Abstracts
Chronic wounds have always been a tough fight in clinical practice, which can not only make patients suffer from pain physically and mentally but also impose a heavy burden on the society. More than one factor is relevant to each step of the development of chronic wounds. Along with the in-depth research, we have realized that figuring out the pathophysiological mechanism of chronic wounds is the foundation of treatment, while wound infection is the key point concerned. The cause of infection should be identified and prevented promptly once diagnosed. This paper mainly describes the mechanism, diagnosis and therapeutic strategies of chronic wound infection, and will put an emphasis on the principle of debridement.
Keywords: Wound infection, Debridement, Treatment, Therapeutic strategy
Introduction
With the improvement of living conditions, chronic wounds are gradually replaced with acute wounds and becoming a major wound type. Generally, chronic wounds are those which fail to progress through the normal stage of wound healing. Different underlying internal and external factors should be taken into consideration.1 A wound occurs when normal skin tissue structure and function are damaged due to different causes, which provides the possibility of bacterial colonization. Wound infection is one of the most common and important wound complications. The relationship of chronic wound and wound infection reflects mutual causality. Proper treatment targeting on wound infection can shorten the process of wound healing, while delayed treatment might enlarge the wound area or even lead to further aggravation, such as a systematic infection.
Mechanisms of chronic wound infection
Skin, as the frontier line of immune defense, can effectively resist the invasion of microorganisms. Once formed, wound is susceptible to infection due to the loss of the innate barrier function of the skin and dermal appendages, which will accelerate the process of bacterial colonization.2 Since bacterial colonization is a typical characteristic of chronic wounds, infection might occur when there are over 1×105 colony forming units per gram tissue.3 Infection can inhibit normal wound healing procedures in multiple ways.
Generally, chronic wound infection involves more than one bacteria species, in which Staphylococcus, Pseudomonas, and Stenotrophomonas are most frequently found.4 Toxic substances such as bacterial endotoxin and exotoxin can induce non-specific and specific immune responses, while immune cells like neutrophils and macrophages play vital parts in those processes. Numerous neutrophils continue to exist in chronic wounds, subsequently lead to pathologic inflammation and delayed healing process.5 Moreover, neutrophils, along with other immune cells that are recruited to the wound bed, can produce excessive protease that can degrade extracellular matrix. Unfortunately, this will cause further tissue damage and delayed reepithelization.6,7
Blood circulation and oxygen tension are also key points of wound healing. Oxygen is an essential requirement of cellular metabolism and other normal cell functions. Oxygen tension of wound tissue depends on blood perfusion, capillary density, local pressure of blood oxygen, local oxygen consumption and so on. Furthermore, it is suggested that wound healing processes related to oxygen can be distinctly accelerated at a higher pO2 level than other tissue.8 Besides, immune cells that lead to persistent inflammation of chronic wounds can also release cytokines which cause vasoconstriction, finally reduce oxygen tension of wound site. Meanwhile, metabolism of immune cells and microorganism from the wound site also contributes to the utilization of oxygen. Hypoxia in wounds will inhibit normal progress of wound healing conversely.
The existence of biofilm in chronic wounds is increasingly recognized by wound practitioners in recent years. Compared with their planktonic state, microorganisms are more likely to aggregate together and grow in communities on the surface naturally. The basic composition of biofilm is microorganism communities and a hydrated matrix of extracellular polymeric substances including lipids, proteins and polysaccharides. What's more, the formation of biofilm can be partially determined by biofilm-forming potential, while synergy and antagonism should also be taken into account. A growing number of researches have confirmed the existence of biofilm in chronic wounds. Once biofilm is established, biofilm-grown bacteria will gain several advantages including enhanced resistance to antimicrobial therapy and immune response, an increased ability of proliferation, and so on. Hence, biofilm is undoubtedly a key factor of chronic wound infection.2,9
Diagnosis of chronic wound infection
So far, we fail to come up with a consensus internationally accepted for the diagnosis of wound infection, yet multiple assessment methods are available for identifying the occurrence of wound infection. Diagnosis of wound infection, generally speaking, derives from clinical presentation and laboratory tests.
Symptoms of wound site are essential for the diagnosis of wound infection. Multiple characteristics are associated with superficial infection, including redness, swelling, heat, tenderness, fluctuation and dysfunction. However, the obvious redness and fluctuation may not often occur in deep wound infection, where a large-scale of swelling constantly takes place. Occasionally, the pus contained will show up after aspiration in the most painful and swollen area. The traits of exudate (volume, color, odor, etc.) vary a lot since infection behaves differently in wounds in aspects of location, extent and pathogen.10 Newly formed epithelialization breakdown and increasing pain in ulcer area are wound infection indicators that are most diagnostically significant. Accompany with worsening wound infection, patients may experience the onset of systematic symptoms, such as fever, chills, headache, muscle pain, and even have a risk of sepsis.
Relevant results of laboratory tests include the increasing leucocyte count, C-reaction protein and procalcitonin. Nevertheless, decreasing leucocyte count represents gram-negative bacterial infection in some patients, with signs like hypothermia and disturbance of consciousness. Simultaneously, bacterial culture and wound biopsy are also diagnostic basis of vital importance. Over 1×105 colony forming units per gram tissue of subeschar bacterial load suggests a high risk of wound infection.11
Early and accurate biofilm diagnosis is essential in consideration of the high incidence of wound biofilm formation, whereas no such diagnostic criteria of biofilm are universally acknowledged. The most frequently used indirect clinical indications of wound biofilm including excessive moisture, poor-quality granulation tissue, signs of local infection, history of antibiotic failure, culture-negative results despite a high suspicion of infection, and failure to heal after dressing all underlying comorbidities. Fibrin and slough are often mistaken for biofilm extracellular polymeric substances, which require clinicians to distinguish precisely. Laboratory tests are irreplaceable in the diagnosis of biofilm. Due to the poor sensitivity and specificity of wound swab, it is recommended that samples from biopsy and sharp debridement be applicable to the tests because of the higher accuracy. The use of scanning electron microscopy and confocal laser scanning microscopy are the most reliable diagnostic techniques.12,13
It should be noted that the great majority of patients with chronic wounds are old people or people with systematic disorders. The results of their laboratory tests may be atypical, which reminds us to make a diagnosis with the aid of other clinical and assistant examination. Therefore, infection in chronic wounds can be generally prompted by wound characteristics, as well as confirmed by systematic symptoms and laboratory tests.
Therapeutic strategies of chronic wound infection
Etiology of chronic wound infection
The underlying causes of wound infection are diverse from each other, yet chronic wounds are susceptible to infection conventionally. Acute skin infection such as furuncle and cellulitis can be converted into chronic infection if not controlled appropriately. Patients with systematic disorders such as immune deficiency, diabetes mellitus and hemopathy can probably suffer from a delay in wound healing. Etiology of wound infection can be distinctly identified when it comes to iatrogenic factors such as surgery, radiotherapy, chemotherapy, implants, extravasation of drugs, and the usage of immunosuppressant.14,15 Diabetes, pressure injury and trauma are responsible for the chronicity of wound infection.16,17 Great importance should be attached to the detection of etiology in the initial assessment of wound infection. There is no doubt that any wound therapy intervention is based on the etiology of chronic wound. An incorrect recognition of the etiology may lead to severe consequences.
Debridement principles of chronic wound infection
Traditional surgical debridement refers to the removal of devitalized tissue that impedes the growth of normal tissue, so as to reduce the risk of infection and promote normal wound healing. Multiple techniques of debridement mainly include surgical debridement, mechanical debridement, biologic debridement (autolytic, enzymatic, honey and maggot therapies), and adjunctive modalities (hydrosurgery, ultrasound, negative pressure wound therapy).18 Debridement holds an irreplaceable position in the treatment of chronic wound. Prompt and adequate debridement is capable of reducing the burden of infection, which may shorten the process of wound healing subsequently.
It should be noted that debridement principles of chronic wound are partially different from general surgical wound, due to its complicated etiology. For instance, chronic wounds caused by major arterial insufficiency are not appropriate to a thorough debridement; tissue fibrosis, as the major pathological characteristic, is to blame for its impediment to the common wound healing process, but should not be completely removed via surgical debridement in most cases. Therefore, debridement of chronic wound infection belongs to traditional surgical debridement, yet has its own distinction.
In terms of the combination of surgical debridement principles and clinical practice of chronic wound infection, we come up with a “3D principle” in the debridement of chronic wound, which refers to “drainage”, “disruption” and “division”.
Drainage–establishment of the unobstructed drainage
Drainage is an age-old topic throughout the history of surgery. The ancient Egyptian made the very first drainage dating from about 2000 BC, which has become a crucial approach of the prevention and treatment of infection. The purpose of drainage contains draining the infectious fluid and liquefied necrotic tissue, so as to remove the bacterial culture medium, reduce bioburden and alleviate the stimulation of inflammation to the surrounding tissue. Conventionally, drainage is split into two types-passive drainage and active drainage. Passive drainage, in which drainage tube and gauze are most commonly used, is generally dependent on the pressure difference and gravity. Active drainage is often driven by external force such as negative pressure; the representative modality is negative pressure wound therapy.19 Drainage is of vital importance in wound bed preparation, and to go further, it is an indispensable part of the whole treatment procedure of chronic wound.
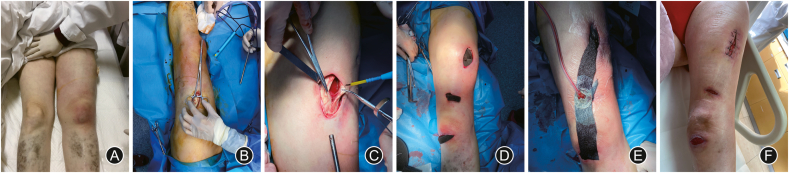
Surgical debridement is one of the most frequently-used, efficient and cost-effective techniques to build up a fluent drainage. Surgical debridement allows direct removal of the devitalized tissue, which provides the fastest route to a prepared wound bed.20 In view of certain blocked infection sites, it is necessary to make an incision in order to ensure fluent drainage. Nevertheless, we ought to lay extra emphasis on the purpose of debridement in our clinical practice of chronic wound infection. For instance, once infection occurs to leg ulcer caused by arterial occlusive diseases in lower extremity, the one and only purpose of debridement is to establish fluent drainage. Though debridement is required for the infected ulcer, removal of all unhealthy tissue in the wound bed or surrounding tissue is hardly recommended, otherwise the necrotic area will expand in no time (Fig. 1).
Fig. 1.
A 50-year-old female with systemic lupus erythematosus was diagnosed as having necrotizing fasciitis. (A) The left thigh was red and swollen. (B) The necrosis area extended to the knee. (C) Debridement revealed the necrotic fascia. (D) & (E) Negative pressure wound therapy was applied through three incisions for five weeks. (F) The patient recovered well and was soon discharged.
Meanwhile, many other key points should be mentioned in the debridement of chronic wound infection.
The premise of adequate drainage is to retain a good command of anatomy, which can minimize the amount of viable tissue sacrificed and maintain basic biological function. Diabetic foot ulcers, which usually located below the ankle, often present with severe infection and necrosis, thus require precise debridement. Hence, with the guidance of anatomic basis and the assurance of blood supply, we suppose to remove necrotic tissue and bone, irrigate the ulcer thoroughly, do further incision if necessary and protect basic biological function of foot and ankle if possible.21,22 Indeed, with numerous underlying lesions involved, multidisciplinary management is highly recommended in the treatment of diabetic foot ulcers, which includes endocrinology, angiology, orthopedics, etc. Stage 4 pressure injury, the most severe pressure injury among all, involves a loss of full-thickness tissue, with exposed facia, muscle, or bone. In case of further infection and necrosis, both debridement and pressure reduction are indispensable. The reduction of pressure, friction, and shear are preconditions, while debridement and drainage are essential conditions of pressure injury treatment. What's more, treatment of pressure ulcer-related osteomyelitis often requires a combination of antibiotic therapy and management mentioned above.23,24
Disruption–adjustment of the wound environment
Theoretically, wound infection is a sort of immunological imbalance generated by increasing wound bacterial load. In terms of pathophysiology, the imbalance represents that the wound environment is conductive to bacterial reproduction instead of tissue regeneration. In the medical history of human fighting against microorganisms, antibiotics always play a crucial but passive role. Generally speaking, antibacterial therapy is required in the treatment of wound infection, whereas the widespread use of antibiotics has brought about the emergence of drug-resistant bacteria.25 Once infected by this kind of bacteria, it not only will postpone the healing process, but also will worsen the infection with newly formed epithelialization breakdown. Hence, a novel thought has formed, whether or not we can adjust the wound milieu to an environment which is detrimental for bacterial proliferation.
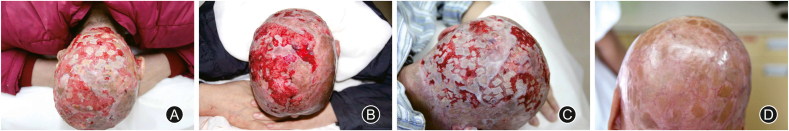
The pH value of wound microenvironment, as an influencing factor of wound healing, was demonstrated in 1970s and has gain increasing attention. As is known to all, an acidic milieu with a pH value of 4–6 is found on the skin surface under normal circumstance, while most human pathogenic bacteria require pH values above 6 for their metabolism and proliferation. As the innate barrier function of skin and dermal appendages are broken and underlying tissue becomes exposed, elements including redundant nutrient, suitable moisture, and appropriate pH value compose the ideal environment for bacterial colonization.26 It has been suggested that alkaline milieu is associated with chronic wound healing and ammonia is to blame for the rising pH value. Thus, the pH value of wound environment brings inspiration to the treatment for some kinds of nonhealing wounds in our clinical practice. With regard to persistent wound infection, the environment of bacteria growth might be destroyed by means of an acidic dressing. Adopting negative pressure wound therapy as an adjuvant therapy, the infection will be distinctly relieved, which is a driving force for the process of wound healing.26, 27, 28 (Fig. 2).
Fig. 2.
A 45-year-old female with scalp avulsion sustained infection caused by multidrug-resistant Staphylococcus aureus after skin grafting. (A) The granulation tissue was swelling with large amounts of purulent exudate. (B) Wound bed preparation: wound irrigation and wet compress with gauze soaked with 20% glacial acetic acid for the first 3 days, and negative pressure wound therapy after the first two procedures. (C) Split-thickness skin grafting. (D) The patient was completely healed.
Biofilms have been implicated in most chronic infections, which has brought new thoughts to the treatment of nonhealing wounds. Study has shown that 60% of chronic wounds were characterized as containing biofilm, while only 6% in acute wounds.29 Once formed, biofilms are found tightly attached to wounds and can be hardly removed. Meanwhile, biofilm-grown bacteria will gain several advantages including enhanced resistance to antimicrobial therapy and immune response, which leads to a delay in wound healing.2 As a result, the removal of biofilm is one of the key points of modifying the wound milieu. Maintenance “desloughing” will help to maintain a healthy wound bed and facilitate wound healing.30 Moreover, other adjuvant therapies are required due to the ability of regeneration of biofilm, such as the application of wound irrigation and antimicrobial dressing.13,31
Division–isolate the source of infection
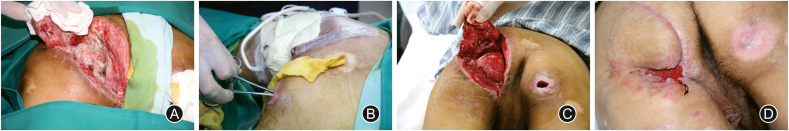
Miscellaneous causal factors are responsible for nonhealing wounds, whereas chronic wounds with specific source of infection can achieve satisfactory therapeutic effects when the source is isolated or completely removed. The control of infection can be increasingly tough on patients with comorbidities, including diabetes mellitus, hemopathy, nephropathy, immune diseases, tumor, and a long-term use of immunosuppressant or glucocorticoids. Consequently, the treatment of comorbidities are supposed to proceed concurrently while managing the chronic wounds.14 The mostly found source of infection in chronic wounds contains foreign body left after trauma or surgery, implants, stoma, etc. Once the foreign body is suspectable to chronic infection, it is significant to identify the trauma history and figure out whether the foreign body exists, sometimes with the help of imaging examination. Incomplete debridement is particularly responsible for the chronicity of an acute wound. One of the most common iatrogenic wounds is generated from the foreign body reaction to suture materials. Non-absorbable sutures may cause suture granulomas and have a bigger potential of chronic infection.32,33 Implants including prosthesis, external and internal fixation, etc., are potential the sources of chronic infection, due to bacterial adhesion to the implant surface or other basic conditions of the patient.34 The isolation of the infection source in peristomal wound infection is troublesome under some circumstances, since the stoma itself as a persistent source of infection cannot be completely removed through debridement. A feasible plan should be work out according to the characteristics of the stoma, in order to manage the infection apart from the stoma and keep it under control. Likewise, wounds closed to perineum should also be paid enough attention to (Fig. 3).
Fig. 3.
A 23-year-old male with pressure injury went through local flap transfer but had poor wound healing. (A) The flap was lifted and the wound was completely exposed. (B) The wound edge closed to anus was stitched temporarily to reduce the contamination of feces. Negative pressure wound therapy was also used for wound bed preparation. (C) The wound bed had less necrotic tissue and more granulation tissue. (D) The flap was almost healed and the patient was soon discharged.
As mentioned above, the “3D principle” is available for the debridement of various types of chronic wound infection, yet the principle is recommended to be applied flexibly due to the different etiology of infection. This treatment strategy cannot only reflect the surgical principles but also accord with the pathologic characteristics of chronic wound diseases, and thus deserves further application in clinical practice.
Antimicrobial agents and dressings
Once wound infection is confirmed, early and accurate anti-infection treatment is essential in spite of debridement, which contains the systematic and local application of antimicrobial substances. An adequate short-term anti-infection therapy is supposed to be carried out according to the results of bacterial culture and drug sensitive tests, as soon as the apparent symptoms and signs appear. The International Working Group on the Diabetic Foot Guidance recommends 6 weeks of antibiotic therapy for patients with diabetic foot osteomyelitis but no resection of the infected bone. Notwithstanding, our clinical practice and researches suggest that long-term antibiotic therapy is not beneficial for the treatment.35,36 Up till now, quinolones, tetracyclines, aminoglycosides, and cephalosporins have been merely applied to antibiotics-containing wound dressings. Unfortunately, adverse effects of bacterial resistance can be induced in case of the improper use of antibiotics, while 70% of bacteria that is responsible for wound infections are resistant to at least one of the most frequently-used antibiotics.37 Local antibiotics can hardly reach a high level of blood concentration, thus should be put into use prudently. Broadly speaking, dressings with antiseptic active ingredients include antibiotics-containing wound dressings (e.g. silver sulfadiazine), physical antisepsis-containing wound dressings (e.g. silver, PHMB), antimicrobial enzyme-containing wound dressings (e.g. lysozyme), chemical antisepsis-containing wound dressings (e.g. Chlorhexidine, sodium hypochlorite, Octenidine), honey,38 compound dressings, etc. Wound dressings can be mainly divided into categories of gauzes, sponges, hydrofibers,39 hydrocolloids, alginates, collagens, hydrogels, films, and so on. Silver-containing dressings are the most commonly-used wound dressings nowadays, due to silver's broad-spectrum antimicrobial activity. The mechanisms of antibacterial effect of silver ions have been recorded in studies explicitly. Silver ions, once bind to peptide glycans of the membranes of bacteria and transported into the cell, will uncouple the respiratory chain from oxidative phosphorylation and affect other numerous bacterial cell functions. In addition, bacteria in biofilms can interact with silver dressings directly, which offers a vital approach for the treatment of wound biofilms.40, 41, 42
Other treatments
In addition to the treatment strategies mentioned above, systematic supportive therapy is an essential integrant of the treatment of chronic wound infection. A majority of patients with chronic wounds have underlying comorbidities include malnutrition, long-time stay in bed, diabetic patients with poor glucose control, long-term steroid therapy, long-term targeted drug therapy, vascular disease without optimal treatment or necessary control, etc.13,14 Hence, it is highly required to improve the basic physical condition holistically, which includes providing nutrition support, decreasing pressure designedly, regulating drug dose under the guidance of doctors from relevant department, improving blood circulation, and so on.43, 44, 45
Meanwhile, many other adjunctive therapies have admirable effects on chronic wound infection, especially infection produced by multidrug-resistant bacteria. Oxygen therapy, mainly contains systematic oxygen therapy and regional oxygen therapy, can directly impact wound healing process by restoring cell metabolism, stimulating angiogenesis and tissue repair.8,46,47 During the process of hyperbaric oxygen therapy, the high blood-to-tissue oxygen pressure gradient can improves the tissue oxygen pressure to 500 mmHg and reduce inflammation by inhibiting the formation of inflammatory cytokines and decreasing leucocyte chemotaxis and adhesion.48 Additionally, hyperbaric oxygen therapy is able to suppress the growth of anaerobic bacteria by providing an oxygen-rich environment, which may contribute to the control of wound infection.49
Nowadays, light therapy has been widely recognized as a potential treatment for various abnormal body conditions. Antimicrobial blue light, with a spectrum of 400 nm–470 nm, has a non-antibiotic strategy against infection. Rather than the traditional mechanism of antibiotics, blue light take effect by stimulating the endogenous porphyrins of microorganisms to the triplet state, which will ultimately lead to damage of DNA, RNA, proteins, and lipids. Moreover, pathogens most frequently found in biofilm such as Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, etc. can be effectively inactivated.50,51 Consequently, irradiation with blue light may lead to a satisfying effect in the treatment of wound infection.
Recently, the recognition of antimicrobial peptides (AMPs) has been improved due to the increase in the incidence of antibiotic resistance. Antimicrobial peptides, both natural and synthetic, have broad-spectrum antibacterial property and are one of the major options to overcome antibiotic resistance. The main mechanism of AMPs against pathogen is to destroy the integrity of cell membrane by membrane permeabilization. Other mechanisms of AMPs found recent years include membrane destabilization, inhibition of macromolecular (e.g. DNA, RNA, protein) synthesis, and intracellular translocation of the peptide.52 Moreover, some AMPs can also act as wound healing peptides, thus displaying the property of reducing the pro-inflammatory response and promoting cell migration and proliferation.53 In comparison with traditional antibiotics, AMPs have more antimicrobial mechanisms, and are able to take effect rapidly but less likely to trigger drug resistance. Furthermore, some of the AMPs have the potential to work as anti-biofilm agents with high clinical value.52,54,55 In general, AMPs have a promising prospect in the treatment of chronic wound infection.
Conclusion
The etiology of chronic wound infection is associated with both endogenous and exogenous factors. Endogenous factors comprise diabetes, blood diseases, immune diseases, impaired blood circulation and so on; exogenous factors include severe trauma, pressure injury, iatrogenic implants, etc. Great importance should be laid on the prevention of patients with high risks of nonhealing wounds, and adequate treatment should be provided instantly in case of serious consequences. After identifying the underlying etiology, a combination of debridement and anti-infection treatment should be taken as a full-course and thorough wound therapy. Debridement, as a crucial procedure, is supposed to be acted up to the “3D principle”. Additionally, accurate assessment of the characteristics and laboratory tests of the wound are definitely the basis of selecting antimicrobial agents. Adjunctive therapies can also be applied to promote wound healing. With the increasing recognition of chronic wounds and the development of wound dressings as well as adjunctive modalities, there is a vast potential for future development of chronic wound prevention and treatment, yet more strenuous endeavor should be made for further achievement.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81671917), and the Natural Science Foundation of Shanghai (19ZR1432200).
Ethical statement
Not applicable.
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Peer review under responsibility of Chinese Medical Association.
Author contributions
Ting Xie contributed to the conception of the study; Yi-Fan Liu contributed significantly to analysis and manuscript preparation; Peng-Wen Ni performed data collection and analysis; Yao Huang helped perform the analysis with constructive discussions.
References
- 1.Menke N.B., Ward K.R., Witten T.M., et al. Impaired wound healing. Clin Dermatol. 2007;25:19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Percival S.L., Hill K.E., Williams D.W., et al. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012;20:647–657. doi: 10.1111/j.1524-475X.2012.00836.x. [DOI] [PubMed] [Google Scholar]
- 3.Withycombe C., Purdy K.J., Maddocks S.E. Micro-management: curbing chronic wound infection. Mol Oral Microbiol. 2017;32:263–274. doi: 10.1111/omi.12174. [DOI] [PubMed] [Google Scholar]
- 4.Rahim K., Saleha S., Zhu X., et al. Bacterial contribution in chronicity of wounds. Microb Ecol. 2017;73:710–721. doi: 10.1007/s00248-016-0867-9. [DOI] [PubMed] [Google Scholar]
- 5.Tanno H., Kawakami K., Kanno E., et al. Invariant NKT cells promote skin wound healing by preventing a prolonged neutrophilic inflammatory response. Wound Repair Regen. 2017;25:805–815. doi: 10.1111/wrr.12588. [DOI] [PubMed] [Google Scholar]
- 6.Wilgus T.A., Roy S., McDaniel J.C. Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care. 2013;2:379–388. doi: 10.1089/wound.2012.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdollahi M., Ng T.S., Rezaeizadeh A., et al. Insulin treatment prevents wounding associated changes in tissue and circulating neutrophil MMP-9 and NGAL in diabetic rats. PloS One. 2017;12 doi: 10.1371/journal.pone.0170951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard M.A., Asmis R., Evans K.K., et al. Oxygen and wound care: a review of current therapeutic modalities and future direction. Wound Repair Regen. 2013;21:503–511. doi: 10.1111/wrr.12069. [DOI] [PubMed] [Google Scholar]
- 9.Wolcott R.D., Rumbaugh K.P., James G., et al. Biofilm maturity studies indicate sharp debridement opens a time- dependent therapeutic window. J Wound Care. 2010;19:320–328. doi: 10.12968/jowc.2010.19.8.77709. [DOI] [PubMed] [Google Scholar]
- 10.Glaudemans A.W., Uckay I., Lipsky B.A. Challenges in diagnosing infection in the diabetic foot. Diabet Med. 2015;32:748–759. doi: 10.1111/dme.12750. [DOI] [PubMed] [Google Scholar]
- 11.Daeschlein G. Antimicrobial and antiseptic strategies in wound management. Int Wound J. 2013;10(Suppl 1):9–14. doi: 10.1111/iwj.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalf D.G., Bowler P.G., Hurlow J. A clinical algorithm for wound biofilm identification. J Wound Care. Mar 2014;23:137–138. doi: 10.12968/jowc.2014.23.3.137. 140-2. [DOI] [PubMed] [Google Scholar]
- 13.Schultz G., Bjarnsholt T., James G.A., et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25:744–757. doi: 10.1111/wrr.12590. [DOI] [PubMed] [Google Scholar]
- 14.Mueck K.M., Kao L.S. Patients at high-risk for surgical site infection. Surg Infect. 2017;18:440–446. doi: 10.1089/sur.2017.058. [DOI] [PubMed] [Google Scholar]
- 15.Cheng B., Tian J., Peng Y., et al. Iatrogenic wounds: a common but often overlooked problem. Burns Trauma. 2019;7:18. doi: 10.1186/s41038-019-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandell J.C., Khurana B., Smith J.T., et al. Osteomyelitis of the lower extremity: pathophysiology, imaging, and classification, with an emphasis on diabetic foot infection. Emerg Radiol. 2018;25:175–188. doi: 10.1007/s10140-017-1564-9. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira W.F., Silva P.M.S., Silva R.C.S., et al. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J Hosp Infect. 2018;98:111–117. doi: 10.1016/j.jhin.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Anghel E.L., DeFazio M.V., Barker J.C., et al. Current concepts in debridement: science and strategies. Plast Reconstr Surg. 2016;138:82s–93s. doi: 10.1097/prs.0000000000002651. [DOI] [PubMed] [Google Scholar]
- 19.Robinson J.O. Surgical drainage: an historical perspective. Br J Surg. 1986;73:422–426. doi: 10.1002/bjs.1800730603. [DOI] [PubMed] [Google Scholar]
- 20.Hoppe I.C., Granick M.S. Debridement of chronic wounds: a qualitative systematic review of randomized controlled trials. Clin Plast Surg. 2012;39:221–228. doi: 10.1016/j.cps.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Blume P., Wu S. Updating the diabetic foot treatment algorithm: recommendations on treatment using advanced medicine and therapies. Wounds. 2018;30:29–35. [PubMed] [Google Scholar]
- 22.Elraiyah T., Domecq J.P., Prutsky G., et al. A systematic review and meta-analysis of debridement methods for chronic diabetic foot ulcers. J Vasc Surg. 2016;63:37S–45S. doi: 10.1016/j.jvs.2015.10.002. e1-2. [DOI] [PubMed] [Google Scholar]
- 23.Andrianasolo J., Ferry T., Boucher F., et al. Pressure ulcer-related pelvic osteomyelitis: evaluation of a two-stage surgical strategy (debridement, negative pressure therapy and flap coverage) with prolonged antimicrobial therapy. BMC Infect Dis. 2018;18:166. doi: 10.1186/s12879-018-3076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blenman J., Marks-Maran D. Pressure ulcer prevention is everyone's business: the PUPS project. Br J Nurs. 2017;26:S16–s26. doi: 10.12968/bjon.2017.26.6.S16. [DOI] [PubMed] [Google Scholar]
- 25.Friedman N.D., Temkin E., Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect. 2016;22:416–422. doi: 10.1016/j.cmi.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Schneider L.A., Korber A., Grabbe S., et al. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res. 2007;298:413–420. doi: 10.1007/s00403-006-0713-x. [DOI] [PubMed] [Google Scholar]
- 27.Sharpe J.R., Booth S., Jubin K., et al. Progression of wound pH during the course of healing in burns. J Burn Care Res. 2013;34:e201–208. doi: 10.1097/BCR.0b013e31825d5569. [DOI] [PubMed] [Google Scholar]
- 28.Kruse C.R., Singh M., Targosinski S., et al. The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: in vitro and in vivo study. Wound Repair Regen. 2017;25:260–269. doi: 10.1111/wrr.12526. [DOI] [PubMed] [Google Scholar]
- 29.James G.A., Swogger E., Wolcott R., et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 30.Percival S.L., Suleman L. Slough and biofilm: removal of barriers to wound healing by desloughing. J Wound Care. 2015;24(498):500–503. doi: 10.12968/jowc.2015.24.11.498. 506-510. [DOI] [PubMed] [Google Scholar]
- 31.Klasinc R., Augustin L.A., Below H., et al. Evaluation of three experimental in vitro models for the assessment of the mechanical cleansing efficacy of wound irrigation solutions. Int Wound J. 2018;15:140–147. doi: 10.1111/iwj.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohtani H. Granuloma cells in chronic inflammation express CD205 (DEC205) antigen and harbor proliferating T lymphocytes: similarity to antigen-presenting cells. Pathol Int. 2013;63:85–93. doi: 10.1111/pin.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ergin O.N., Demirel M., Ozmen E. An exceptional case of suture granuloma 30 years following an open repair of achilles tendon rupture: a case report. J Orthop Case Rep. 2017;7:50–53. doi: 10.13107/jocr.2250-0685.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16:397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 35.Abbas M., Uckay I., Lipsky B.A. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expet Opin Pharmacother. 2015;16:821–832. doi: 10.1517/14656566.2015.1021780. [DOI] [PubMed] [Google Scholar]
- 36.Lipsky B.A., Aragon-Sanchez J., Diggle M., et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. Jan 2016;32(Suppl 1):45–74. doi: 10.1002/dmrr.2699. [DOI] [PubMed] [Google Scholar]
- 37.Negut I., Grumezescu V., Grumezescu A.M. Treatment strategies for infected wounds. Molecules. 2018;23:2392. doi: 10.3390/molecules23092392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Kased R.F., Amer R.I., Attia D., et al. Honey-based hydrogel: in vitro and comparative in vivo evaluation for burn wound healing. Sci Rep. 2017;7:9692. doi: 10.1038/s41598-017-08771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metcalf D.G., Bowler P.G. Clinical impact of an anti-biofilm Hydrofiber dressing in hard-to-heal wounds previously managed with traditional antimicrobial products and systemic antibiotics. Burns Trauma. 2020;8 doi: 10.1093/burnst/tkaa004. tkaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kramer A., Dissemond J., Kim S., et al. Consensus on wound antisepsis: update 2018. Skin Pharmacol Physiol. 2018;31:28–58. doi: 10.1159/000481545. [DOI] [PubMed] [Google Scholar]
- 41.Holt K.B., Bard A.J. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+ Biochemistry. 2005;44:13214–13223. doi: 10.1021/bi0508542. [DOI] [PubMed] [Google Scholar]
- 42.Kostenko V., Lyczak J., Turner K., et al. Impact of silver-containing wound dressings on bacterial biofilm viability and susceptibility to antibiotics during prolonged treatment. Antimicrob Agents Chemother. 2010;54:5120–5131. doi: 10.1128/aac.00825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pugliese D.J. Infection in venous leg ulcers: considerations for optimal management in the elderly. Drugs Aging. 2016;33:87–96. doi: 10.1007/s40266-016-0343-8. [DOI] [PubMed] [Google Scholar]
- 44.Hsu C.C., Kwan G.N., Singh D., et al. Angioplasty versus stenting for infrapopliteal arterial lesions in chronic limb-threatening ischaemia. Cochrane Database Syst Rev. 2018;12:Cd009195. doi: 10.1002/14651858.CD009195.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaul E. Assessment and management of pressure ulcers in the elderly: current strategies. Drugs Aging. 2010;27:311–325. doi: 10.2165/11318340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 46.Bozok S., Ilhan G., Yilmaz Y., et al. Protective effects of hyperbaric oxygen and iloprost on ischemia/reperfusion-induced lung injury in a rabbit model. Eur J Med Res. 2012;17:14. doi: 10.1186/2047-783x-17-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santema K.T.B., Stoekenbroek R.M., Koelemay M.J.W., et al. Hyperbaric oxygen therapy in the treatment of ischemic lower- extremity ulcers in patients with diabetes: results of the DAMO2CLES multicenter randomized clinical trial. Diabetes Care. 2018;41:112–119. doi: 10.2337/dc17-0654. [DOI] [PubMed] [Google Scholar]
- 48.Memar M.Y., Yekani M., Alizadeh N., et al. Hyperbaric oxygen therapy: antimicrobial mechanisms and clinical application for infections. Biomed Pharmacother. 2019;109:440–447. doi: 10.1016/j.biopha.2018.10.142. [DOI] [PubMed] [Google Scholar]
- 49.Steiner T., Seiffart A., Schumann J., et al. Hyperbaric oxygen therapy in necrotizing soft tissue infections: a retrospective study. Adv Exp Med Biol. 2018;1072:263–267. doi: 10.1007/978-3-319-91287-5_42. [DOI] [PubMed] [Google Scholar]
- 50.Ferrer-Espada R., Liu X., Goh X.S., et al. Antimicrobial blue light inactivation of polymicrobial biofilms. Front Microbiol. 2019;10:721. doi: 10.3389/fmicb.2019.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amin R.M., Bhayana B., Hamblin M.R., et al. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: in vitro and in vivo studies. Laser Surg Med. 2016;48:562–568. doi: 10.1002/lsm.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sierra J.M., Fuste E., Rabanal F., et al. An overview of antimicrobial peptides and the latest advances in their development. Expet Opin Biol Ther. 2017;17:663–676. doi: 10.1080/14712598.2017.1315402. [DOI] [PubMed] [Google Scholar]
- 53.Gomes A., Teixeira C., Ferraz R., et al. Wound-healing peptides for treatment of chronic diabetic foot ulcers and other infected skin injuries. Molecules. 2017;22:1743. doi: 10.3390/molecules22101743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy R., Tiwari M., Donelli G., et al. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9:522–554. doi: 10.1080/21505594.2017.1313372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu Z., Deslouches B., Walton W.G., et al. Enhanced biofilm prevention activity of a SPLUNC1-derived antimicrobial peptide against Staphylococcus aureus. PloS One. 2018;13 doi: 10.1371/journal.pone.0203621. doi:10.1371/journal.pone.0203621. [DOI] [PMC free article] [PubMed] [Google Scholar]