Abstract
The Fontan procedure is a palliative cardiac surgery performed for children with a single functional ventricle, and its aim is to divert central venous return to the pulmonary artery without passing through the right ventricle. We herein report the first case of endometrial cancer after the Fontan procedure that was successfully treated with laparoscopic hysterectomy. A 38-year-old female who underwent the Fontan procedure at the age of 23 presented with abnormal genital bleeding and was diagnosed with endometrial cancer. Computed tomography revealed distinctive venous vasculature in the pelvis: most of the venous return from the left lower extremity occurred via the extremely distended left ovarian vein. Laparoscopic total hysterectomy and bilateral salpingo-oophorectomy were performed. The pneumoperitoneum was maintained at 8–10 mmHg during the operation so that the high intra-abdominal pressure would not interfere with venous return. In addition, the left ovarian vein was first test clamped to ensure that circulation was maintained and was then resected. Although the volume of blood loss reached 358 ml due to high venous pressure, her postoperative course was favorable, and she had no signs of recurrence at the 12-month follow-up. Our case suggests that laparoscopic hysterectomy might be safe and feasible for patients with endometrial cancer after the Fontan procedure, as long as a preoperative study of pelvic vascularization and intraoperative monitoring of the circulation is carried out.
Keywords: Endometrial cancer, Fontan procedure, Laparoscopic hysterectomy
Introduction
Endometrial cancer is the seventh most common malignancy in women worldwide, and its incidence is increasing in developed countries [1]. Endometrial cancer is often diagnosed at an early stage because it frequently causes vaginal bleeding. Thus, surgery plays a major role in the treatment of endometrial cancer, and minimally invasive surgery, such as laparoscopic surgery and robot-assisted surgery, has recently become increasingly popular [2].
The Fontan procedure is a palliative cardiac surgery performed for children with a single functional ventricle, and its aim is to divert central venous return to the pulmonary artery without passing through the right ventricle. Recently, some studies have raised a concern about increased cancer-related mortality in adult Fontan survivors [3] and an elevated cancer risk in patients with congenital heart disease [4], although survival rates after the Fontan procedure are almost 80% at 20 years [5, 6]. The treatment of cancer, especially with laparoscopic surgery, after the Fontan procedure is challenging due to the unique hemodynamics of Fontan circulation. Pneumoperitoneum with carbon dioxide and the Trendelenburg position result in an increase in intra-abdominal and thoracic pressure, thereby compromising central venous return and reducing cardiac output. To date, an inadequate number of cases of laparoscopic surgery in patients with Fontan physiology have been accumulated [7–10], and there have been no cases in gynecological surgery. We herein describe the first case of endometrial cancer after the Fontan procedure that was successfully treated with laparoscopic hysterectomy.
Case report
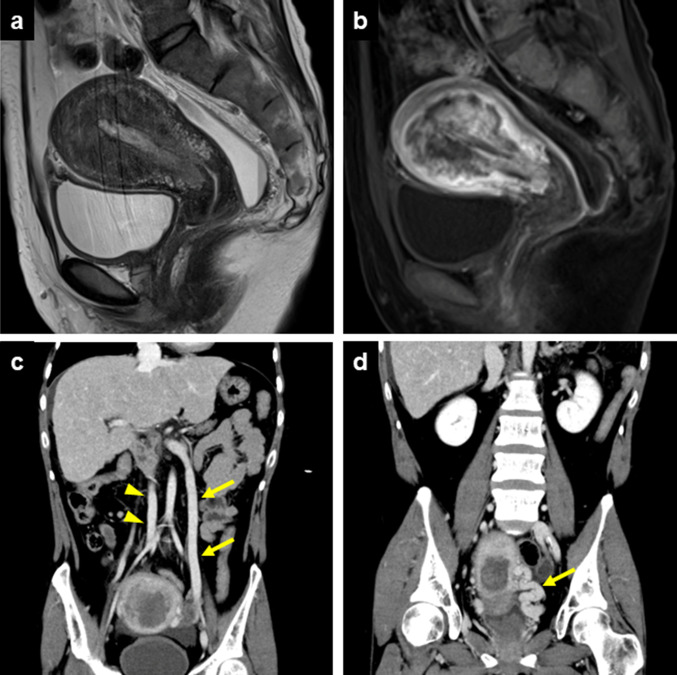
The patient was a 38-year-old nulligravida female. Shortly after birth, she was diagnosed with pulmonary atresia and a left ventricular type single ventricle, and the Blalock-Taussig shunt was performed. She underwent the Bjork operation when she was 16 years old and finally underwent a total cavopulmonary connection type Fontan procedure at the age of 23. Fifteen years later, she presented with abnormal vaginal bleeding, and a biopsy of the uterine endometrium showed endometrial carcinoma, grade 1 (G1). Gadolinium-enhanced magnetic resonance imaging suggested endometrial cancer that diffusely invaded the myometrium (Fig. 1a and b). Contrast-enhanced computed tomography (CT) showed no lymph node swelling or metastasis. In addition, CT revealed abnormalities in the venous system characteristic of this patient; the right external iliac vein and the left common iliac vein were extremely hypoplastic, and the left ovarian vein was as distended as the inferior vena cava (Fig. 1c). Furthermore, the left uterine vein was also markedly distended (Fig. 1d). Based on these findings, Fig. 2 described the patient's assumed venous perfusion: venous blood flowed from the left lower limb to enter the inferior vena cava via the external iliac vein, internal iliac vein, uterine vein, gonadal vein and renal veins.
Fig. 1.
Images from magnetic resonance imaging (a and b) and computed tomography (c and d). a Sagittal T2-weighed image. A diffuse, low-intensity area was detected in the uterine corpus, suggesting invasive endometrial carcinoma. b Sagittal dynamic contrast-enhanced T1-weighted image at 20 s. A nonhomogeneous, early-enhanced lesion invaded the myometrium. c Coronal image. The left ovarian vein (arrows) was as distended as the inferior vena cava (arrowheads). d Coronal image. The left uterine vein was markedly distended (arrow)
Fig. 2.
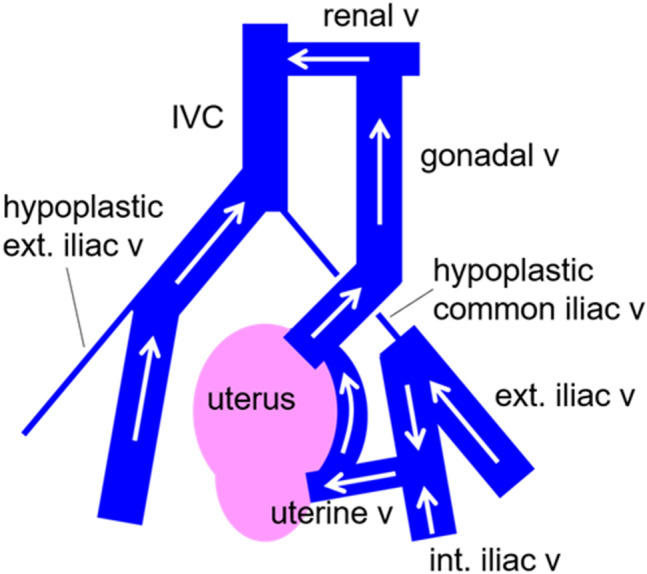
Scheme of the expected venous return from both lower limbs in the present case. v vein, IVC inferior vena cava, ext. external, int. internal
After a thorough preoperative evaluation by a multidisciplinary team, her cardiac function was found to be well preserved with an ejection fraction of 61%, and total laparoscopic hysterectomy and bilateral salpingo-oophorectomy were performed. The patient was ventilated with a positive end-expiratory pressure of 5 cmH2O, and the pneumoperitoneum was maintained between 8 and 10 mmHg. There was concern as to whether severing the left ovarian vein would have a serious effect on systemic circulatory dynamics because the left ovarian vein appeared to be the main venous return from the left lower limb. Thus, before cutting the left ovarian vein, the vessel was temporarily clamped with a bulldog clip to ensure that there was no change in circulatory dynamics (Fig. 3). The left uterine vein was dilated as shown in the preoperative CT images. In order to reduce bleeding, it was first ligated and then cut. In the process of cutting the cardinal ligament and vagina, a large amount of bleeding was observed, and eventually, the volume of blood loss reached 358 ml. During the operation, central venous pressure was monitored continuously, and intermittent transesophageal echocardiography examinations were carried out to check cardiac function. The operative time was 371 min, and her postoperative course was generally favorable, except for a port site infection.
Fig. 3.
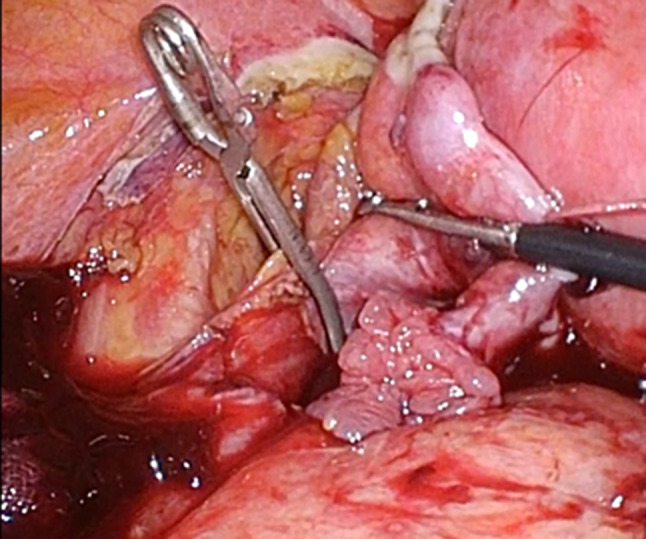
Intraoperative image. The left ovarian vein was temporarily clamped with a bulldog clip
Histopathologically, the cancer cells displayed a glandular architecture with partially squamous differentiation, and there was mild nuclear atypia (Fig. 4). The cytodiagnosis of ascites was negative. Because the tumor invasion had reached the serosa, the final diagnosis was endometrial carcinoma, G1, FIGO stage IIIA (pT3aNXM0). We recommended adjuvant systemic chemotherapy; however, the patient did not agree after careful consideration. At the 12-month follow-up, she had no signs of recurrence.
Fig. 4.
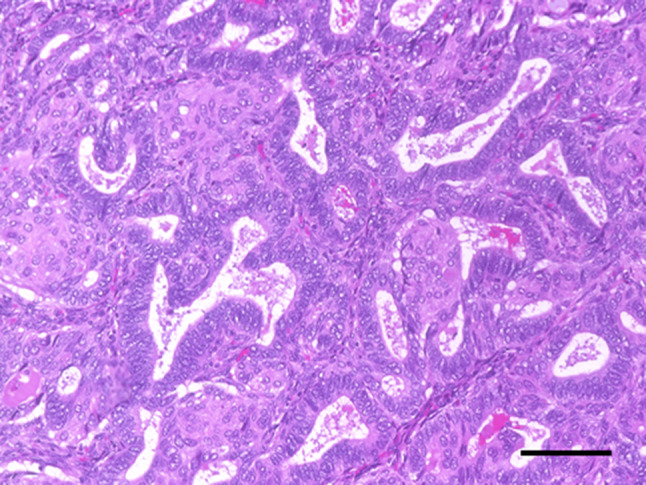
Microscopic findings of the uterine corpus. Cancer cells displayed a glandular architecture with partially squamous differentiation, and nuclear atypia was mild (× 200, bar 100 µm)
Discussion
To the best of our knowledge, this is the first case report of endometrial cancer successfully treated by laparoscopic surgery in a patient with Fontan physiology. It has been 50 years since the Fontan procedure was first introduced in 1971 [11]. During this time, the procedure has undergone various improvements and has become the standard surgical treatment for patients with a single functional ventricle.
The most frequent cancer that occurs after the Fontan procedures is hepatocellular carcinoma [12]. The elevated central venous pressure and decreased cardiac output in Fontan physiology cause chronic liver congestion, thereby leading to the development of hepatocellular carcinoma. The annual incidence of new-onset hepatocellular cancer in the fourth decade after surgery was 8.83% [12]. Consequently, cancer-related mortality in adult Fontan survivors notably increased after age 40 [3]. Moreover, patients with congenital heart disease appear to have significantly increased cancer risk, probably because of their frequent exposure to medical radiation and unelucidated genetic background [4]. At present, cases of malignancy other than liver cancer in patients with Fontan circulation are extremely rare. However, since a number of patients who underwent Fontan surgery in the 1970s and 1980s have reached the age of 40, the number of cancer patients with Fontan circulation may gradually increase in the future.
The standard treatment for endometrial cancer is surgical removal of the uterus and adnexa, and the laparoscopic approach is now widespread because of its feasibility, safety and efficacy in terms of minimal invasiveness and risk of recurrence [13, 14]. However, laparoscopic surgery in patients with Fontan circulation is hemodynamically challenging and requires special considerations. The most important concern is increased intra-abdominal pressure due to pneumoperitoneum, which impairs venous return [7]. In addition, increasing intra-abdominal pressure, the Trendelenburg position, and positive-pressure ventilation all contribute to increased intrathoracic pressure [8]. Both increased intraabdominal and thoracic pressure compromise venous return and decrease cardiac output because in Fontan circulation, central venous blood is drained directly to pulmonary arteries, which is maintained only by the pressure gradient between them. Therefore, both the pneumoperitoneum and positive end-expiratory pressure should be set appropriately. Moreover, intraoperative central venous pressure monitoring and cardiac function assessment by transesophageal echocardiography are recommended.
In the present case, the patient had very distinctive venous vasculature in the pelvis. It is uncertain whether this abnormality was related to congenital heart disease. However, it is quite possible that frequent cardiac catheterization or central venous catheterization by venous cutdown since childhood resulted in venous stenosis or subocclusion. A preoperative CT scan showed that most of the venous return from the left lower extremity occurred via the left ovarian vein. Since a sudden reduction in venous return in patients with Fontan circulation directly induces circulatory failure, we temporarily clamped the left ovarian vein to see if it affected the systemic circulatory dynamics. When performing laparoscopic hysterectomy in post-Fontan procedure patients, the possibility of such pelvic venous abnormalities should be considered, and a thorough preoperative imaging evaluation should be performed to confirm vascularization.
Increased venous bleeding due to high central venous pressure, which is characteristic of patients with Fontan circulation, should also be noted. Generally, laparoscopic surgery has the great advantage that insufflation pressure can promote hemostasis. Although high intra-abdominal pressure would be detrimental to patients with Fontan circulation, appropriate pneumoperitoneum should be used effectively to reduce venous bleeding.
When offering cancer treatment to post-Fontan patients, we must carefully consider whether the benefit of each treatment greatly outweighs the risk of associated complications. In our case, retroperitoneal lymphadenectomy was abandoned because the therapeutic value of lymphadenectomy had not yet been established in endometrial cancer [15], and it seemed unreasonable to perform it with the risk of uncontrolled heavy bleeding due to increased venous pressure. In addition, there are few reports and many unknowns regarding cardiac safety following systemic chemotherapy in patients with Fontan circulation [16, 17]. For endometrial cancer, it may be difficult to use doxorubicin, which is cardiotoxic, and cisplatin, which requires a large infusion load.
In conclusion, in order to provide patients with the benefits of laparoscopic surgery, the characteristic hemodynamics of Fontan circulation must be fully understood and measures taken accordingly. Our case suggests that laparoscopic hysterectomy might be safe and feasible in patients with endometrial cancer after the Fontan procedure, as long as a preoperative study of pelvic vascularization and intraoperative monitoring of the circulation is carried out.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Written informed consent was obtained from the patient for the publication of this case report and its accompanying images.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Scalici J, Laughlin BB, Finan MA, et al. The trend towards minimally invasive surgery (MIS) for endometrial cancer: an ACS-NSQIP evaluation of surgical outcomes. Gynecol Oncol. 2015;136:512–515. doi: 10.1016/j.ygyno.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Ohuchi H, Inai K, Nakamura M, et al. Mode of death and predictors of mortality in adult Fontan survivors: A Japanese multicenter observational study. Int J Cardiol. 2019;276:74–80. doi: 10.1016/j.ijcard.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S, Gurvitz MZ, Beausejour-Ladouceur V, et al. Cancer risk in congenital heart disease-what is the evidence? Can J Cardiol. 2019;35:1750–1761. doi: 10.1016/j.cjca.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Khairy P, Fernandes SM, Mayer JE, Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 6.Downing TE, Allen KY, Glatz AC, et al. Long-term survival after the Fontan operation: Twenty years of experience at a single center. J Thorac Cardiovasc Surg. 2017;154(243–253):e242. doi: 10.1016/j.jtcvs.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 7.McClain CD, McGowan FX, Kovatsis PG. Laparoscopic surgery in a patient with Fontan physiology. Anesth Analg. 2006;103:856–858. doi: 10.1213/01.ane.0000237294.88298.8e. [DOI] [PubMed] [Google Scholar]
- 8.Pans SJ, van Kimmenade RR, Ruurda JP, et al. Haemodynamics in a patient with Fontan physiology undergoing laparoscopic cholecystectomy. Neth Heart J. 2015;23:383–385. doi: 10.1007/s12471-015-0704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelico R, Lisignoli V, Monti L, et al. Laparoscopic liver resection for hepatocellular carcinoma in Fontan-associated chronic liver disease. The first case report. Int J Surg Case Rep. 2019;59:144–147. doi: 10.1016/j.ijscr.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokota Y, Noda T, Kobayashi S, et al. A case report of Fontan procedure-related hepatocellular carcinoma: pure laparoscopic approach by low and stable pneumoperitoneum. BMC Surg. 2020;20:80. doi: 10.1186/s12893-020-00741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohuchi H, Hayama Y, Nakajima K, et al. Incidence, predictors, and mortality in patients with liver cancer after fontan operation. J Am Heart Assoc. 2021;10:e016617. doi: 10.1161/JAHA.120.016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331–5336. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost JA, Webster KE, Bryant A, et al. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. 2017;10:CD07585. doi: 10.1002/14651858.CD007585.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh C, Youn JK, Han JW, et al. Hepatocellular carcinoma after the Fontan procedure in a 16-year-old girl: A case report. Medicine (Baltimore) 2016;95:e4823. doi: 10.1097/MD.0000000000004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maehara N, Niizuma H, Nakamura T, et al. Chemoradiotherapy of spinal extradural Ewing sarcoma after the Fontan procedure. Pediatr Int. 2020;62:1197–1199. doi: 10.1111/ped.14258. [DOI] [PubMed] [Google Scholar]