Abstract
Sepsis is a life-threatening clinical syndrome and one of the most challenging health problems in the world. Pathologically, sepsis and septic shock are caused by a dysregulated host immune response to infection, which can eventually lead to multiple organ failure and even death. As an adaptor transporter between the endoplasmic reticulum and Golgi apparatus, stimulator of interferon response cGAMP interactor 1 (STING1, also known as STING or TMEM173) has been found to play a vital role at the intersection of innate immunity, inflammation, autophagy, and cell death in response to invading microbial pathogens or endogenous host damage. There is ample evidence that impaired STING1, through its immune and non-immune functions, is involved in the pathological process of sepsis. In this review, we discuss the regulation and function of the STING1 pathway in sepsis and highlight it as a suitable drug target for the treatment of lethal infection.
Keywords: Sepsis, STING1, Cell death, Inflammation, Immunity
Abbreviations: STING1, stimulator of interferon response cGAMP interactor 1; PAMPs, pathogen-associated molecular patterns; DAMPs, damage-associated molecular patterns; PRRs, pattern-recognizing receptors; LPS, lipopolysaccharides; HMGB1, high-mobility group box 1; type I IFNs, type I interferons; ER, endoplasmic reticulum; CGAS, cyclic GMP-AMP synthase; mtDNA, mitochondrial DNA; nDNA, nuclear DNA; cGAMP, cyclic guanosine monophosphate-adenosine monophosphate; CDNs, cyclic dinucleotides; EGFR, epidermal growth factor receptor; cfDNA, cell-free DNA; TLR9, toll-like receptor 9; IRF3, interferon regulatory factor 3; ALK, ALK receptor tyrosine kinase; AKT, serine-threonine kinase; CLP, cecal ligation and puncture; AIM2, absent in melanoma 2; caspase, CASP; IFNAR1, interferon alpha and beta receptor subunit 1; CXCL10, C-X-C motif chemokine ligand 10; TBK1, TANK-binding kinase 1; DMXAA, 5,6-dimethylxanthenone-4-acetic acid; TNFα, tumor necrosis factor α; IL, interleukin; CCL2, C-C motif chemokine ligand 2; NF-κB, nuclear factor-κB; SQSTM1, sequestosome 1; RIPK, receptor interacting serine/threonine-protein kinase; MLKL, mixed-lineage kinase domain-like pseudokinase; GSDMD, gasdermin D; GSDMD-N, N-terminal fragment of GSDMD; GPX4, glutathione peroxidase 4; PLCG1, phospholipase C gamma 1
Introduction
Sepsis is a challenging clinical syndrome and the most common reason for admission to an intensive care unit. A major public health concern, sepsis, accounted for 48.9 million cases and 11 million global deaths (19.7% of all) in 2017.1 An international task force has updated the definition of sepsis, describing it as a life-threatening organ dysfunction caused by a dysregulated host response to bacteria, viruses, or other pathogen infections.2 Despite its complex pathophysiological mechanism, sepsis is characterized by early excessive inflammation and late immunosuppression, which are related to cell death and various abnormalities in the coagulation response.2, 3, 4 During sepsis, innate immune cells (e.g., macrophages, monocytes, and neutrophils) are activated to trigger inflammation by sensing pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) through multiple pattern-recognizing receptors (PRRs). PAMPs are components or products of microorganisms and include microbial genomes (DNA and RNA) and lipopolysaccharides (LPS, a constituent of the outer membrane components of gram-negative bacteria).5,6 In contrast, DAMPs are endogenous molecules driven by the host cell and include proteins (such as high-mobility group box 1[HMGB1]) and nonproteins (such as host DNA and ATP). PAMPs and DAMPs can activate various innate immune pathways and have become important therapeutic targets for sepsis.7, 8, 9
Stimulator of interferon response cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) interactor 1 (STING1, also known as STING, TMEM173, MITA, or MPYS) was originally identified by multiple groups as a key adaptor protein in producing type I interferons (type I IFNs) in macrophages or monocytes during DNA-induced immune responses.10, 11, 12, 13 Subsequent studies revealed an immune-independent function of STING1 in promoting autophagy14 and various kinds of cell death (e.g., apoptosis, necroptosis, pyroptosis, ferroptosis, mitotic cell death, and immunogenic cell death) in various cells.15 As a crucial part of the host immune defense, an aberrated activation of STING1 might induce an imbalance in the inflammation immune network.16 Consequently, a hyperactivation of the STING1 signaling pathway plays an indispensable role in the development of various inflammatory diseases, such as Aicardi-Goutieres syndrome, COPA syndrome, and systemic lupus erythematosus.17 Similarly, preclinical and clinical studies suggest that an excessive activation of STING1 is a pathogenic event of sepsis. In contrast, the pharmacological or genetic inhibition of the STING1 pathway can protect mice from polymicrobial sepsis and lethal endotoxemia.18, 19, 20 In this review, we highlight recent findings revealing how STING1 networks enforce septic response, and discuss the potential of STING1 as a drug target in lethal infection.
STING1 and DNA signals in sepsis
STING1 is an evolutionarily conserved transmembrane protein normally expressed on the endoplasmic reticulum (ER) membrane in immune and non-immune cells.21 As a key ER-associated adaptor protein, STING1 can be activated by cytoplasmic DNA produced from pathogens or hosts to trigger strong type I IFNs and inflammation responses.17 Mechanically, cyclic GMP-AMP synthase (CGAS, also known as MB21D1), the upstream PRR of STING1, detects and binds DNA from invading pathogens (e.g., viruses and bacteria) or damaged hosts (including mitochondrial DNA [mtDNA] and nuclear DNA [nDNA]), leading to the production of a second messenger, cGAMP.21 In addition, bacterial-derived cyclic dinucleotides (CDNs, such as cyclic-di-GMP and cyclic-di-AMP) can bypass the CGAS-dependent pathway to directly induce STING1 activation.22 Thus, STING1 can be activated in a CGAS-dependent and -independent manner.
Sepsis is related to host DNA damage and subsequent DNA release from multiple sources.23 Extensive cell death becomes the major source of released DNA during sepsis.24 Circulating cell-free DNA (cfDNA) released by host cells has been shown to be significantly elevated and causes inflammation and organ failure in septic mice and patients.7,8,19 Circulating mtDNA is a marker of mortality in intensive care unit patients that is associated with the development and prognosis of sepsis,25, 26, 27 and it initiates inflammation and subsequent immunosuppression, leading to organ dysfunction and lung injury.28, 29, 30 In addition to mtDNA, elevated nDNA levels are also associated with the development of sepsis in patients.7,31 These findings suggest that elevated cfDNA levels in the early stages of sepsis may represent a candidate biomarker for the severity of sepsis.
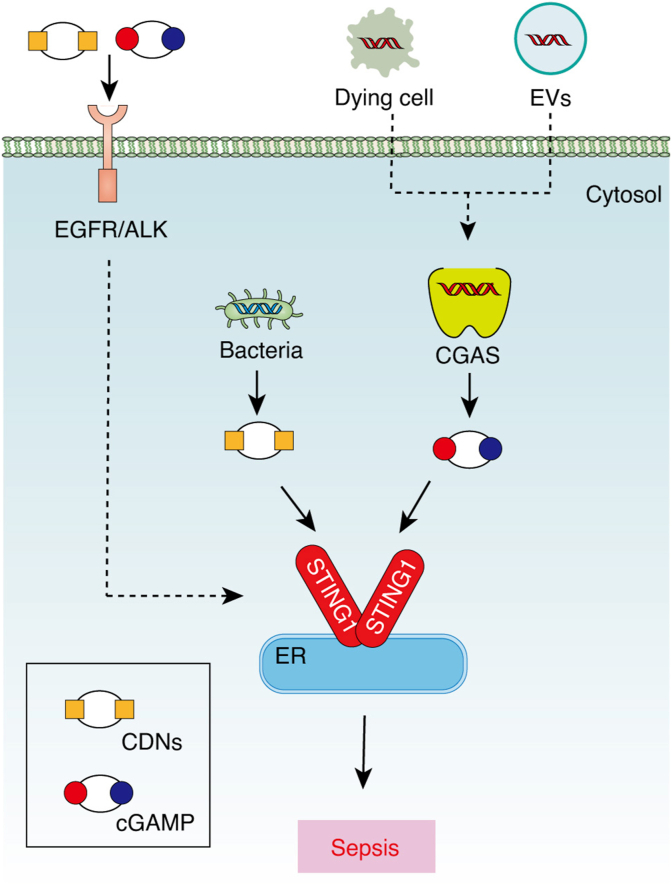
At least two DNA sensor or receptor pathways mediate mtDNA activity in sepsis. On the one hand, circulating mtDNA can activate toll-like receptor 9 (TLR9) and inflammasomes, contributing to sepsis.32,33 On the other hand, STING1 is also required for an mtDNA-induced inflammatory response in sepsis.20 STING1-deficient mice exhibit a reduced mtDNA-induced inflammatory response and acute lung injury. Additional studies have shown that the function of STING1 in sepsis seems to be parallel to the level of cfDNA. Compared with moderate sepsis, cfDNA in severe sepsis is more elevated. Therefore it is not surprising that STING1 knockout mice are only protected from severe sepsis and not moderate sepsis.19 Functionally, host genomic DNA can activate interferon regulatory factor 3 (IRF3)-dependent gene transcription in macrophages in a STING1-dependent manner. Considering that nDNA and mtDNA can transfer between cells and activate STING1 through the debris of dying cells or DNA-containing extracellular vesicles,34,35 it is possible that STING1 senses the cfDNA after having been taken up by cells (Fig. 1). Alternatively, a plasma membrane receptor-dependent pathway can mediate exogenous DNA signals to activate STING1.18 In particular, CDNs and cGAMP can activate the CGAS/STING1 pathway through transmembrane epidermal growth factor receptor (EGFR) and ALK receptor tyrosine kinase (ALK) in macrophages or monocytes. Although ALK may not be a direct adaptor for cytosolic STING1 due to a lack of direct interaction between ALK and STING1, serine-threonine kinase (AKT) can act as a downstream signal of ALK and promotes STING1 activation through protein phosphorylation (Fig. 1). Pharmacologically inhibiting the ALK-AKT-STING1 pathway by LDK378 protects against cecal ligation and puncture (CLP)-induced sepsis in mice,36,37 suggesting a potential drug target for treating sepsis.
Fig. 1.
STING1 senses extracellular DNA signals in sepsis. During sepsis, extracellular DNA may enter cells through the endocytosis of dying cells or DNA-containing EVs, leading to CGAS/STING1 activation, while extracellular DNA signals, including CDNs and cGAMP, may promote STING1 activation through EGFR/ALK-dependent transmembrane receptors.
Abbreviations: ALK: ALK receptor tyrosine kinase; CDNs: cytosolic cyclic dinucleotides; cGAMP: cyclic GMP-AMP; CGAS: cyclic GMP-AMP synthase; EGFR: epidermal growth factor receptor; ER: endoplasmic reticulum; EVs: extracellular vesicles; STING1: stimulator of interferon response cGAMP interactor 1.
Although bacterial infection is the most important cause of sepsis, with immune cell death being attributed to bacterial DNA, the DNA exhibits a less lethal effect in mouse polymicrobial sepsis.38 Blocking the recognition of bacterial DNA alleviates inflammation, implying that the DNA plays a major role in priming the immune system. Most bacteria release DNA into cells via endocytosis or phagocytosis to bind TLR9,39,40 whereas some bacteria, such as Francisella tularensis, can enter macrophages and release DNA directly into the cytosol to activate the absent in melanoma 2 (AIM2) inflammasome for sepsis.41 Whether STING1 is involved in sensing bacterial DNA-related TLR9 or AIM2 pathways during sepsis remains to be further clarified.
The mtDNA might directly contribute to inflammation through a cytoplasmic signaling pathway during sepsis. Impaired mitochondria produce more cytosolic mtDNA serving as DAMPs, which activates the NLR family pyrin domain containing 3 (NLRP3) inflammasome and subsequent caspase 1 (CASP1) in macrophages, ultimately releasing cytokines similar to a sepsis condition.42 Accordingly, the depletion of CGAS inhibits mtDNA-mediated STING1 and NLRP3 activation, thereby protecting against LPS-induced acute lung injury.43 These findings prove the new connection between the STING1 pathway and NLRP3 inflammasome in causing sepsis.
Taken together, elevated cfDNA signals, including mtDNA and nDNA, could trigger a STING1-dependent inflammatory response during sepsis. The inhibition of DNA sensing by cfDNA scavengers or deoxyribonuclease (DNASE1) can effectively inhibit a severe sepsis-related cytokine storm and organ dysfunction, indicating the novel therapeutics of targeting cfDNA/STING1 in sepsis.20,44, 45, 46
STING1 and type I IFNs in sepsis
Type I IFNs are essential cytokines in promoting and regulating immune and inflammatory responses as well as for controlling several types of cell death (e.g. apoptosis, necroptosis, and pyroptosis), which are crucial for septic response.47 Mounting evidence suggests that type I IFNs are essential effectors in LPS- or virus-induced lethal sepsis,48,49 and their expression is driven by the transcription factor IRF3.50,51 The pharmacologic or genetic inhibition of type I IFN receptors also ameliorates LPS- and CLP-induced sepsis in mice.52,53 Neutralizing type I IFN signaling could be a therapeutic option for patients with acute sepsis.54,55 Mechanically, type I IFNs could induce HMGB1 release, leading to caspase 11 (CASP11)-dependent pyroptosis in macrophages and hepatocytes, thus mediating bacterial infection-induced lethal coagulation.56,57 Given that sepsis mortality often occurs after a prolonged period of immunosuppression rather than exaggerated inflammation,58 type I IFNs might play a different role in the later stage of sepsis compared to a role in innate and adaptive immune responses. However, in a low-lethality sepsis model, interferon alpha and beta receptor subunit 1 (IFNAR1)-deficient mice and chimeric mice lacking IFNAR1 in hematopoietic cells display increased mortality after CLP, with elevated peritoneal bacterial counts and reduced C-X-C motif chemokine ligand 10 (CXCL10)-dependent peritoneal neutrophil recruitment and function.59 These data indicate a host defense role of type I IFNs in sepsis.
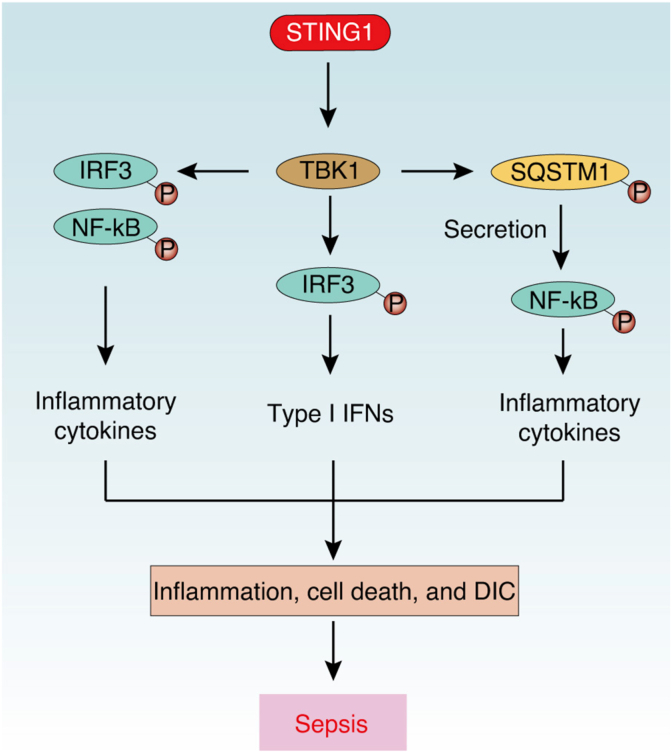
STING1 plays a well-known role in driving IRF3-dependent type I IFN production. After binding with cGAMP, STING1 undergoes a change to an active state, thus translocating to the Golgi apparatus and activating the transcription factor IRF3 by recruitment of a phosphokinase, TANK-binding kinase 1 (TBK1).60 Subsequently, active IRF3 translocate the nucleus to induce transcription of type I IFN genes, which are involved in immune responses in infection and antitumor immunity. As expected, STING1 has been found to contribute to type I IFN-mediated septic response. In human abdominal sepsis, the expression of STING1 in peripheral blood mononuclear cells and intestinal cells is increased, which is associated with intestinal inflammation in patients with sepsis.20 During lethal sepsis in mice, STING1 is activated by cfDNA to promote IRF3-dependent type I IFN expression.19,20 STING1-deficient septic mice have reduced inflammation, tissue damage, and bacterial translocation, and STING1 agonist (5,6-dimethylxanthenone-4-acetic acid [DMXAA]) can aggravate CLP-induced intestinal cell apoptosis and systemic inflammation.20 In addition, the administration of DMXAA causes shock-like symptoms and significant mortality in mice, whereas STING1- or IFNAR1-deficient mice are completely resistant to mortality after DMXAA injection.61 Considering that the STING1-IRF3 axis is involved in inflammasome activation and cell death as described below,43,62 the activation of STING1 might contribute to lethal sepsis through type I IFN-dependent inflammation (Fig. 2).
Fig. 2.
STING1-mediated type I IFNs and inflammatory cytokines in sepsis. During sepsis, activated STING1 recruits and phosphorylates TBK1, which further activates IRF3- and NF-κB-dependent type I IFN and inflammatory cytokine production. Meanwhile, activated TBK1 could promote phosphorylation and secretion of SQSTM1, which further activates NF-κB.
Abbreviations: DIC: disseminated intravascular coagulation; IRF3: interferon regulatory factor 3; NF-κB: nuclear factor kappa B complex; SQSTM1: sequestosome 1; STING1: stimulator of interferon response cGAMP interactor 1; TBK1: TANK binding kinase 1.
Of note, STING1 might regulate coagulation activation in lethal infections independent of type I IFN signaling. The loss of IFNAR1 and IRF3 in mice only slightly reduces the animals' death but fail to affect the activation of coagulation in CLP-induced sepsis mice.63 Meanwhile, type I IFNs do not induce the release of coagulation factor III (F3), which is the principal initiator of coagulopathy in sepsis in primary and immortalized monocytes or macrophages. These findings suggest that the involvement of the STING1/IRF3/type I IFN axis may contribute to an earlier step of sepsis by promoting inflammation rather than the coagulation pathway. Nevertheless, type I IFNs play an important role in establishing and regulating the host's defense against microbial infections.
STING1 and cytokine storms in sepsis
The initiation of inflammation is important to eradicate infection and repair tissue damage, whereas an excessive inflammatory response (or cytokine storm) can cause disseminated intravascular coagulation, tissue injury, organ dysfunction, and death in inflammatory diseases, such as severe COVID-19.64, 65, 66, 67, 68, 69 During sepsis, the production of inflammatory cytokines, such as tumor necrosis factor α (TNFα), interleukin 1 (IL1), interleukin 6 (IL6), C-C motif chemokine ligand 2 (CCL2), and CXCL10, are controlled by various transcriptional factors, especially nuclear factor-κB (NF-κB).58,70,71
In addition to IRF3, STING1 activation could also recruit transforming growth factor β-activated kinase (TAK) and the inhibitor of NF-κB kinase (IKK) complexes thereby activating NF-κB–mediated production of inflammatory cytokines such as TNFα, IL6, CXCL10, and CCL5.60,72 In an acute pancreatitis model, STING1 knockout mice develop less inflammation than control mice, while the STING1 agonist DMXAA increases TNFα expression and worsens acute pancreatitis.34 Consistent with this, systemic DMXAA administration results in the release of TNFα and IL6 as well as shock-like symptoms in mice, and TNFα is essential for strong necroptosis after the activation of STING1 in macrophages.61 These data suggest STING1 may act as an enhancer of inflammation by promoting pro-inflammatory cytokine production, leading to a cytokine storm and sepsis.
During human abdominal sepsis, STING1 signaling and STING1-induced inflammatory cytokines (IL1, IL6, and TNFα) are activated in peripheral blood mononuclear cells.20 DMXAA treatment activates the STING1/IRF3 or NF-κB pathway and increases the levels of serum and intestinal cytokines (IL6 and TNFα) and intestinal epithelial cell apoptosis in CLP-induced septic mice,20 whereas STING1 deficiency significantly reduces the levels of serum IL6, interleukin 12B, and CCL2 in CLP-induced severe sepsis in mice.19 These data indicate that STING1 activation contributes to an inflammatory response in polymicrobial sepsis. Moreover, STING1-mediated NLRP3 inflammasome activation triggers the expression of cytokines (interleukin 1 beta [IL1β], TNFα, CCL2, and HMGB1) as well as apoptosis and pyroptosis in mice.62 ALK-dependent STING1 activation is also required for LPS-induced activation of the IRF3 and NF-κB pathway and the subsequent cytokine storms and systemic coagulation in mice.36,37 Meanwhile, STING1 deficiency can reduce the expression of ALT and F3 in CLP-induced septic mice.20,63 Thus, STING1 is an important mediator of polymicrobial sepsis- and endotoxemia-induced inflammation, coagulation, and tissue damage (Fig. 2).
Inflammation is the double-edged sword of infection-related immunity, which depends on the type of signals involved. STING1 has also been suggested to be a negative regulator in some chronic inflammatory diseases, such as inflammatory bowel disease. STING1-deficient mice are prone to intestinal inflammation in response to moderate dextran sulfate sodium stimulation.73,74 STING1 deficiency impairs the production of interleukin 10 (IL10),74 which is an anti-inflammatory cytokine regulated by IRF3 and NF-κB.75 These results suggest a protective effect of STING1-dependent IL10 secretion for gut homeostasis by preventing an excessive inflammatory response. Overall, STING1 signaling may play a paradoxical role in acute and chronic inflammatory response, depending on the induction of anti-inflammatory and pro-inflammatory cytokines.
STING1 and autophagy in sepsis
Autophagy is a lysosome-dependent degradation process and includes three types: macroautophagy, microautophagy, and chaperone-mediated autophagy.76 Macroautophagy (hereafter referred to as autophagy), the most studied form of autophagy, is closely related to inflammation and immunity.77 After the onset of sepsis, autophagy is induced by PAMPs, DAMPs, and pro-inflammatory cytokines,5,78,79 generally conferring protective effects on multiple tissues.80, 81, 82, 83 The pro-survival role of autophagy in sepsis is attributed to the clearance of invasive microbes,78 the balancing of an anti- and pro-inflammatory response,80 the maintenance of lipid metabolism,84 the quality control of mitochondria,42 and the prevention of cell death.83,85 Genetically, some autophagy-related (ATG) genes fine-tune these processes.86 For example, deficiencies in beclin1 (BECN1, also known as ATG6) or microtubule-associated protein 1 light chain 3 alpha (also known as ATG8) result in an exaggerated inflammatory response with the development of organ injury and death.81,87 PTEN-induced kinase 1 and Parkin RBR E3 ubiquitin protein ligase (PRKN/PARK2)-mediated mitophagy is important for mitochondria integrity and inflammation control in sepsis.80,88
Accumulating evidence has demonstrated that STING1 can induce autophagy through a canonical autophagy induction process, which depends on the inactivation of mechanistic target of rapamycin (MTOR) kinase and the activation of BECN1, or through a noncanonical autophagy induction process, which is independent of unc-51–like autophagy activating kinase 1 (ULK1), BECN1, and phosphatidylinositol 3-kinase catalytic subunit type 3 (PIK3C3).14,89, 90, 91 STING1-mediated autophagy is involved in multiple biological processes, including the removal of bacteria and viruses.89,92 The function of STING1 in autophagy is conserved in invertebrates and vertebrates.14,92
STING1-induced autophagy can also prevent the overactivation of STING1 by sequestosome 1 (SQSTM1/p62), a well-known autophagy selective receptor that induces lysosomal degradation, whereas reduced SQSTM1 expression or pharmacological inhibition of autophagy exaggerates STING1-mediated type I IFN and cytokine production.72,93 Thus, SQSTM1-mediated autophagic degradation of STING1 may act as a negative controller of the STING1 pathway, protecting the overwhelming inflammatory response. Notably, IFN-dependent C-terminal tail region and IRF3 are not required for STING1-mediated autophagy.14 Given that IFN-dependent STING1 signaling may originate from the evolution of vertebrates only of which STING1 contains C-terminal tail domain recruiting TBK1 and IRF3,94 the IFN-independent autophagy induction of STING1 may be a primordial function of the CGAS pathway for antimicrobial infection. Thus, it is possible that STING1-induced autophagy can prevent cytokine storms and sepsis not only through degrading STING1 but also through the clearance of pathogens. Considering that the pharmacological activation of autophagy could prevent sepsis95,96 and pharmacological inhibition of autophagy worsens septic response,97 it is recommended that STING1-mediated autophagy be activated to treat sepsis.
In contrast to the intracellular function of SQSTM1 in inflammation, extracellular SQSTM1 was recently found to act as a lethal inflammatory mediator of sepsis after STING1 activation.98 The activation of STING1 and subsequent TBK1 induces SQSTM1 phosphorylation, expression, and subsequent secretion in macrophages. The extracellular SQSTM1 binding the insulin receptor on plasma membrane activates NF-κB (Fig. 2), causing polarization of pro-inflammatory macrophages, and finally leading to septic death in mice through excessive inflammation and coagulation. These data suggest multifaceted roles for SQSTM1 as an autophagy receptor or pro-inflammatory mediator in sepsis. More efforts should be made to illustrate the contributions of STING1 and SQSTM1-induced inflammatory response and autophagy to sepsis and other diseases.
STING1 and cell death in sepsis
Regulated cell death (RCD) is caused by the activation of one or more signal transduction modules. RCD includes but is not limited to apoptosis, necroptosis, pyroptosis, and ferroptosis.99,100 Since increased cell death is observed in non-immune and immune cells during sepsis, RCD is considered to play an important role in organ dysfunction and immune dysregulation.24,101,102 Recent evidence indicates that STING1 mediates various types of RCD and hence contributes to inflammation, immunosuppression, and the coagulation activation of sepsis, as described below.
STING1-mediated apoptosis in sepsis
Apoptosis is generally a form of immune-silent RCD, in which the cellular membranes remain intact and intracellular proteins and organelles are removed without alarming the immune system. Apoptotic pathways engage mitochondria or death receptor-mediated signal transduction, resulting in the activation of a series of apoptotic effectors, such as caspase 3 (CASP3), caspase 6 (CASP6), and caspase 7 (CASP7), which ultimately leads to nonlytic cell death.103
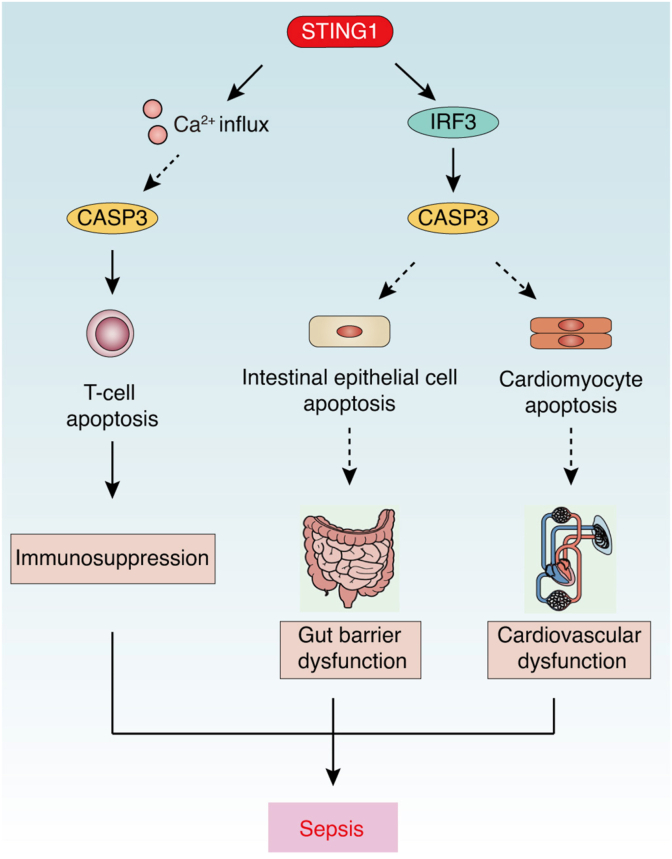
Apoptosis is one of the most well-described mechanisms leading to sepsis-induced organ dysfunction in gut and respiratory epithelial cells, cardiomyocytes, and endothelial cells.24 Recently, several studies have linked STING1 with non-immune cell apoptosis in sepsis. Increased intestinal permeability after CLP induces intestinal epithelial cell (IEC) apoptosis and is blocked in STING1 knockout mice, while treatment with the STING1 agonist DMXAA aggravates sepsis-induced IEC apoptosis and abdominal sepsis.20 These findings indicate that STING1-mediated apoptosis favors the pathogenesis of sepsis by damaging the gut barrier. In addition to gut, STING1 also participates in heart tissue apoptosis.104 During sepsis, LPS-induced STING1 upregulation contributes to apoptosis and subsequent heart injuries in mice, while this process can be inhibited by the administration of selenium, which inactivates the STING1 pathway. In addition, STING1 deficiency significantly reduces the level of apoptotic cardiomyocytes in LPS-treated mice and decreases the activation of CASP3 and reduces the ratio of BCL2-associated X, apoptosis regulator (BAX) to BCL2 apoptosis regulator (BCL2).62 Given that STING1-mediated apoptosis is often involved in the regulation of ER stress, IRF3, or BAX function in non-immune cells,15 it is possible the same mechanism is involved in non-immune cell apoptosis during sepsis. Overall, STING1 is directly involved in the apoptosis of non-immune cells and the damage to the intestinal barrier and heart tissue during sepsis (Fig. 3).
Fig. 3.
STING1-mediated apoptosis in sepsis. During sepsis, activated STING1 triggers T cell apoptosis via a Ca2+- and CASP3-dependent pathway, causing immunosuppression or triggering apoptosis in non-immune cells (such as intestinal epithelial cells and cardiomyocytes) via an IRF3-dependent pathway, causing tissue dysfunction (such as gut barrier or cardiovascular dysfunction).
Abbreviations: Ca2+: calcium; CASP: caspase; F3: coagulation factor III; IRF3: interferon regulatory factor 3; STING1: stimulator of interferon response cGAMP interactor 1.
The loss of immune cells through apoptosis is one of the known mechanisms responsible for impaired immune defenses in acute critical illness, including septic shock.105 Extensive apoptosis in lymphocytes and dendritic cells could release a large quantity of DAMPs, including HMGB1, cfDNA, histones, and neutrophil extracellular traps, leading to cytokine storms, immunosuppression, and coagulation activation and finally causing multiple organ failure and death.106 STING1 activation by agonists has been shown to trigger apoptosis in B cells and T cells,107, 108, 109, 110, 111 indicating that STING1 activation is highly pro-apoptotic in lymphocytes and may contribute to lymphocyte apoptosis in sepsis. Indeed, splenic CD4 T cells experience STING1-mediated apoptosis during sepsis, leading to immunodeficiency in endotoxemia mice.112 Specifically, LPS-induced cleaved CASP3 is increased in a STING1-dependent manner, which could be blocked by the competitive interaction of the Notch intracellular signaling domain with the CDN-binding domain of STING1, thereby limiting the activation of STING1 by cGAMP and the blockage of STING1-mediated T cell apoptosis (Fig. 3). Interestingly, STING1 activation promotes T cell apoptosis by the regulation of calcium (Ca2+) homeostasis and ER stress independently of IFN signaling.109 Further research may focus on the pro-apoptotic effect of STING1 in lymphocytes during sepsis, which is independent of IFN.
In summary, STING1-mediated apoptosis can lead to tissue damage induced by non-immune cell death and immunosuppression induced by T cell death. Since many anti-apoptotic strategies have successfully reduced mortality after sepsis,113,114 it is possible to treat sepsis by inhibiting STING1-mediated apoptosis.
STING1-mediated necroptosis in sepsis
Necroptosis is a programmed form of necrosis, of which the core regulator is composed of three proteins: receptor interacting serine/threonine kinase 1 (RIPK1), receptor interacting serine/threonine kinase 3 (RIPK3), and mixed-lineage kinase domain-like pseudokinase (MLKL). After activation by death receptors or PRRs, RIPK1 phosphorylates MLKL through RIPK3. MLKL then oligomerizes and destroys the plasma membrane, leading to necroptotic cell death.115 Necroptosis acts as a trigger of inflammation in many diseases.116,117 Increased necroptosis is also implicated in sepsis through promoting the release or activation of inflammatory effectors, such as HMGB1,118, 119, 120 TNF-related apoptosis-inducing ligand,121 and gasdermin D (GSDMD).122 STING1 signals can trigger necroptosis and promote inflammation by initiating MLKL expression or inducing MLKL phosphorylation.123,124 As expected, TNF and necroptotic signaling is increased in STING1 agonist-induced sterile shock in mice; however, STING1-mediated necroptosis may have a limited role in the production of inflammatory cytokines in blood.61 The precise role of STING1 in necroptosis during sepsis needs to be further studied.
STING1-mediated pyroptosis in sepsis
Pyroptosis is a form of lytic pro-inflammatory RCD driven by N-terminal fragment of GSDMD (GSDMD-N)- or N-terminal fragment of gasdermin E (GSDME/DFNA5-N)-dependent pore formation, and it occurs in various immune and non-immune cells.125 The induction of pyroptosis requires the activation of inflammasomes, which are multiprotein intracellular complexes that detect PAMPs and DAMPs.126,127 Canonical inflammasome complexes, such as NLRP3 and AIM2, activate the CASP1-mediated secretion of IL1 family cytokines (e.g., IL1β and interleukin 18 [IL18]) and cleavage of GSDMD that produces GSDMD-N.128 Alternatively, cytoplasmic LPS-induced activation of CASP11 (also known as caspase 4 [CASP4] or caspase 5 [CASP5] in humans) triggers noncanonical inflammasome-mediated GSDMD-N production.129 In contrast, the production of GSDME-N is mediated by CASP3.130 These findings indicate that the caspase family is involved in apoptosis and non-apoptotic cell death.
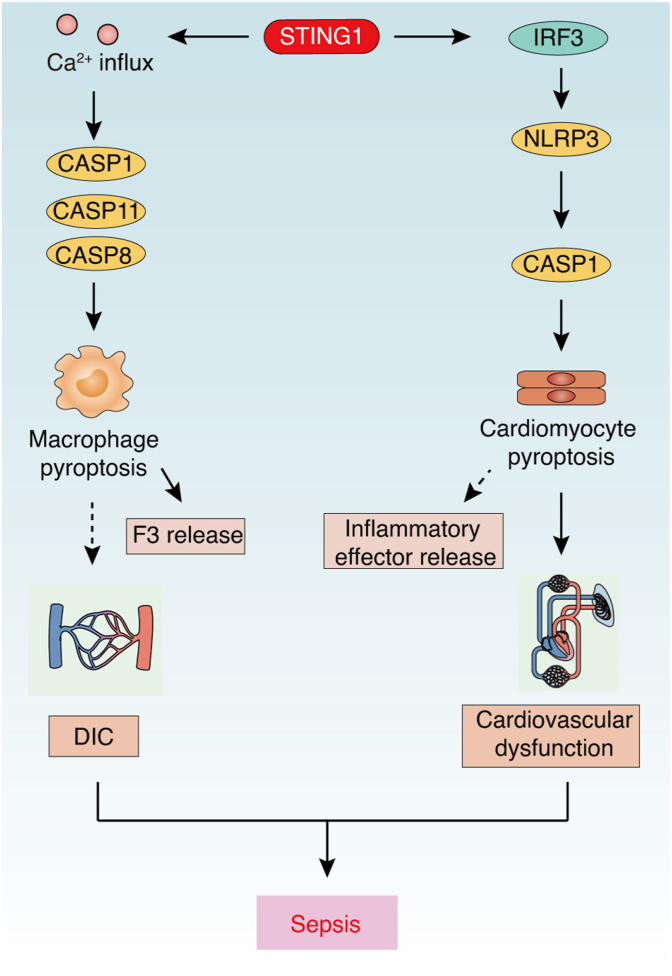
Although adequate pyroptosis can protect against bacterial infection, hyperactivated pyroptosis can rupture the plasma membrane, resulting in the release of abundant inflammatory effectors for organ dysfunction in sepsis.126,131, 132, 133 SQSTM1 can also be passively released through GSDMD-dependent pyroptosis, acting as an extracellular mediator of septic death in myeloid cells.98,134 The chemical inhibition of GSDMD directly is able to reduce the release of inflammatory mediators, including SQSTM1, during sepsis.98,135 The activation and function of pyroptosis in sepsis is further regulated by STING1. For example, cytosolic DNA signals can activate STING1-mediated pyroptosis by an IRF3-dependent transcriptional upregulation of NLRP343 or lysosomal cell death-related potassium efflux to activate NLRP3 inflammasomes.136 In sepsis, a STING1 deficiency reduces the protein levels of NLRP3 and CASP1 in LPS-treated septic mice, whereas IRF3 activation by STING1 could increase the expression of NLRP3 and CASP1 and subsequent pytoptosis in neonatal rat cardiomyocytes.62 Thus, an IRF3-dependent pathway downstream of STING1 might be involved in pytoptosis and heart injury during sepsis. Further, type I IFNs could induce HMGB1 release, leading to CASP11-dependent GSDMD activation in macrophages,56 whereas heparin can inhibit this process to prevent sepsis.137 These data indicate that STING1-mediated type I IFNs might also contribute to noncanonical inflammasome-mediated GSDMD activation, pyroptosis, and tissue injury (Fig. 4).
Fig. 4.
STING1-mediated pyroptosis in sepsis. During sepsis, activated STING1 triggers Ca2+-mediated pyroptosis in macrophages, leading to F3 release and coagulation. However, STING1 also activates IRF3/NLRP3 axis-mediated CASP1-dependent pyroptosis in cardiomyocytes, leading to cardiovascular dysfunction.
Abbreviations: Ca2+: calcium; CASP: caspase; DIC: disseminated intravascular coagulation; F3: coagulation factor III; IRF3: interferon regulatory factor 3; NLRP3: NLR family pyrin domain containing 3; STING1: stimulator of interferon response cGAMP interactor 1.
Coagulation is characterized as a driver of multiple organ failure in sepsis.64 GSDMD-N–mediated pore formation could release F3, leading to coagulation, although it may occur in a pyroptosis-dependent and -independent manner.138, 139, 140 STING1-dependent GSDMD activation has been shown to trigger F3 release during sepsis.63 Global depletion or conditional ablation of STING1 in myeloid cells blocks GSDMD-N production and protects mice against CLP-induced F3 release in the blood, coagulation activation, and finally death. STING1-mediated ER-stress and Ca2+ influx are required for CASP activation and subsequent GSDMD-mediated F3 release in macrophages. Moreover, ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 (ATP2A2) and inositol 1,4,5-trisphosphate receptor type 1 (ITPR1), by controlling Ca2+ transport between the ER and cytoplasm, regulate GSDMD-N formation and subsequent F3 release and coagulation activation in sepsis. In contrast, a deficiency of CGAS, IFNAR1, and IRF3 fails to affect CLP-induced coagulation activation in mice, indicating that STING1-mediated GSDMD-dependent coagulation may depend on Ca2+ elevation, rather than on the IRF3/type I IFN pathway (Fig. 4). In fact, STING1 has been widely associated with ER homeostasis and Ca2+ signals.89,109,141,142 Since ER-related Ca2+ signals play a broad role in various types of cell death,143 we speculate that Ca2+ signals are an important downstream event of STING1 activation, which not only regulates pyrolysis, but also regulates apoptosis and other cell deaths during sepsis.
STING1-mediated ferroptosis in sepsis
Ferroptosis is an iron-dependent oxidative RCD triggered by lipid peroxidation, which is negatively regulated by certain antioxidant systems, especially the solute carrier family 7 member 11 (SLC7A11)-glutathione–glutathione peroxidase 4 (GPX4) axis.144, 145, 146 Excessive or defective ferroptotic cell death is associated with a growing list of diseases involving dysregulated inflammation and immune response.147,148 In sepsis mouse models, the pharmacological inhibition of ferroptosis can protect CLP- or LPS-induced tissue injury via a decreased inflammation response,149,150 suggesting a role for ferroptosis in sepsis. However, the conditional depletion of GPX4 in myeloid cells increases lipid peroxidation-dependent pyroptosis, rather than ferroptosis, leading to a systemic inflammatory response and multiple organ dysfunctions in polymicrobial sepsis mice.151 In contrast, a lipid peroxidizing enzyme, arachidonate 5-lipoxygenase (ALOX5), mediates lipid peroxidation that is responsible for CASP11 inflammasome activation and pyroptosis in macrophages, which favors LPS-induced sepsis.127 This process is further enhanced by the phosphorylation of phospholipase C gamma 1 (PLCG1) activation and Ca2+ elevation.151 Considering the contribution of Ca2+ influx in GSDMD activation as we described earlier, we hypothesize that sepsis-induced lipid peroxidation activates the PLCG1/Ca2+ pathway and subsequent pyroptosis. In fact, a recent study demonstrated that NADPH oxidase-mediated lipid peroxidation could activate phospholipase C-dependent Ca2+ influx, which further promotes NLRP3 inflammasome and CASP1 activation via reactive oxygen species in mitochondria, leading to GSDMD-mediated pyroptosis.152 These data indicate a crosstalk effect of lipid peroxidation between ferroptosis and pyrolysis during sepsis. STING1 activation can induce lipid peroxidation and ferroptosis in pancreatic cancer cells through STING1-dependent autophagic degradation of GPX4.153,154 Moreover, STING1-mediated lipid peroxidation contributes to intestinal ischemia-reperfusion injury, which is a systemic inflammatory response syndrome.155 STING1 deficiency can reduce the levels of lipid peroxidation biomarkers in macrophages and intestinal tissues, and a lipid peroxidation inhibitor, liproxstatin-1, can reverse the lung and liver cell death induced by STING1 activation. In contrast, GPX4 depletion increases lipid peroxidation in macrophages and limits STING1 activation by lipid product 4-hydroxynonenal-mediated carbonylation, indicating a dual regulation between STING1 and lipid peroxidation.156 Further study of the relationship between STING1 and lipid peroxidation may expand the role of STING1-mediated ferroptosis in sepsis.
Conclusions and prospects
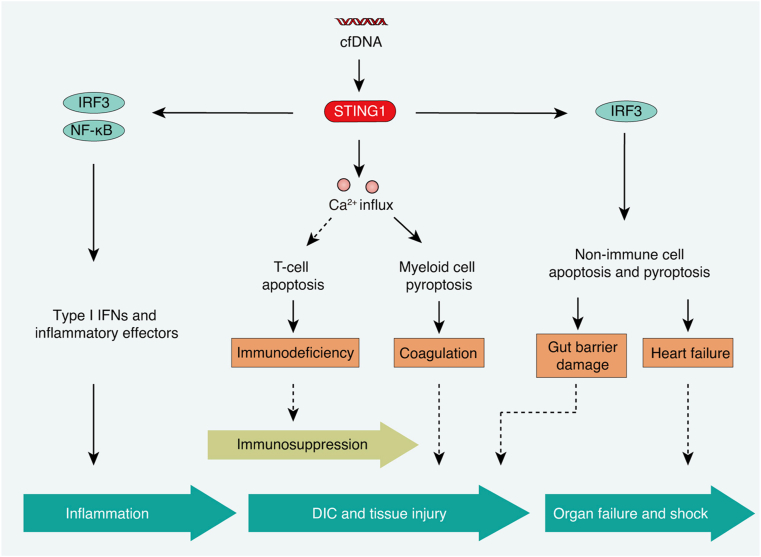
In this review, we summarize the multiple functions of STING1 in the pathogenesis of sepsis (Fig. 5). In the early stages of sepsis, STING1 activation initiates the production and secretion of type I IFNs or inflammatory cytokines and effectors, leading to an overwhelming inflammatory response. In the later stage of sepsis, STING1 activation induces Ca2+-dependent apoptosis in T cells, GSDMD-dependent pyroptosis in myeloid cells, and IRF3-dependent apoptosis and pyroptosis in non-immune cells, which leads to immunosuppression, coagulation, and tissue injury. Several questions remain to be answered: Can STING1-dependent autophagy play the role of a negative regulatory loop in sepsis? Is STING1-mediated necroptosis, ferroptosis, and other types of cell death related to sepsis? Is there any direct link between STING1 and lipid peroxidation in sepsis? How does STING1 activation regulate Ca2+ signaling during sepsis?
Fig. 5.
The network of STING1 in the pathological process of sepsis. Activated STING1 triggers the production of type I IFNs and inflammatory cytokines, apoptosis, and pyroptosis, contributing to different stages in the pathological process of sepsis, such as inflammation, immunosuppression, DIC and tissue injury, organ failure, and shock.
Abbreviations: Ca2+: calcium; cfDNA: circulating cell-free DNA; DIC: disseminated intravascular coagulation; IRF3: interferon regulatory factor 3; NF-κB: nuclear factor kappa B complex; STING1: stimulator of interferon response cGAMP interactor 1.
The multifunctional role of STING1 in sepsis suggests that the chemical inhibition of STING1 might be a novel therapeutic strategy for treating sepsis. Although STING1 agonists have been used as drugs for the clinical treatment of cancer and as antiviral therapy in recent years, few STING1 inhibitors have been developed so far. Future work may focus on the development of new antagonists that directly target the processes of STING1 activation, including DNA sensing, ER transport to the Golgi apparatus, and the recruitment of downstream proteins, such as IRF3, NF-κB, and NLRP3. STING1 activation is tightly controlled by posttranslational modification, such as palmitoylation, which has been proved to be effectively targeted by covalent small molecules that inhibit STING1-induced immune response and T cell death.110,157 Therefore, it is possible to develop inhibitors that affect the posttranslational modification of STING1. In addition, the atypical role of STING1 in autophagy and Ca2+ signaling has been confirmed. The combination of genetic technology and chemical screening in these areas may help identify new drugs for the treatment of sepsis and other STING1-related diseases.
Funding
Nil.
Ethical statement
Not applicable.
Declaration of competing interest
The authors declare no conflicts of interest or financial interests.
Acknowledgments
We thank Dave Primm (Department of Surgery, University of Texas Southwestern Medical Center) for his critical reading of the manuscript.
Footnotes
Peer review under responsibility of Chinese Medical Association.
Author contributions
Ruo-Xi Zhang wrote the manuscript. Rui Kang and Dao-Lin Tang edited the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
References
- 1.Rudd K.E., Johnson S.C., Agesa K.M., et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) J Am Med Assoc. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gando S., Shiraishi A., Yamakawa K., et al. Role of disseminated intravascular coagulation in severe sepsis. Thromb Res. 2019;178:182–188. doi: 10.1016/j.thromres.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Deng W., Zhu S., Zeng L., et al. The circadian clock controls immune checkpoint pathway in sepsis. Cell Rep. 2018;24:366–378. doi: 10.1016/j.celrep.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D., Kang R., Coyne C.B., et al. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentile L.F., Moldawer L.L. DAMPs, PAMPs, and the origins of SIRS in bacterial sepsis. Shock. 2013;39:113–114. doi: 10.1097/SHK.0b013e318277109c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmermans K., Kox M., Scheffer G.J., et al. Plasma nuclear and mitochondrial DNA levels, and markers of inflammation, shock, and organ damage in patients with septic shock. Shock. 2016;45:607–612. doi: 10.1097/SHK.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 8.Di Caro V., Walko T.D., 3rd, Bola R.A., et al. Plasma mitochondrial DNA--a novel DAMP in pediatric sepsis. Shock. 2016;45:506–511. doi: 10.1097/SHK.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comish P.B., Carlson D., Kang R., et al. Damage-associated molecular patterns and the systemic immune consequences of severe thermal injury. J Immunol. 2020;205:1189–1197. doi: 10.4049/jimmunol.2000439. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun W., Li Y., Chen L., et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci U S A. 2009;106:8653–8658. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong B., Yang Y., Li S., et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Gui X., Yang H., Li T., et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567:262–266. doi: 10.1038/s41586-019-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R., Kang R., Tang D. The STING1 network regulates autophagy and cell death. Signal Transduct Target Ther. 2021;6:208. doi: 10.1038/s41392-021-00613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber G.N. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15:760–770. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decout A., Katz J.D., Venkatraman S., et al. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021 doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng L., Kang R., Zhu S., et al. ALK is a therapeutic target for lethal sepsis. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aan5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heipertz E.L., Harper J., Walker W.E. STING and TRIF contribute to mouse sepsis, depending on severity of the disease model. Shock. 2017;47:621–631. doi: 10.1097/SHK.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 20.Hu Q., Ren H., Li G., et al. STING-mediated intestinal barrier dysfunction contributes to lethal sepsis. EBioMedicine. 2019;41:497–508. doi: 10.1016/j.ebiom.2019.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopfner K.P., Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol. 2020;21:501–521. doi: 10.1038/s41580-020-0244-x. [DOI] [PubMed] [Google Scholar]
- 22.Ahn J., Barber G.N. STING signaling and host defense against microbial infection. Exp Mol Med. 2019;51:1–10. doi: 10.1038/s12276-019-0333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruda M.C., Ruggeberg K.G., O'Sullivan P., et al. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb(R) sorbent porous polymer beads. PloS One. 2018;13 doi: 10.1371/journal.pone.0191676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinheiro da Silva F., Nizet V. Cell death during sepsis: integration of disintegration in the inflammatory response to overwhelming infection. Apoptosis. 2009;14:509–521. doi: 10.1007/s10495-009-0320-3. [DOI] [PubMed] [Google Scholar]
- 25.Nakahira K., Kyung S.Y., Rogers A.J., et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001577. discussion e1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuels D.C., Hulgan T., Fessel J.P., et al. Mitochondrial DNA haplogroups and delirium during sepsis. Crit Care Med. 2019;47:1065–1071. doi: 10.1097/CCM.0000000000003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrington J.S., Huh J.W., Schenck E.J., et al. Circulating mitochondrial DNA as predictor of mortality in critically ill patients: a systematic review of clinical studies. Chest. 2019;156:1120–1136. doi: 10.1016/j.chest.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faust H.E., Reilly J.P., Anderson B.J., et al. Plasma mitochondrial DNA levels are associated with ARDS in trauma and sepsis patients. Chest. 2020;157:67–76. doi: 10.1016/j.chest.2019.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer S.T., Franken L., Adamzik M., et al. Mitochondrial DNA: an endogenous trigger for immune paralysis. Anesthesiology. 2016;124:923–933. doi: 10.1097/ALN.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 30.Weiss S.L., Zhang D., Bush J., et al. Persistent mitochondrial dysfunction linked to prolonged organ dysfunction in pediatric sepsis. Crit Care Med. 2019;47:1433–1441. doi: 10.1097/CCM.0000000000003931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawkins R.B., Stortz J.A., Holden D.C., et al. Persistently increased cell-free DNA concentrations only modestly contribute to outcome and host response in sepsis survivors with chronic critical illness. Surgery. 2020;167:646–652. doi: 10.1016/j.surg.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q., Raoof M., Chen Y., et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuji N., Tsuji T., Ohashi N., et al. Role of mitochondrial DNA in septic AKI via toll-like receptor 9. J Am Soc Nephrol. 2016;27:2009–2020. doi: 10.1681/ASN.2015040376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Q., Wei Y., Pandol S.J., et al. STING signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology. 2018;154:1822–1835. doi: 10.1053/j.gastro.2018.01.065. e1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torralba D., Baixauli F., Villarroya-Beltri C., et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat Commun. 2018;9:2658. doi: 10.1038/s41467-018-05077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang R., Xie Y., Zhang Q., et al. Intracellular HMGB1 as a novel tumor suppressor of pancreatic cancer. Cell Res. 2017;27:916–932. doi: 10.1038/cr.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge W., Hu Q., Fang X., et al. LDK378 improves micro- and macro-circulation via alleviating STING-mediated inflammatory injury in a Sepsis rat model induced by Cecal ligation and puncture. J Inflamm. 2019;16:3. doi: 10.1186/s12950-019-0208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plitas G., Burt B.M., Nguyen H.M., et al. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med. 2008;205:1277–1283. doi: 10.1084/jem.20080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hemmi H., Takeuchi O., Kawai T., et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 40.Tsujimoto H., Ono S., Matsumoto A., et al. A critical role of CpG motifs in a murine peritonitis model by their binding to highly expressed toll-like receptor-9 on liver NKT cells. J Hepatol. 2006;45:836–843. doi: 10.1016/j.jhep.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 41.Fernandes-Alnemri T., Yu J.W., Juliana C., et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakahira K., Haspel J.A., Rathinam V.A., et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ning L., Wei W., Wenyang J., et al. Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung injury induced by lipopolysaccharide. Clin Transl Med. 2020;10:e228. doi: 10.1002/ctm2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dawulieti J., Sun M., Zhao Y., et al. Treatment of severe sepsis with nanoparticulate cell-free DNA scavengers. Sci Adv. 2020;6 doi: 10.1126/sciadv.aay7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee Y.Y., Park H.H., Park W., et al. Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials. 2021;267:120389. doi: 10.1016/j.biomaterials.2020.120389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laukova L., Konecna B., Babickova J., et al. Exogenous deoxyribonuclease has a protective effect in a mouse model of sepsis. Biomed Pharmacother. 2017;93:8–16. doi: 10.1016/j.biopha.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Li Y., Guo X., Hu C., et al. Type I IFN operates pyroptosis and necroptosis during multidrug-resistant A. baumannii infection. Cell Death Differ. 2018;25:1304–1318. doi: 10.1038/s41418-017-0041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karaghiosoff M., Steinborn R., Kovarik P., et al. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat Immunol. 2003;4:471–477. doi: 10.1038/ni910. [DOI] [PubMed] [Google Scholar]
- 49.Pinto A.K., Ramos H.J., Wu X., et al. Deficient IFN signaling by myeloid cells leads to MAVS-dependent virus-induced sepsis. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker W.E., Bozzi A.T., Goldstein D.R. IRF3 contributes to sepsis pathogenesis in the mouse cecal ligation and puncture model. J Leukoc Biol. 2012;92:1261–1268. doi: 10.1189/jlb.0312138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heipertz E.L., Harper J., Goswami D.G., et al. IRF3 signaling within the mouse stroma influences sepsis pathogenesis. J Immunol. 2021;206:398–409. doi: 10.4049/jimmunol.1900217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karimi Y., Poznanski S.M., Vahedi F., et al. Type I interferon signalling is not required for the induction of endotoxin tolerance. Cytokine. 2017;95:7–11. doi: 10.1016/j.cyto.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 53.de Weerd N.A., Vivian J.P., Nguyen T.K., et al. Structural basis of a unique interferon-beta signaling axis mediated via the receptor IFNAR1. Nat Immunol. 2013;14:901–907. doi: 10.1038/ni.2667. [DOI] [PubMed] [Google Scholar]
- 54.Rackov G., Shokri R., De Mon M.A., et al. The role of IFN-beta during the course of sepsis progression and its therapeutic potential. Front Immunol. 2017;8:493. doi: 10.3389/fimmu.2017.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huys L., Van Hauwermeiren F., Dejager L., et al. Type I interferon drives tumor necrosis factor-induced lethal shock. J Exp Med. 2009;206:1873–1882. doi: 10.1084/jem.20090213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang X., Cheng X., Tang Y., et al. The role of type 1 interferons in coagulation induced by gram-negative bacteria. Blood. 2020;135:1087–1100. doi: 10.1182/blood.2019002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kader M., El Andaloussi A., Vorhaour J., et al. Interferon type I regulates inflammasome activation and high mobility group box 1 translocation in hepatocytes during ehrlichia-induced acute liver injury. Hepatol Commun. 2021;5:33–51. doi: 10.1002/hep4.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hotchkiss R.S., Moldawer L.L., Opal S.M., et al. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly-Scumpia K.M., Scumpia P.O., Delano M.J., et al. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med. 2010;207:319–326. doi: 10.1084/jem.20091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balka K.R., Louis C., Saunders T.L., et al. TBK1 and IKKepsilon act redundantly to mediate STING-induced NF-kappaB responses in myeloid cells. Cell Rep. 2020;31:107492. doi: 10.1016/j.celrep.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 61.Brault M., Olsen T.M., Martinez J., et al. Intracellular nucleic acid sensing triggers necroptosis through synergistic type I IFN and TNF signaling. J Immunol. 2018;200:2748–2756. doi: 10.4049/jimmunol.1701492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li N., Zhou H., Wu H., et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215. doi: 10.1016/j.redox.2019.101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H., Zeng L., Xie M., et al. TMEM173 drives lethal coagulation in sepsis. Cell Host Microbe. 2020;27:556–570. doi: 10.1016/j.chom.2020.02.004. e556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levi M., van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Lopez-Otin C., Kroemer G. Hallmarks of health. Cell. 2021;184:33–63. doi: 10.1016/j.cell.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 66.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang D., Comish P., Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen R., Huang Y., Quan J., et al. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wool G.D., Miller J.L. The impact of COVID-19 disease on platelets and coagulation. Pathobiology. 2020:1–13. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang N., Wu R., Tang D., et al. The BET family in immunity and disease. Signal Transduct Target Ther. 2021;6:23. doi: 10.1038/s41392-020-00384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu R., Chen F., Wang N., et al. ACOD1 in immunometabolism and disease. Cell Mol Immunol. 2020;17:822–833. doi: 10.1038/s41423-020-0489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prabakaran T., Bodda C., Krapp C., et al. Attenuation of cGAS-STING signaling is mediated by a p62/SQSTM1-dependent autophagy pathway activated by TBK1. EMBO J. 2018;37 doi: 10.15252/embj.201797858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Canesso M.C.C., Lemos L., Neves T.C., et al. The cytosolic sensor STING is required for intestinal homeostasis and control of inflammation. Mucosal Immunol. 2018;11:820–834. doi: 10.1038/mi.2017.88. [DOI] [PubMed] [Google Scholar]
- 74.Ahn J., Son S., Oliveira S.C., et al. STING-dependent signaling underlies IL-10 controlled inflammatory colitis. Cell Rep. 2017;21:3873–3884. doi: 10.1016/j.celrep.2017.11.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang E.Y., Guo B., Doyle S.E., et al. Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J Immunol. 2007;178:6705–6709. doi: 10.4049/jimmunol.178.11.6705. [DOI] [PubMed] [Google Scholar]
- 76.Klionsky D.J., Abdel-Aziz A.K., Abdelfatah S., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1) Autophagy. 2021;17:1–382. doi: 10.1080/15548627.2020.1797280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deretic V., Saitoh T., Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakagawa I., Amano A., Mizushima N., et al. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- 79.Djavaheri-Mergny M., Amelotti M., Mathieu J., et al. Regulation of autophagy by NFkappaB transcription factor and reactives oxygen species. Autophagy. 2007;3:390–392. doi: 10.4161/auto.4248. [DOI] [PubMed] [Google Scholar]
- 80.Kang R., Zeng L., Xie Y., et al. A novel PINK1- and PARK2-dependent protective neuroimmune pathway in lethal sepsis. Autophagy. 2016;12:2374–2385. doi: 10.1080/15548627.2016.1239678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun Y., Yao X., Zhang Q.J., et al. Beclin-1-Dependent autophagy protects the heart during sepsis. Circulation. 2018;138:2247–2262. doi: 10.1161/CIRCULATIONAHA.117.032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stana F., Vujovic M., Mayaki D., et al. Differential regulation of the autophagy and proteasome pathways in skeletal muscles in sepsis. Crit Care Med. 2017;45:e971–e979. doi: 10.1097/CCM.0000000000002520. [DOI] [PubMed] [Google Scholar]
- 83.Carchman E.H., Rao J., Loughran P.A., et al. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology. 2011;53:2053–2062. doi: 10.1002/hep.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chung K.W., Kim K.M., Choi Y.J., et al. The critical role played by endotoxin-induced liver autophagy in the maintenance of lipid metabolism during sepsis. Autophagy. 2017;13:1113–1129. doi: 10.1080/15548627.2017.1319040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lim J., Park H., Heisler J., et al. Autophagy regulates inflammatory programmed cell death via turnover of RHIM-domain proteins. Elife. 2019;8 doi: 10.7554/eLife.44452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie Y., Kang R., Sun X., et al. Posttranslational modification of autophagy-related proteins in macroautophagy. Autophagy. 2015;11:28–45. doi: 10.4161/15548627.2014.984267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie J.M., Li B., Yu H.P., et al. TIGAR has a dual role in cancer cell survival through regulating apoptosis and autophagy. Canc Res. 2014;74:5127–5138. doi: 10.1158/0008-5472.CAN-13-3517. [DOI] [PubMed] [Google Scholar]
- 88.Piquereau J., Godin R., Deschenes S., et al. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy. 2013;9:1837–1851. doi: 10.4161/auto.26502. [DOI] [PubMed] [Google Scholar]
- 89.Moretti J., Roy S., Bozec D., et al. STING senses microbial viability to orchestrate stress-mediated autophagy of the endoplasmic reticulum. Cell. 2017;171:809–823 e813. doi: 10.1016/j.cell.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weber M.M., Lam J.L., Dooley C.A., et al. Absence of specific Chlamydia trachomatis inclusion membrane proteins triggers premature inclusion membrane lysis and host cell death. Cell Rep. 2017;19:1406–1417. doi: 10.1016/j.celrep.2017.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu D., Wu H., Wang C., et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 2019;26:1735–1749. doi: 10.1038/s41418-018-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamashiro L.H., Wilson S.C., Morrison H.M., et al. Interferon-independent STING signaling promotes resistance to HSV-1 in vivo. Nat Commun. 2020;11:3382. doi: 10.1038/s41467-020-17156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonugunta V.K., Sakai T., Pokatayev V., et al. Trafficking-mediated STING degradation requires sorting to acidified endolysosomes and can Be targeted to enhance anti-tumor response. Cell Rep. 2017;21:3234–3242. doi: 10.1016/j.celrep.2017.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu X., Wu F.H., Wang X., et al. Molecular evolutionary and structural analysis of the cytosolic DNA sensor cGAS and STING. Nucleic Acids Res. 2014;42:8243–8257. doi: 10.1093/nar/gku569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsieh C.H., Pai P.Y., Hsueh H.W., et al. Complete induction of autophagy is essential for cardioprotection in sepsis. Ann Surg. 2011;253:1190–1200. doi: 10.1097/SLA.0b013e318214b67e. [DOI] [PubMed] [Google Scholar]
- 96.Cho H.I., Kim S.J., Choi J.W., et al. Genipin alleviates sepsis-induced liver injury by restoring autophagy. Br J Pharmacol. 2016;173:980–991. doi: 10.1111/bph.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takahashi W., Watanabe E., Fujimura L., et al. Kinetics and protective role of autophagy in a mouse cecal ligation and puncture-induced sepsis. Crit Care. 2013;17:R160. doi: 10.1186/cc12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou B., Liu J., Zeng L., et al. Extracellular SQSTM1 mediates bacterial septic death in mice through insulin receptor signalling. Nat Microbiol. 2020;5:1576–1587. doi: 10.1038/s41564-020-00795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Galluzzi L., Vitale I., Aaronson S.A., et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang D., Kang R., Berghe T.V., et al. The molecular machinery of regulated cell death. Cell Res. 2019 doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hotchkiss R.S., Monneret G., Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Girardot T., Rimmele T., Venet F., et al. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis. 2017;22:295–305. doi: 10.1007/s10495-016-1325-3. [DOI] [PubMed] [Google Scholar]
- 103.Tang D., Kang R., Berghe T.V., et al. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X., Yang B., Cao H.L., et al. Selenium supplementation protects against lipopolysaccharide-induced heart injury via sting pathway in mice. Biol Trace Elem Res. 2021;199:1885–1892. doi: 10.1007/s12011-020-02295-5. [DOI] [PubMed] [Google Scholar]
- 105.Hotchkiss R.S., Nicholson D.W. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 106.Cheng Z., Abrams S.T., Toh J., et al. The critical roles and mechanisms of immune cell death in sepsis. Front Immunol. 2020;11:1918. doi: 10.3389/fimmu.2020.01918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang C.H., Zundell J.A., Ranatunga S., et al. Agonist-mediated activation of STING induces apoptosis in malignant B cells. Canc Res. 2016;76:2137–2152. doi: 10.1158/0008-5472.CAN-15-1885。. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Larkin B., Ilyukha V., Sorokin M., et al. Cutting edge: activation of STING in T cells induces type I IFN responses and cell death. J Immunol. 2017;199:397–402. doi: 10.4049/jimmunol.1601999。. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu J., Chen Y.J., Dobbs N., et al. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J Exp Med. 2019;216:867–883. doi: 10.1084/jem.20182192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu J., Dobbs N., Yang K., et al. Interferon-independent activities of mammalian STING mediate antiviral response and tumor immune evasion. Immunity. 2020;53:115–126 e115. doi: 10.1016/j.immuni.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gulen M.F., Koch U., Haag S.M., et al. Signalling strength determines proapoptotic functions of STING. Nat Commun. 2017;8:427. doi: 10.1038/s41467-017-00573-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Long J., Yang C., Zheng Y., et al. Notch signaling protects CD4 T cells from STING-mediated apoptosis during acute systemic inflammation. Sci Adv. 2020;6 doi: 10.1126/sciadv.abc5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ayala A., Perl M., Venet F., et al. Apoptosis in sepsis: mechanisms, clinical impact and potential therapeutic targets. Curr Pharmaceut Des. 2008;14:1853–1859. doi: 10.2174/138161208784980617. [DOI] [PubMed] [Google Scholar]
- 114.Hotchkiss R.S., Coopersmith C.M., Karl I.E. Prevention of lymphocyte apoptosis--a potential treatment of sepsis? Clin Infect Dis. 2005;41(Suppl 7):S465–S469. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- 115.Vandenabeele P., Galluzzi L., Vanden Berghe T., et al. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 116.Pasparakis M., Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 117.Jorgensen I., Rayamajhi M., Miao E.A. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17:151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoo H., Im Y., Ko R.E., et al. Association of plasma level of high-mobility group box-1 with necroptosis and sepsis outcomes. Sci Rep. 2021;11:9512. doi: 10.1038/s41598-021-88970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y., Xu Q., Wang Y., et al. Necroptosis is active and contributes to intestinal injury in a piglet model with lipopolysaccharide challenge. Cell Death Dis. 2021;12:62. doi: 10.1038/s41419-020-03365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kang R., Chen R., Zhang Q., et al. HMGB1 in health and disease. Mol Aspect Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoo H., Lee J.Y., Park J., et al. Association of plasma level of TNF-related apoptosis-inducing ligand with severity and outcome of sepsis. J Clin Med. 2020;9 doi: 10.3390/jcm9061661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen H., Li Y., Wu J., et al. RIPK3 collaborates with GSDMD to drive tissue injury in lethal polymicrobial sepsis. Cell Death Differ. 2020;27:2568–2585. doi: 10.1038/s41418-020-0524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang R., Li C., Qiao P., et al. OGG1-initiated base excision repair exacerbates oxidative stress-induced parthanatos. Cell Death Dis. 2018;9:628. doi: 10.1038/s41419-018-0680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luo W., Wang Y., Zhang L., et al. Critical role of cytosolic DNA and its sensing adaptor STING in aortic degeneration, dissection, and rupture. Circulation. 2020;141:42–66. doi: 10.1161/CIRCULATIONAHA.119.041460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Broz P., Pelegrin P., Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2019 doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- 126.Chen R., Zeng L., Zhu S., et al. cAMP metabolism controls caspase-11 inflammasome activation and pyroptosis in sepsis. Sci Adv. 2019;5 doi: 10.1126/sciadv.aav5562. eaav5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen R., Zhu S., Zeng L., et al. AGER-mediated lipid peroxidation drives caspase-11 inflammasome activation in sepsis. Front Immunol. 2019;10:1904. doi: 10.3389/fimmu.2019.01904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shi J., Zhao Y., Wang K., et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 129.Kayagaki N., Stowe I.B., Lee B.L., et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y., Gao W., Shi X., et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 131.Kim M.J., Bae S.H., Ryu J.C., et al. SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy. 2016;12:1272–1291. doi: 10.1080/15548627.2016.1183081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee S., Nakahira K., Dalli J., et al. NLRP3 inflammasome deficiency protects against microbial sepsis via increased lipoxin B4 synthesis. Am J Respir Crit Care Med. 2017;196:713–726. doi: 10.1164/rccm.201604-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Y., Jing Y.Y., Zeng C.Y., et al. Scutellarin suppresses NLRP3 inflammasome activation in macrophages and protects mice against bacterial sepsis. Front Pharmacol. 2017;8:975. doi: 10.3389/fphar.2017.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zou B., Liu J., Klionsky D.J., et al. Extracellular SQSTM1 as an inflammatory mediator. Autophagy. 2020 doi: 10.1080/15548627.2020.1843253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rathkey J.K., Zhao J., Liu Z., et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gaidt M.M., Ebert T.S., Chauhan D., et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171:1110–1124 e1118. doi: 10.1016/j.cell.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tang Y., Wang X., Li Z., et al. Heparin prevents caspase-11-dependent septic lethality independent of anticoagulant properties. Immunity. 2021;54:454–467. doi: 10.1016/j.immuni.2021.01.007. e456. [DOI] [PubMed] [Google Scholar]
- 138.Wu C., Lu W., Zhang Y., et al. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity. 2019;50:1401–1411 e1404. doi: 10.1016/j.immuni.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang X., Cheng X., Tang Y., et al. Bacterial endotoxin activates the coagulation cascade through gasdermin D-dependent phosphatidylserine exposure. Immunity. 2019;51:983–996. doi: 10.1016/j.immuni.2019.11.005. e986. [DOI] [PubMed] [Google Scholar]
- 140.Wu R., Wang N., Comish P.B., et al. Inflammasome-dependent coagulation activation in sepsis. Front Immunol. 2021;12:641750. doi: 10.3389/fimmu.2021.641750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Srikanth S., Woo J.S., Wu B., et al. The Ca(2+) sensor STIM1 regulates the type I interferon response by retaining the signaling adaptor STING at the endoplasmic reticulum. Nat Immunol. 2019;20:152–162. doi: 10.1038/s41590-018-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Y., Chen W., Wang Y. STING is an essential regulator of heart inflammation and fibrosis in mice with pathological cardiac hypertrophy via endoplasmic reticulum (ER) stress. Biomed Pharmacother. 2020;125:110022. doi: 10.1016/j.biopha.2020.110022. [DOI] [PubMed] [Google Scholar]
- 143.Marchi S., Patergnani S., Missiroli S., et al. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018;69:62–72. doi: 10.1016/j.ceca.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 144.Kuang F., Liu J., Tang D., et al. Oxidative damage and antioxidant defense in ferroptosis. Front Cell Dev Biol. 2020;8:586578. doi: 10.3389/fcell.2020.586578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tang D., Kroemer G. Ferroptosis. Curr Biol. 2020;30:R1292–R1297. doi: 10.1016/j.cub.2020.09.068. [DOI] [PubMed] [Google Scholar]
- 146.Chen X., Kang R., Kroemer G., et al. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 147.Chen X., Kang R., Kroemer G., et al. Ferroptosis in infection, inflammation, and immunity. J Exp Med. 2021:218. doi: 10.1084/jem.20210518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tang D., Chen X., Kang R., et al. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li N., Wang W., Zhou H., et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med. 2020;160:303–318. doi: 10.1016/j.freeradbiomed.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 150.Wei S., Bi J., Yang L., et al. Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice. Clin Transl Med. 2020;10:e173. doi: 10.1002/ctm2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kang R., Zeng L., Zhu S., et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe. 2018;24:97–108. doi: 10.1016/j.chom.2018.05.009. e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li J., Wang X., Mei K.C., et al. Lateral size of graphene oxide determines differential cellular uptake and cell death pathways in Kupffer cells, LSECs, and hepatocytes. Nano Today. 2021;37 doi: 10.1016/j.nantod.2020.101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li C., Zhang Y., Liu J., et al. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 2021;17:948–960. doi: 10.1080/15548627.2020.1739447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kuang F., Liu J., Li C., et al. Cathepsin B is a mediator of organelle-specific initiation of ferroptosis. Biochem Biophys Res Commun. 2020;533:1464–1469. doi: 10.1016/j.bbrc.2020.10.035. [DOI] [PubMed] [Google Scholar]
- 155.Wu J., Liu Q., Zhang X., et al. STING-dependent induction of lipid peroxidation mediates intestinal ischemia-reperfusion injury. Free Radic Biol Med. 2021;163:135–140. doi: 10.1016/j.freeradbiomed.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 156.Jia M., Qin D., Zhao C., et al. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat Immunol. 2020;21:727–735. doi: 10.1038/s41590-020-0699-0. [DOI] [PubMed] [Google Scholar]
- 157.Haag S.M., Gulen M.F., Reymond L., et al. Targeting STING with covalent small-molecule inhibitors. Nature. 2018;559:269–273. doi: 10.1038/s41586-018-0287-8. [DOI] [PubMed] [Google Scholar]