Abstract
A nested PCR (nPCR) assay for the detection of canine coronavirus (CCV) in fecal samples is described. The target sequence for the assay was a 514-bp fragment within the spike (S) glycoprotein gene. The sensitivity of the assay is extremely high, detecting as little as 25 50% tissue culture infective doses per g of unprocessed feces. A clinical trial using dogs challenged orally with CCV SA4 and CCV NVSL was used to compare viral isolation and the nPCR assay as detection techniques over a 2-week period of infection. Virus isolation detected CCV shedding from day 4 to 9 postchallenge, while the nPCR assay detected CCV shedding from day 4 to 13 postchallenge. Cloning and sequencing of the nPCR assay product enabled investigation of the evolutionary relationships between strains within the S gene. The simple and rapid procedure described here makes this assay an ideal alternative technique to electron microscopy and viral isolation in cell culture for detection of CCV shedding in feces. The described assay also provides a method of identifying new strains of CCV without the complicated and time-consuming practice of raising antibodies to individual strains. This is illustrated by the identification, for the first time, of an Australian isolate of CCV (UWSMN-1).
Canine coronavirus (CCV) is a single-stranded, positive-sense RNA viral pathogen of dogs that usually produces symptoms varying from mild to moderate gastroenteritis (1–3, 20, 26). In young or stressed animals, or in combination with other pathogens such as canine parvovirus, symptoms are more severe or fatal (1). Serological testing of antibodies by serum neutralization (15) or indirect enzyme-linked immunosorbent assay (ELISA) (19, 27) provides an indication of the exposure of an animal to CCV. Detection of anti-CCV immunoglobulin M (IgM) and anti-CCV IgG class immunoglobulins by indirect ELISA (16, 26) enables current or previous exposure to CCV, respectively, to be determined. However, definitive identification of CCV-induced disease can only be established by the identification of CCV shedding in feces by either electron microscopy or virus isolation in cell culture. This situation is further complicated by the fact that many workers have experienced difficulties in cultivating coronaviruses in vitro (5, 29).
The PCR has been utilized as a detection technique for canine viral pathogens such as canine parvovirus from feces (10, 22, 28). A nested PCR (nPCR) assay has also been described for feline infectious peritonitis virus (7), a closely related coronavirus, and more recently an nPCR assay for the detection of CCV based on primers to the transmembrane protein M gene has been described (17).
The S gene of the coronavirus family has a variable region close to the 5′ end and is involved in antigenic differences between strains (for a review, see reference 20). Recombinant strains of coronavirus exist that have a spike (S) gene originally derived from coronaviruses of other species (9). While coronaviruses are known to undergo frequent recombination events in vitro (12, 13), the frequency of these occurrences in the field is unknown, but such events are suspected to be an important means of avoiding host immunity (9).
In Australia, as elsewhere, field samples of CCV have been found to be difficult to culture, with several failed attempts having been reported (6, 14, 21). Despite identification of CCV and coronavirus-like particles with electron microscopic studies of fecal samples from Australian dogs (6, 14, 21), the prevalence of CCV in the Australian dog population has only recently been firmly established using indirect ELISA to detect anti-CCV IgG and IgM antibodies (16). However, without cultivation of CCV, determination of specific strains responsible for enteric outbreaks is difficult. Thus, based on known DNA sequences of the CCV S protein gene (30), we describe here the development of an nPCR assay for the detection and identification of different CCV strains from feces. This has allowed detection of a novel CCV isolate from an Australian dog with fatal gastroenteritis.
MATERIALS AND METHODS
Virus and cells.
Crandell feline kidney (CRFK) cells originally derived from domestic cat kidney (4) were obtained from Fort Dodge Laboratories, Fort Dodge, Iowa. CCV strains NVSL, SA4 and TN449 were also obtained from Fort Dodge Laboratories (from master seed stock) and were used at passage <20.
Cell culture.
CRFK cells were propagated in growth medium containing essential minimum Earl salts medium (EMEM) (Trace Biosciences, Sydney, Australia), 2 mM l-glutamine, 0.05% lactalbumin hydrolysate and 10% fetal bovine serum, not inactivated (FBSNI) (CSL Biosciences, Melbourne, Australia). Maintenance medium for maintaining confluent cells consisted of EMEM, 2 mM l-glutamine, 0.05% lactalbumin hydrolysate and 5% FBSNI.
CCV clinical trial.
Specific-pathogen-free dogs were maintained for a period of 1 year and were bled weekly. Titers of antibody to CCV were determined by indirect ELISA, with all dogs found seronegative to CCV over the 52-week period (data not shown). At 52 weeks, each animal was challenged orally with a viral titer of 108 50% tissue culture infective doses (TCID50)—an equal mixture of CCV SA4 and CCV NVSL.
Specimen processing.
Feces were collected daily, 2 days before oral challenge, and for a period of 14 days postchallenge. Fecal samples were prepared for both virus isolation and RNA extraction as a 10% suspension in maintenance media. The sample was centrifuged for 5 min at 3,000 × g before serial filtration through 0.8-, 0.45-, and 0.2-μm-pore-size Minisart filters (Sartorius AG, Goettingen, Germany), and stored at −70°C.
Field survey.
Feces were collected from 15 dogs suspected of having CCV infection. In the case of field sample UWSMN-1, blood, feces, and fresh and formalin-fixed tissue specimens were collected at necropsy following fatal gastroenteritis in an 8-week-old pup.
Virus isolation.
Virus isolation and titration were performed by inoculating 10−1 to 10−7 dilutions of processed fecal samples onto 96-well microtitration cell culture plates (Nunc, Roskilde, Denmark) seeded with CRFK cells at 80% confluency and incubated for 48 h at 37°C in a CO2 (4 to 6%) incubator. Plates were fixed with 80% acetone for 30 min at −20°C, dried at room temperature, and stained with 50 μl of 1:100 anti-CCV direct fluorescent antibody conjugate (American BioResearch, Sevierville, Tenn.) per well for 30 min at 37°C in a CO2 (4 to 6%) incubator. The fluorescent antibody was decanted, and plates were washed three times with rinse buffer (27 μM Na2CO3, 100 μM NaHCO3 and 36 μM NaCl) before being scored for the presence of CCV. The tissue culture infectious dose (TCID50 per milliliter) at 50% was determined as previously described (8).
RNA isolation.
RNA was isolated from the processed fecal samples using Total RNA isolation reagent (Advanced Biotechnologies, Surrey, United Kingdom), following the manufacturer's instructions. cDNA synthesis was performed using avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wis.) according to the manufacturer's instructions, with the exception of using 1 μl of RNA preparation with 125 pmol of CCVR1 primer in place of oligo(dT) primer.
nPCR assay.
PCR primers were chosen for conserved sites flanking regions of variability on the basis of mismatch to other CCV and feline coronavirus (FoCV) species within the S gene. First-round PCR was performed using primers CCVF1 (TAATGTGACACAAYTGCCTGGCAATG [positions 201 to 227]) and CCVR1 (CTGTAGAAACTYGACTCACTCACTG [positions 1261 to 1286]). Primers CCVF2 (GTACTGGCAATGCAMGWGGTAAACC [positions 403 to 428]) and CCVR2 (ACRTTGGTNGCATAGCCAGTGCA [positions 895 to 917]) were used for second-round amplification. Numbering is from the 5′ end of the S gene of CCV-K378, according to that of Wesseling et al. (30) (note: Y = C or T; R = A or G; N = A, G, C, or T; W = A or T). PCRs were performed in a 50-μl reaction volume containing 5 μl of cDNA, 25 mM MgCl2, 5 μl of 10× PCR buffer (containing 100 mM Tris-HCl and 500 mM KCl), 54 pmol of each primer, a 200 μM concentration of each deoxynucleoside triphosphate, and 3.5 U of Ampli Taq Gold enzyme (Perkin-Elmer, Foster City, Calif.). The temperature regimen consisted of a 94°C 10-min denaturation cycle followed by 94°C for 25 s, 58°C for 30 s, and 72°C for 2 min, for 33 cycles. An elongation step of 72°C for 5 min ended the PCR. The product of the first-round PCR was diluted 1:50 with nuclease-free water (Promega), and 5 μl was used in the second-round amplification. Cycling conditions for the second PCR were identical to those described for the first-round amplification.
The final amplified products were detected by electrophoresis through a 1.2% agarose gel in 1× TBE (90 mM Tris-borate and 2 mM EDTA) running buffer. The gel was then stained in a solution of ethidium bromide (10 g/ml) and photographed under UV light.
The specificity of the designated primers was examined using CCV-TN449-infected CRFK cells 48 h postinfection and noninfected CRFK control cells. Optimization of the nPCR assay was performed using feces from dogs, 5 days post-CCV SA4 and CCV NVSL oral challenge as a positive control and using feces from pre-viral challenge specific-pathogen-free dogs as a negative control. The analytical sensitivity of the assay was determined using CCV serially diluted in feces from noninfected pups.
DNA sequencing.
The PCR products were purified from agarose gels using a Bresa-Clean DNA purification kit (Geneworks, Adelaide, Australia). DNA was cloned using the pGEM-T easy Vector system II (Promega) according to the manufacturer's instructions. Nucleotide sequencing was performed in both orientations by automated sequencing at Newcastle DNA (University of Newcastle, Newcastle, Australia).
Sequence analysis.
Alignments of the DNA sequences from different CCV strains were made using the PILEUP program of the Genetics Computer Group package through the Australian National Genomic Information Service (Sydney University, Sydney, Australia). Percentage identities were calculated using the HOMOLOGIES program from the same package. A DNA parsimony phylogenetic tree was constructed by first using the Genetics Computer Group implementation of the PHYLIP package, ESEQBOOT, to generate 1,000 bootstrap sampling data sets and then analyzing these with the EDNAPARS program to find the most parsimonious trees. A consensus tree was constructed from these data using the ECONSENSE program of the PHYLIP package.
Nucleotide sequence accession number.
The sequence of the S gene product for CCV UWSMN-1 has been deposited in GenBank and assigned accession number AF327928.
RESULTS
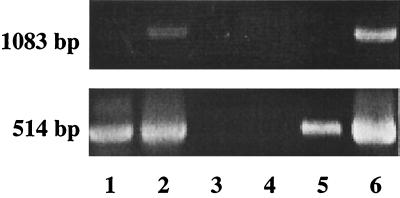
The nPCR assay demonstrated increased sensitivity in comparison to PCR performed using only first-round primers CCVF1 and CCVR1 (Fig. 1). After second-round amplification, the nested product appears as a 514-bp fragment (Fig. 1).
FIG. 1.
Comparison of PCR and nPCR amplification of different cell culture-propagated CCV strains, processed as described in Materials and Methods. First-round amplification resulted in the predicted 1,083-bp product, while second-round amplification produced a 514-bp product. Lane 1, CCV SA4; lane 2, CCV NVSL; lane 3, water control; lane 4, negative control (noninfected CRFK cells); lane 5, CCV TN449 (low titer); lane 6, CCV TN449 (high titer).
Serial dilutions of CCV-infected fecal samples were used to determine the limit of sensitivity of the nPCR assay, which was found to be 2.5 TCID50 per reaction (Table 1). A detection limit of 2.5 TCID50 of virus per reaction approximately corresponds to a viral titer of 25 TCID50 per g of unprocessed feces. The primers were designed on conserved regions within the S protein gene of a consensus of CCV and FoCV. The assay demonstrated the ability to detect serological variants of CCV, including CCV strains TN449, SA4, and NVSL (Fig. 1).
TABLE 1.
Detection limit of nPCR assay determined with CCV-spiked fecal samples as described in Materials and Methods
| Amt of virus (TCID50)/reaction | nPCR assaya |
|---|---|
| 250 | + |
| 40 | + |
| 25 | + |
| 4 | + |
| 2.5 | + |
| 0.4 | − |
The nPCR assay was scored as either positive (+) or negative (−).
To confirm that the nPCR assay could be used as a suitable diagnostic field test, we compared virus isolation and the nPCR assay using experimentally infected pups over a 2-week period post-viral challenge. Virus isolation technique detected CCV in the feces of the two dogs examined from day 4 postchallenge up to day 9 (Table 2). The nPCR assay also detected the commencement of CCV shedding again from day 4 up to day 9 but could also detect viral shedding intermittently up to day 13. On nine occasions, CCV was detected by the nPCR assay when viral particles were not detected by isolation techniques (Table 2).
TABLE 2.
Comparison of virus isolation and nPCR assay as detection methodsa
| Animal and detection method | Result at day postchallenge
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| Dog 167 | ||||||||||||||||||
| VIb | 0 | 0 | 0 | 0 | 0 | 0 | 1.6 | 2.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| PCRc | − | − | − | − | − | − | + | + | + | + | + | + | − | + | − | + | − | |
| Dog 173 | ||||||||||||||||||
| VI | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 | 1.6 | 2.4 | 0.4 | 1.0 | 0.6 | 0 | 0 | 0 | 0 | 0 | |
| PCR | − | − | − | − | − | − | + | + | + | + | + | + | − | + | + | + | − | |
Two dogs were challenged orally with 108 virus particles at day 0.
VI, virus isolation. Virus isolation titers are given in log TCID50 per milliliter.
The nPCR assay was scored as either positive (+) or negative (−).
Comparison of the nPCR assay and viral isolation techniques was also performed in a preliminary field screen of dogs demonstrating signs of potential CCV infection or of animals from sites of recent gastroenteric disease outbreaks. Of the 15 dogs examined in the preliminary field screen, all dogs were found negative for CCV by virus isolation in CRFK cells. In contrast, the nPCR assay detected CCV in the feces from 1 of the 15 dog field samples. Isolate UWSMN-1 was obtained from an 8-week-old pup presented with fatal gastroenteritis during an outbreak of diarrhea in commercial breeding premises.
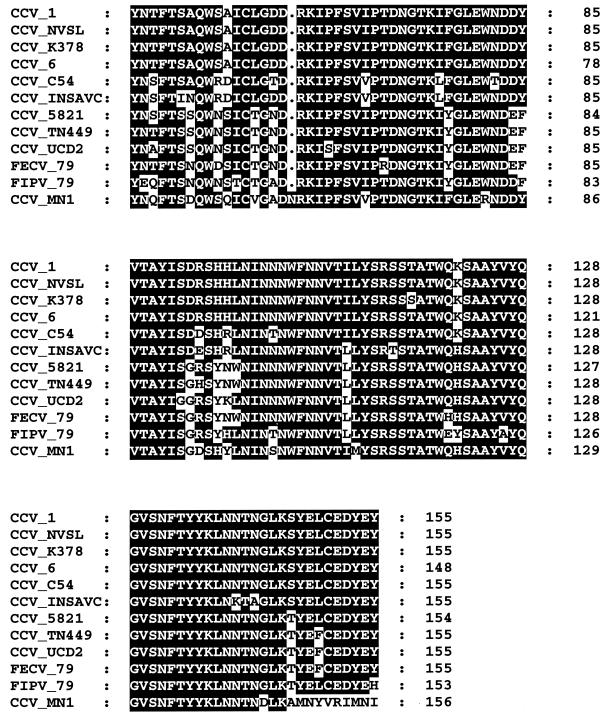
To establish nPCR assay specificity for CCV, sequencing of the nPCR product from field sample UWSMN-1 and positive control samples (CCV TN449, CCV NVSL, and CCV SA4) was performed. Sequencing identified CCV S-gene-specific sequences (Fig. 2). UWSMN-1 contains a codon (AAC) insertion at position 82, which results in the deduced insertion of an asparagine residue between aspartic acid and arginine residues which are conserved in other CCV and FoCV strains. UWSMN-1 also contains a 1-bp deletion at position 444 (Fig. 2) in relation to other CCV strains, and this leads to the 10 deduced amino acids at the end of the sequence being very different from those in other CCVs (Fig. 3).
FIG. 2.
Nucleotide and amino acid sequences of the nPCR assay S gene product for CCV UWSMN-1.
FIG. 3.
Amino acid sequence alignment of nPCR assay S gene product for CCV 1–71 (AF116246), CCV NVSL (AF116244), CCV K378 (X77047), CCV 6 (A22882), CCV C54 (A22886), CCV INSAVC (D13096), CCV 5821 (AB017789), CCV TN449 (AF116245), CCV UCD2 (AF116245), FoCV (FECV) 79–1683 (X80799), feline infectious peritonitis virus 79–1146 (X06170), and CCV UWSMN-1 (AF327928) (GenBank accession numbers are denoted in parentheses). Shaded regions indicate amino acid residues conserved among the different CCV and FoCV strains. Variable regions of the consensus sequence are indicated by white boxing.
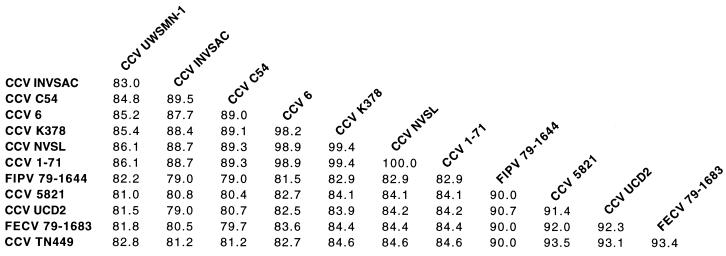
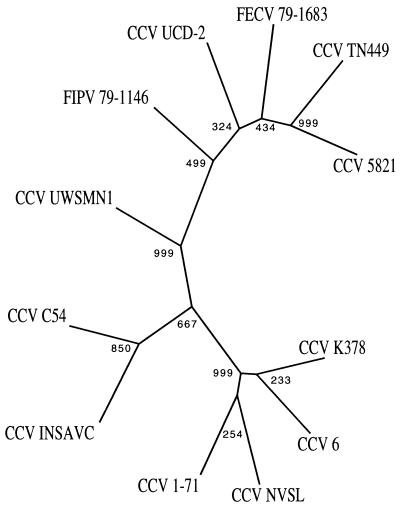
To examine the relationships between the Australian strain of CCV and other previously described strains of CCV within the S gene, the percentage identities between the different strains of CCV were calculated (Fig. 4). The Australian field sample had the least amount of identity to any of the other strains, with only 86.1% homology to its most closely related strains, CCV NVSL and CCV 1-71 (Fig. 4). Phylogenetic analysis was performed to create an unrooted tree of the relationships between different CCV strains. This tree is based on DNA rather than protein parsimony as it was felt that the frameshift mutation in the Australian isolate sequences mentioned above would bias the relationships. The tree (Fig. 5) shows that the S gene sequences of most of the coronavirus strains cluster into two main clades that correspond to typical CCV and FoCV types. Note that S gene sequences of several CCV strains (UCD-2, TN449, and 5821) are grouped with the FoCVs in this tree. This close relationship between S gene sequences of some CCV and FoCV strains has previously been noted, and it has been proposed that this has arisen from recombination between CCV and FoCV strains (9, 11, 29). Interestingly, the Australian field isolate forms a discrete branch which is intermediate between those of the CCV and FoCV S gene sequences.
FIG. 4.
Percentage of nucleotide identity between the S gene variable region sequence of CCV strain UWSMN-1 and other CCV and FoCV strains denoted in Fig. 3.
FIG. 5.
Phylogenic tree of CCV and FoCV S gene sequences based on DNA parsimony using the PHYLIP package as described in the Materials and Methods. Bootstrap values indicate the number of times out of 1,000 iterations that a branch point was identified.
DISCUSSION
The nPCR assay described in this study was based on primer sites within conserved regions of the S gene that flank a region of variability. It was hypothesized that by basing primer design on conserved regions, the assay would be able to detect a variety of serologically different CCV strains. This proved correct as the assay was able to detect several different strains of CCV, including TN449, NVSL, SA4, and an Australian field strain (UWSMN-1). Sequencing confirmed the assay's ability to amplify CCV-specific products, and the relatively low identity between the Australian field strain and the other well-characterized strains further demonstrates that the assay is able to detect diverse strains of CCV, while still providing informative sequence data for distinguishing closely related strains. The sensitivity of this nPCR assay is comparable to that of other coronavirus nPCR assays (7, 17). With a detection limit of 25 TCID50 per g of unprocessed feces, the assay is approximately 4 × 104 times more sensitive than electron microscopy, which has a reported detection limit of approximately 106 particles per g of unprocessed feces (18). Sequencing of the PCR product positively identifies CCV-specific products, avoiding the possibility of false-positive results commonly associated with electron microscopy.
Epidemics of CCV in kennels have been reported worldwide (3, 16, 19, 24), and chronic infection of animals has been proposed as a source for persistent reinfection in colonies (25). CCV has been demonstrated to be shed intermittently (26). We detected CCV shedding from day 4 postchallenge up to day 9 by virus isolation, whereas intermittent viral shedding up to 13 days postchallenge was detected by the nPCR assay. The ability of the nPCR assay to detect viral shedding beyond the detection limit of viral isolation demonstrates the increased sensitivity of the assay over viral isolation techniques in the detection of field isolates.
The application of the nPCR assay to the preliminary field screen further demonstrates the advantages of the nPCR assay as a CCV detection technique. In the example of case UWSMN-1, the animal presented with fatal gastroenteritis, unlike some previous descriptions of the mild disease normally associated with CCV infection. Without detection of viral shedding, it is difficult to conclude whether CCV is the causative agent of gastroenteritis, as mixed viral infections may occur (1, 16). CCV infection may also be confused with canine parvovirus infections as they display broadly similar clinical and pathological features. To further clarify the presence of CCV infection we examined fecal and intestinal cellular material for the presence of CCV by both virus isolation and nPCR assay techniques. Virus isolation failed to identify CCV, as did further attempts to cultivate CCV from these samples. However, CCV was identified by the nPCR assay. The identification of CCV shedding identifies CCV as a possible cause of canine gastroenteritis in Australia.
It is known that coronavirus recombinations occur frequently in vitro (12, 13). There is also growing evidence that coronavirus recombinations also occur in the field, although the frequency of these events is unknown. FoCV type II strains (79–1683 and 79–1146) have been demonstrated as arising from double recombination events between FoCV type I strains and CCV (9). The FoCV type II strains have CCV-like S genes, and the authors speculated that the transfer of these genes may provide some sort of growth advantage or escape from immune response (9). Recently, the S gene of CCV strain UCD-1 was shown to be more closely related to those of porcine transmissible gastroenteritis virus rather than CCV strains (29). CCV 5821 was also found to have an S gene more closely related to those of FoCV (11), suggesting that recombinant CCV strains may also occur in the field. Therefore, one possible explanation for the intermediate relationship of the Australian CCV strain UWSMN-1 revealed in our phylogenetic tree is that the S genes of this strain have arisen by homologous recombination between CCV and FoCV. However, if this were a relatively recent event then these sequences would be expected to share discrete blocks of homology with either the typical CCV or FoCV S sequences. As indicated in Fig. 3, this is not the case, as the differences between UWSMN-1 and the CCVs and FoCVs are widely dispersed, and therefore UWSMN-1 does not appear to simply represent a chimera between CCV and FoCV sequences. This finding is more consistent with the Australian field isolate being relatively distantly related to other CCVs. It is tempting to speculate that UWSMN-1 could represent sequences from the coronavirus-like particles that have been described in two electron microscopic studies in Australia rather than classic CCVs. However, confirmation of these notions will require further investigation, including sequencing of other regions from these isolates and viral isolation. The assay described herein provides a diagnostic test that can be used to diagnose CCV infection and monitor the divergence and evolution of field strains responsible for epidemic outbreaks, without the need for cultivation or raising of antibodies against specific strains.
ACKNOWLEDGMENTS
M. J. Naylor was supported by a Cooperative Education for Enterprise Development grant funded by Fort Dodge Australia Pty Ltd and the University of Western Sydney, Nepean, Australia.
REFERENCES
- 1.Appel M J G. Does canine coronavirus augment the effects of subsequent parvovirus infection? Vet Med Small Anim Clin. 1988;83:360–366. [Google Scholar]
- 2.Binn L N, Lazar E, Keenan K P, Huxsoll D, Marchwicki R H, Strano A J. Recovery and characterization of a coronavirus from military dogs with diarrhea. Proc Annu Meet U S Anim Health Assoc. 1975;78:359–366. [PubMed] [Google Scholar]
- 3.Carmichael, L. E. 1978. Infectious canine enteritis caused by corona-like virus: current status and request for information. Baker Inst. Lab. Report. Series 2, number 9.
- 4.Crandell R A, Fabricant C G, Nelson Rees W A. Development, characterisation, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK) In Vitro. 1973;9:176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- 5.DeGroot R J, Horzinek M C. Feline infectious peritonitis. In: Siddell S G, editor. The Coronaviridae. New York, N.Y: Plenum Press; 1995. pp. 293–315. [Google Scholar]
- 6.Finlaison D S. Faceal viruses of dogs—an electron microscope study. Vet Microbiol. 1995;46:295–305. doi: 10.1016/0378-1135(95)00094-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamble D A, Lobbiani A, Gramegna M, Moore L E, Colucci G. Development of a nested PCR assay for detection of feline infectious peritonitis virus in clinical specimens. J Clin Microbiol. 1997;35:673–675. doi: 10.1128/jcm.35.3.673-675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaskell R M, Gaskell C J, Dennis D E, Wooldbridge M J A. Efficacy of an inactivated feline calicivirus (FCV) vaccine against challenge with United Kingdom strains and its interaction with the FCV carrier state. Res Vet Sci. 1982;32:23–26. [PubMed] [Google Scholar]
- 9.Herrewegh A A P M, Smeenk I, Horzinek M C, Rottier P J M, de Groot R J. Feline coronavirus type II strains 79–1683 and 79–1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J Virol. 1998;72:4508–4514. doi: 10.1128/jvi.72.5.4508-4514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Wang J, Graham D Y, Estes M K. Detection of Norwalk virus in stool by polymerase chain reaction. J Clin Microbiol. 1992;30:2529–2534. doi: 10.1128/jcm.30.10.2529-2534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokubu T, Taharaguchi S, Katayama S, Hatano M, Takahashi T, Iwamoto K, Masubuchi K, Yamanaka M, Sasaki O, Inaba Y. Nucleotide sequence of the spike protein of canine coronavirus strain 5821. J Jpn Vet Med Assoc. 1998;51:251–255. [Google Scholar]
- 12.Lai M M C, Baric R C, Makino S, Keck J G, Engbert J, Leibowitz J L, Stohlman S A. Recombination between nonsegmented RNA genomes of murine coronavirus. J Virol. 1985;56:449–456. doi: 10.1128/jvi.56.2.449-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makino S, Keck J G, Stohlman S A, Lai M M C. High-frequency RNA recombination of murine coronavirus. J Virol. 1986;57:729–739. doi: 10.1128/jvi.57.3.729-737.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall J A, Healy D S, Studdert M J, Scott P C, Kennett M L, Ward B K, Gust I D. Viruses and virus-like particles in the faeces of dogs with and without diarrhoea. Aust Vet J. 1984;61:33–38. doi: 10.1111/j.1751-0813.1984.tb07186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mochizuki M, Sugiura R, Akuzawa M. Micro-neutralization test with canine coronavirus for detection of coronavirus antibodies in dogs and cats. Jpn J Vet Sci. 1987;49:563–565. doi: 10.1292/jvms1939.49.563. [DOI] [PubMed] [Google Scholar]
- 16.Naylor M J, Monckton R P, Lehrbach P R, Deane E M. Canine coronavirus in Austalian dogs. Aust Vet J. 2001;79:27–30. doi: 10.1111/j.1751-0813.2001.tb10718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pratelli A, Tempesta M, Greco G, Martella V, Buonavoglia C. Development of a nested PCR for the detection of canine coronavirus. J Virol Methods. 1999;80:11–15. doi: 10.1016/S0166-0934(99)00017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratelli A, Buonavoglia D, Martella V, Tempesta M, Lavazza A, Buonavoglia C. Diagnosis of canine coronavirus infection using nested-PCR. J Virol Methods. 2000;84:91–94. doi: 10.1016/S0166-0934(99)00134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimmelzwaan G F, Groen J, Egberink H, Borst G H A, UytdeHaag F G C M, Osterhaus A D M E. The use of enzyme-linked immunosorbent assay systems for serology and antigen detection in parvovirus, coronavirus and rotavirus infections in dogs in The Netherlands. Vet Microbiol. 1991;26:25–40. doi: 10.1016/0378-1135(91)90039-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saif L J. Coronavirus immunogens. Vet Microbiol. 1993;37:285–297. doi: 10.1016/0378-1135(93)90030-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnagl R D, Holmes I H. Coronavirus-like particles in stools from dogs, from some country areas of Australia. Vet Rec. 1978;102:528–529. doi: 10.1136/vr.102.24.528. [DOI] [PubMed] [Google Scholar]
- 22.Schunck B, Kraft W, Truyen U. A simple touch-down polymerase chain reaction for the detection of canine parvovirus and feline panleukopenia virus in feces. J Virol Methods. 1995;55:427–433. doi: 10.1016/0166-0934(95)00069-3. [DOI] [PubMed] [Google Scholar]
- 23.Spaan W J M, Cavanagh D, Horzinek M C. Coronaviruses: structure and genome expression. J Gen Virol. 1988;69:2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- 24.Tennant B J, Gaskell R M, Jones R C, Gaskell C J. Prevalence of antibodies to four major canine viral disease in dogs in a Liverpool hospital population. J Small Anim Pract. 1991;32:175–179. [Google Scholar]
- 25.Tennant B J, Gaskell R M, Jones R C, Gaskell C J. Studies on the epizootiology of canine coronavirus. Vet Rec. 1993;132:7–11. doi: 10.1136/vr.132.1.7. [DOI] [PubMed] [Google Scholar]
- 26.Tennant B J, Gaskell R M, Kelly D F, Carter S D. Canine coronavirus infection in the dog following oronasal inoculation. Res Vet Sci. 1991;51:11–18. doi: 10.1016/0034-5288(91)90023-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuchiya K, Horimoto T, Azetaka M, Takahashi E, Konishi S. Enzyme-linked immunosorbent assay for the detection of canine coronavirus and its antibody in dogs. Vet Microbiol. 1991;26:41–51. doi: 10.1016/0378-1135(91)90040-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uwatoka K, Sunairi M, Nakajima M, Yamaura K. Rapid method utilizing the polymerase chain reaction for detection of canine parvovirus in feces of diarrheic dogs. Vet Microbiol. 1995;43:315–323. doi: 10.1016/0378-1135(94)00102-3. [DOI] [PubMed] [Google Scholar]
- 29.Wesley R D. The S gene of canine coronavirus, strain UCD-1, is more closely related to the S gene of transmissible gastroenteritis virus than to that of feline infectious peritonitis virus. Virus Res. 1999;61:145–152. doi: 10.1016/S0168-1702(99)00032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesseling J G, Vennema H, Godeke G J, Horzinek M C, Rottier P J M. Nucleotide sequence and expression of the spike (S) gene of canine coronavirus and comparison with S proteins of feline and porcine coronaviruses. J Gen Virol. 1994;75:1789–1794. doi: 10.1099/0022-1317-75-7-1789. [DOI] [PubMed] [Google Scholar]