Abstract
The ability of MALDI-TOF for the identification of nontuberculous mycobacteria (NTM) has improved recently thanks to updated databases and optimized protein extraction procedures. Few multicentre studies on the reproducibility of MALDI-TOF have been performed so far, none on mycobacteria. The aim of this study was to evaluate the reproducibility of MALDI-TOF for the identification of NTM in 15 laboratories in 9 European countries. A total of 98 NTM clinical isolates were grown on Löwenstein-Jensen. Biomass was collected in tubes with water and ethanol, anonymized and sent out to the 15 participating laboratories. Isolates were identified using MALDI Biotyper (Bruker Daltonics). Up to 1330 MALDI-TOF identifications were collected in the study. A score ≥ 1.6 was obtained for 100% of isolates in 5 laboratories (68.2–98.6% in the other). Species-level identification provided by MALDI-TOF was 100% correct in 8 centres and 100% correct to complex-level in 12 laboratories. In most cases, the misidentifications obtained were associated with closely related species. The variability observed for a few isolates could be due to variations in the protein extraction procedure or to MALDI-TOF system status in each centre. In conclusion, MALDI-TOF showed to be a highly reproducible method and suitable for its implementation for NTM identification.
Subject terms: Clinical microbiology, Infectious-disease diagnostics
Introduction
All species from the Mycobacterium genus except for Mycobacterium tuberculosis complex and Mycobacterium leprae are commonly known as non-tuberculous mycobacteria (NTM)1. This is an ever-growing group of bacteria (https://lpsn.dsmz.de/genus/mycobacterium) widely distributed in the environment2. The recommendation of the American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA) and the British Thoracic Society is to provide species-level identification of NTM isolates from clinical origin in order to elucidate their clinical significance3,4.
In recent years, the identification of NTM in clinical laboratories has improved by the application of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). On the one hand, improved commercial databases have increased the number of species that can be reliably identified by this system5,6. Moreover, in MALDI Biotyper system (Bruker Daltonics) the log(score) values ≥ 1.8 and ≥ 1.6 have been accepted for high-confidence and low-confidence identification of mycobacteria isolates, respectively, in order to increase their identification rate without loss of diagnostic accuracy5. Furthermore, several protein extraction procedures have been tested in order to efficiently break the cell wall of mycobacteria and extract their proteins7–10. This has been achieved by the use of chemical reagents (acetonitrile and formic acid) and mechanical disruption applying silica beads and vortexing, bead beating or, in some cases, by sonication11–13. Thanks to the previous procedures, now it is possible to obtain a rapid and reliable identification of NTM by MALDI-TOF MS.
There are few studies that have addressed the inter-laboratory reproducibility of MALDI-TOF MS so far, none on mycobacteria. Mainly they have focused on other bacterial species such as Staphylococcus aureus14, Escherichia coli15, nonfermenting bacteria16, anaerobic bacteria17 and mold18. They concluded that MALDI-TOF MS is a highly reproducible method between different laboratories, and attribute minor errors to the instruments or databases used.
The aim of this study was to evaluate the reproducibility of MALDI-TOF MS and performance of sonication procedure for identification of NTM in different laboratories from 9 European Economic Area (EEA) countries.
Results
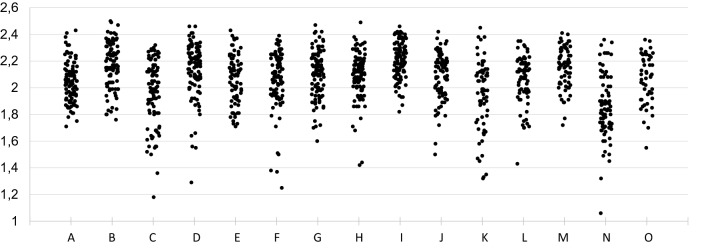
A total of nine participating centres analysed all 98 mycobacterial isolates. In the other 6 laboratories, a lower number of isolates was analysed due to loss of samples during transport or during processing (Table 1). This resulted in a total of 1330 MALDI-TOF MS identifications performed. Globally, a log(score) ≥ 1.60 was obtained for 100% of the isolates in 5 laboratories, and between 68.2 and 98.6% of the cases in the other 10 centres (Table 1). The identification obtained by MALDI-TOF MS was 100% correct to complex-level in 12 laboratories (97.4–99.0% in the other 3 centres). Regarding species-level accuracy, identification was 100% correct in 8 participating centres (96.1–99.0% in the other 7 laboratories). Twelve (80.0%) centres reached a mean log(score) ≥ 2.0 in their analysis. Considering all 1330 identifications, 1243 (93.5%) obtained log(score) above 1.60. From these isolates with an accurate identification, 1239 (99.7%) were correct at complex-level and 1232 (99.1%) at species-level. Among the nine centres with all isolates analysed, Fleiss Kappa’s index (k = 0.917, p < 0.0001, confidence interval [CI] 95% 0.908–0.927) showed a highly accurate agreement between participants. Distribution of isolates by centres and according to the MALDI-TOF MS score is shown in Fig. 1.
Table 1.
Identification results for all participating centres.
| Centre | Isolates analysed | Log(score) ≥ 1.60 (%) | Complex-level correct (%) | Species-level correct (%) | Mean log(score) |
|---|---|---|---|---|---|
| A | 98 | 100 | 100 | 100 | 2.04 |
| B | 98 | 100 | 100 | 100 | 2.17 |
| C | 98 | 87.8 | 100 | 100 | 1.96 |
| Da | 98 | 96.9 | 100 | 98.9 | 2.14 |
| E | 98 | 78.6 | 97.4 | 96.1 | 2.06 |
| F | 98 | 94.9 | 100 | 98.9 | 2.07 |
| G | 98 | 100 | 99 | 99 | 2.10 |
| H | 98 | 98 | 100 | 100 | 2.09 |
| I | 98 | 100 | 100 | 100 | 2.21 |
| J | 86 | 93 | 100 | 98.7 | 2.09 |
| K | 85 | 68.2 | 98.3 | 96.5 | 1.95 |
| L | 73 | 98.6 | 100 | 97.2 | 2.06 |
| M | 73 | 100 | 100 | 100 | 2.16 |
| N | 81 | 88.9 | 100 | 100 | 1.86 |
| O | 50 | 98 | 100 | 100 | 2.06 |
| Total | 1330 | 93.5 | 99.7 | 99.1 | 2.07 |
aMycoEX extraction procedure performed.
Figure 1.
Distribution of isolates by centres and MALDI-TOF MS log(score).
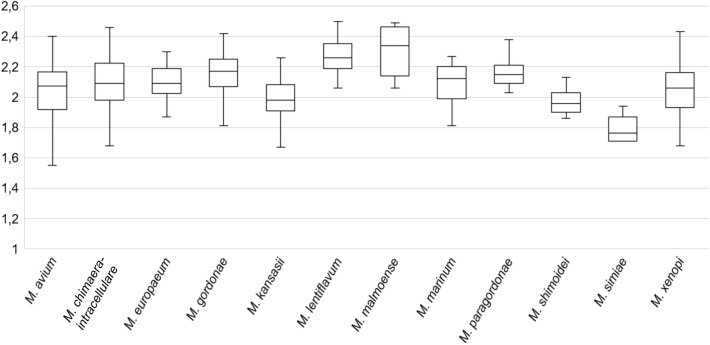
Among slow growing mycobacteria species, Mycobacterium simiae obtained the lowest log(score): mean of 1.76 (range 1.29–1.94), as observed in Fig. 2. The remaining species of this group obtained their identification with log(score) close to 2.0, with Mycobacterium lentiflavum (mean 2.25; range 1.87–2.50) and Mycobacterium malmoense (mean 2.27; range 1.47–2.49) yielding the highest log(score). Among isolates with log(score) < 1.60 or no peaks (87/1330; 6.5%), 68.1% were slow growing species.
Figure 2.
Log(score) obtained in all centres for slow growing species.
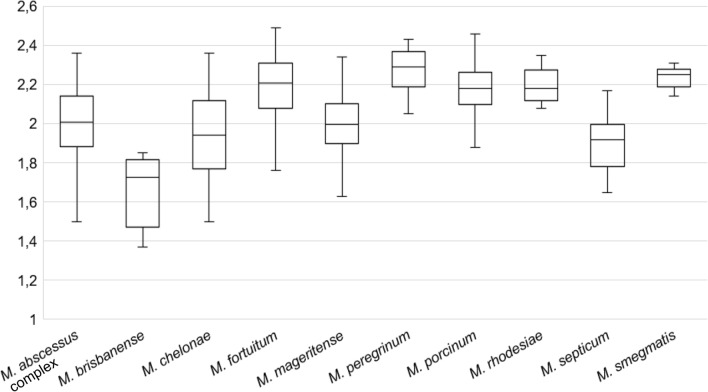
Regarding rapid growing species, Mycobacterium brisbanense obtained log(score) lower than 1.80 (mean 1.66; range 1.37–1.85) in most of participating centres, as observed in Fig. 3. The species with higher log(score) were those belonging to Mycobacterium fortuitum complex (M. fortuitum, Mycobacterium peregrinum and Mycobacterium porcinum; mean 2.17; range 1.36–2.49), Mycobacterium rhodesiae (mean 2.19; range 2.08–2.35) and Mycobacterium smegmatis (mean 2.22; range 2.03–2.31).
Figure 3.
Log(score) obtained in all centres for rapid growing species.
According to their Kappa indexes, 21 out of the 22 species analysed obtained a high level of accuracy among laboratories, with a total concordance for M. lentiflavum, M. malmoense, Mycobacterium shimoidei, Mycobacterium mageritense, M. peregrinum, M. rhodesiae and M. smegmatis (Table 2). The species with lowest Kappa value was M. simiae (K = 0.748). Globally, 13 isolates obtained a misidentification in at least one centre. Among these, eight isolates were misidentified in all three replicated spots, and five obtained a misidentification in one of the three replicated spots (Table 3). Nine of the 13 misidentifications were related with rapid-growing mycobacteria: these involved close related species from Mycobacterium chelonae-abscessus complex and M. fortuitum complex. The other four strains were slow-growing mycobacteria, specifically from Mycobacterium avium complex and the species Mycobacterium gordonae, Mycobacterium kansasii and Mycobacterium europaeum. Regarding log(score) cut-offs, eight strains involved MALDI-TOF MS log(score) in high confidence category (score ≥ 1.80), and two of them above 2.00.
Table 2.
Mycobacterial species included in the study and Kappa index obtained.
| Slow growing species | Rapid growing species | ||||
|---|---|---|---|---|---|
| Species | N | Kappa index | Species | N | Kappa index |
| M. avium | 8 | 0.970 | M. abscessus complex | 13 | 0.968 |
| M. chimaera-intracellulare | 11 | 0.877 | M. brisbanense | 1 | 0.874 |
| M. europaeum | 3 | 0.872 | M. chelonae | 4 | 0.906 |
| M. gordonae | 12 | 0.942 | M. fortuitum | 11 | 0.977 |
| M. kansasii | 7 | 0.983 | M. mageritense | 3 | 1.000 |
| M. lentiflavum | 6 | 1.000 | M. peregrinum | 2 | 1.000 |
| M. malmoense | 1 | 1.000 | M. porcinum | 4 | 0.970 |
| M. marinum | 1 | 0.874 | M. rhodesiae | 1 | 1.000 |
| M. paragordonae | 2 | 0.940 | M. septicum | 1 | 0.874 |
| M. shimoidei | 2 | 1.000 | M. smegmatis | 1 | 1.000 |
| M. simiae | 1 | 0.748 | |||
| M. xenopi | 3 | 0.960 | |||
Table 3.
Isolates that obtained some misidentification.
| Strain number | Species | Centres with misidentification reported | Species identified | Number of replicated spots misidentified | Log(score) |
|---|---|---|---|---|---|
| 4 | M. chelonae | 1 | M. salmoniphilum | 3/3 | 1.85–2.08 |
| 5 | M. paragordonae | 1 | M. gordonae | 3/3 | 1.94–2.09 |
| 6 | M. abscessus complex | 1 | M. fortuitum | 3/3 | 1.76–1.80 |
| 32 | M. chimaera-intracellulare | 1 | M. marseillense | 1/3 | 1.84 |
| 42 | M. chelonae | 2 | M. immunogenum | 3/3 | 1.67–1.82 |
| 1 | M. salmoniphilum | 1/3 | 1.96 | ||
| 45 | M. abscessus complex | 1 | M. kansasii | 3/3 | 1.44–1.60 |
| 56 | M. abscessus complex | 1 | M. fortuitum | 1/3 | 1.91 |
| 59 | M. abscessus complex | 1 | M. immunogenum | 3/3 | 1.45–1.66 |
| 1 | Mixed species | 3/3 | |||
| 64 | M. septicum | 1 | M. peregrinum | 3/3 | 1.62–1.70 |
| 1 | M. neworleansense | 1/3 | 1.71 | ||
| 67 | M. kansasii | 2 | M. gastri | 1/3 | 1.75–1.95 |
| 74 | M. europaeum | 1 | M. avium | 3/3 | 1.66–1.71 |
| 94 | M. porcinum | 1 | M. peregrinum | 1/3 | 1.80 |
| 99 | M. porcinum | 1 | M. peregrinum | 1/3 | 1.88 |
| 1 | M. neworleansense | 1/3 | 1.97 |
Discussion
Although MALDI-TOF MS has become a reference method for the identification of mycobacteria species in clinical microbiology laboratories, few studies have evaluated its inter-laboratory reproducibility so far and none of them has focussed on mycobacteria. This study collected identification data from 15 different laboratories that blindly analysed up to 98 mycobacterial strains, resulting in a total of 1330 MALDI-TOF MS identifications. As a result, more than 99% of isolates that obtained a satisfactory log(score) ≥ 1.60 were accurately identified at the species level. Besides, most of the isolates analysed in each participating centre reached a log(score) higher than 1.60 by MALDI-TOF MS. In fact, up to five of the participating laboratories obtained a log(score) above 1.60 in all isolates they analysed. The achievement of suitable MALDI-TOF MS log(score) in mycobacteria has improved over the last years thanks to optimized protein extraction protocols11 and the updating of the commercial databases, providing reliable identification that can be trusted by clinicians.
In this study, the sonication procedure was evaluated in 14 centres, leaving only one laboratory—proficient in the use of MALDI-TOF MS for the identification of mycobacteria isolates—that applied MycoEX protocol. The identification provided by this laboratory showed no important differences in comparison with other participants, who applied the sonication method (Table 1). Moreover, the model and the technical specifications of the mass spectrometer for spectra acquisition were common in all participating laboratories. In addition, all centres obtained mycobacterial isolates from the same culture, without need of growing them in each laboratory. All of this led to a few possible variables that could have interfered in the results obtained between different centres: (1) transportation and storage time of samples; (2) the technical experience of the personnel performing the protein extraction protocol19, although all participants had previous experience with MALDI-TOF MS; (3) the calibration and maintenance status of mass spectrometer in each participating laboratory.
Regarding differences between mycobacterial species, the score values observed could have been greatly influenced by the number of isolates included for each species and their representation in database. Thus, species with lower log(score) were M. simiae, M. brisbanense and Mycobacterium septicum, for which one isolate was included (Table 2). Although M. simiae and M. septicum are well represented in MALDI-TOF MS database, it is possible that the isolates included in this study had different intrinsic characteristics than those in the database. In the case of M. brisbanense, only two spectra are included in current database. Conversely, species with higher log(score) and identification rate were M. malmoense (n = 1) and M. lentiflavum (n = 6) for slow-growing mycobacteria and M. fortuitum complex (n = 11), M. rhodesiae (n = 1) and M. smegmatis (n = 1) for rapid-growing. With the exception of M. rhodesiae, all previous species are widely represented in database used.
Mycobacterial misidentifications by MALDI-TOF MS have been reported in literature, even with high log(score)20. Fortunately, this is not a common phenomenon. Misidentifications usually arise due to some species being very closely related or pertaining to the same mycobacterial complex. In this study, three replicates were used for analysis of each isolate. Although more than 99% of isolates have been correctly identified at species-level, a total of 13 different isolates obtained at least one misidentification in some replicates by one of the participating centres (Table 3). Some of the misidentifications reported involved only one of these three replicates, so they could probably have been caused by technical issues during spectra acquisition in this spot. When three replicates are used, same identification in two of them may be considered as reliable identification. According to this statement, only 10 (0.008%) isolates from 1243 analyses were definitely misidentified (Table 3).
Most of the misidentifications obtained were reported as other species of the same group, except strain number 56 (M. abscessus complex), which was identified as M. fortuitum in one spot (score = 1.91). Regarding misidentifications involving all three replicates, most of them were from rapid-growing species, such as M. chelonae and M. abscessus. Although these two species were not confused, some laboratories identified one of these isolates as Mycobacterium salmoniphilum and Mycobacterium immunogenum, two species closely related to M. chelonae21,22. The most controversial misidentifications were the following: (1) one centre that identified a M. abscessus isolate as M. kansasii, even though it obtained a low log(score) (1.60); (2) a M. abscessus with mixed species identified in three replicates, maybe due to contamination of tubes or MALDI plate spots during preparation of sample; and (3) one M. europaeum identified as M. avium in one centre. For the lattermost misidentification, it could not be definitively determined if it was a MALDI-TOF MS misidentification or due to contamination. In all cases, sample interchange was ruled out.
In summary, MALDI-TOF MS demonstrated an extraordinary inter-laboratory reliability for the identification of NTMs, reaching a high concordance among identifications obtained in participating centres. Besides, sonication of the bacterial pellet has shown to be a good alternative to the MycoEX procedure20. Misidentifications were scarce and they were considered as minor errors since the identifications provided by the MALDI-TOF MS corresponded to species from the same complex. Although little improvements can still be made in databases, MALDI-TOF MS showed to be a highly reproducible technique, and therefore, it could be globally implemented for rapid, accurate and first line identification method for mycobacteria in clinical microbiology laboratories.
Methods
Mycobacterial strains and participating centres
A total of 98 NTM clinical isolates were analysed in 15 laboratories from 9 European Economic Area (EEA) countries (Austria, Belgium, Croatia, France, Germany, Ireland, Norway, Spain and United Kingdom). The strains were recovered from frozen stocks and grown on Löwenstein-Jensen (Becton Dickinson, Franklin Lakes, NJ) in the Department of Clinical Microbiology and Infectious Diseases of the Hospital General Universitario Gregorio Marañón (Madrid, Spain). They encompassed 22 different mycobacterial species (Table 2), previously identified by sequencing of 16S rRNA and/or hsp65 genes. A 10 µl loop was used to collect biomass from colonies in tubes with 300 µl water and 900 µl ethanol, sent out to the participating laboratories and blindly analysed.
MALDI-TOF MS protein extraction protocol
In 14 centres, the protein extraction procedure was performed by sonication as previously described11,23 and the remaining laboratory applied Myco-EX protocol recommended by the manufacturer. Upon arrival in the laboratory, the tubes with the samples were centrifuged 5 min at 14,000 rpm and the pellet was resuspended in 300 µl of high-performance liquid chromatography (HPLC) or sterile Milli-Q water, after which they were heat inactivated at 95 °C for 30 min. After the addition of 900 µl of ethanol, the tubes were centrifuged for 2 min at 14,000 rpm and the supernatant was discarded. The pellet was dried for a few minutes at room temperature. Then, a spatula tip with 0.5 mm diameter glass or zirconia/silica beads (BioSpec products, USA) and 50 µl of acetonitrile were added. The tubes were vortexed for 10 s. In 14 laboratories, an additional step of 15 min sonication was performed. After this, 50 µl of formic acid were added and the tubes were vortexed again for 10 s. Finally, the samples were centrifuged for 2 min at 14,000 rpm and 1 µl of the supernatant was deposited by triplicate onto the MALDI plate (Bruker Daltonics, Bremen, Germany). After drying, 1 µl of HCCA (α-cyano-4-hydroxycinnamic acid) matrix was added to the spots and dried at room temperature.
MALDI-TOF MS analysis
In all participating centres, the MALDI-TOF system used was a Biotyper microflex LT (Bruker Daltonics). The software used was FlexControl v3.0 with the Mycobacteria Library v4.0. The spectra were obtained in the positive linear mode, over a mass/charge (m/z) ratio of 2000–20,000 Da and the accelerating voltage was 20 kV. The samples were measured in automatic mode using a nitrogen laser at 40 shots per second, with a total of 240 laser shots collected per spot. The log(score) cut-offs used were those recommended by the manufacturer: < 1.60 as not reliable identification, 1.60–1.79 as low confidence identification and a log(score) ≥ 1.80 as high confidence identification.
Statistical analysis
Fleiss Kappa index was applied to measure the concordance of the results from different centres regarding the reference method (low < 0.40; moderate 0.41–0.60; accurate 0.61–0.80; very accurate > 0.81). In order to evaluate the same number of isolates, only those centres with all isolates analysed were selected for statistics using SPSS 28.0 software (IBM, NY, USA).
Acknowledgements
All the authors have participated in the implementation of MALDI-TOF MS for mycobacterial identification. G. Greub and B. Rodríguez-Sánchez are members of the European Study Group for Genomic and Molecular Diagnosis (ESGMD) Executive Committee.
Author contributions
D.R.T.: experimentation, formal analysis, data collection, validation, visualization, writing (original draft preparation and review/editing). F.A., I.M., J.A.O.C., R.G., J.V.I., A.V.D.B., G.H.A., C.B., D.O.H., J.J.P., G.T., G.B., P.J.C., M.G., J.G.M., G.G., J.H., A.I., M.C.M.G., M.O., B.P., A.B.P., M.J.R.S.: analysis of isolates, writing and review/editing. L.Q.: experimentation, submission of isolates, data collection. B.R.S.: conceptualization, project administration, formal analysis, supervision, validation, visualization, original draft preparation and review/editing.
Funding
This study was supported by the ESCMID Grant 2018 and the projects PI15/01073, PI18/00997 and PI18/01068 from the Health Research Fund (Instituto de Salud Carlos III. Plan Nacional de I + D + I 2013–2016) of the Carlos III Health Institute (ISCIII, Madrid, Spain) partially financed by the European Regional Development Fund (FEDER) ‘A way of making Europe’. David Rodríguez-Temporal was funded by the Intramural Program of the Gregorio Marañón Health Research Institute. Belén Rodríguez-Sánchez is a recipient of a Miguel Servet contract (CPII19/00002) supported by the Health Research Fund.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
David Rodriguez-Temporal, Email: david.rodriguez@iisgm.com.
Belén Rodríguez-Sánchez, Email: mbelen.rodriguez@iisgm.com.
References
- 1.Porvaznik I, Solovic I, Mokry J. Non-tuberculous mycobacteria: Classification, diagnostics, and therapy. Adv. Exp. Med. Biol. 2017;944:19–25. doi: 10.1007/5584_2016_45. [DOI] [PubMed] [Google Scholar]
- 2.van Ingen J. Microbiological diagnosis of nontuberculous mycobacterial pulmonary disease. Clin. Chest Med. 2015;36:43–54. doi: 10.1016/j.ccm.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Griffith DE, et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 4.Haworth CS, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD) Thorax. 2017;72:ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Sanchez B, et al. Evaluation of MALDI biotyper mycobacteria library v3.0 for identification of nontuberculous mycobacteria. J. Clin. Microbiol. 2016;54:1144–1147. doi: 10.1128/JCM.02760-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Temporal D, Perez-Risco D, Struzka EA, Mas M, Alcaide F. Impact of updating the MALDI-TOF MS database on the identification of nontuberculous mycobacteria. J. Mass Spectrom. 2017;52:597–602. doi: 10.1002/jms.3944. [DOI] [PubMed] [Google Scholar]
- 7.Tudo G, et al. Implementation of MALDI-TOF MS technology for the identification of clinical isolates of Mycobacterium spp. in mycobacterial diagnosis. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:1527–1532. doi: 10.1007/s10096-015-2381-2. [DOI] [PubMed] [Google Scholar]
- 8.Lotz A, et al. Rapid identification of mycobacterial whole cells in solid and liquid culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2010;48:4481–4486. doi: 10.1128/JCM.01397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saleeb PG, Drake SK, Murray PR, Zelazny AM. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2011;49:1790–1794. doi: 10.1128/JCM.02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Temporal D, Perez-Risco D, Struzka EA, Mas M, Alcaide F. Evaluation of two protein extraction protocols based on freezing and mechanical disruption for identifying nontuberculous mycobacteria by matrix-assisted laser desorption ionization-time of flight mass spectrometry from liquid and solid cultures. J. Clin. Microbiol. 2018 doi: 10.1128/JCM.01548-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alcaide F, et al. How to: identify non-tuberculous Mycobacterium species using MALDI-TOF mass spectrometry. Clin. Microbiol. Infect. 2018;24:599–603. doi: 10.1016/j.cmi.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Marekovic I, et al. Evaluation of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry in identification of nontuberculous mycobacteria. Chemotherapy. 2016;61:167–170. doi: 10.1159/000442517. [DOI] [PubMed] [Google Scholar]
- 13.Ceyssens PJ, et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for combined species identification and drug sensitivity testing in mycobacteria. J. Clin. Microbiol. 2017;55:624–634. doi: 10.1128/JCM.02089-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker J, Fox A, Edward-Jones V, Gordon D. Intact cell mass spectrometry (ICMS) used to type methicillin-resistant Staphylococcus aureus: Media effects and inter-laboratory reproducibility. J. Microbiol. Methods. 2002;48:117–126. doi: 10.1016/s0167-7012(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 15.Wunschel SC, et al. Bacterial analysis by MALDI-TOF mass spectrometry: An inter-laboratory comparison. J. Am. Soc. Mass Spectrom. 2005;16:456–462. doi: 10.1016/j.jasms.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Mellmann A, et al. High interlaboratory reproducibility of matrix-assisted laser desorption ionization-time of flight mass spectrometry-based species identification of nonfermenting bacteria. J. Clin. Microbiol. 2009;47:3732–3734. doi: 10.1128/JCM.00921-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veloo ACM, et al. A multi-center ring trial for the identification of anaerobic bacteria using MALDI-TOF MS. Anaerobe. 2017;48:94–97. doi: 10.1016/j.anaerobe.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Lau AF, et al. Multicenter study demonstrates standardization requirements for mold identification by MALDI-TOF MS. Front. Microbiol. 2019;10:2098. doi: 10.3389/fmicb.2019.02098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veloo AC, Elgersma PE, Friedrich AW, Nagy E, van Winkelhoff AJ. The influence of incubation time, sample preparation and exposure to oxygen on the quality of the MALDI-TOF MS spectrum of anaerobic bacteria. Clin. Microbiol. Infect. 2014;20:O1091–1097. doi: 10.1111/1469-0691.12644. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Temporal D, Rodriguez-Sanchez B, Alcaide F. Evaluation of MALDI biotyper interpretation criteria for accurate identification of nontuberculous mycobacteria. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.01103-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RW, et al. Mycobacterium immunogenum sp. nov., a novel species related to Mycobacterium abscessus and associated with clinical disease, pseudo-outbreaks and contaminated metalworking fluids: an international cooperative study on mycobacterial taxonomy. Int. J. Syst. Evol. Microbiol. 2001;51:1751–1764. doi: 10.1099/00207713-51-5-1751. [DOI] [PubMed] [Google Scholar]
- 22.Whipps CM, Butler WR, Pourahmad F, Watral VG, Kent ML. Molecular systematics support the revival of Mycobacterium salmoniphilum (ex Ross 1960) sp. nov., nom. rev., a species closely related to Mycobacterium chelonae. Int. J. Syst. Evol. Microbiol. 2007;57:2525–2531. doi: 10.1099/ijs.0.64841-0. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor JA, et al. Improved matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS)-based identification of Mycobacterium spp. by use of a novel two-step cell disruption preparatory technique. J. Clin. Microbiol. 2016;54:495–496. doi: 10.1128/JCM.02998-15. [DOI] [PMC free article] [PubMed] [Google Scholar]