | 78.0 [70.0, 87.0] | <0.001 |
| Smoking | 794 (2.2%) | 4 (0.8) | 304 (5.4) | 258 (2.0) | 1,407 (2.7) | 45 (1.8) | <0.001 |
| Drinking | 2,697 (7.4) | 0 (0.0) | 84 (1.5) | 1,024 (7.9) | 1,193 (8.1) | 172 (6.9) | <0.001 |
| Diabetes | 3,931 (10.8) | 3 (0.6) | 170 (3.0) | 1,101 (8.5) | 2,267 (15.3) | 390 (15.6) | <0.001 |
| Hypertension | 8,498 (23.4) | 0 (0.0) | 187 (3.3) | 2,205 (17.0) | 4,993 (33.7) | 1,113 (44.6) | <0.001 |
| Hyperlipidemia | 459 (1.3) | 0 (0.0) | 22 (0.4) | 153 (1.2) | 250 (1.7) | 34 (1.4) | <0.001 |
| Heart disease | 2,452 (6.7) | 3 (0.6) | 75 (1.3) | 359 (2.8) | 1,446 (9.6) | 571 (20.1) | <0.001 |
| Cancer | 824 (2.3) | 6 (1.3) | 41 (0.7) | 257 (2.0) | 427 (2.9) | 93 (3.7) | <0.001 |
| COPD | 850 (2.3) | 0 (0.0) | 14 (0.2) | 125 (1.0) | 495 (3.3) | 216 (8.7) | <0.001 |
| Tuberculosis | 506 (1.4) | 1 (0.2) | 57 (1.0) | 157 (1.2) | 229 (1.5) | 62 (2.5) | <0.001 |
| Chronic kidney disease | 924 (2.5) | 1 (0.2) | 78 (1.4) | 315 (2.4) | 421 (2.8) | 109 (4.4) | <0.001 |
| Liver disease | 1,189 (3.3) | 4 (0.8) | 186 (3.3) | 511 (3.9) | 413 (2.8) | 75 (3.0) | <0.001 |
| Intracerebral hemorrhage | 178 (0.5) | 0 (0.0) | 8 (0.1) | 42 (0.3) | 187 (0.6) | 41 (1.6) | <0.001 |
| Asthma | 386 (1.1) | 3 (0.6) | 52 (0.9) | 134 (1.0) | 165 (1.1) | 32 (1.3) | 0.48 |
| History of stroke | 1,160 (3.2) | 0 (0.0) | 8 (0.1) | 136 (1.1) | 668 (4.5) | 348 (13.9) | <0.001 |
| Fever | 24,026 (66.1) | 266 (56.2) | 3,725 (66.1) | 8,799 (67.9) | 9,831 (66.4) | 1,405 (56.3) | <0.001 |
| Cough | 22,525 (62.0) | 209 (44.2) | 3,345 (59.4) | 8,164 (63.0) | 9,345 (63.1) | 1,462 (58.6) | <0.001 |
| Hemoptysis | 1,504 (4.1) | 7 (1.5) | 200 (3.6) | 480 (3.7) | 679 (4.6) | 138 (5.5) | <0.001 |
| Muscle soreness | 4,006 (11.0) | 11 (2.3) | 563 (10.0) | 1,457 (11.3) | 1,737 (11.7) | 238 (9.5) | <0.001 |
| Fatigue | 13,986 (38.5) | 46 (9.7) | 1,857 (33.0) | 4,909 (37.9) | 6,230 (42.1) | 944 (37.8) | <0.001 |
| Running nose | 845 (2.3) | 30 (6.3) | 128 (2.3) | 272 (2.1) | 357 (2.4) | 58 (2.3) | <0.001 |
| Pharyngalgia | 2,537 (7.0) | 13 (2.7) | 417 (7.4) | 903 (7.0) | 1,034 (7.0) | 170 (6.8) | 0.005 |
| Shortness of breath | 14,445 (39.7) | 25 (5.3) | 1,699 (30.2) | 5,084 (39.3) | 6,622 (44.7) | 1,015 (40.6) | <0.001 |
| Chest pain | 2,328 (6.4) | 9 (1.9) | 345 (6.1) | 810 (6.3) | 999 (6.7) | 165 (6.6) | <0.001 |
| Anorexia | 4,882 (13.4) | 11 (2.3) | 582 (10.3) | 1,567 (12.1) | 2,260 (15.3) | 462 (18.5) | <0.001 |
| Nausea and vomiting | 3,821 (10.5) | 39 (8.2) | 604 (10.7) | 1,283 (9.9) | 1,614 (10.9) | 281 (11.3) | 0.019 |
| Diarrhea | 4,762 (13.1) | 24 (5.1) | 770 (13.7) | 1,675 (12.9) | 1,993 (13.5) | 300 (12.0) | <0.001 |
| Stomachache | 1,988 (5.5) | 15 (3.2) | 311 (5.5) | 621 (4.8) | 860 (5.8) | 81 (7.2) | <0.001 |
| Deadache | 2,773 (7.6) | 17 (3.6) | 458 (8.1) | 1,026 (7.9) | 1,091 (7.4) | 181 (7.2) | 0.002 |
| Dizziness | 2,093 (5.8) | 6 (1.3) | 343 (6.1) | 717 (5.5) | 854 (5.8) | 173 (6.9) | <0.001 |
| Disturbance of consciousness | 404 (1.1) | 2 (0.4) | 26 (0.5) | 77 (0.6) | 187 (1.3) | 112 (4.5) | <0.001 |
| BWC, >10 ×109 cells/L | 1,858 (7.37) | 5 (3.5) | 180 (4.7) | 478 (5.4) | 975 (9.2) | 220 (12.1) | <0.001 |
| LC, <0.8 ×109 cells/L | 6,354 (20.84) | 6 (3.8) | 419 (9.3) | 1,863 (17.0) | 3,313 (25.9) | 753 (36.3) | <0.001 |
| Platelets, <100 ×109 cells/L | 1,284 (4.39) | 3(1.9) | 81 (1.8) | 364 (3.3) | 655 (5.1) | 181 (8.7) | <0.001 |
| Hemoglobin, <90 g/L | 1,196 (3.93) | 5 (3.2) | 69 (1.5) | 342 (3.1) | 572 (4.5) | 208 (10.0) | <0.001 |
| Albumin, <35g/L | 8,005 (29.01) | 1 (0.7) | 382 (9.3) | 2,015 (20.4) | 4,585 (39.7) | 1,022 (53.6) | <0.001 |
| ALT, >40 u/L | 6,386 (23.05) | 20 (13.2) | 1,156 (28.2) | 2,480 (25.0) | 2,480 (21.4) | 250 (13.0) | <0.001 |
| Prothrombin time, >16s | 1,769 (7.03) | 15 (11.3) | 233 (6.4) | 536 (6.1) | 774 (7.2) | 211 (11.6) | <0.001 |
| D-D dimer, >0.5 μg/L | 13,211 (36.34) | 29 (23.1) | 1,083 (28.6) | 3,567 (38.01) | 6,935 (60.85) | 1,597 (82.83) | <0.001 |
| Die | 2,492 (6.9) | 4 (0.8) | 64 (1.1) | 435 (3.4) | 1,452 (9.8) | 539 (21.6) | <0.001 |
COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; BWC, blood white cells; LC, lymphocyte; ALT, Alanine aminotransferase. Data are reported as a number of patients and percentage in the age group (%) for categorical variables and median (interquartile range [IQR]) for continuous variables.
Analysis of Risk Factors for Death in Patients With COVID-19
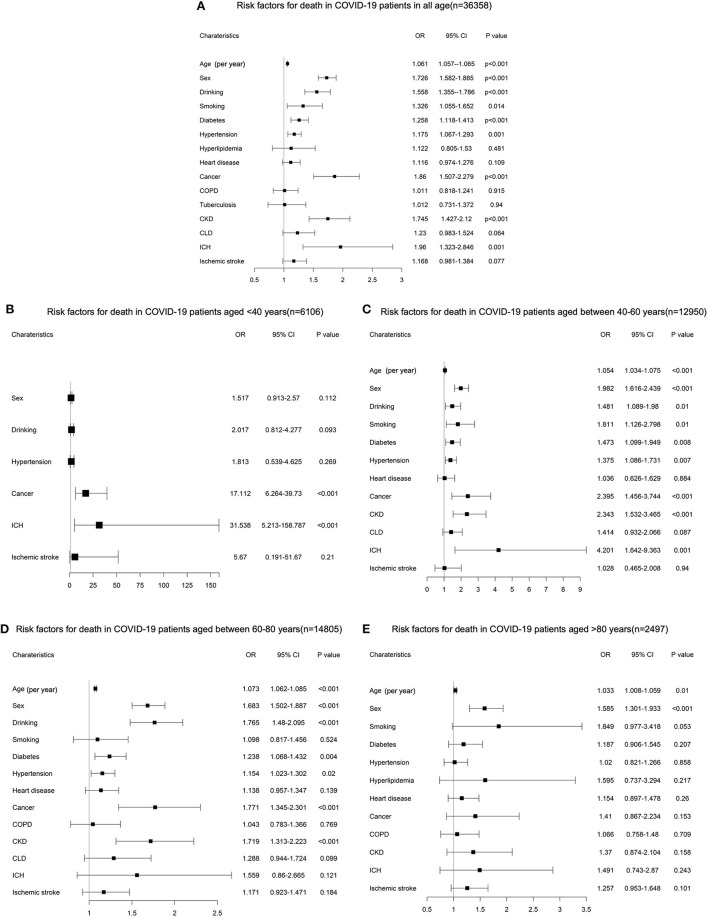
To investigate the risk factors for death in patients with COVID-19, we performed a bilateral logistic regression analysis. After adjusting for age, sex, and comorbidities, multivariable logistic regression analysis showed that greater age (OR = 1.061 [95% CI 1.057–1.065], p < 0.001), male gender (OR = 1.726 [95% CI 1.582–1.885], p < 0.001)], alcohol consumption (OR = 1.558 [95% CI 1.355–1.786], p < 0.001), smoking (OR = 1.326 [95% CI 1.055–1.652], p = 0.014), hypertension (OR = 1.175 [95% CI 1.067–1.293], p = 0.001), diabetes (OR = 1.258 [95% CI 1.118–1.413], p < 0.001), cancer (OR = 1.86 [95% CI 1.507–2.279], p < 0.001), chronic kidney disease (CKD) (OR = 1.745 [95% CI 1.427–2.12], p < 0.001), and intracerebral hemorrhage (ICH) (OR = 1.96 [95% CI 1.323–2.846], p = 0.001) were independent risk factors for death of COVID-19 (Figure 2A).
Figure 2.
(A) Multivariable logistic regression analysis for risk factors of death in the entire cohort of patients with coronavirus disease 2019 (COVID-19). (B) Multivariable logistic regression analysis for risk factors of death in COVID-19 patients aged <40 years. (C) Multivariable logistic regression analysis for risk factors of death in COVID-19 patients aged between 40 and 60 years. (D) Multivariable logistic regression analysis for risk factors of death COVID-19 patients aged between 60 and 80 years. (E) Multivariable logistic regression analysis for risk factors of death COVID-19 patients aged >80 years.
The differences among age groups were found when results were stratified by age groups. For patients aged <40 years, only cancer (OR = 17.112 [95% CI 6.264–39.73], p < 0.001) and ICH (OR = 31.538 [95% CI 5.213–158.787], p < 0.001) were associated with higher odds of death (Figure 2B). For people aged 40–60 years, risk factors are almost the same for all patients, but cancer (OR = 2.395 [95% CI 1.456–3.744], p < 0.001), CKD (OR = 2.343 [95% CI 1.532–3.465], p < 0.001), and ICH (OR = 4.201 [95% CI 1.642–9.363], p = 0.001) had an obvious higher OR for death (Figure 2C). For people aged 60–80 years, older age (OR = 1.073 [95% CI 1.062–1.085], p < 0.001), male gender (OR = 1.683 [95% CI 1.502–1.887], p < 0.001), alcohol consumption (OR = 1.765 [95% CI 1.48–2.095], p < 0.001), diabetes (OR = 1.238 [95% CI 1.068–1.432], p = 0.004), hypertension (OR = 1.154 [95% CI 1.023–1.302], p = 0.02), cancer (OR = 1.771 [95% CI 1.345–2.301], p < 0.001), and CKD (OR = 1.719 [95% CI 1.313–2.223], p < 0.001) were associated with higher odds of death (Figure 2D). For people aged >80 years, only age (OR = 1.033 [95% CI 1.008–1.059], p = 0.01) and male gender (OR = 1.585 [95% CI 1.301–1.933], p < 0.001) were associated with higher odds of death (Figure 2E).
Baseline Characteristics of Deceased Patients With COVID-19 in Different Age Groups
There were 2,495 (6.85%) patients who died during their hospitalization. Among them, men were over-represented across all age groups. Patients aged <40 (10.6 and 1.5%) and >80 (7.1 and 3.3%) years were less likely to smoke and drink. The proportion of patients with diabetes, hypertension, hyperlipidemia, COPD, tuberculosis, CKD, prior stroke increased with age groups, but patients in the 40–60 years group had higher rates of CKD and CLD. Patients in <40 age group had significant higher rates of ICH (4.5% vs. 1.6%, 1.1%, 2.6%), and cancer (9.1% vs. 4.8%, 4.9%, 5.0%) although without statistically significant.
Fever (62.1 and 64% vs. 79.5 and 75.4%), cough (56.1 and 61.8% vs. 71.0 and 65.6%), and shortness of breath (48.5 and 53.2% vs. 60.5 and 60.7%) were less common in patients aged <40 years and > 80 years, and patients aged >80 years showed a significantly increased incidence of disturbance of consciousness (11.3 vs. 6.1%, 5.5, 6.7%).
Regarding laboratory test results, leukocytosis was more common in deceased patients with COVID-19 in the group aged 60–80 years old. Lymphopenia, eosinophil, and basophilopenia were seen most frequently in the group aged 40–80 years. A significantly higher proportion of decease patients younger than 40 years of age had reduced hemoglobin (17 vs. 11.0%, 10.9, 11.0%). Reduced albumin was more common in deceased patients aged >60 (68.9 and 67.8% vs. 38.3 and 53.1%). The proportion of cases with increased D-D dimer rose with increasing age groups of deceased patients with COVID-19 (Table 2).
Table 2.
Baseline characteristics and outcomes of deceased patients with COVID-19 in different age groups.
| characteristic | Overall | ≤40 | >40, ≤60 | >60, ≤80 | >80 | p |
|---|---|---|---|---|---|---|
| n | 2,492 | 66 | 435 | 1,452 | 539 | |
| Age | 71.0 [63.0, 79.0] | 34.0 [30.0, 38.0] | 55.0 [50.0, 57.0] | 70.0 [66.0, 75.0] | 85.0 [83.0, 89.0] | <0.001 |
| Sex (female/male) | 958/1,534 (38.4/61.6) | 25/41 (37.9/62.1) | 153/282 (35.2/64.8) | 558/894 (38.4/61.6) | 222/317 (41.2/58.8) | 0.297 |
| Systolic pressure | 125 [105.0, 140.0] | 120 [104.2,132.2] | 122 [100.5,135.0] | 125.5 [103.0, 140.0] | 128 [112.5, 141.0] | 0.003 |
| diastolic pressure | 80.0 [70.2, 93.0] | 83.0 [78.0, 93.2] | 81.0 [74.0, 98.0] | 80.0 [71.0, 94.0] | 78.00 [70.0, 87.0] | <0.001 |
| Smoking | 295 (11.8) | 7 (10.6) | 59 (13.6) | 191 (13.2) | 38 (7.1) | 0.001 |
| Drinking | 109 (4.4) | 1 (1.5) | 25 (5.7) | 65 (4.5) | 18 (3.3) | 0.198 |
| Diabetes | 458 (18.4) | 1 (1.5) | 68 (15.6) | 289 (19.9) | 100 (18.6) | 0.001 |
| Hypertension | 987 (39.6) | 5 (7.6) | 124 (28.5) | 597 (41.1) | 261 (48.4) | <0.001 |
| Hyperlipidemia | 48 (1.9) | 0 (0.0) | 8 (1.8) | 28 (1.9) | 12 (2.2) | 0.666 |
| Heart disease | 355 (14.2) | 2 (3.0) | 21 (4.8) | 197 (13.6) | 135 (25.0) | <0.001 |
| Cancer | 125 (5.0) | 6 (9.1) | 21 (4.8) | 71 (4.9) | 27 (5.0) | 0.497 |
| COPD | 126 (5.1) | 0 (0.0) | 5 (1.1) | 63 (4.3) | 58 (10.8) | <0.001 |
| Tuberculosis | 49 (2.0) | 0 (0.0) | 8 (1.8) | 24 (1.7) | 17 (3.2) | 0.109 |
| Chronic kidney disease | 142 (5.7) | 2 (3.0) | 30 (6.9) | 77 (5.3) | 33 (6.1) | 0.451 |
| Liver disease | 103 (4.1) | 2 (3.0) | 29 (6.7) | 54 (3.7) | 18 (3.3) | 0.033 |
| Intracerebral hemorrhage | 40 (1.6) | 3 (4.5) | 7 (1.6) | 16 (1.1) | 14 (2.6) | 0.026 |
| Asthma | 34 (1.4) | 1 (1.5) | 6 (1.4) | 21 (1.4) | 6 (1.1) | 0.953 |
| History of stroke | 202 (8.1) | 1 (1.5) | 9 (2.1) | 94 (6.5) | 98 (18.2) | <0.001 |
| Faver | 1,827 (73.3) | 41 (62.1) | 346 (79.5) | 1,095 (75.4) | 345 (64.0) | <0.001 |
| Cough | 1,632 (65.5) | 37 (56.1) | 309 (71.0) | 953 (65.6) | 333 (61.8) | 0.008 |
| Hemoptysis | 118 (4.7) | 2 (3.0) | 21 (4.8) | 70 (4.8) | 25 (4.6) | 0.926 |
| Muscle soreness | 252 (10.1) | 10 (15.2) | 52 (12.0) | 142 (9.8) | 48 (8.9) | 0.212 |
| Fatigue | 1,049 (42.1) | 20 (30.3) | 179 (41.1) | 626 (43.1) | 224 (41.6) | 0.203 |
| Running nose | 66 (2.6) | 1 (1.5) | 10 (2.3) | 34 (2.3) | 21 (3.9) | 0.229 |
| Pharyngalgia | 170 (6.8) | 3 (4.5) | 32 (7.4) | 102 (7.0) | 33 (6.1) | 0.743 |
| Shortness of breath | 1,463 (58.7) | 32 (48.5) | 263 (60.5) | 881 (60.7) | 287 (53.2) | 0.006 |
| Chest pain | 169 (6.8) | 4 (6.1) | 36 (8.3) | 98 (6.7) | 31 (5.8) | 0.476 |
| Anorexia | 457 (18.3) | 9 (13.6) | 72 (16.6) | 264 (18.2) | 112 (20.8) | 0.254 |
| Nausea and vomiting | 292 (11.7) | 14 (21.2) | 50 (11.5) | 163 (11.2) | 65 (12.1) | 0.104 |
| Diarrhea | 369 (14.8) | 7 (10.6) | 72 (16.6) | 218 (15.0) | 72 (13.4) | 0.404 |
| Stomachache | 155 (6.2) | 5 (7.6) | 24 (5.5) | 84 (5.8) | 42 (7.8) | 0.343 |
| Deadache | 197 (7.9) | 1 (1.5) | 36 (8.3) | 121 (8.3) | 39 (7.2) | 0.214 |
| Dizziness | 184 (7.4) | 4 (6.1) | 25 (5.7) | 101 (7.0) | 54 (10.0) | 0.052 |
| Disturbance of consciousness | 187 (7.5) | 4 (6.1) | 24 (5.5) | 98 (6.7) | 61 (11.3) | 0.002 |
| BWCs, >10 ×109/L | 541 (31.3) | 8(17.4) | 79 (26.0) | 363 (35.2) | 91 (26.2) | <0.001 |
| LCs, <0.8 ×109/L | 1,209 (61.4) | 23 (43.4) | 200 (56.5) | 758 (64.7) | 228 (58.3) | 0.002 |
| Platelets, <100 ×109/L | 340 (17.3) | 10 (18.9) | 47 (13.2) | 207 (17.7) | 76 (19.5) | 0.132 |
| Hemoglobin, <90 g/L | 219 (11.1) | 9 (17.0) | 39 (11.0) | 128 (10.9) | 43 (11.0) | <0.001 |
| Albumin, <35 g/L | 1,201 (65.1) | 18 (38.3) | 173 (53.1) | 753 (68.9) | 257 (67.8) | <0.001 |
| ALT, >40 u/L | 537 (29.1) | 15 (31.9) | 101 (31.0) | 344 (31.4) | 77 (20.3) | <0.001 |
| Prothrombin time, >16s | 303 (18.4) | 9 (18.8) | 47 (16.3) | 184 (18.9) | 63 (18.6) | 0.803 |
| D-D dimer, >0.5μg/L | 1,477(81.6) | 27(51.92) | 229(70.9) | 910(84.34) | 311(87.36) | <0.001 |
COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; BWC, blood white cells; LC, lymphocyte; ALT, alanine aminotransferase. Data are reported as number of patients and percentage in the age group (%) for categorical variables and median (IQR) for continuous variables.
Age-Related Complications in Deceased Patients With COVID-19
Patients with COVID-19 are apt to die from multiple complications. The comparison between complications in deceased patients according to age groups is shown in Table 3. The most common complications included ARDS (41.3%), respiratory failure (71.4%), and extrapulmonary complications, such as acute myocardial infarction (2.1%), acute myocardial injury (15.3%), arrhythmia (5.2%), acute kidney injury (19.4%), acute liver injury (19.0%), gastrointestinal bleeding (2.0%), acute ischemic stroke (1.5%), sepsis (10.9%), and coagulopathy (22.5%). The incidence of most complications increased with increasing age, with respiratory failure (p = 0.005), acute myocardial infarction (p = 0.049), acute myocardial injury (p = 0.001), AKI (p = 0.014), acute liver injury (p = 0.039), and acute ischemic stroke (p = 0.018) attaining statistical significance. However, arrhythmias (7.6% vs. 2.5, 5.6, 6.1%), gastrointestinal bleeding (4.5% vs. 2.3, 1.9, 1.5%), and sepsis (21.2% vs. 11.0, 10.8, 10.0) were more common in deceased patients with COVID-19 patients aged <40 years, although only arrhythmia reaching statistical significance (p = 0.039).
Table 3.
Age-related complications in deceased patients with COVID-19.
| Characteristic | Total | ≤40 | >40, ≤60 | >60, ≤80 | >80 | p |
|---|---|---|---|---|---|---|
| n | 2,492 | 66 | 435 | 1,452 | 539 | |
| ARDS | 1,028 (41.3) | 23 (34.8) | 160 (36.8) | 614 (42.3) | 231 (42.9) | 0.116 |
| Respiratory failure | 1,779 (71.4) | 39 (59.1) | 288 (66.2) | 1,058 (72.9) | 394 (73.1) | 0.005 |
| Acute myocardial infarction | 51(2.0) | 0 (0.0) | 5 (1.1) | 30 (2.1) | 16 (3.0) | 0.144 |
| Acute myocardial injury | 381 (15.3) | 5 (7.6) | 44 (10.1) | 232 (16.0) | 100 (18.6) | 0.001 |
| Arrhythmia | 130 (5.2) | 5 (7.6) | 11 (2.5) | 81 (5.6) | 33 (6.1) | 0.039 |
| Acute kidney injury | 483 (19.4) | 5 (7.6) | 71 (16.3) | 290 (20.0) | 117 (21.7) | 0.014 |
| Acute liver injury | 474 (19.0) | 9 (13.6) | 87 (20.0) | 296 (20.4) | 82 (15.2) | 0.039 |
| Gastrointestinal bleeding | 49 (2.0) | 3 (4.5) | 10 (2.3) | 28 (1.9) | 8 (1.5) | 0.364 |
| Acute ischemic stroke | 38 (1.5) | 0 (0.0) | 5 (1.1) | 17 (1.2) | 16 (3.0) | 0.018 |
| Sepsis | 273 (11.0) | 14 (21.2) | 48 (11.0) | 157 (10.8) | 54 (10.0) | 0.054 |
| Coagulopathy | 560 (22.5) | 16 (24.2) | 81 (18.6) | 335 (23.1) | 128 (23.7) | 0.201 |
COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; Data are reported as number of patients and percentage in the age group (%).
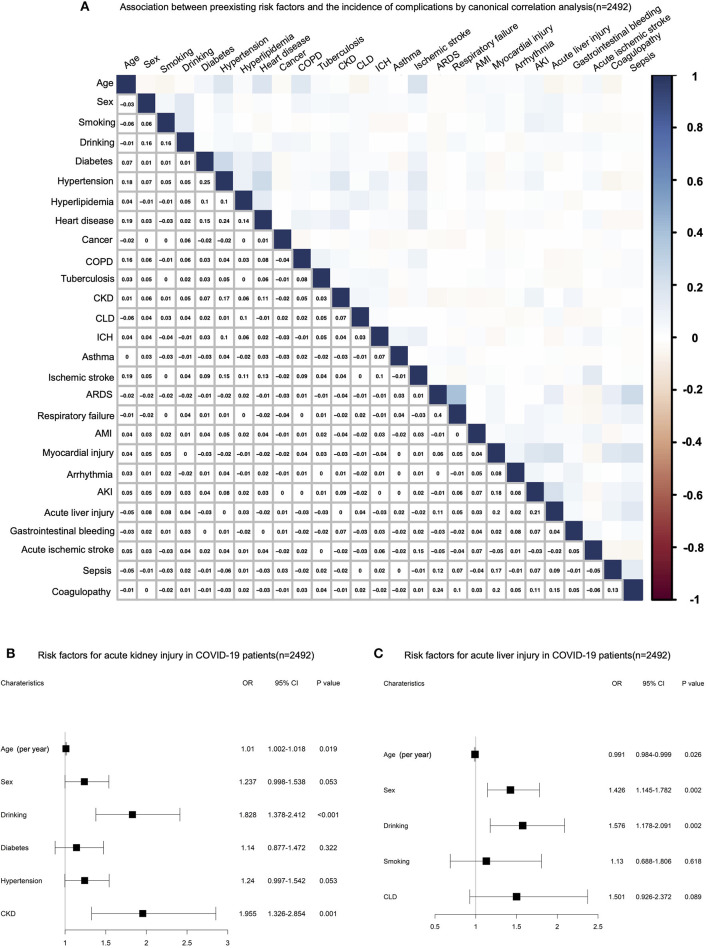
Associations Between Risk Factors and Complications in Deceased Patients With COVID-19
We further investigated the relationship between preexisting risk factors and the incidence of complications. By canonical correlation analysis, we found a relatively poor correlation between pre-existing risk factors and complications. Higher correlation coefficients (R) were found between male gender (0.075) and alcohol consumption (0.077) with acute liver injury, alcohol consumption (0.092), hypertension (0.077), and history of CKD (0.089) with acute renal failure (Figure 3A). Multivariable logistic regression analysis was used to verify the above correlation. After adjusted for age, sex, and comorbidities, we found younger age (OR = 0.991 [95% CI 0.984–0.999], p = 0.026), male gender (OR = 1.426 [95% CI 1.145–1.782], p = 0.002), and alcohol consumption (OR = 1.576 [95% CI 1.178–2.091], p = 0.002) were independent risk factors for acute liver injury (Figure 3B), and greater age (OR = 1.01 [95% CI 1.002–1.018], p = 0.019), alcohol consumption (OR = 1.828 [95% CI 1.378–2.412], p < 0.001), and history of chronic kidney (OR = 1.955 [95% CI 1.326–2.854], p = 0.001) were independent risk factors for acute renal failure (Figure 3C).
Figure 3.
(A) Canonical correlation analysis (CCA) for the relationship between preexisting risk factors and the incidence of complications. (B) Multivariable logistic regression analysis for risk factors of acute kidney injury (AKI) in deceased patients with COVID-19. (C) Multivariable logistic regression analysis for risk factors of acute liver injury in deceased patients with COVID-19.
Discussions
The COVID-19 pandemic has killed millions of people all over the world, and has frequently overwhelmed healthcare systems in the hardest-hit areas (1). This study provides detailed clinical data on a large sample of patients with COVID-19 in Wuhan, China, the original disease epicenter. As with any infectious disease without specific treatments, host factors are the key determinants of disease severity and prognosis for COVID-19. Identification of risk factors would help to optimize patient management and reduce mortality in future pandemics of the respiratory virus (24).
In this study, we found that greater age, male gender, alcohol consumption, smoking, hypertension, diabetes, cancer, CKD, and ICH were each independent risk factors for death following hospitalization for COVID-19. This finding was in accord with previous large sample studies (7, 25). Among these risk factors, age proved to be the most important, in this study, we get an OR of 1.061, but while all other variables are dichotomies, age was treated as a continuous variable, an OR of 1.061 means that for each additional year of age, the risk of death increased by 6.1%. The various age-related changes in the geriatric population include deterioration in lung capacity and muscle atrophy, which propagate to physiological impairments, such as reductions of lung reserve, airway clearance, and the defensive barrier function (6). Age-related immune system remodeling, or immunosenescence, is considered to be the major reason for the increased susceptibility of the elderly to viral infection (26–28). Further, preexisting comorbidities are more common in elderly patients. The disparities in the nature of preexisting comorbidities and pathophysiological changes across age groups indicated that patients in different age groups have different risk factors.
With this in mind, we examined differences in clinical manifestations, risk factors, complications, and clinical outcomes among different age groups. Different mortality rates may indicate the possible existence of different risk factors, and the differences in mortality are the main basis for our group setting. Previous studies suggested that children and teenagers (<18) show differences in clinical features and outcomes from adults with COVID-19 (29). In this study, only 473 patients aged <18 were hospitalized due to COVID-19, and they had a less incidence in almost all the symptoms, and only 4 patients died, children seem to have a lower risk of infection than adults and have a lower mortality rate. Similar to pediatric patients, previous studies have shown that people younger than 40 are less likely to become infected with COVID-19, and those infected have a lower mortality rate (14, 26). However, recent national data showed an increase in new infections among young people, an uptick that could not be fully explained by increased testing in these groups (30, 31). The increased infection rate among the young continues to cause mounting deaths in young people. With fewer comorbidities, the cause of death among young people, who presumably possess a stronger immune system, is perplexing. Our analysis revealed cancer and cerebral hemorrhage as the main comorbidities associated with death among patients with COVID-19 younger than 40 years. Due to the small sample size for the patient with cancer and cerebral hemorrhage, though we have more than 6,000 cases in this age group, the CI 95% was broad, but very high OR still indicated that cancer and cerebral hemorrhage greatly increased the risk of death. In this group of patients, increasing age appeared to have little effect on the risk of death. This suggests that the reduced daily-life physical abilities due to disability or reduced immunity caused by radiation and chemotherapy for tumors may be the major factors imparting a greater risk of death among young patients with COVID-19.
Previous studies have found that the mortality of patients with COVID-19 begins to increase significantly with age after 40 years old (14), and patients aged 40–80 years were the most influenced by preexisting comorbidities. The risk factors for this age group were similar to those for all patients, probably because they comprised the majority of the entire sample of patients with COVID-19. Patients older than 80 years of age have a particularly high mortality rate (26). In this study, we found that the commonly-reported symptoms of COVID-19, such as fever and cough, had a lesser incidence in the groups older than 80, while falls and compromised mental status on admission were more prevalent. Indeed, advanced age can make the diagnosis more complex, as elderly adults with COVID-19 frequently have atypical manifestations, thus calling for caution in the diagnosis of COVID-19 (5). For people aged >80 years, although there was a higher percentage of patients with multiple comorbidities, older age and male gender were the only factors associated with higher odds of death. Notably, frequent comorbidities, such as hypertension and diabetes were not independently associated with mortality in elderly patients over 80 years of age, as noted in previous studies with smaller sample sizes (32, 33). This result indicates that age-related debilitation and decline in immunity may be the major causes of COVID-19 mortality in the elderly.
Multi-system extrapulmonary complications of COVID-19 had been reported (4, 34), but the differences in the incidence of complications in patients of different age groups have been unknown. In this study, we determined the age-dependence of the prevalence of critical complications in patients dying after hospitalization for COVID-19. We found that coagulopathy, AKI, acute myocardial injury, and acute liver injury were the most common complications occurring after COVID-19 infection. The incidence of most complications increased with age, the new finding was that arrhythmias, gastrointestinal bleeding, and sepsis had a higher incidence in younger patients dying with COVID-19. Disparities by age group in the incidence of extrapulmonary complications provide further evidence that COVID-19 is disproportionally affected by age.
Since complications happened during COVID-19 could be extremely life-threatening, studying the association between previous comorbidities of patients with COVID-19 and present complications may help to take informed measures to reduce mortality, but the association have not been reported. In this study, we found a relatively poor correlation between preexisting risk factors and specific complications. Except for age, sex, and alcohol consumption, we found that only a history of a chronic kidney was independent risk factor for acute renal failure. These results might suggest that extrapulmonary complications are likely related to direct organ damage caused by SARS-CoV-2 and its systemic consequences.
While being the largest cohort study of its type, this study had several limitations. First, it was a retrospective study, thus calling for the identification of primary exposure and outcome, as well as confounding factors, based only upon hospital electronic records from administrative datasets, which may vary in accuracy and reliability among the hospitals. Second, we reviewed complications of deceased in-patients with COVID-19 but were not able to collect corresponding data from patients surviving the disease. Therefore, the incidence of extrapulmonary complications and the association between extrapulmonary complications and the risk of death cannot be explained in this study. Third, we did not study the time from infection to death by methods, such as survival analysis, where age might play a role. Our study demonstrated the effect of age on clinical symptoms, complications, and outcomes, but we did not detail differences among the age groups. In addition, we cannot address the possibility that treatment protocols may have differed among the hospitals based on local experience.
In conclusion, we have described the disproportionally effects of age on the clinical manifestations, risk factors, complications, and outcomes of patients with COVID-19. For the first time, we examined risk factors in patients of different age groups with different mortality. Different from patients aged 40–80 years whose risk factors for death were mainly metabolic comorbidities, cancer and intracerebral hemorrhage were first found to be the main independent risk factors in patients aged <40 years. Age and male gender were the main risk factors for death in patients aged >80 years. We described the incidence of the most common complications of COVID-19 in deceased patients in different age groups, and found that arrhythmias, gastrointestinal bleeding, and sepsis had a higher incidence in younger patients dying with COVID-19. While the association between preexisting risk factors and complications have not been reported, and in this study, we reported a poor correlation. This information gives clinicians a better insight into the effect of age on the clinical manifestations, risk factors, and prognosis of COVID-19, which may guide appropriate remedial measures.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
HZ, YW, MW, GL, SX, and WW: concept and design. HZ, YW, YH, XLiu, ML, YT, XLi, and GY: acquisition, analysis, or interpretation of data. HZ, YW, MW, and WW: drafting of the manuscript. ML, YT, XLi, and GY: statistical analysis. SX and WW: obtained funding. YH, XLiu, YT, and GL: administrative, technical, or material support. MW and WW: supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by a COVID-19 Emergency Project from Wuhan Science and Technology Bureau (2020020401010096) and Ministry of Science and Technology of People's Republic of China (2020YFC0841301). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of Interest
ML, YT, XLi, and GY are employed by Winning Health Technology Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Xiaosong Yu, Mingming Hao, Zhiyuan Wen, Jiangwei Guo, Mingzhu Shao, and Yusen Fu for the work in data extraction and preprocessing.
Glossary
Abbreviations
- COVID-19
the coronavirus disease 2019
- ARDS
acute respiratory distress syndrome
- AMI
acute myocardial infarction
- AIS
acute ischemic stroke
- AKI
acute kidney injury
- CKD
chronic kidney disease
- CLD
chronic liver disease.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.757459/full#supplementary-material
References
- 1.Wang C, Wang Z, Wang G, Lau JY, Zhang K, Li W. COVID-19 in early 2021: current status and looking forward. Signal Transduct Target Ther. (2021) 6:114. 10.1038/s41392-021-00527-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. (2020) 17:e1003321. 10.1371/journal.pmed.1003321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman KE, Magder LS, Baghdadi JD, Pineles L, Levine AR, Perencevich EN, et al. Impact of sex and metabolic comorbidities on COVID-19 mortality risk across age groups: 66,646 inpatients across 613 U.S. hospitals. Clin Infect Dis. (2020) 73:e4113–23. 10.1093/cid/ciaa1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan X, Zhang S, Xu J, Zhou M, Huang Q, Duan L, Zet al. Comparison of clinical characteristics among younger and elderly deceased patients with COVID-19: a retrospective study. Aging. (2020) 13:16–26. 10.18632/aging.202139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Belda AB, Fernandez-Garces M, Mateo-Sanchis E, Madrazo M, Carmona M, Piles-Roger L, et al. COVID-19 in older adults: What are the differences with younger patients? Geriatr Gerontol Int. (2021) 21:60–5. 10.1111/ggi.14102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim JP, Low KYH, Lin NJJ, Lim CZQ, Ong SWX, Tan WYT, et al. Predictors for development of critical illness amongst older adults with COVID-19: Beyond age to age-associated factors. Arch Gerontol Geriatr. (2021) 94:104331. 10.1016/j.archger.2020.104331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cates J, Lucero-Obusan C, Dahl RM, Schirmer P, Garg S, Oda G, et al. Risk for in-hospital complications associated with COVID-19 and influenza—Veterans Health Administration, United States, October 1, 2018-May 31, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1528–34. 10.15585/mmwr.mm6942e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamwal S, Gautam A, Elsworth J, Kumar M, Chawla R, Kumar P. An updated insight into the molecular pathogenesis, secondary complications and potential therapeutics of COVID-19 pandemic. Life Sci. (2020) 257:118105. 10.1016/j.lfs.2020.118105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. (2020) 192:E583. 10.1503/cmaj.200685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kordzadeh-Kermani E, Khalili H, Karimzadeh I. Pathogenesis, clinical manifestations and complications of coronavirus disease 2019 (COVID-19). Future Microbiol. (2020) 15:1287–305. 10.2217/fmb-2020-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae S, Kim SR, Kim MN, Shim WJ, Park SM. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart. (2021) 107:373–80. 10.1136/heartjnl-2020-317901 [DOI] [PubMed] [Google Scholar]
- 12.Monpara JD, Sodha SJ, Gupta PK. COVID-19 associated complications and potential therapeutic targets. Eur J Pharmacol. (2020) 886:173548. 10.1016/j.ejphar.2020.173548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiruneh SA, Tesema ZT, Azanaw MM, Angaw DA. The effect of age on the incidence of COVID-19 complications: a systematic review and meta-analysis. Syst Rev. (2021) 10:80. 10.1186/s13643-021-01636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. (2020) 41:145–51. 10.3760/cma.j.issn.0254-6450.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 15.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. (2012) 307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 17.Severe Covid GG, Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, et al. Genomewide association study of severe covid-19 with respiratory failure. N Engl J Med. (2020) 383:1522–34. 10.1056/NEJMoa2020283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siew ED, Ikizler TA, Matheny ME, Shi Y, Schildcrout JS, Danciu I., et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. (2012) 7:712–9. 10.2215/CJN.10821011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D. Si, B. Du,, L. Ni, B. Yang, H. Sun, N. Jiang, et al. Death, discharge and arrhythmias among patients with COVID-19 and cardiac injury. CMAJ 192 (2020) E791-E798. 10.1503/cmaj.200879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Si D, Du B, Ni L, Yang B, Sun H, Jiang N, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mesnier J, Cottin Y, Coste P, Ferrari E, Schiele F, Lemesle G, et al. Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: a registry study. Lancet Public Health. (2020) 5:e536–42. 10.1016/S2468-2667(20)30188-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. (2020) 77:1–7. 10.1001/jamaneurol.2020.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giammaria D, Pajewski A. Can early treatment of patients with risk factors contribute to managing the COVID-19 pandemic? J Glob Health. (2020) 10:010377. 10.7189/jogh.10.010377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman KE, Magder LS, Baghdadi JD, Pineles L, Levine AR, Perencevich EN, et al. Impact of sex and metabolic comorbidities on COVID-19 mortality risk across age groups: 66,646 inpatients across 613 U.S. hospitals. Clin Infect Dis. (2020) 73:e4113–23. 10.1093/cid/ciaa1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. (2021) 65:101205. 10.1016/j.arr.2020.101205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Driscoll M, Ribeiro Dos Santos G, Wang L, Cummings DAT, Azman AS, Paireau J, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. (2021) 590:140–5. 10.1038/s41586-020-2918-0 [DOI] [PubMed] [Google Scholar]
- 28.Channappanavar R, Perlman S. Age-related susceptibility to coronavirus infections: role of impaired and dysregulated host immunity. J Clin Invest. (2020) 130:6204–13. 10.1172/JCI144115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. (2020) 4:653–61. 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finelli L, Gupta V, Petigara T, Yu K, Bauer KA, Puzniak LA. Mortality Among US Patients Hospitalized With SARS-CoV-2 Infection in 2020. JAMA Netw Open. (2021) 4:e216556. 10.1001/jamanetworkopen.2021.6556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manivannan M, Jogalekar MP, Kavitha MS, Maran BAV, Gangadaran P. A mini-review on the effects of COVID-19 on younger individuals. Exp Biol Med. (2021) 246:293–7. 10.1177/1535370220975118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Covino M, De Matteis G, Polla DAD, Santoro M, Burzo ML, Torelli E, et al. Predictors of in-hospital mortality AND death RISK STRATIFICATION among COVID-19 PATIENTS aged >/= 80 years old. Arch Gerontol Geriatr. (2021) 95:104383. 10.1016/j.archger.2021.104383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Covino M, De Matteis G, Santoro M, Sabia L, Simeoni B, Candelli M, et al. Clinical characteristics and prognostic factors in COVID-19 patients aged >/=80 years. Geriatr Gerontol Int. (2020) 20:704–8. 10.1111/ggi.13960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murk W, Gierada M, Fralick M, Weckstein A, Klesh R, Rassen JA. Diagnosis-wide analysis of COVID-19 complications: an exposure-crossover study. CMAJ. (2021) 193:E10–8. 10.1503/cmaj.201686 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study were available from the corresponding author upon reasonable request.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.