Abstract
Purpose
To investigate the clinical value of urine interleukin-18 (IL-8), neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) for the early diagnosis of acute kidney injury (AKI) in patients with ureteroscopic lithotripsy (URL) related urosepsis.
Methods
A retrospective study was carried out in 157 patients with urosepsis after URL. The patients were divided into AKI group and non-AKI group according to the Kidigo guideline and urine IL-8, NGAL and KIM-1 levels were detected by enzyme-linked immunosorbent assay at 0, 4, 12, 24 and 48 h after the surgery. Receiver operating characteristic curve (ROC) was used to evaluate the diagnostic value of these three biomarkers for postoperative AKI.
Results
The level of urine IL-8, NGAL and KIM-1 in AKI group was significantly higher than that in non-AKI group at 4, 12, 24 and 48 h (p < 0.01). The ROC analysis showed the combined detection of urine IL-8, NGAL and KIM-1 at 12 h had a larger area under curve (AUC) than a single marker (0.997, 95% CI: 0.991–0.998), and the sensitivity and specificity were 98.2% and 96.7%, respectively. Pearson correlation analysis showed that the levels of urine NGAL at 4, 12, 24 and 48 h in AKI patients were positively correlated with the levels of urine KIM-1 and IL-18 (p < 0.01).
Conclusion
AKI could be quickly recognized by the elevated level of urine IL-8, NGAL and KIM-1 in patients with URL-related urosepsis. Combined detection of the three urine biomarkers at 12 h after surgery had a better diagnostic performance, which may be an important reference for the early diagnosis of AKI.
Keywords: Urosepsis, Ureteroscopic lithotripsy, Acute kidney injury, Early diagnosis, Interleukin-18, Neutrophil gelatinase-associated lipocalin, Kidney injury molecule-1
Introduction
With the advances of endoscopic technology, ureteroscopic lithotripsy (URL) has been widely used in the treatment of urinary calculus, but urosepsis becomes one of the most serious risks of URL, which may occur during or within a few hours after the surgery. The reported morbidity and mortality of URL-related urosepsis are 5%–20% and 40%–50%, respectively.1 Continuous hypertensive perfusion of renal pelvis during URL is the direct cause of bacteremia, toxemia, together with acute kidney injury (AKI), so patients with URL-related urosepsis are at higher risk for developing AKI.2 Since most patients with unilateral urinary calculi have no abnormalities in traditional AKI indicators such as serum creatinine (Scr) due to contralateral renal compensation, early intervention is often ignored.
Recent studies have shown that the level of urinary biomarkers like interleukin-18 (IL-8), neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) increases in the early stage of AKI, prior to creatinine elevation and histological changes, which may contribute to the early detection of AKI.3, 4, 5 In this study, we evaluated the diagnostic value of urine IL-8, NGAL and KIM-1 for AKI in patients with URL-related urosepsis, aimed to provide sensitive and specific biomarkers for the early diagnosis of AKI.
Methods
General materials
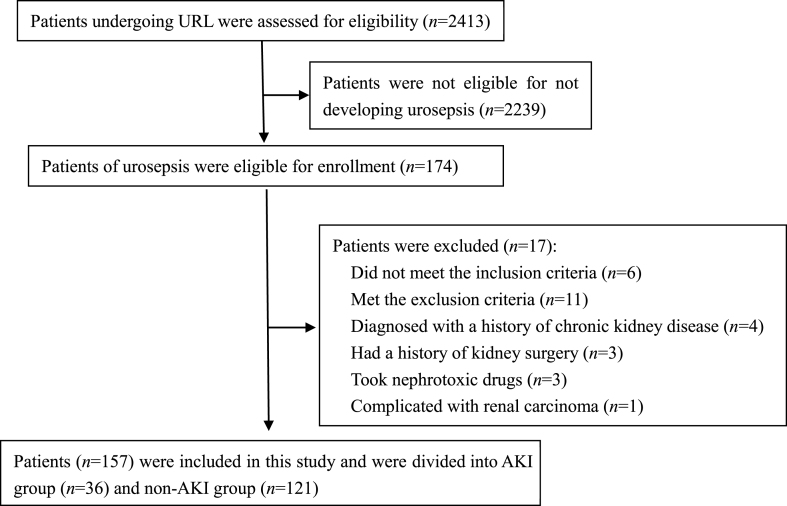
From January 2019 to April 2021, our hospital admitted 2413 patients with URL, of which 174 cases (7.21%) developed urosepsis after surgery, and a total of 157 cases (62 males and 95 females, aged 29–76 years, with an average age of (51.06 ± 10.92) years) were included in this study (Fig. 1). All the subjects signed the informed consents, and the study was approved by the ethics committee of our hospital.
Fig. 1.
Flowchart of subject enrollment. URL: ureteroscopic lithotripsy; AKI: acute kidney injury.
The inclusion criteria: (1) patients who underwent ureteroscopic holmium laser lithotripsy (including transurethral or percutaneous nephrostomy) and were diagnosed with kidney stones or ureteral stones; (2) preoperative Scr and urea nitrogen were normal, and urine test showed no proteinuria, estimated glomerular filtration rate was >60 mL/min/1.73 m,2 perioperative blood glucose and blood pressure were controlled at normal levels, and hemoglobin >10/L; (3) urosepsis was diagnosed by evidence of urinary infection combined with sequential organ failure assessment score ≥2, according to the Chinese guidelines for emergency treatment of sepsis/septic shock.6
The exclusion criteria: (1) patients diagnosed with renal disfunction or a history of kidney disease by preoperative examination; (2) patients with previous kidney surgery or recent use of nephrotoxic drugs; (3) patients with malignant tumors, autoimmune diseases or severe liver diseases.
Research method
Data collection and grouping
The clinical data of a total of 157 patients complicated with postoperative urosepsis were retrospectively reviewed, including age, sex, body mass index, blood pressure and chronic diseases. These patients were divided into AKI group (n = 36) and non-AKI group (n = 121), according to the evaluation of postoperative renal function.
The diagnostic criteria for AKI were referred to Kidigo clinical practice guidelines for acute kidney injury, 2012: sudden decrease of renal function within 48 h, manifested by increased Scr ≥26.5 μmol/L from the absolute value, or ≥50% from the base value, or urine volume maintained at <0.5 mL/(kg·h) for >6 h.
Detection of urine biomarkers
Urine samples (8 mL) of the 157 subjects were collected at 0, 4, 12, 24 and 48 h after URL, centrifuged at 3500 r/min for 10 min, and the supernatant was frozen at −80 °C for detection. The levels of IL-18, NGAL and KIM-1 in urine samples were tested by enzyme-linked immunosorbent assay, and the kits were provided by Shanghai Kexing Biotechnology Company (Shanghai, China).
Statistical method
SPSS 20.0 statistical software was used to analyze the data. The measurement data were expressed as ±s, and independent-sample t-test was used for comparison between two groups. The enumeration data were expressed as rate (%), and χ2 test was used for the comparison between groups. Receiver operating characteristic (ROC) curves were used to analyze the early diagnostic value of urine IL-18, NGAL and KIM-1 levels for AKI. The correlation analysis was completed by Pearson correlation analysis. p<0.05 was considered to indicate a statistically significant difference.
Results
The clinical data were compared between AKI group and non-AKI group
There was no statistical significance in gender, age, body mass index, systolic blood pressure, diastolic blood pressure and basic diseases between the two groups (p > 0.05) (Table 1).
Table 1.
Comparison of the clinical data between AKI group and non-AKI group.
| Variables | AKI group (n = 36) | Non-AKI group (n = 121) | χ2/t value | p value |
|---|---|---|---|---|
| Male, n (%) | 14 (38.9) | 48 (39.7) | 1.685 | 0.172 |
| Age (years) | 52.83 ± 10.21 | 49.84 ± 11.36 | 1.189 | 0.247 |
| BMI (kg/m2) | 25.62 ± 2.69 | 24.75 ± 2.33 | 0.484 | 0.599 |
| Systolic pressure (mmHg) | 131 ± 12.06 | 133.70 ± 11.74 | 0.897 | 0.351 |
| Diastoblic pressure (mmHg) | 79.30 ± 9.75 | 80.52 ± 9.63 | 0.812 | 0.392 |
| History of diabetes, n (%) | 9 (25.0) | 24 (19.8) | 1.134 | 0.325 |
| History of hypertension, n (%) | 7 (19.4) | 22 (18.2) | 0.581 | 0.473 |
| History of CHD, n (%) | 8 (22.2) | 24 (19.8) | 0.352 | 0.462 |
AKI: acute kidney injury; BMI: body mass index; CHD: coronary heart disease.
Levels of urine IL-18, NGAL and KIM-1 at different time points were compared between AKI group and non-AKI group
The levels of urine IL-18, NGAL and KIM-1 at 4, 12, 24 and 48 h after URL in AKI group were higher than those in non-AKI group, with statistical significance (each p < 0.01). There was no significant difference between these three urine biomarkers in AKI group and non-AKI group immediately after URL (p>0.05) (Table 2).
Table 2.
Comparison of urine IL-18, NGAL and KIM-1 levels between AKI group and non-AKI group at different time.
| Variables | AKI group | Non-AKI group | χ2/t | p value |
|---|---|---|---|---|
| IL-18 (pg/mL) | ||||
| 0 h | 13.92 ± 6.81 | 12.30 ± 5.45 | 0.923 | 0.328 |
| 4 h | 18.56 ± 7.76 | 14.10 ± 6.23 | 9.230 | <0.01 |
| 12 h | 31.29 ± 10.55 | 15.35 ± 7.24 | 9.398 | <0.01 |
| 24 h | 33.82 ± 10.85 | 19.42 ± 7.80 | 8.177 | <0.01 |
| 48 h | 34.76 ± 11.13 | 21.82 ± 8.12 | 8.09 | <0.01 |
| NGAL (ng/mL) | ||||
| 0 h | 490.12 ± 412.76 | 430.64 ± 335.85 | 1.431 | 0.235 |
| 4 h | 578.42 ± 496.75 | 448.85 ± 349.46 | 9.835 | <0.01 |
| 12 h | 845.77 ± 675.41 | 451.21 ± 341.13 | 10.763 | <0.01 |
| 24 h | 890.52 ± 763.85 | 482.64 ± 383.34 | 10.527 | <0.01 |
| 48 h | 912.50 ± 804.06 | 541.63 ± 432.48 | 10.439 | <0.01 |
| KIM-1 (pg/mL) | ||||
| 0 h | 18.13 ± 4.81 | 17.09 ± 4.24 | 0.851 | 0.343 |
| 4 h | 25.15 ± 8.69 | 19.52 ± 4.37 | 7.820 | <0.01 |
| 12 h | 50.03 ± 12.02 | 23.82 ± 7.53 | 9.963 | <0.01 |
| 24 h | 48.57 ± 11.16 | 24.32 ± 7.69 | 8.729 | <0.01 |
| 48 h | 46.97 ± 10.77 | 24.99 ± 7.79 | 8.634 | <0.01 |
IL-18: interleukin-18; NGAL: neutrophil gelatinase-associated lipocalin; KIM-1: kidney injury molecule-1; AKI: acute kidney injury.
Value of urine IL-18, NGAL, KIM-1 levels and combined detection for early diagnosis of AKI were evaluated
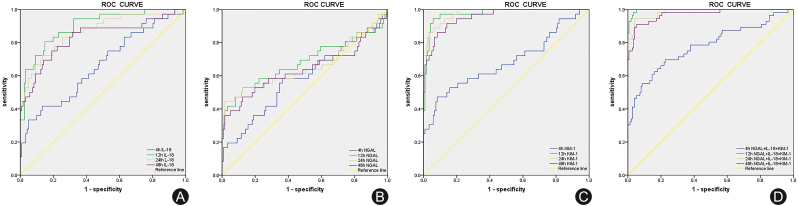
The ROC curves were drawn based on the three urine biomarkers at different time points in AKI group and non-AKI group. The area under curve (AUC) of urinary IL-18, NGAL and KIM-1 levels at the hour 12 for the diagnosis of AKI were 0.881 (95% CI: 0.815–0.947), 0.681 (95% CI:0.585–0.777) and 0.968 (95% CI: 0.937–0.996), and the best cut-off values were 26.44 pg/mL, 1110.62 ng/mL and 33.39 pg/mL, respectively. The AUC of combined detection of urinary IL-18, NGAL and KIM-1 at the hour 12 for diagnosis of AKI was 0.997 (95% CI: 0.991–0.998), showing the best sensitivity (98.2%) and specificity (96.7%) (Table 3 and Fig. 2).
Table 3.
Value of urine IL-18, NGAL, KIM-1 levels and combined detection for early diagnosis of AKI.
| Variables | The optimum cutoff value | AUC (95% CI) | Sensitivity | Specificity |
|---|---|---|---|---|
| IL-18 (pg/mL) | ||||
| 4 h | 16.73 | 0.681 (0.594–0.769) | 0.661 | 0.695 |
| 12 h | 26.44 | 0.881 (0.815–0.947) | 0.786 | 0.947 |
| 24 h | 30.08 | 0.859 (0.795–0.923) | 0.679 | 0.927 |
| 48 h | 28.31 | 0.822 (0.753–0.892) | 0.768 | 0.768 |
| NGAL (ng/mL) | ||||
| 4 h | 984.41 | 0.578 (0.482–0.674) | 0.286 | 0.947 |
| 12 h | 1110.62 | 0.681 (0.585–0.777) | 0.375 | 0.987 |
| 24 h | 945.00 | 0.677 (0.580–0.774) | 0.500 | 0.881 |
| 48 h | 989.69 | 0.671 (0.575–0.768) | 0.518 | 0.861 |
| KIM-1 (pg/mL) | ||||
| 4 h | 24.12 | 0.721 (0.629–0.813) | 0.571 | 0.874 |
| 12 h | 33.39 | 0.968 (0.937–0.996) | 0.946 | 0.907 |
| 24 h | 36.23 | 0.967 (0.94–0.989) | 0.893 | 0.940 |
| 48 h | 32.86 | 0.947 (0.913–0.982) | 0.929 | 0.854 |
| IL-18+ NGAL+ KIM-1 4 h | – | 0.779 (0.699–0.859) | 0.696 | 0.775 |
| IL-18+ NGAL+ KIM-1 12 h | – | 0.997 (0.991–0.998) | 0.982 | 0.967 |
IL-18: interleukin-18; NGAL: neutrophil gelatinase-associated lipocalin; KIM-1: kidney injury molecule-1; AKI: acute kidney injury; AUC: area under curve; CI: confidential interval.
Fig. 2.
(A) ROC curve of urine IL-18 level for the diagnosis of AKI; (B) ROC curve of urine NGAL level for the diagnosis of AKI; (C) ROC curve of urine KIM-1 level for the diagnosis of AKI; (D) ROC curve of combined detection of the three urine biomarkers for the diagnosis of AKI.
3.4. Correlation analysis of urine IL-18, NGAL and KIM-1 levels at different detection periods
Pearson correlation analysis showed that the levels of urine NGAL at the hour 4, 12, 24 and 48 in AKI patients were positively correlated with the levels of KIM-1 and IL-18 (p < 0.01), but there was no significant correlation at the 0 h (p > 0.05) (Table 4).
Table 4.
Correlation analysis of the levels of urine IL-18, NGAL and KIM-1 at different time.
| Variables |
KIM-1 |
IL-18 |
||
|---|---|---|---|---|
| R |
p |
r |
p |
|
| NGAL (h) | ||||
| 0 h | 0.106 | 0.259 | 0.096 | 0.277 |
| 4 h | 0.691 | <0.01 | 0.713 | <0.01 |
| 12 h | 0.738 | <0.01 | 0.725 | <0.01 |
| 24 h | 0.682 | <0.01 | 0.696 | <0.01 |
| 48h | 0.697 | <0.01 | 0.725 | <0.01 |
Discussion
URL is the most commonly used endoscopic treatment for urinary calculus, but the intraoperative high perfusion pressure of pelvis is the direct cause of bacteria or toxins entering the blood, so URL-related urosepsis occurs sometimes and becomes one of the most risky complications of URL.7 Urosepsis, which accounts for 5% of all sepsis, is a life-threatening organ dysfunction caused by urinary tract infection, and AKI is one of the most common organ injuries caused by urosepsis.8, 9, 10 In this study, the incidence of urosepsis after URL surgery was 7.21%, of which the incidence of AKI was 22.9%, which was similar to the reported literature. Theoretically, removing blockages through the URL will allow the blocked side of the kidney to recover immediately and the overall kidney function will be better than before, but if sepsis occurs during the recovery phase, sequential organ dysfunction and infection of the kidney itself may significantly increase the risk of AKI. Therefore, urologists need to pay close attention to AKI caused by URL-related urosepsis, and early identification and intervention are very important.
Scr or cystatin C, as a traditional indicator of renal function, can usually be identified 48–72 h after histological changes, but these two indicators are not suitable for the early diagnosis of AKI. Recent studies have found that some urine biomarkers such as NGAL, KIM-1 and IL-18 increase at 24–72 h before histological changes, which can provide important evidence for the early diagnosis of AKI.11,12 NGAL is a reactive protein isolated from neutrophils and is mainly expressed in renal distal epithelial cells, which can directly reflect the degree of kidney injury and can be significantly increased in early stage of AKI.13 KIM-1, as a member of the immunoglobulin gene superfamily, is not expressed in normal renal tissues, but is highly expressed in damaged renal proximal tubular epithelial cells, which is involved in early kidney injury, repair and fibrosis of renal interstitium.14 IL-18 is a pro-inflammatory cytokine produced by monocyte macrophages, and is synthesized by proximal renal tubule cells during kidney injury, which acts as an effector of inflammatory response.15 This study showed that in patients with URL-related urosepsis, the levels of IL-18, NGAL and KIM-1 in AKI group were significantly higher than those in non-AKI group at 4, 12, 24 and 48 h after surgery, indicating that urine IL-18, NGAL and KIM-1 can predict AKI as early as 4 h after URL. In the study of AKI caused by urinary obstruction, it was found that urine NGAL decreased rapidly within 6 h after the removal of obstruction. Therefore, urine NGAL is considered to be a sensitive biomarker for monitoring kidney injury and postoperative recovery in urinary obstruction.16 However, this study suggested that although the urine blockage was eliminated through the URL, high levels of urine IL-18, NGAL and KIM-1 persisted in patients with urosepsis, suggesting that patients with urosepsis were at higher risk of kidney injury. In fact, we also detected these three urine markers in patients without urosepsis and no significant changes were found, so these data were not included in this study. Therefore, we speculate that for patients with URL-related urosepsis, the risk of AKI may be related to the infection of the kidney itself or to the secondary kidney injury caused by sepsis during the recovery of the renal function.
Early studies on urine biomarkers of AKI focused on patients undergoing cardiac surgery, severe infections and ICU. The purpose of these studies was not only to find the etiology of AKI, but also to identify it earlier. Besides the urine markers in this study, urine N-acetyl-beta-glucosamynidase, tissue transglutaminase (tTG), transforming growth factor-β (TGF-β), Matrix metalloproteinases (MMPs), and so on are also the research hotspots.17, 18, 19, 20 This suggests that a single marker is not enough to monitor the occurrence and outcome of AKI accurately, and combined markers may be more effective. For example, urine IL-18 combined with KIM-1 is considered to have the best diagnostic value for AKI that already exists, and urine NGAL combined with IL-18 is the best marker for the diagnosis of early AKI, while urine Nacetyl-beta-glucosamynidase combined with KIM-1 and IL-18 is an ideal marker for the prognosis of AKI.21,22 In this study, the ROC curve showed that the combined marker of IL-18, NGAL and KIM-1 at hour 12 had the largest AUC for the diagnosis of AKI with the best sensitivity and specificity, which was helpful for the early diagnosis of AKI caused by sepsis. Urine collection can be performed at 12 h after URL to assess AKI risk.
In summary, urosepsis is one of the most serious complications after URL, which is associated with a high risk of AKI. Urine IL-18, NGAL and KIM-1 levels can rapidly predict the postoperative AKI, and combined detection of the three markers has better sensitivity and specificity. Further study of urine markers, especially combined detection of multiple markers, is necessary in the etiological diagnosis and treatment outcomes of AKI.
Funding
This article was sponsored by Medical Research Foundation of Chongqing, China (2019MSXM034).
Ethical statement
The study was performed in accordance with the Declaration of Helsinki and approved by the institutional committee on research ethics (approval certificate no if you have).
Declaration of competing interest
The authors declare no competing interest.
Footnotes
Peer review under responsibility of Chinese Medical Association.
Author contributions
Dan Tan: data analysis and paper writing. Liang Zhao: ELISA and data collection. Wei Peng, Fang-Hao Wu, Guo-Bin Zhang, Bo Yang: surgery performing and sample collection. Wen-Qian Huo: experimental design and text editing.
References
- 1.Shafi H., Moazzami B., Pourghasem M., et al. An overview of Treatment options for urinary stones. Caspian J Intern Med. 2016;7:1–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y., Liao B., Feng S., et al. Comparison of safety and efficacy in preventing postoperative infectious complications of a 14/16F ureteral access sheath with a 12/14F ureteral access sheath in flexible ureteroscopic lithotripsy. J Endourol. 2018;32:923–927. doi: 10.1089/end.2018.0222. [DOI] [PubMed] [Google Scholar]
- 3.Abu Zeid A.M., Mohammed D.Y., AbdAlazeem A.S., et al. Urinary NGAL incorporation into Renal Angina Index for early detection of acute kidney injury in critically ill children. J Clini Nephrol. 2019;3:93–99. doi: 10.29328/journal.jcn.1001032. [DOI] [Google Scholar]
- 4.Assadi F., Sharbaf F.G. Urine KIM-1 as a potential biomarker of acute renal injury after circulatory collapse in children. Pediatr Emerg Care. 2019;35:104–107. doi: 10.1097/PEC.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 5.Miao N., Yin F., Xie H., et al. The cleavage of gasdermin D by caspase-11 promotes tubular epithelial cell pyroptosis and urinary IL-18 excretion in acute kidney injury. Kidney Int. 2019;96:1105–1120. doi: 10.1016/j.kint.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Yu X.Z., Yao Y.M., Zhou R.B. Guidelines for emergency treatment of sepsis/septic shock in China. J Clinl Emerg (China) 2018;19:567–577. [Google Scholar]
- 7.Farag M., Timm B., Davis N., et al. Pressurized-bag irrigation versus hand-operated irrigation pumps during ureteroscopic laser lithotripsy: comparison of infectious complications. J Endourol. 2020;34:914–918. doi: 10.1089/end.2020.0148. [DOI] [PubMed] [Google Scholar]
- 8.Petejova N., Martinek A., Zadrazil J., et al. Acute kidney injury in septic patients treated by selected nephrotoxic antibiotic agents-pathophysiology and biomarkers-A review. Int J Mol Sci. 2020;21:7115. doi: 10.3390/ijms21197115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kellum JA, Wen X, Caestecker MP, et al. Sepsis-associated acute kidney injury: a problem deserving of new solutions. Nephron. 201;143:174-178. 10.1159/000500167. [DOI] [PMC free article] [PubMed]
- 10.Zhu Z., Cui Y., Zeng H., et al. The evaluation of early predictive factors for urosepsis in patients with negative preoperative urine culture following mini-percutaneous nephrolithotomy. World J Urol. 2020;38:2629–2636. doi: 10.1007/s00345-019-03050-9. [DOI] [PubMed] [Google Scholar]
- 11.Beker B.M., Corleto M.G., Fieiras C., et al. Novel acute kidney injury biomarkers: their characteristics, utility and concerns. Int Urol Nephrol. 2018;50:705–713. doi: 10.1007/s11255-017-1781-x. [DOI] [PubMed] [Google Scholar]
- 12.Moledina D.G., Hall I.E., Thiessen-Philbrook H., et al. Performance of serum creatinine and kidney injury biomarkers for diagnosing histologic acute tubular injury. Am J Kidney Di. 2017;70:807–816. doi: 10.1053/j.ajkd.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S.A., Noel S., Kurzhagen J.T., et al. CD4(+) T cell-derived NGAL modifies the outcome of ischemic acute kidney injury. J Immunol. 2020;204:586–595. doi: 10.4049/jimmunol.1900677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreucci M., Faga T., Pisani A., et al. The ischemic/nephrotoxic acute kidney injury and the use of renal biomarkers in clinical practice. Eur J Intern Med. 2017;39:1–8. doi: 10.1016/j.ejim.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Yamanishi K., Mukai K., Hashimoto T., et al. Physiological and molecular effects of interleukin-18 administration on the mouse kidney. J Transl Med. 2018;16:51. doi: 10.1186/s12967-018-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karabay E., Yucetas U., Aytac Ates H., et al. Neutrophil gelatinase-associated lipocalin is an early marker of renal damage in lower urinary tract obstruction in rats. Arch Esp Urol. 2020;73:554–560. [PubMed] [Google Scholar]
- 17.Bayless R.L., Moore A.R., Hassel D.M., et al. Equine urinary N-acetyl-beta-D-glucosaminidase assay validation and correlation with other markers of kidney injury. J Vet Diagn Invest. 2019;31:688–695. doi: 10.1177/1040638719867124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CHiggins C.E., Tang J., Mian B.M., et al. TGF-beta1-p53 cooperativity regulates a profibrotic genomic program in the kidney: molecular mechanisms and clinical implications. Faseb J. 2019;33:10596–10606. doi: 10.1096/fj.201900943R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J., Ren P., Wang Y., et al. Serum Matrix metalloproteinase-7 level is associated with fibrosis and renal survival in patients with IgA nephropathy. Kidney Blood Press Res. 2017;42:541–552. doi: 10.1159/000477132. [DOI] [PubMed] [Google Scholar]
- 20.Kim J.Y., Wee Y.M., Choi M.Y., et al. Urinary transglutaminase 2 as a potent biomarker to predict interstitial fibrosis and tubular atrophy of kidney allograft during early posttransplant period in deceased donor kidney transplantation. Ann Surg Treat Res. 2019;97:27–35. doi: 10.4174/astr.2019.97.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrezenmeier E.V., Barasch J., Budde K., et al. Biomarkers in acute kidney injury-pathophysiological basis and clinical performance. Acta Physiol. 2017;219:554–572. doi: 10.1111/apha.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakal U., Sarac M., Tartar T., et al. A study of the utility of novel non-invasive urinary and serum biomarkers of blunt kidney injury in a rat model: NGAL, KIM-1, and IL-18. Cent Eur J Immunol. 2019;44:219–225. doi: 10.5114/ceji.2019.89592. [DOI] [PMC free article] [PubMed] [Google Scholar]