Abstract
[11C]UCB-J is a PET radioligand that binds to the presynaptic vesicle glycoprotein 2A. Therefore, [11C]UCB-J PET may serve as an in vivo marker of synaptic integrity. The main objective of this study was to evaluate the quantitative accuracy and the 28-day test–retest repeatability (TRT) of various parametric quantitative methods for dynamic [11C]UCB-J studies in Alzheimer’s disease (AD) patients and healthy controls (HC). Eight HCs and seven AD patients underwent two 60-min dynamic [11C]UCB-J PET scans with arterial sampling over a 28-day interval. Several plasma-input based and reference-region based parametric methods were used to generate parametric images using metabolite corrected plasma activity as input function or white matter semi-ovale as reference region. Different parametric outcomes were compared regionally with corresponding non-linear regression (NLR) estimates. Furthermore, the 28-day TRT was assessed for all parametric methods. Spectral analysis (SA) and Logan graphical analysis showed high correlations with NLR estimates. Receptor parametric mapping (RPM) and simplified reference tissue model 2 (SRTM2) BPND, and reference Logan (RLogan) distribution volume ratio (DVR) regional estimates correlated well with plasma-input derived DVR and SRTM BPND. Among the multilinear reference tissue model (MRTM) methods, MRTM1 had the best correspondence with DVR and SRTM BPND. Among the parametric methods evaluated, spectral analysis (SA) and SRTM2 were the best plasma-input and reference tissue methods, respectively, to obtain quantitatively accurate and repeatable parametric images for dynamic [11C]UCB-J PET.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13550-021-00874-8.
Keywords: Alzheimer’s disease, [11C]UCB-J, Parametric methods, PET, SV2A
Background
Abnormal brain deposits of amyloid β (Aβ), aggregation of tau into neurofibrillary tangles (NFTs) and synaptic loss are neuropathological hallmarks of Alzheimer’s disease (AD) [1]. According to new AD models, the accumulation of abnormal proteins and synaptic loss occur many years before the onset of AD [2, 3]. Previous studies reported that lower synaptic density in the hippocampus and cerebral cortex is associated with cognitive impairment in AD patients [4, 5]. In these papers synaptic density was measured post-mortem; however, recently, it became possible to quantify synaptic density in-vivo using PET with the ligand [11C]UCB-J which targets the synaptic vesicle protein 2A (SV2A).
SV2A is a glycoprotein present in the membrane of presynaptic vesicles and is located in synapses throughout the brain. There are three different isoforms of synaptic vesicle proteins: SV2A, SV2B and SV2C [6]. SV2 proteins are involved in vesicle transport in the synapse and are essential for the function of our nervous system. Although the specific physiological role of SV2A is still unclear, SV2A is thought to be involved in the exocytosis of neurotransmitters and plays an important role in regulating/modulating synaptic function6. Since SV2A is highly expressed throughout the brain, it is a suitable target for positron emission tomography (PET) imaging when aiming to assess synaptic integrity in vivo.
(R)-1-((3-([11C]methyl)pyridin-4-yl)methyl)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one ([11C]UCB-J) is a PET ligand with high affinity and specificity for SV2A [7, 8]. [11C]UCB-J enables in vivo imaging of synaptic density in neurological diseases. Previous studies have found that [11C]UCB-J kinetics can be best described by a reversible one-tissue compartment model with additional blood volume fraction as a parameter (1T2k_VB) [9, 10]. Moreover, the white matter semi-ovale (SO), has been validated as a suitable reference region for quantification of synaptic density in the brain [11].
To date, the (regional) kinetics of [11C]UCB-J have mainly been assessed using conventional tracer kinetic models based on predefined regions of interest (ROI). In addition to ROI approaches, voxel-wise analysis, using quantitative parametric images can give additional information when a specific signal is not homogenous in a region and gets diluted when evaluated at a regional level. Therefore, it is important to obtain quantitatively accurate parametric maps. In a recent study evaluating various parametric methods for dynamic [11C]UCB-J PET, Mertens et al. reported that the simplified reference tissue model 2 (SRTM2) was the preferred parametric method for voxel-wise analysis using white matter SO as reference region [12]. However, this study was performed only in healthy controls (HCs) and no test–retest repeatability (TRT) data was assessed to evaluate the performance of the parametric methods. In a recent other study [10], we assessed TRT at regional level for conventional kinetic models for [11C]UCB-J in a 28-day time period in order to closely mimic the condition of the clinical drug intervention design [13]. The expected effect size was 25% in a 28-day time period [13]. The aim of the current study was to validate various parametric methods to obtain quantitatively accurate [11C]UCB-J parametric images in both HCs and mild to moderate AD patients. In addition, to assess the TRT for these parametric methods over a 28-day period in order to closely mimic the clinical drug intervention design.
Methods
Participants
Eight HCs with an average age of 63.1 ± 6.3 years and MMSE score of 29.4 ± 0.9 and seven mild to moderate AD patients with an average age of 64.3 ± 8.3 years and MMSE score of 24.1 ± 1.8 from the Amsterdam University Medical Center (Amsterdam UMC) participated in the study. All AD patients had a diagnosis of probable AD defined by National Institute on Aging—Alzheimer’s Association (NIA-AA) [14]. All AD patients had positive Aβ biomarkers either determined by Aβ42 in cerebrospinal fluid (CSF) (Aβ42 < 813 pg/mL) [15]) or visual read of an amyloid-β PET scan, and a Mini-Mental State Examination (MMSE) score between 18–26. All HCs were cognitively normal without cognitive complaints, absence of significant impairment in cognitive functions or activities of daily living and the MMSE score was ≥ 27. The Medical Review and Ethics Committee (MREC) of Foundation BEBO in Assen approved the current study and local feasibility was confirmed by the MREC of Amsterdam UMC. Furthermore, all subjects had to provide written informed consent prior to enrolment.
Data acquisition
3D T1 weighted MRI scans were obtained using a 3.0 T Philips Ingenuity Time-of-Flight PET/MR scanner at Amsterdam UMC for all participants for brain tissue segmentation and PET co-registration. Each participant underwent two dynamic PET scans on the same PET/CT system with an interval of 28 days. The mean interval and SD between the test and retest scans was 28.3 ± 1.3 days. PET scans were acquired on the Ingenuity TF PET/CT scanner (Amsterdam UMC, Philips Medical Systems, Best, the Netherlands). In short, prior to each PET scan, a low-dose computed tomography (CT) was acquired for attenuation correction. The low-dose CT was followed by a dynamic PET scan after a bolus injection of 347 MBq ± 41 [11C]UCB-J. During scanning, the head of the subjects was stabilized to reduce movement artefacts. More specifically, subjects were positioned within the center of axial and transaxial fields of view, such that the orbito-meatal line was parallel to the detectors with the use of laser beams, this way motion during the PET acquisition was checked every now and then and was minimized as much as possible. The scan duration was 90 min for HC and 60 min for AD patients. Based on the results of previous studies [9, 10], only 60 min scan data will be used for the present study. The PET list mode data were rebinned into a total of 19 frames (1 × 15, 3 × 5, 3 × 10, 4 × 60, 2 × 150, 2 × 300 and 4 × 600 s) followed by a reconstruction using 3D RAMLA with a matrix size of 128 × 128 × 90 and a final voxel size of 2 × 2 × 2 mm3, including all usual corrections for dead time, decay, attenuation, randoms and scatter.
Continuous arterial blood sampling using an online detection system [16] was acquired continuously over 30 min for AD patients and HCs. At set time points (5, 10, 15, 20, 40, 50, 60 for AD patient and, 5, 10, 15, 20, 40, 50, 60, 75 and 90 min for HCs) manual blood samples were collected (5–7 mL each), to estimate the plasma-to-whole-blood ratios and to measure plasma metabolite fractions. The continuous online blood sampler data were calibrated using the manual whole blood activity and corrected for metabolites, plasma to whole blood ratios and delay, using information measured from manual samples. This resulted in a metabolite corrected arterial plasma input function. Manual blood samples were collected in heparin tubes and centrifuged for 5 min at 5000 r/min.
Data analysis
Image processing
The 3D T1 weighted MR images were co-registered onto the dynamic PET scan using VINCI v 2.56 software (Max Plank Institute, Cologne, Germany). Sixty eight regions of interest (ROIs) as in Hammers template [17] were defined on the co-registered MRI using PVElab [18]. Corresponding regional time activity curves (TACs) were extracted by superimposing these 68 ROIs onto the dynamic PET scan.
Kinetic analysis (non-linear regression)
Earlier studies have determined that a 1T2k_VB best describes the in vivo kinetics of [11C]UCB-J [9, 10]. Therefore, 1T2k_VB model was used to estimate the kinetic parameters such as, distribution volume ratio (DVR), total volume of distribution (VT) and rate of influx of radioligand from blood to tissue (K1). The regional non-linear regression (NLR) estimates were obtained with a home-developed MATLAB script and served as a gold standard to evaluate settings and validate different parametric methods. Furthermore, reference tissue-based parametric methods were validated against corresponding kinetic parameters, such as binding potential (BPND) and R1 (influx of the tracer into the ROI relative to the reference region) derived by using simplified reference tissue model (SRTM). White matter SO was considered as the reference region.
Parametric images
Parametric images were obtained by using an in-house built software tool (PPET) [19]. Various linearization methods were evaluated: plasma input Logan (Logan) [20], reference Logan (RLogan) [21] and five versions of the multilinear reference tissue model (MRTMo, MRTM1, MRTM2, MRTM3, MRTM4) [22–24]. Furthermore, basis function methods such as receptor parametric mapping (RPM) [25], SRTM2 [26] and spectral analysis (SA) [27] were validated. Plasma input based Logan and SA were studied to generate VT and/or K1 images. To produce the BPND and R1 images, RLogan, MRTMo-4, RPM and SRTM2 were evaluated. For SA, a linear regression fitting model (weighted residual sum of squares) was used. Equation 1 illustrates the weighting factors estimation [28].
| 1 |
The outcome represents the variances calculated for each frame and is based on the whole scanner true counts (T), decay correction factor () and frame length (). Whole scanner true counts were obtained from the scanner acquisition statistics, or estimated using (not-corrected) total counts in each frame. The decay correction factor was calculated using the formula stated by Yaqub et al. [28] and frame length (in seconds) is an acquisition parameter. Weighting factors for each frame were calculated using . The above mentioned models were fitted to the data using non-linear least squares in combination with these weighting factors.
Different start times (t*) were evaluated by comparing the estimated regional parametric values to the reference standard (parametric values estimated using plasma input based 1T2k_VB model) for linearization methods. However, in case of parametric methods using basis function approach, an initial estimate of the θ1, θ2, and θ3, was derived using the equations described by Gunn et al. 1997 [25]. These values were further adapted to obtain the optimal settings by comparing the regional parametric estimates to the reference standard. Furthermore, the weighting factors used in the implementation of the parametric methods were based on the study by Yaqub et al. [28].
Statistical analysis
To evaluate the correspondence between parametric methods estimates and NLR estimates across all ROIs and across all subjects, coefficients of determination (r2) and the slopes of the regression line were calculated. Equation 2 was used to calculate the TRT for each parametric method in native space for regional values. Furthermore, all parametric images were warped to Montreal Neurological Institute (MNI152) space, with Statistical Parametric Mapping (SPM) version 12 software (Welcome Trust Center for Neuroimaging, University College London, UK). Transformation matrixes derived from warping the co-registered MRI scans to MNI were used for this purpose. The warped images were used to calculate absolute TRT repeatability (average and SD) using Eq. 3 for each parametric method. The bias to assess overestimation or underestimation was calculated using Eq. 4. Furthermore, the intraclass correlation coefficient (ICC) was analysed using an average-measurement, absolute-agreement, 2-way mixed-effects model for each parametric method.
| 2 |
| 3 |
| 4 |
Results
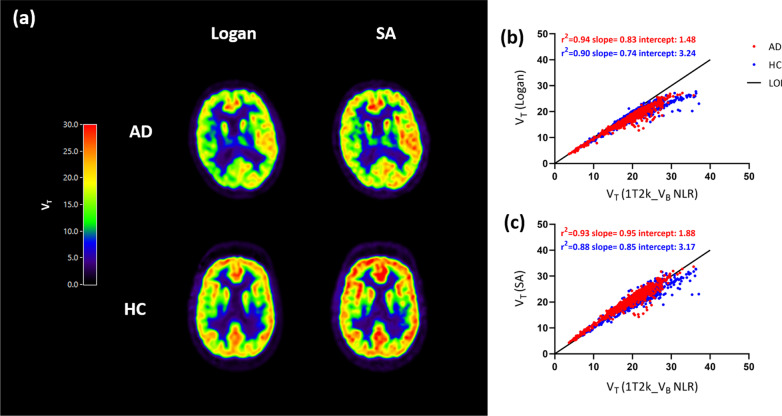
The optimal settings for the various parametric imaging methods are presented in Table 1. Parametric images for VT derived from Logan and SA are presented in Fig. 1a, separately for a typical AD and HC subject. Regional VT values obtained from SA (AD: r2 = 0.93, slope = 0.95; HC: r2 = 0.88, slope = 0.85) and Logan (AD: r2 = 0.94, slope = 0.83; HC: r2 = 0.90, slope = 0.74) had a good correspondence with VT values derived from 1T2k_VB using test and retest data (Fig. 1b, c). Coefficients of determination and slopes for SA and Logan VT with corresponding NLR estimates are presented in Additional file 1: Fig. 1, separately for test and retest data for each group. Furthermore, scatterplots for VT obtained with SA with corresponding NLR VT estimates are presented in Additional file 2: Fig. 2, separately color-coded for each subject. In Additional files 3, 4: Figs. 3 & 4, scatterplots for VT and K1 obtained with SA with corresponding NLR VT and K1 estimates are presented, separately color-coded for each region. K1 estimates obtained from SA showed an underestimation of 14.1% ± 4.7 for AD patients and 13.2% ± 4.9 for HCs when compared to NLR derived K1 estimates (Additional file 12: Table 1). Coefficients of determination and slopes for each subject for parameters obtained from SA are presented in Additional file 13: Table 2.
Table 1.
The optimal settings used for different parametric methods
| Parametric method | Interval (min) | Basis function range (min−1) | Number of basis functions |
|---|---|---|---|
| Spectral analysisa | 0–60 | 0.01–0.1 | 50 |
| Logana | 10–60 | – | – |
| Reference Loganb | 30–60 | – | – |
| RPMb | 0–60 | 0.01–0.1 | 30 |
| SRTM2b | 0–60 | 0.01–0.1 | 30 |
| MRTMob | 10–60 | – | – |
| MRTM1b | 10–60 | – | – |
| MRTM2b | 10–60 | – | – |
| MRTM3b | 10–60 | – | – |
| MRTM4b | 10–60 | – | – |
MRTM: multilinear reference tissue model; RPM: receptor parametric mapping; SRTM2: simplified reference tissue model 2. White matter semi-ovale was used as reference region
aPlasma input based implementations
bReference tissue based implementations
Fig. 1.
a Logan and SA derived VT parametric images for a typical AD patient and HC subject. Coefficients of determination for regional VT estimates of all Hammers template regions for all the subjects obtained using b Logan and c SA with corresponding NLR VT estimates. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity
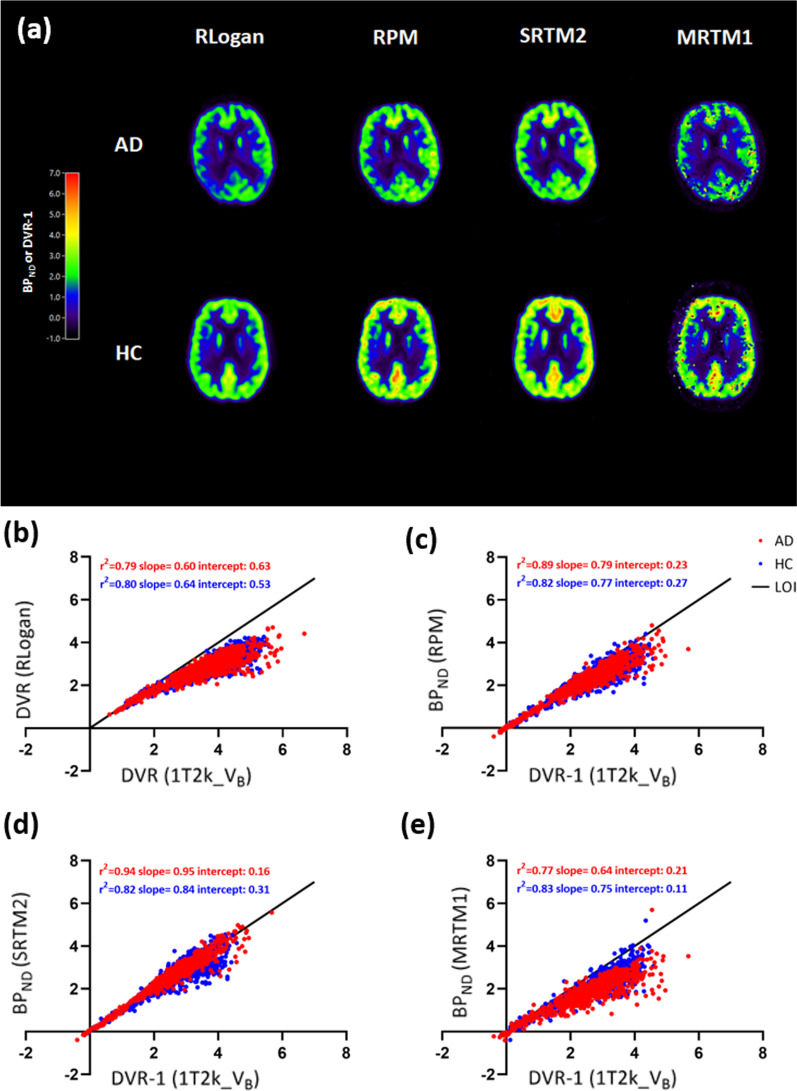
Figure 2a shows the DVR and BPND parametric images derived from RLogan, RPM, SRTM2 and MRTM1 implementations. RLogan, RPM and STRM2 provided visually good quality parametric images.
Fig. 2.
a RLogan, RPM, SRTM2, and MRTM1 DVR/BPND parametric images for a typical AD patient and HC. Scatterplots for regional DVR/BPND estimates of all Hammers template regions for all the subjects obtained using b RLogan, c RPM, d SRTM2, and e MRTM1 with corresponding NLR estimates. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity; BPND: binding potential; DVR: distribution volume ratio; NLR: non-linear regression; RLogan: reference Logan; RPM: receptor parametric mapping; SRTM2: simplified reference tissue model 2
Coefficients of determination (r2) and slopes for each parametric implementation with their corresponding NLR estimates using 60 min data are presented in Table 2, 3, separately for AD patients and HCs. RLogan derived DVR correlated well with NLR derived DVR (AD: r2 = 0.79, slope = 0.60; HC: r2 = 0.80, slope = 0.64) (Fig. 2b) and SRTM BPND (AD: r2 = 0.83, slope = 0.75; HC: r2 = 0.82, slope = 0.78). RPM BPND correlated well with indirect measure of BPND (DVR-1) (AD: r2 = 0.89, slope = 0.79; HC: r2 = 0.82, slope = 0.77) (Fig. 2c) and SRTM BPND (AD: r2 = 0.97, slope = 1.02; HC: r2 = 0.92, slope = 1.00) using test and retest data. SRTM2 BPND showed the best correlations with DVR-1 (AD: r2 = 0.94, slope = 0.95; HC: r2 = 0.82, slope = 0.84) (Fig. 2d) and SRTM BPND (AD: r2 = 0.91, slope = 1.18; HC: r2 = 0.81, slope = 1.02). Furthermore, R1 obtained from both RPM (AD: r2 = 0.99, slope = 0.99; HC: r2 = 0.99, slope = 1.01) and SRTM2 (AD: r2 = 0.97, slope = 1.01; HC: r2 = 0.98, slope = 1.01) had an excellent correlation with NLR derived SRTM R1. Coefficients of determination (r2) and slopes for parameters obtained from RPM and SRTM2 are presented in Additional file 13: Table 2 and Additional file 14: Table 3 for all subjects separately to illustrate inter-subject variability. Furthermore, the % bias (mean + SD) of micro/macro-parameters estimated using the parametric methods of interest with respect to the corresponding 1T2k_VB and SRTM estimates are presented in Additional file 12: Table 1 and Additional file 15: Table 4.
Table 2.
Coefficients of determination (r2) and slope of parametric [11C]UCB-J VT, K1 and BPND against corresponding 1T2k_VB estimates using 60 min data
| HC | AD | |||
|---|---|---|---|---|
| r2 | Slope | r2 | Slope | |
| Spectral analysisa VT | 0.88 | 0.85 | 0.93 | 0.95 |
| Spectral analysis a K1 | 0.94 | 0.85 | 0.97 | 0.84 |
| Logan a VT | 0.90 | 0.74 | 0.94 | 0.83 |
| Reference Loganb DVR | 0.80 | 0.64 | 0.79 | 0.60 |
| RPMb BPND | 0.82 | 0.77 | 0.89 | 0.79 |
| SRTM2b BPND | 0.82 | 0.84 | 0.94 | 0.95 |
| MRTMob BPND | 0.79 | 0.62 | 0.76 | 0.57 |
| MRTM1b BPND | 0.83 | 0.75 | 0.77 | 0.64 |
| MRTM2b BPND | 0.59 | 0.78 | 0.40 | 1.13 |
| MRTM3b BPND | 0.75 | 0.64 | 0.63 | 0.51 |
| MRTM4b BPND | 0.85 | 0.81 | 0.56 | 0.87 |
BPND: binding potential; MRTM: multilinear reference tissue model; NLR: non-linear regression; RPM: receptor parametric mapping; SRTM2: simplified reference tissue model 2.
aPlasma input implementations
bReference region implementations
Table 3.
Coefficients of determination (r2) and slope of parametric [11C]UCB-J DVR, BPND and R1 against corresponding SRTM estimates
| HC | AD | |||
|---|---|---|---|---|
| r2 | Slope | r2 | Slope | |
| Reference Loganb DVR | 0.82 | 0.78 | 0.83 | 0.75 |
| RPMb BPND | 0.92 | 1.00 | 0.97 | 1.02 |
| RPMb R1 | 0.99 | 1.01 | 0.99 | 0.99 |
| SRTM2b BPND | 0.81 | 1.02 | 0.91 | 1.18 |
| SRTM2b R1 | 0.98 | 1.01 | 0.97 | 1.01 |
| MRTMo BPNDb | 0.81 | 0.76 | 0.85 | 0.73 |
| MRTMo b R1 | 0.40 | 0.70 | 0.34 | 0.67 |
| MRTM1b BPND | 0.85 | 0.91 | 0.87 | 0.83 |
| MRTM1b R1 | 0.93 | 1.09 | 0.91 | 1.02 |
| MRTM2b BPND | 0.53 | 0.89 | 0.43 | 1.41 |
| MRTM2b R1 | 0.93 | 1.10 | 0.94 | 1.02 |
| MRT3b BPND | 0.80 | 0.80 | 0.70 | 0.66 |
| MRTM3b R1 | 0.02 | 0.11 | 0.00 | 0.05 |
| MRTM4b BPND | 0.87 | 1.01 | 0.60 | 1.06 |
| MRTM4b R1 | 0.05 | 0.08 | 0.00 | − 0.06 |
BPND: binding potential; MRTM: multilinear reference tissue model; NLR: non-linear regression; RPM: receptor parametric mapping; SRTM2: simplified reference tissue model 2
aPlasma input implementations
bReference region implementations
Among the MRTM methods, MRTM1 had the best correspondence with DVR (AD: r2 = 0.77, slope = 0.64; HC: r2 = 0.83, slope = 0.75) (Fig. 2e) and SRTM BPND (AD: r2 = 0.87, slope = 0.83; HC: r2 = 0.85, slope = 0.91). Most of the MRTM parametric images were noisy and had qualitatively unreliable parametric images. Coefficients of determination and slopes for DVR and BPND obtained from RLogan, RPM, SRTM2 and MRTM1 with corresponding NLR estimates are presented in Additional file 5: Fig. 5, separately for test and retest data for each subject group. Furthermore, scatterplots for BPND estimates obtained using RPM and SRTM2 against corresponding NLR VT estimates are presented in Additional files 6, 7: Figs. 6 & 7, separately color-coded for each subject. In addition, scatterplots of the parameters obtained from SRTM2 with corresponding NLR estimates are presented in Additional files 8, 9: Figs. 8 & 9, separately color-coded for each region.
The TRT of different parametric methods using 60 min data for whole brain grey matter are presented in Table 4 separately for AD patients and HCs. Most of the evaluated parametric methods had comparable TRTs as the NLR counterpart methods. TRT for a few other brain regions for parameters estimates using SA, SRTM2, and RPM are presented in Additional files 15, 16, 17, 18: Tables 4, 5, 6, 7. ICC for each parametric method and associated parametric estimation(s) are presented in Table 5. In addition, the TRT voxel-wise images (average and SD) calculated for HCs and AD patients are presented in Additional files 10, 11: Figs. 10 & 11.
Table 4.
TRT values estimated for whole brain (grey matter) are presented for each parametric method
| TRT whole brain (grey matter) | ||||
|---|---|---|---|---|
| HC | AD | |||
| Mean | SD | Mean | SD | |
| SA VT | − 9 | 7 | 2 | 9 |
| SA K1 | 1 | 12 | 6 | 13 |
| Logan VT | − 5 | 5 | 3 | 8 |
| RLogan DVR | − 3 | 6 | 6 | 9 |
| RPM DVR (BPND + 1) | − 5 | 8 | 6 | 13 |
| RPM R1 | − 3 | 7 | 3 | 11 |
| SRTM2 DVR (BPND + 1) | − 2 | 10 | 6 | 10 |
| SRTM2 R1 | − 2 | 7 | 3 | 9 |
| MRTM1 DVR (BPND + 1) | − 4 | 6 | 8 | 13 |
| MRTM1 R1 | 0 | 12 | 1 | 10 |
TRT for NLR VT and K1 was − 8% ± 4., − 2% ± 14 for HCs and 3% ± 8, 3% ± 14 for AD patients, respectively. TRT for Plasma-input DVR was − 7% ± 6 for HCs and 7% ± 13 for AD patients. TRT for SRTM derived DVR (BPND + 1) and R1 was − 6% ± 7, − 2% ± 6 for HCs and was − 5% ± 9, 2% ± 10 for AD patients, respectively
Table 5.
ICC values estimated for each parametric method and associated parametric estimation(s) separately for HC’s and AD patients
| HC | AD | |||
|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | |
| SA VT | 0.97 | 0.95–0.98 | 0.98 | 0.96–0.99 |
| SA K1 | 0.97 | 0.91–0.98 | 0.95 | 0.92–0.97 |
| Logan VT | 0.98 | 0.97–0.99 | 0.98 | 0.96–0.98 |
| RLogan DVR | 0.96 | 0.92–0.98 | 0.96 | 0.94–0.98 |
| RPM BPND | 0.96 | 0.93–0.98 | 0.96 | 0.94–0.98 |
| RPM R1 | 0.96 | 0.93–0.98 | 0.96 | 0.93–0.97 |
| SRTM2 BPND | 0.97 | 0.95–0.98 | 0.97 | 0.95–0.98 |
| SRTM2 R1 | 0.97 | 0.94–0.98 | 0.96 | 0.93–0.97 |
| MRTM1 BPND | 0.96 | 0.93–0.97 | 0.95 | 0.92–0.97 |
| MRTM1 R1 | 0.95 | 0.91–0.97 | 0.96 | 0.93–0.97 |
SA, RPM and SRTM2 parametric implementations were also evaluated using 90 min data for HCs only. Coefficients of determination (r2) and slopes for these parametric implementations with their corresponding NLR estimates using 90 min data are presented in Additional files 19, 20: Tables 8 & 9. Regional VT values obtained from SA (HC: r2 = 0.90, slope = 0.84) had a good correspondence with VT values derived from 1T2k_VB. Furthermore, RPM BPND correlated well with DVR-1 (HC: r2 = 0.90, slope = 0.79) and SRTM BPND (HC: r2 = 0.97, slope = 0.99). SRTM2 BPND showed the good correlations with DVR-1 (HC: r2 = 0.89, slope = 0.82) and SRTM BPND (HC: r2 = 0.93, slope = 1.00). The TRT of SA, RPM and SRTM2 parametric methods using 90 min data for whole brain grey matter are presented in Additional file 21: Table 10 for HCs.
Discussion
Since disease-specific signal is not homogenous throughout the ROI, relying solely on regional analyses may potentially result in loss of significant signal differences due to spatial dilution. Therefore, voxel-level analyses are necessary to obtain quantitatively accurate parametric images. The present study showed that SA performed better than Logan and in case of reference region based implementations, SRTM2 performs the best for [11C]UCB-J quantification.
VT values obtained with SA proved to have a better correspondence with VT values derived from 1T2k_VB compared to Logan. Logan showed an underestimation when compared to regional NLR estimates. The underestimation either could be explained by statistical noise [27], or by the fact that Logan implementation does not account for blood volume fraction (VB). This bias was partly compensated by assuming that VB is constant but since VB varied between subjects and different brain regions, no VB corrections was applied while performing the Logan analysis. Alternatively, SA is a plasma-input based basis function approach that also accounts for VB, the correspondence with NLR estimates is much better when compared to Logan. Moreover, SA also generates parametric images for K1, which also had good correspondence with NLR K1 estimates (Table 2).
Regarding reference tissue methods, MRTMo-4, RLogan, RPM and SRTM2 were evaluated. For RLogan DVR, an underestimation of 21.1% ± 9.3 for AD patients and 20.7% ± 8.4 for HCs was observed when compared to plasma input (NLR) DVR and 9.7% ± 8.2 for AD patients and 8.8% ± 7.0 for HCs when compared to SRTM (NLR) BPND. This could be explained by the noise induced negative biases of RLogan [29]. Normally, the disadvantage of using graphical methods such as RLogan is that the noise in the TACs is elevated due to a correlation of errors in the dependent and independent variable of the RLogan equation, which results in an underestimation of DVR. RPM BPND also presented an underestimation of 11.0% ± 10.8 for AD patients and 12.2% ± 12.9 for HCs when compared to plasma input (NLR) derived DVR, but had excellent correspondence with SRTM (NLR). In our recent study [10], a good correlation between regional estimates of SRTM (NLR) BPND and plasma input (NLR) DVR was observed but also with an underestimation of approximately 25% [10]. White matter SO showed different kinetics than the rest of the brain which could possibly explain this underestimation. Normally, an ideal reference region has a K1’/k2’ equal to the K1/k2 of the other brain regions, thus assuming that non-displaceable binding is equal between the reference region and other brain regions. Furthermore, an ideal reference region also has a faster efflux rate (k2’) than the apparent efflux rate of other brain regions (k2a = k2/(1 + BPND)), which makes the three parameters of interest (R1, k2’ and k2a) identifiable in the SRTM equation (see Eq. 5).
| 5 |
For white matter SO, however, k2’ was equal to k2a, which would make the (k2′–k2a) non identifiable. This might be the reason for SRTM not performing well for [11C]UCB-J, which could also imply that using plasma input (NLR) DVR an indirect measure of BPND is a better reference for parametric methods validation.
A recent study by Rossano et al. [30] observed differences in white matter kinetics and found approximately 20% higher non-displaceable uptake in the white matter compared to grey matter regions. This finding indicates that even though white matter is deprived of specific binding, [11C]UCB-J kinetics in the non-displaceable compartment may be different in white matter than in grey matter regions, which also makes the use of white matter SO as a reference region challenging. The differences in kinetics of white and grey matter could be explained by the differences in the tissue itself. Namely, grey matter is composed of neurons and glial cells, while white matter mainly consists of myelinated axons and oligodendroglia. White matter contains a higher concentration of lipids compared to grey matter due to the myelin that is present around the axons [31]. This could explain the lower k2’ that is observed in white matter SO. The observed 20% higher non-displaceable binding in white matter SO by Rossano et al. [30] also supports the idea that even without specific binding, [11C]UCB-J may behave differently in white and grey matter. However, even if there are subtle differences between the two tissue types, as long as these differences are consistent within and between groups, white matter SO could be used as a normalisation region, which seems to be the situation in the presented study group.
In the current study, SRTM2 showed good correspondence with plasma-input (NLR) derived DVR and SRTM (NLR) BPND. SRTM2 BPND had an overestimation of 1.7% ± 8.8 for AD patients and an underestimation of 3.2% ± 13.4 for HCs when compared to DVR. Furthermore, SRTM2 had an overestimation of 24.0% ± 17.2 for AD patients and 16.8% ± 17.0 for HCs when compared with SRTM BPND. SRTM2 is an adaptation of RPM, in which the tracer efflux rate constant k2’ from the reference region is fixed to a certain value by running RPM twice. In the second run, k2’ is fixed to the median (all voxels with BPND higher than 3) from the first run. The threshold of 3 for SRTM2 was obtained by assessing multiple values against the estimated parameter from gold standard (NLR regression model). Voxels with relatively high BPND values were used to identify the voxels that constitutes to the specific signal. These voxels were used to obtain the median k2’ value to perform SRTM2. Since the k2’ was fixed, the number of parameters were reduced from three to two, which makes the fits more reliable, since there are less parameters to estimate [26]. SRTM2 seems to perform better for [11C]UCB-J. SRTM2 BPND had a good correspondence with plasma input DVR, possibly due to more reliable fits. However, since SRTM2 is based on the SRTM model, the parameter estimations could still be effected by the non-identifiable k2’.
Among all MRTM implementations, MRTM1 BPND showed moderate correspondence with plasma input (NLR) DVR-1 with an underestimation of 25.6% ± 19 for AD patients and 20.6% ± 19 for HCs. All other MRTM images were noisy and presented quantitatively inaccurate parametric images. The lower performance of the MRTM methods could be explained by noise, as previous studies observed that noise could result in additional parameter bias in (multi) linearized methods [23, 29].
In the current study, coefficients of determination of blood-based and reference-tissue based parametric methods were validated against regional parametric estimates using NLR based models, separately for HCs and AD patients. In this regards, the performance of the blood based parametric methods (SA and Logan) was slightly better for AD patients when compared to HC subjects (Table 2). In case of reference region based parametric methods, RLogan, and MRTMo had a comparable performance between the two subject groups (Tables 2, 3) but for RPM and SRTM2 a slightly better performance for AD patients compared to HCs was observed again. Although in certain scenarios, the coefficients of determination for the comparisons of the parametric methods with the gold standard were slightly better for AD patients, there is no clear indications that any of the parametric methods actually perform better for AD patients than HCs.
Irrespective of the scan duration (90 min or 60 min), similar coefficients of determination and slopes were observed when comparing the parametric estimations for SA, RPM, and SRTM2 with the respective NLR estimations. However, the TRT slightly improved when using 90 min data for these parametric methods than when using 60 min scan data. Unfortunately, 90 min scan data was only available for HCs in the current study, therefore, this assessment was not possible to be performed on AD patients scan data. Further research on use of 90 min scan data is warranted as an improved reproducibility of the parametric estimation for these methods will be beneficial for drug intervention studies.
A recent study by Mertens et al. [12] also evaluated different parametric methods for [11C]UCB-J. They concluded that both SRTM2 and MRTM1 provided good parametric maps compared to RLogan. They also observed that SRTM2 BPND had the best correspondence with plasma input derived DVR. The current study also showed that BPND estimates derived from SRTM2 had the best correspondence with NLR DVR estimates. However, the performance of MRTM1 and RLogan was not similar as described by Mertens et al. [12]. One explanation could be that Mertens et al. [12] used a fixed value for the tracer efflux rate constant (k2′) when using RLogan and MRTM1 as well. So therefore, the number of parameters was limited to two, which led to less noise on the parameter estimations and eventually led to better correspondence with NLR. The current study did not use a fixed value for k2′ for RLogan and MRTM1 implementations, thereby possibly explaining the observed difference in performance.
In a previous study from our group with regional NLR analysis, TRT for NLR VT and K1 was − 8% ± 4.3, − 2% ± 14.3 for HCs and 3% ± 8.1, 3% ± 13.6 for AD patients, respectively. TRT for Plasma-input (NLR) DVR was − 7% ± 6.2 for HCs and 7% ± 12.7 for AD patients. TRT for SRTM (NLR) derived DVR and R1 was − 6% ± 6.9, − 2% ± 6.3 for HCs and was − 5% ± 9.3, 2% ± 10.0 for AD patients, respectively. The HCs showed systematically lower values for the parameters VT, DVR and BPND for the retest scan. The reason for finding a negative bias in the retest scan for HCs is still unclear. No technical errors (e.g. related to data acquisition or data processing), diurnal variations or changes in food intake were detected that could explain this underestimation. In the current study, a similar pattern was observed with the parametric methods too but the negative bias was slightly smaller than with conventional methods. The TRT for SA VT was almost similar to the TRT of whole brain VT estimated by NLR. Even though, SA K1 had an underestimation with reference to the NLR K1 estimates but the TRT for SA K1 was slightly better for HCs when compared to the TRT of NLR K1. This could be because SA approach is more robust to noise than the NLR fitting algorithm. Furthermore, whole brain TRT for SRTM2 derived BPND was similar to SRTM (NLR) derived BPND. The TRT for SRTM2 R1 was slightly better for both groups compared to SRTM (NLR) derived R1. RLogan DVR had a remarkably better TRT than plasma input (NLR) DVR for both groups, however, there was an underestimation of 21.1% ± 9.3 for AD patients and 20.7% ± 8.4 for HCs when compared to plasma input (NLR) DVR and an underestimation of 9.8% ± 8.2 for AD patients and 8.8% ± 7.0 for HCs when compared to SRTM (NLR) DVR (BPND + 1), which makes RLogan not an optimal parametric method for quantification of.
In general, whole brain TRT was much better for HCs compared to AD patients for all evaluated parametric methods (Table 4). The larger variability of TRT in AD patients could be expected as the decrease in the synaptic density depends on the disease severity and the degree of atrophy that might vary from patient to patient. Although, in case of controls one would expect these differences to be minimal (no disease specific effects) and thereby a lower variability.
[11C]UCB-J will ultimately be used to determine synaptic density in the brain but it is also important to identify other parameters effecting the in vivo tracer kinetics such as influx and efflux of the tracer. The specific binding of the tracer is not just dependent on the availability of the receptors/targets of interest but also on the availability of the tracer. Therefore, in this study the focus was also to obtain quantitatively accurate K1 and R1 maps. As, they could help to monitor the delivery of the tracer such as influx and efflux which is of course beneficial to monitor changes in blood flow but also its effect on tracer delivery and clearance, changes that can be caused by the treatment/drug i.e. the response of a treatment, or by progression of disease.
One of the main limitations of the study was that no challenge study was performed to validate the use of white matter SO as reference region. Koole et al. [11] had validated the white matter SO as reference region for [11C]UCB-J PET in young healthy adults. However, the current study included relatively old HC subjects and AD patients. Since the SO is located in subcortical white matter, and white matter changes are prevalent findings in the elderly [32], re-validation of the reference region for [11C]UCB-J PET should be considered in further studies in older HC subjects and in patient groups. Another limitation of this study is that a few other plasma-input based voxel-level methods were not evaluated in this study, such as multilinear analysis [24], Empirical Bayesian Estimation in Graphical Analysis [33] and the Variational Bayesian inference method [34]. Similarly, for the reference region-based voxel-level methods, the reference region version of the Likelihood Estimation in Graphical Analysis [35] was not evaluated in this study. These methods could also be considered in further studies.
Conclusions
Among the parametric approaches assessed in the current study, both SA and SRTM2 were the optimal plasma-input and reference tissue parametric methods (in comparison to the 1T2k_VB model), respectively, to obtain quantitatively accurate and repeatable parametric images for [11C]UCB-J.
Supplementary Information
Additional file 1. Coefficients of determination for VTestimated using Logan and SA separately for (a), (c) test and (b), (d) retest data with corresponding NLR (1T2k_VB) VT estimates. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity.
Additional file 2. Scatterplots for VT estimated using SA for test and retest scans, separately for (a) HCs and (b) AD patients with corresponding NLR (1T2k_VB) VT estimates and separately color-coded for each subject. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity.
Additional file 3. Scatterplot for VT estimated using SA with corresponding NLR (1T2k_VB) VT estimates separately color-coded for each region.
Additional file 4. Scatterplot for K1 estimated using SA with corresponding NLR (1T2k_VB) K1 estimates separately color-coded for each region.
Additional file 5. Coefficients of determination for RLogan, RPM, SRTM2, and MRTM1 separately for (a), (c), (e), (g) test and (b), (d), (f), (h) retest data with corresponding NLR (1T2k_VB) estimates. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity; BPND: binding potential; DVR: distribution volume ratio; NLR: non-linear regression; RLogan: reference Logan; RPM: receptor parametric mapping; SRTM2: simplified reference tissue model 2; MRTM1: multilinear reference tissue model 1.
Additional file 6. Scatterplots for BPND estimated using RPM for test and retest scans, separately for (a) HCs and (b) AD patients with corresponding NLR (1T2k_VB) DVR-1 estimates and separately color-coded for each subject. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity.
Additional file 7. Scatterplots for BPND estimated using SRTM2 for test and retest scans, separately for (a) HCs and (b) AD patients with corresponding NLR (1T2k_VB) DVR-1 estimates and separately color-coded for each subject. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity.
Additional file 8. Scatterplot for BPND estimated using SRTM2 with corresponding NLR (1T2k_VB) DVR-1 estimates separately color-coded for each region.
Additional file 9. Scatterplot for R1 estimated using SRTM2 with corresponding NLR (SRTM) R1 estimates separately color-coded for each region.
Additional file 10. TRT repeatability voxel-wise image (average and SD) for AD patients separately for SA VT, RPM BPND and SRTM2 BPND.
Additional file 11. TRT repeatability voxel-wise image (average and SD) for HCs separately for SA VT, RPM BPND and SRTM2 BPND.
Additional file 12. The % bias (mean + SD) estimated for the parametric methods of interest against corresponding 1T2k_VB estimates using 60 minutes data.
Additional file 13. Coefficients of determination (r2) and slopes of parametric [11C]UCB-J VT, K1 and BPND against corresponding 1T2k_VB estimates separately for all subjects. All the Hammers ROIs were included for this analysis. Regional parametric values from both test and retest scans were pulled together for these comparisons.
Additional file 14. Coefficients of determination (r2) and slopes of parametric [11C]UCB-J BPND and R1 against corresponding SRTM estimates separately for all subjects. All the Hammers ROIs were included for this analysis. Regional parametric values from both test and retest scans were pulled together for these comparisons.
Additional file 15. The % bias (mean + SD) estimated for the parametric methods of interest against corresponding SRTM estimates using 60 minutes data.
Additional file 16. TRT (%) values estimated for specific brain regions (grey matter) are presented for SA VT and K1.
Additional file 17. TRT (%) values estimated for specific brain regions (grey matter) are presented for SRTM2 BPND and R1.
Additional file 18. TRT (%) values estimated for specific brain regions (grey matter) are presented for RPM BPND and R1.
Additional file 19. Coefficients of determination (r2) and slopes of parametric [11C]UCB-J VT, K1 and BPND against corresponding 1T2k_VB estimates for HCs using 90 minutes data. All the Hammers ROIs were included for this analysis.
Additional file 20. Coefficients of determination (r2) and slopes of parametric [11C]UCB-J BPND and R1 against corresponding SRTM estimates using 90 minutes data. All the Hammers ROIs were included for this analysis.
Additional file 21. TRT (%) values estimated for whole brain (grey matter) are presented for each parametric method using 90 minutes data.
Abbreviations
- 2T4k_VB
Reversible 2 tissue compartment model with blood volume correction
- [11C]UCB-J
(R)-1-((3-([11C]methyl)pyridin-4-yl)methyl)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one
- AD
Azheimer’s disease
- Amsterdam UMC
Amsterdam University Medical Center
- BPND
Binding potential
- CT
Computed tomography
- DVR
Distribution volume ratio
- HC
Healthy control
- K1
Rate of influx of radioligand from blood to tissue
- Logan
Plasma input Logan
- MMSE
Mini-Mental State Examination
- MREC
Medical Review and Ethics Committee
- MRTM
Multilinear reference tissue model
- NFTs
Neurofibrillary tangles
- NIA-AA
National Institute on Aging—Alzheimer’s Association
- NLR
Nonlinear regression
- OSEM
Ordered-subsets-expectation–maximization
- PET
Positron emission tomography
- Rlogan
Reference Logan
- ROI
Regions of interest
- RPM
Receptor parametric mapping
- SA
Spectral analysis
- SO
Semi-ovale
- SRTM2
Simplified reference tissue model 2
- SUVr
Standardized uptake value ratio
- SV2A
Synaptic vesicle protein 2A
- TAC
Time activity curve
- TOF
Time of flight
- TRT
Test–retest repeatability
- VT
Total volume of distribution
Authors' contributions
HT, SG, SS, MR, MI, BL and RB contributed to the concept and design of the study. EC, EW, PKF, AG, DVG, PaS, PhS, RS, AW and BB acquisition of the data. HT, SG and RB contributed to the analysis and interpretation of the data. HT drafted the manuscript. HT, SG, EC, EW, PKF, AG, DVG, PDD AW, BB, EV, SV, and RB read, critically reviewed, and approved the manuscript. All authors read and approved the final manuscript.
Funding
This research was made possible by Rodin Therapeutics Inc. by providing funding for the study.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Medical Review and Ethics Committee (MREC) of Foundation BEBO in Assen approved the current study and local feasibility was confirmed by the MREC of Amsterdam UMC. All subjects signed an informed consent form prior to participation.
Consent for publication
Not applicable.
Competing interests
No potential competing of interest relevant to this article exist.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimer's Dementia. 2012;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer's disease. Neuron. 2013;80(6):1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pozueta J, Lefort R, Shelanski ML. Synaptic changes in Alzheimer's disease and its models. Neuroscience. 2013;251:51–65. doi: 10.1016/j.neuroscience.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 4.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27(5):457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 5.Hamos JE, DeGennaro LJ, Drachman DA. Synaptic loss in Alzheimer's disease and other dementias. Neurology. 1989;39(3):355–361. doi: 10.1212/WNL.39.3.355. [DOI] [PubMed] [Google Scholar]
- 6.Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 1994;14(9):5223–5235. doi: 10.1523/JNEUROSCI.14-09-05223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madeo M, Kovacs AD, Pearce DA. The human synaptic vesicle protein, SV2A, functions as a galactose transporter in Saccharomyces cerevisiae. J Biol Chem. 2014;289(48):33066–33071. doi: 10.1074/jbc.C114.584516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabulsi NB, Mercier J, Holden D, Carre S, Najafzadeh S, Vandergeten MC, et al. Synthesis and preclinical evaluation of 11C-UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med. 2016;57(5):777–784. doi: 10.2967/jnumed.115.168179. [DOI] [PubMed] [Google Scholar]
- 9.Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8(348):348ra96. doi: 10.1126/scitranslmed.aaf6667. [DOI] [PubMed] [Google Scholar]
- 10.Tuncel H, Boellaard R, Coomans EM, de Vries EF, Glaudemans AW, Feltes PK, et al. Kinetics and 28-day test-retest repeatability and reproducibility of [(11)C]UCB-J PET brain imaging. J Cereb Blood Flow Metab. 2021;41(6):1338–1350. doi: 10.1177/0271678X20964248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koole M, van Aalst J, Devrome M, Mertens N, Serdons K, Lacroix B, et al. Quantifying SV2A density and drug occupancy in the human brain using [(11)C]UCB-J PET imaging and subcortical white matter as reference tissue. Eur J Nucl Med Mol Imaging. 2019;46(2):396–406. doi: 10.1007/s00259-018-4119-8. [DOI] [PubMed] [Google Scholar]
- 12.Mertens N, Maguire RP, Serdons K, Lacroix B, Mercier J, Sciberras D, et al. Validation of parametric methods for [(11)C]UCB-J PET imaging using subcortical white matter as reference tissue. Mol Imag Biol. 2020;22(2):444–452. doi: 10.1007/s11307-019-01387-6. [DOI] [PubMed] [Google Scholar]
- 13.Alkermes I. A 28 day parallel group study to assess the effects of RDN-929 U.S. National Library of Medicine2019. Updated 20 Feb 2020. https://clinicaltrials.gov/ct2/show/record/NCT03963973?view=record.
- 14.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tijms BM, Willemse EAJ, Zwan MD, Mulder SD, Visser PJ, van Berckel BNM, et al. Unbiased approach to counteract upward drift in cerebrospinal fluid amyloid-beta 1–42 analysis results. Clin Chem. 2018;64(3):576–585. doi: 10.1373/clinchem.2017.281055. [DOI] [PubMed] [Google Scholar]
- 16.Boellaard R, van Lingen A, van Balen SC, Hoving BG, Lammertsma AA. Characteristics of a new fully programmable blood sampling device for monitoring blood radioactivity during PET. Eur J Nucl Med. 2001;28(1):81–89. doi: 10.1007/s002590000405. [DOI] [PubMed] [Google Scholar]
- 17.Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19(4):224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbol S, Frokjaer VG, et al. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24(4):969–979. doi: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Boellaard R, Yaqub M, Lubberink M, Lammertsma A. PPET: a software tool for kinetic and parametric analyses of dynamic PET studies. NeuroImage. 2006;31.
- 20.Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10(5):740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 21.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Ichise M, Ballinger JR. From graphical analysis to multilinear regression analysis of reversible radioligand binding. J Cereb Blood Flow Metab. 1996;16(4):750–752. doi: 10.1097/00004647-199607000-00028. [DOI] [PubMed] [Google Scholar]
- 23.Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23(9):1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- 24.Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab. 2002;22(10):1271–1281. doi: 10.1097/01.WCB.0000038000.34930.4E. [DOI] [PubMed] [Google Scholar]
- 25.Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6(4):279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22(12):1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham VJ, Jones T. Spectral analysis of dynamic PET studies. J Cereb Blood Flow Metab. 1993;13(1):15–23. doi: 10.1038/jcbfm.1993.5. [DOI] [PubMed] [Google Scholar]
- 28.Yaqub M, Boellaard R, Kropholler MA, Lammertsma AA. Optimization algorithms and weighting factors for analysis of dynamic PET studies. Phys Med Biol. 2006;51(17):4217–4232. doi: 10.1088/0031-9155/51/17/007. [DOI] [PubMed] [Google Scholar]
- 29.Slifstein M, Laruelle M. Effects of statistical noise on graphic analysis of PET neuroreceptor studies. J Nucl Med. 2000;41(12):2083–2088. [PubMed] [Google Scholar]
- 30.Rossano S, Toyonaga T, Finnema SJ, Naganawa M, Lu Y, Nabulsi N, et al. Assessment of a white matter reference region for (11)C-UCB-J PET quantification. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2019:271678x19879230. [DOI] [PMC free article] [PubMed]
- 31.O'Brien JS, Sampson EL. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J Lipid Res. 1965;6(4):537–544. doi: 10.1016/S0022-2275(20)39619-X. [DOI] [PubMed] [Google Scholar]
- 32.Xiong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011:617927. doi: 10.4061/2011/617927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanderigo F, Ogden RT, Bertoldo A, Cobelli C, Mann JJ, Parsey RV. Empirical Bayesian estimation in graphical analysis: a voxel-based approach for the determination of the volume of distribution in PET studies. Nucl Med Biol. 2010;37(4):443–451. doi: 10.1016/j.nucmedbio.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castellaro M, Rizzo G, Tonietto M, Veronese M, Turkheimer FE, Chappell MA, et al. A Variational Bayesian inference method for parametric imaging of PET data. Neuroimage. 2017;150:136–149. doi: 10.1016/j.neuroimage.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Zanderigo F, Ogden RT, Parsey RV. Reference region approaches in PET: a comparative study on multiple radioligands. J Cereb Blood Flow Metab. 2013;33(6):888–897. doi: 10.1038/jcbfm.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Coefficients of determination for VTestimated using Logan and SA separately for (a), (c) test and (b), (d) retest data with corresponding NLR (1T2k_VB) VT estimates. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity.
Additional file 2. Scatterplots for VT estimated using SA for test and retest scans, separately for (a) HCs and (b) AD patients with corresponding NLR (1T2k_VB) VT estimates and separately color-coded for each subject. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity.
Additional file 3. Scatterplot for VT estimated using SA with corresponding NLR (1T2k_VB) VT estimates separately color-coded for each region.
Additional file 4. Scatterplot for K1 estimated using SA with corresponding NLR (1T2k_VB) K1 estimates separately color-coded for each region.
Additional file 5. Coefficients of determination for RLogan, RPM, SRTM2, and MRTM1 separately for (a), (c), (e), (g) test and (b), (d), (f), (h) retest data with corresponding NLR (1T2k_VB) estimates. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity; BPND: binding potential; DVR: distribution volume ratio; NLR: non-linear regression; RLogan: reference Logan; RPM: receptor parametric mapping; SRTM2: simplified reference tissue model 2; MRTM1: multilinear reference tissue model 1.
Additional file 6. Scatterplots for BPND estimated using RPM for test and retest scans, separately for (a) HCs and (b) AD patients with corresponding NLR (1T2k_VB) DVR-1 estimates and separately color-coded for each subject. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity.
Additional file 7. Scatterplots for BPND estimated using SRTM2 for test and retest scans, separately for (a) HCs and (b) AD patients with corresponding NLR (1T2k_VB) DVR-1 estimates and separately color-coded for each subject. AD: Alzheimer’s disease; HC: healthy control; LOI: line of identity.
Additional file 8. Scatterplot for BPND estimated using SRTM2 with corresponding NLR (1T2k_VB) DVR-1 estimates separately color-coded for each region.
Additional file 9. Scatterplot for R1 estimated using SRTM2 with corresponding NLR (SRTM) R1 estimates separately color-coded for each region.
Additional file 10. TRT repeatability voxel-wise image (average and SD) for AD patients separately for SA VT, RPM BPND and SRTM2 BPND.
Additional file 11. TRT repeatability voxel-wise image (average and SD) for HCs separately for SA VT, RPM BPND and SRTM2 BPND.
Additional file 12. The % bias (mean + SD) estimated for the parametric methods of interest against corresponding 1T2k_VB estimates using 60 minutes data.
Additional file 13. Coefficients of determination (r2) and slopes of parametric [11C]UCB-J VT, K1 and BPND against corresponding 1T2k_VB estimates separately for all subjects. All the Hammers ROIs were included for this analysis. Regional parametric values from both test and retest scans were pulled together for these comparisons.
Additional file 14. Coefficients of determination (r2) and slopes of parametric [11C]UCB-J BPND and R1 against corresponding SRTM estimates separately for all subjects. All the Hammers ROIs were included for this analysis. Regional parametric values from both test and retest scans were pulled together for these comparisons.
Additional file 15. The % bias (mean + SD) estimated for the parametric methods of interest against corresponding SRTM estimates using 60 minutes data.
Additional file 16. TRT (%) values estimated for specific brain regions (grey matter) are presented for SA VT and K1.
Additional file 17. TRT (%) values estimated for specific brain regions (grey matter) are presented for SRTM2 BPND and R1.
Additional file 18. TRT (%) values estimated for specific brain regions (grey matter) are presented for RPM BPND and R1.
Additional file 19. Coefficients of determination (r2) and slopes of parametric [11C]UCB-J VT, K1 and BPND against corresponding 1T2k_VB estimates for HCs using 90 minutes data. All the Hammers ROIs were included for this analysis.
Additional file 20. Coefficients of determination (r2) and slopes of parametric [11C]UCB-J BPND and R1 against corresponding SRTM estimates using 90 minutes data. All the Hammers ROIs were included for this analysis.
Additional file 21. TRT (%) values estimated for whole brain (grey matter) are presented for each parametric method using 90 minutes data.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.