Abstract
Cardiac metastases from head and neck cancers are sometimes found at autopsy, but are rarely found before death; therefore, case reports are uncommon. In this report, we describe a case of cardiac metastasis from head and neck cancer. Although asymptomatic at the time of detection, positron emission tomography–computed tomography was effective in ascertaining the diagnosis. However, patients with cardiac metastases usually have a poor prognosis, and unfortunately, the patient died shortly after detection. At autopsy, the patient had a “hyperdense armored heart” owing to a huge pericardial metastases. Here, we report the imaging and autopsy findings of a hyperdense armored heart owing to cardiac metastases from head and neck cancer.
Keywords: Head and neck cancer, Cardiac neoplasm, Metastasis, Cardiac tamponade
Introduction
It is very rare that cardiac metastases from head and neck cancer are found during treatment. Because of the silent nature of cardiac metastases, the diagnosis is often delayed. We present a unique case in which a squamous cell carcinoma (SCC) of the gingiva metastasized to the heart and presented with hyperdense armored heart (HAH), which is usually reported on post-mortem imaging computed tomography (PMCT) or autopsy [1].
Case report
A 59 year-old male was referred to our department with the chief complaint of trismus and awareness of an oral mass. A 50 mm diameter mass extended from the left hard palate to the upper gingiva. The histological diagnosis of the biopsy specimen was SCC. Computed tomography (CT), magnetic resonance imaging (MRI), and fluorodeoxyglucose positron emission tomography–computed tomography (FDG PET–CT) were performed and detected the tumor with involvement of the maxilla, hard palate, soft palate, maxillary sinus, temporal muscle, medial pterygoid muscle, lateral pterygoid muscle, and pterygoid process. Multiple lymph node metastases were also identified in the left level Ib and II, and right level II regions. The cancer was staged as carcinoma of the upper gingiva, T4bN2cM0, according to the American Joint Committee on Cancer (AJCC) cancer staging guidelines, 7th edition. Definitive chemoradiation therapy combined with cisplatin was performed. The primary lesion and cervical lymph node metastases both decreased in size, and the patient was followed up.
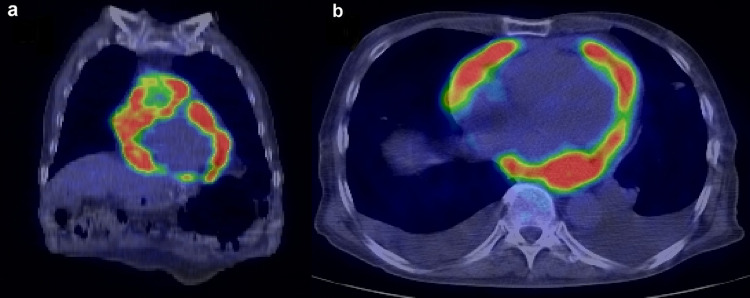
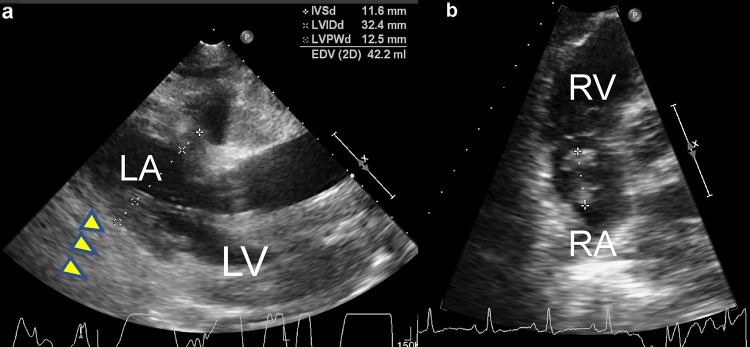
Three months after completing the treatment, follow-up PET–CT was performed. FDG accumulation was minimal in both the primary lesion and cervical lymph node metastases; however, there was marked FDG uptake in the heart, with a standard uptake value (SUV) of 17.0 (Fig. 1). Electrocardiography (ECG) showed atrial fibrillation. Echocardiography demonstrated that the entire pericardium was replaced by a solid mass lesion, and an intracardiac tumor was observed in the right atrium (Fig. 2). These masses were highly suspicious for metastatic disease. At this point, the patient had no subjective symptoms. After 2 weeks, he was hospitalized owing to the appearance of malaise, and he died of rapid deterioration of his general condition 3 weeks later. At autopsy, huge pericardial metastatic tumors covered the entire circumference of the heart, so-called hyperdense armored heart (HAH) (Fig. 3). The heart weighed 1800 g and showed marked congestion, and hemorrhagic infarction was seen in the right lung. A 5 mm diameter left adrenal grand metastasis was found incidentally. Histology of the cardiac tumor and the adrenal gland tumor revealed SCC. Because of the HAH, circulatory failure owing to a highly constrictive pericarditis-like condition was considered the direct cause of death.
Fig. 1.
Positron emission tomography–computed tomography findings. A hypermetabolic lesion is seen in the pericardium. a Coronal plane. b Axial plane
Fig. 2.
Findings during transthoracic echocardiography. a Parasternal long-axis view showing left ventricular diastolic dysfunction because of a thickened pericardial solid mass (yellow arrowheads). b Apical four-chamber view showing a thrombus or tumor in the right atrium. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
Fig. 3.
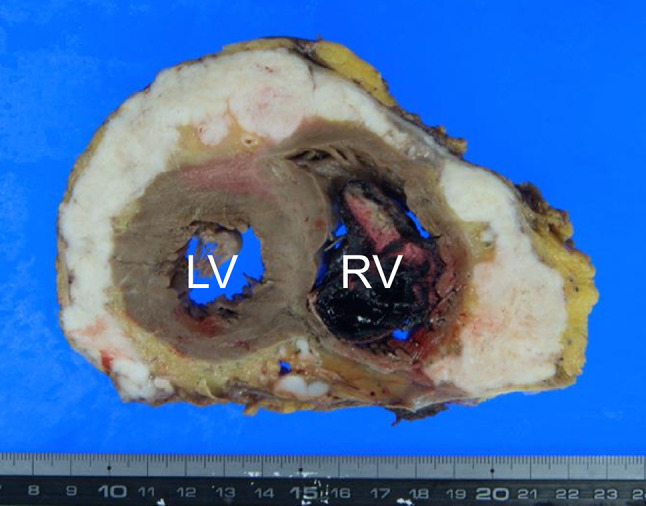
Macroscopic autopsy findings. Huge pericardial metastatic tumors covered the entire circumference of the heart. A thrombus can be seen in the right ventricle. RV right ventricle, LV left ventricle
Discussion
Cardiac metastases are rare. The most common tumors that metastasize to the heart are cancers of the lung, breast, esophagus, malignant lymphoma, leukemia, and melanoma [2]. Additionally, the most common sites of metastases are the pericardium, myocardium, and endocardium, in that order [3]. Cardiac metastases from head and neck SCC are often found at autopsy. Of these, oral cancer accounts for the largest percentage, with 17/23 (73.9%) reported cases [4]. Another autopsy study demonstrated that oral cavity cancer invaded the heart in 4 of 75 cases (5.3%); 22 of 79 (27.8%) for melanoma and 97 of 460 (21.0%) for lung adenocarcinoma, with an overall incidence of 662 of 7289 (9.1%) cases [5]. Considering these autopsy studies, cardiac metastases from oral SCC are likely more common than we have assumed.
Generally, there are no significant clinical findings with cardiac metastases unless more extensive systemic disease is present [6]. In symptomatic cases, heart failure and arrhythmia can have a significant impact on the prognosis; therefore, it is important to establish a definitive diagnosis when patients are asymptomatic.
Imaging for cardiac tumors constitutes echocardiography, CT, MRI, and PET–CT. In this case, we performed echocardiography and PET–CT. Echocardiography showed that the entire pericardial sac was replaced by solid material, suggesting pericardial metastases. PET–CT is now used routinely to detect distant metastases in most head and neck cancers. Rahbar et al. reported that with a cut-off maximum SUV (SUVmax) of 3.5, PET–CT could be used to noninvasively determine tumor malignancies, with a sensitivity of 100% and a specificity of 86% [7]. In our case, SUVmax was high, and with the echocardiography findings and the patient’s clinical course, we concluded that the patient had cardiac metastases.
Once diagnosed, treatment of cardiac metastases is palliative; there is currently no effective treatment. Similarly, in this case, because the patient’s general condition deteriorated rapidly, it was judged that chemotherapy was not indicated, and that palliative care had to be selected. Recently, case reports describing immune checkpoint inhibitor use for cardiac metastasis of head and neck SCC have been reported, but all of the patients had a poor prognosis [8, 9].
In HAH, the epicardium is replaced and surrounded by metastatic tumors and becomes sclerotic, resulting in diastolic dysfunction. Cardiac tamponade owing to hardening of the affected epicardium and myocardium occurred in this case, with subsequent cardiac dilatation failure. Although the patient was initially asymptomatic, severe malaise, dyspnea, and delirium, which were symptoms of right heart failure owing to cardiac dilatation disorder, developed early. Conduction disturbance owing to direct invasion of the conduction system also occurred, which may also have led to the patient’s sudden death. In cardiac tumors, including HAH, although the ECG waveforms are nonspecific and there are no characteristic findings in each region, arrhythmia owing to ST-T change and myocardial conduction disturbance has been observed with myocardial infiltration [10]. Additionally, low potential difference and the appearance of sinus tachycardia that increases with increasing pericardial fluid accumulation, indirectly suggests pericardial metastasis [11]. Atrial fibrillation was seen in this case at diagnosis. Regarding the causes of HAH, most are infectious, but some are of cancerous origin [12]. The majority of HAH owing to pericardial metastases of SCC originate from lung cancer; only one case of HAH has been reported in head and neck cancer [13]. In addition, ours is the first case of asymptomatic HAH, which is a valuable addition to the existing literature.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Written informed consent was obtained from the patient for publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Keitaro Fujii, Email: s51045204@gmail.com.
Takayuki Imai, Email: imai-ta479@miyagi-pho.jp.
Yukinori Asada, Email: asada7@yahoo.co.jp.
Ikuro Sato, Email: sato-ik510@miyagi-pho.jp.
Tomoko Yamazaki, Email: tomoko-yamazaki@miyagi-pho.jp.
Kazuto Matsuura, Email: kmatsuur@east.ncc.go.jp.
References
- 1.Filograna L, Laberke P, Ampanozi G, et al. Role of post-mortem computed tomography (PMCT) in the assessment of the challenging diagnosis of pericardial tamponade as cause of death in cases with hemopericardium. Radiol Med. 2015;120:723–730. doi: 10.1007/s11547-015-0517-1. [DOI] [PubMed] [Google Scholar]
- 2.Patel H, Francke M, Stahura H, et al (2018) Solitary cardiac metastasis from primary oral squamous cell carcinoma presenting as ST-elevation MI. BMJ Case Rep 17:bcr2018224732. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5905818/ [DOI] [PMC free article] [PubMed]
- 3.Hanfling SM (1960) Metastatic cancer to the heart. Review of the literature and report of 127 cases. Cirulation 22:474–483. 10.1161/01.cir.22.3.474 [DOI] [PubMed]
- 4.Kim JK, Sindhu K, Bakst RL (2019) Cardiac Metastasis in a Patient with Head and Neck Cancer: A Case Report and Review of the Literature. Case Rep Otolaryngol 9581259. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6501223/ [DOI] [PMC free article] [PubMed]
- 5.Bussani R, De-Giorgio F, Abbate A, et al (2007) Cardiac metastases. J Clin Pathol 60:27–34. https://jcp.bmj.com/content/60/1/27.long [DOI] [PMC free article] [PubMed]
- 6.Gassman HS, Meadows R Jr, Baker LA (1955) Metastatic tumors of the heart. Am J Med 19:357–365. https://www.amjmed.com/article/0002-9343(55)90124-8/pdf [DOI] [PubMed]
- 7.Rahbar K, Seifarth H, Schäfers M, et al (2012) Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J Nucl Med 53:856–863. https://jnm.snmjournals.org/content/53/6/856.long [DOI] [PubMed]
- 8.Shafiq A, Samad F, Roberts E, et al (2019) Squamous Cell Carcinoma of the Tongue with Metastasis to Myocardium: Report of a Case and Literature Review. Case Rep Cardiol 1649580. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6854178/ [DOI] [PMC free article] [PubMed]
- 9.Mark-Adjeli P, Cirrone J, Gupta R, et al (2019) Tonsillar carcinoma as a rare cause of cardiac metastases. J Community Hosp Intern Med Perspect 9:524–528. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6968673/ [DOI] [PMC free article] [PubMed]
- 10.Cates CU, Virmani R, Vaughn WK, et al (1986) Electrocardiographic markers of cardiac metastasis. Am Heart J 112:1297–1303. https://www.sciencedirect.com/science/article/pii/0002870386903637?via%3Dihub [DOI] [PubMed]
- 11.Refaat MM, Katz WE (2011) Neoplastic pericardial effusion. Clin Cardiol 34:593–598. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6652358/ [DOI] [PMC free article] [PubMed]
- 12.Imazio M, Spodick DH, Brucato A, et al (2010) Controversial issues in the management of pericardial diseases. Circulation 121:916–928. 10.1161/circulationaha.108.844753 [DOI] [PubMed]
- 13.Schwender FT, Wollner I, Kunju LP, et al (2002) Squamous cell carcinoma of the buccal mucosa with metastases to the pericardial cavity, lung and thyroid. Oral Oncol 38:114-116. https://www.sciencedirect.com/science/article/abs/pii/S1368837501000215?via%3Dihub [DOI] [PubMed]