Abstract
Rationale
India is experiencing a regional increase in cases of multidrug-resistant tuberculosis (MDR-TB).
Objectives
Given the complexity of MDR-TB diagnosis and care, we sought to address key knowledge gaps in MDR risk factors, care delays, and drivers of delay to help guide disease control.
Methods
From January 2018 to September 2019, we conducted interviews with adults registered with the National TB Elimination Program for MDR (n = 128) and non–MDR-TB (n = 269) treatment to quantitatively and qualitatively study care pathways. We collected treatment records and GeneXpert-TB/RIF diagnostic reports.
Measurements and Main Results
MDR-TB was associated with young age and crowded residence. GeneXpert rifampicin resistance diversity was measured at 72.5% Probe E. Median time from symptom onset to diagnosis of MDR was 90 days versus 60 days for non-MDR, Wilcoxon P < 0.01. Delay decreased by a median of 30 days among non-MDR patients with wider access to GeneXpert, Wilcoxon P = 0.02. Pathways to care were complex, with a median (interquartile range) of 4 (3–5) and 3 (2–4) encounters for MDR and non-MDR, respectively. Of patients with MDR-TB, 68% had their first encounter in the private sector, and this was associated with a larger number of subsequent healthcare encounters and catastrophic expenditure.
Conclusions
The association of MDR with young age, crowding, and low genotypic diversity raises concerns of ongoing MDR transmission fueled by long delays in care. Delays are decreasing with GeneXpert use, suggesting the need for routine use in presumptive TB. Qualitatively, we identify the need to improve patient retention in the National TB Elimination Program and highlight patients’ trust relationship with private providers.
Keywords: pathways, multidrug-resistant tuberculosis, private sector, delays, National TB Elimination Program
At a Glance Commentary
Scientific Knowledge on the Subject
Multidrug-resistant tuberculosis (MDR-TB) has been a serious concern globally and especially for India, being the country with the highest burden. The pathways to MDR-TB care are complex yet less explored in the Indian context.
What This Study Adds to the Field
The association of MDR with young age, crowding, and low genotypic diversity raises concerns of ongoing MDR transmission fueled by long delays in care. Delays are decreasing with GeneXpert use, suggesting the need for routine use in presumptive TB. Qualitatively, we identify the need to improve patient retention in the National TB Elimination Program and highlight patients’ trust relationship with private providers.
India carries more than a quarter (27%) of the global burden of tuberculosis (TB), with an estimated 2.7 million cases in 2018 (1). Although TB incidence is slowly declining, the emergence and transmission of drug resistance is challenging disease control efforts (2). In 2016, the first Indian national drug resistance survey estimated the proportion of multidrug-resistant TB (MDR-TB, resistant to at least isoniazid and rifampicin) at 2.8% (95% confidence interval [CI], 2.3–3.5%) among new cases and 6.2% (95% CI, 5.5–6.9%) overall, that is, among new or previously treated TB cases (3). Although estimates at the state level were of lower precision than nationally, the survey suggests Maharashtra is an MDR-TB “hotspot” with a proportion (7.7% among new TB; 95% CI, 4.5–10.9%) more than double the national estimate. A 2007 drug resistance survey conducted in Maharashtra reported the proportion of MDR at 2.7% of new cases (4), suggesting a large interval increase between 2007 and 2015. There are also several single health center reports in Mumbai, the largest city in Maharashtra, that support an increasing MDR proportion (5–7).
MDR rates may be rising because of new resistance acquisition (i.e., due to within-patient pathogen evolution under suboptimal antibiotic treatment) or may instead result from primary infection with transmitted MDR-TB. Both of these possibilities are potentiated by delays in MDR diagnosis and effective treatment. There is currently limited quantification of MDR transmission and care delays in India. Available studies report long delays up to 55 days before drug-susceptible TB treatment initiation (8–13). A systematic reassessment of delays and barriers to care is timely given recent TB health system changes. These include the expansion of standardized treatment within the public National TB Elimination Program (NTEP) and the large-scale rollout of molecular TB tests, such as the GeneXpert MTB/RIF (Xpert) assay that can decrease time-to-result, increase diagnostic sensitivity for TB and MDR-TB, and allow us to approximate the genetic epidemiology of rifampicin resistance mutations (14–16). In addition, timely TB care is hampered by the presence of a vast and weakly regulated private healthcare sector in India, with recent efforts focused heavily on improving notification of TB from this sector and referral to the NTEP (17, 18). There has been limited evaluation of care after referral to the NTEP. Here, we undertook a mixed-methods study among patients registered with the NTEP for treatment of MDR-TB and non–MDR-TB with the following objectives: 1) identify risk factors associated with MDR-TB including rifampicin resistance gene (rpoB) diversity, 2) quantify delays to the first healthcare encounter and to diagnosis and initiation of appropriate treatment for TB and MDR-TB, and 3) map patient pathways as they navigate the private/public health system to receive TB and MDR-TB care, and document reasons driving care transitions.
Some of the results of these studies have been previously reported in the form of a preprint (https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3717571).
Methods
Study Setting
We conducted this cross-sectional mixed-methods study from January 2018 to September 2019 in the metropolis Pune Municipal Corporation (PMC) and surrounding industrial belt of Pimpri-Chinchwad Municipal Corporation (PCMC) areas of Maharashtra state. Maharashtra is the second most populous state in India with the 10th highest TB incidence rate (19). The study area spans approximately 7 million population (9,400 people/km2); it includes 564 slums in the PMC region and has a considerable migrant population (20). The region is served by 17 TB units (TUs) under the NTEP; each TU covers a population of 0.3–0.5 million. The PCMC area TUs manage patients from surrounding rural areas. Thus, the study area has urban, industrial, and some rural representation, similar to most Indian states. Until September 2018, Xpert was recommended for retreatment cases or in case of nonresponse to first-line treatment. After that, it was recommended for all sputum smear–positive TB cases and a subset of presumptive TB.
Approvals
We secured approvals from the Indian Ministry of Health and Family Welfare, Central TB Division, the state and local NTEP, Harvard Medical School’s Institutional Review Board, and the institutional ethics committee of Dr. D. Y. Patil Vidyapeeth.
Xpert Probe Binding
Data collection and analysis of Xpert rifampicin resistance gene, rpoB, probe binding are presented separately in Annex 1 in the online supplement.
Participants Excluding Xpert Probe Analysis
We consecutively recruited patients aged ⩾15 years with microbiologically confirmed TB who were registered and treated in the 17 TUs in the PMC and PCMC area. We used NTEP laboratory records to identify 1) patients with rifampicin-resistant TB diagnosed by Xpert (all were confirmed isoniazid resistant by a line probe assay and are hence MDR) and 2) comparator non–MDR-TB cases registered for first-line therapy after confirmation of sputum smear positivity. These patients either were negative for rifampicin resistance by Xpert or were not tested but experienced sputum smear conversion to negative on first-line therapy. Eligible patients for enrollment were contacted by phone or in person with the help of TU staff.
Procedures and Data Collection Excluding Xpert Probe Analysis
After introduction to the study, patients (and guardians for participants aged 15 to <18 yr) were consented; participants aged 15 to <18 years were assented. Study participants underwent a structured interview followed by an in-depth interview for the majority (Annexes E1 and E2). In the structured interview, we collected sociodemographic data including employment, marital status, family type, education, residence type and locality (crowded if residing in slums or huts and noncrowded if residing in a colony/compound, apartment, or a bungalow), average time spent in a crowded locality per day (24 h or <24 h), history of TB, health providers visited, TB diagnosis for the current episode, substance abuse, and comorbidities. Additional data collection from the NTEP treatment cards included the type of treatment regimen, sputum smear status, comorbidities, and substance use. Additional interview procedures are provided in the online supplement.
Quantitative Analysis Excluding Xpert Probe Analysis
Quantitative data was entered in Qualtrics.XM (Qualtrics) and processed using MS-Excel 2013 (Microsoft) and R Statistical Language. We used the chi-square test to compare proportions and the nonparametric Wilcoxon rank-sum to test continuous variables. We performed a multivariable logistic regression analysis to identify risk factors associated with MDR-TB without variable selection unless otherwise stated in the results. We tested for interaction between sex and the variable “24 hours per day spent in crowded locality/slum” and found that this was not statistically significant.
Qualitative Analysis
Data from in-depth interviews was processed using MAXQDA (VERBI, Version 18). Based on the questions used in the interview guide, a coding list was prepared. Thematic analysis was performed by identifying the most commonly reported themes followed by the less commonly reported themes and conducted separately for the MDR and non-MDR groups. Thematic saturation was reached for both groups (21). We used a convergent parallel design. Further details are provided in Annex E1.
Results
Enrollment and Sample Characteristics
During the study period, we identified 448 patients with non–MDR-TB and 235 patients with MDR-TB who met the inclusion criteria and contacted them for enrollment. Of these, 179 patients with non–MDR-TB and 110 patients with MDR-TB were not enrolled. The most common reason for the inability to enroll was the lack of telephone contact numbers or no response (84 [47%] and 51 [46%], respectively) (Annex E3). Death before the telephone contact was noted in 9 (5%) nonenrolled patients with non–MDR-TB and 16 (15%) nonenrolled patients with MDR. The age distribution was similar among nonenrolled patients, patients who died before attempted enrollment, and patients enrolled in this study (Annex E3).
We enrolled a total of 269 patients with non–MDR-TB and 128 patients with MDR-TB detected by Xpert assay (Tables 1 and 2). Of the enrolled patients with TB, 64% were adolescents or young adults (aged 15–35 yr) and 54% were men. A large proportion (75%) resided in crowded localities such as slums or chawls. One-third (33%) reported history of prior TB; alcohol use was reported by 21%; 9% reported diabetes and 5% reported HIV coinfection.
Table 1.
Sociodemographic Characteristics of Study Participants (n = 397)
| Characteristics | Non-MDR (n = 269) | MDR (n = 128) | Total (n = 397) |
|---|---|---|---|
| Age group | |||
| 15–35 yr | 165 (61.3) | 92 (71.9) | 257 (64.7) |
| 36–56 yr | 77 (28.6) | 35 (27.3) | 112 (28.2) |
| >56 yr | 27 (10.0) | 1 (0.8) | 28 (7.1) |
| Sex | |||
| M | 158 (58.7) | 56 (43.8) | 214 (53.9) |
| F | 111 (41.3) | 72 (56.2) | 183 (46.1) |
| Marital status | |||
| Unmarried | 77 (28.6) | 41 (32.0) | 118 (29.7) |
| Married | 176 (65.4) | 80 (62.5) | 256 (64.5) |
| Other (widowed/separated) | 16 (5.9) | 7 (5.4) | 15 (3.8) |
| Family type | |||
| Nuclear | 136 (50.6) | 69 (53.9) | 205 (51.6) |
| Joint and extended | 119 (44.2) | 55 (42.9) | 174 (43.8) |
| Single | 8 (3.0) | 2 (1.6) | 10 (2.5) |
| Stay with friends | 6 (2.2) | 2 (1.6) | 8 (2.0) |
| Education | |||
| Illiterate | 31 (11.5) | 5 (3.9) | 36 (9.1) |
| Primary (1st–4th) | 37 (13.8) | 13 (10.2) | 50 (12.6) |
| Secondary (5th–10th) | 139 (51.7) | 58 (45.3) | 197 (49.6) |
| High secondary (11th/12th) | 34 (12.6) | 25 (19.5) | 59 (14.9) |
| Graduation/PG | 28 (10.3) | 27 (21.1) | 55 (13.9) |
| Occupation | |||
| Unemployed | 55 (20.4) | 35 (27.3) | 90 (22.7) |
| Housewife | 55 (20.4) | 32 (25.0) | 87 (21.9) |
| Student | 25 (9.3) | 23 (18.0) | 48 (12.1) |
| Skilled laborer | 26 (9.7) | 15 (11.7) | 41 (10.3) |
| Other | 108 (40.1) | 23 (17.9) | 131 (32.9) |
| Type of house/locality | |||
| Crowded | 202 (75.1) | 97 (75.7) | 299 (75.3) |
| Noncrowded | 67 (24.9) | 31 (24.3) | 98 (24.7) |
Definition of abbreviation: MDR = multidrug resistant; PG = postgraduation.
Data are shown as n (%).
Table 2.
Self-reported History of TB, Substance Use, and Comorbidities among Study Participants (n = 397)
| Characteristics | Non-MDR (n = 269) | MDR (n = 128) | Total (n = 397) |
|---|---|---|---|
| History of TB | |||
| Yes | 65 (24.1) | 65 (50.7) | 130 (32.9) |
| No | 204 (75.9) | 63 (49.3) | 267 (67.1) |
| Number of previous TB episodes (n = 130) | |||
| 1 | 56 (86.2) | 45 (69.3) | 101 (77.7) |
| 2 | 9 (13.8) | 20 (30.7) | 29 (22.3) |
| Substance use | |||
| Smoking* | |||
| Yes | 27 (10.2) | 9 (7.0) | 36 (9.2) |
| No | 238 (89.8) | 119 (93.0) | 357 (90.8) |
| Chewing tobacco* | |||
| Yes | 78 (29.5) | 23 (18.0) | 101 (25.8) |
| No | 186 (70.5) | 105 (82.0) | 291 (74.2) |
| Alcohol* | |||
| Yes | 60 (22.6) | 25 (19.5) | 85 (21.6) |
| No | 206 (77.4) | 103 (80.5) | 309 (78.4) |
| Comorbidities | |||
| Diabetes | |||
| Yes | 27 (10.0) | 10 (7.8) | 37 (9.3) |
| No | 242 (90.0) | 118 (92.2) | 360 (90.7) |
| HIV* | |||
| Yes | 10 (3.7) | 9 (7.1) | 19 (4.8) |
| No | 259 (96.3) | 117 (92.9) | 376 (95.2) |
| Cancer | |||
| Yes | 0 (0) | 1 (0.8) | 1 (0.3) |
| No | 175 (65.1) | 72 (56.2) | 247 (62.2) |
| Unknown | 94 (34.9) | 55 (43.0) | 149 (37.5) |
Definition of abbreviations: MDR = multidrug-resistant; TB = tuberculosis.
Data are shown as n (%).
Data are missing for some participants.
Risk Factors Associated with MDR-TB
We assessed sociodemographic and medical risk factors for MDR-TB infection using the non–MDR-TB group as a reference (Table 3). After multivariable adjustment, MDR-TB was significantly associated with a prior history of TB (adjusted odds ratio [aOR], 4.57; 95% CI, 2.72–7.70; P < 0.001) and continuous residence in a crowded locality (aOR, 2.02; 95% CI, 1.12–3.63; P = 0.019). Older age was protective of MDR (aOR, 0.71 for every 10-yr increase in age; 95% CI, 0.57–0.89; P = 0.003).
Table 3.
Univariate and Multivariable Logistic Regression Indicating Association of Self-reported Risk Factors and MDR-TB (n = 372)
| Predictors | Crude OR (95% CI) | Crude P Value | Adjusted OR (95% CI) | P Value (Wald) |
|---|---|---|---|---|
| Sex, F | 1.81 (1.17–2.82) | 0.008 | 1.17 (0.63–2.19) | 0.614 |
| Age (10-yr increments) | 0.75 (0.63–0.90) | 0.002 | 0.71 (0.57–0.89) | 0.003 |
| Unemployed* | 2.40 (1.51–3.84) | <0.001 | — | — |
| 24 h/d spent in crowded locality/slum | 2.42 (1.45–4.05) | <0.001 | 2.02 (1.12–3.63) | 0.019 |
| History of TB | 3.46 (2.18–5.49) | <0.001 | 4.57 (2.72–7.70) | <0.001 |
| HIV | 1.95 (0.73–5.2) | 0.180 | 1.96 (0.65–5.95) | 0.235 |
| Diabetes | 0.71 (0.32–1.58) | 0.405 | 1.29 (0.52–3.24) | 0.582 |
| Alcohol use | 0.82 (0.48–1.42) | 0.484 | 2.15 (0.98–4.72) | 0.056 |
| Smoking | 0.63 (0.28–1.43) | 0.271 | 0.65 (0.22–1.92) | 0.440 |
| Chewing tobacco | 0.51 (0.3–0.88) | 0.015 | 0.48 (0.23–0.98) | 0.044 |
| Annual family income† | 1.02 (1.01–1.04) | 0.010 | 1.03 (1.00–1.05) | 0.015 |
Definition of abbreviations: CI = confidence interval; MDR = multidrug-resistant; OR = odds ratio; TB = tuberculosis.
Note: n = 25 had missing data on comorbidities or substance use and were excluded from the regression analysis.
Unemployment was excluded from the multivariable model because of strong collinearity with continuous residence in crowded locality and the latter factor being more proximal in the causal pathway.
Annual family income measured in 10,000 Indian rupee increments.
Xpert rpoB mutation patterns
We examined mutation patterns among a sample of 233 patients with MDR/rifampicin-resistant TB diagnosed contemporaneously in our study catchment area (Annex E1). We calculated the frequency of rpoB probe binding patterns in these 233 patients as an approximate measure of the diversity of circulating rifampicin resistance isolates (Table 4). The majority, 72.5%, had a pattern consistent with an S531L, S531W, or L533P rpoB mutation.
Table 4.
GeneXpert MTB/RIF Failed or Delayed Probe Patterns
| Probe | Binding Failed or Delayed (n = 233) [n (%)] | Mutations Targeted |
|---|---|---|
| Probe E | 169 (72.5) | S531L, S531W, L533P |
| Probe D | 23 (9.9) | H526Y, H526N, H526D, H526C, H526P, H526R, H526L |
| Probe B | 20 (8.6) | Q513K, Q513P, Q513L, D516Y, D516V, D516G, 516-517del |
| Probe A | 13 (5.6) | G510H, L511P, L511R |
| Probe C | 2 (0.9) | S522L S522Q S522W |
| A + D | 4 (1.8) | — |
| A + E | 1 (0.5) | — |
| A + B | 1 (0.5) | — |
Pathways to Care
We collected delays between first symptoms to diagnosis for all enrolled patients using the structured interview (n = 128 MDR, n = 269 non-MDR). We mapped care pathways and more detailed delays for the 128 MDR-TB patients, and for a subset (n = 139) of non-MDR-TB with whom we conducted in-depth interviews. Non-MDR-TB patients interviewed in-depth did not differ in sex, age, time spent in crowded locality or annual family income from patients who were not similarly interviewed (Annex 4).
Time to health system access, diagnosis, and treatment
The median duration between symptom onset and first encounter with the health system was 15 days (interquartile range [IQR], 9–30) for the MDR-TB group and 10 days (6–15) for the non-MDR group (Wilcoxon P < 0.0001) (Table 5). Health system delays between the first encounter and diagnosis were longer among MDR patients, median 80 days (IQR, 44–161), than among non-MDR, median 48 days (IQR, 24–80; Wilcoxon P < 0.0001). Delays between the onset of symptoms and diagnosis were longer for MDR (median 90 days; IQR, 60–180) than for non–MDR-TB (median 60 days; IQR, 30–90; Wilcoxon P < 0.001). For 29% of MDR patients, the total duration from symptom onset to diagnosis was ⩾6 months. Treatment rapidly followed diagnosis for non-MDR patients (median delay 1 day; IQR, 0–4; data available for 16 of 139), but treatment delay was more substantial for MDR (median 8 days; IQR, 3–14 days; data available for 84 of 126; Wilcoxon P < 0.001).
Table 5.
Delays to TB Care
| Delay Type | Symptoms to First Encounter (d) | First Encounter to Diagnosis (d) | Onset of Symptoms to Diagnosis of TB or MDR-TB (d) |
|---|---|---|---|
| Non–MDR-TB (n = 139) | 10 (6–15) | 48 (24–80) | 60 (30–90) |
| MDR-TB (n = 126) | 15 (9–30) | 80 (44–161) | 90 (60–180) |
Definition of abbreviations: MDR = multidrug-resistant; TB = tuberculosis.
Data are shown as median (interquartile range).
Delays and Xpert usage
From September 2018, the Xpert assay was recommended for a wider group of patients including all patients in whom TB was diagnosed and a subset of patients with presumptive TB, namely, children, those coinfected with HIV, and patients with extra pulmonary symptoms. The majority of enrolled MDR patients received diagnoses before that time (115/128), and we could not assess change in delays in this group. For non-MDR patients, we found a significant decrease in delays: from 60 days (IQR, 30–90; N = 220) to 30 days (IQR, 30–60; N = 49; Wilcoxon P = 0.023) before and after the policy change.
Pathways to MDR-TB care
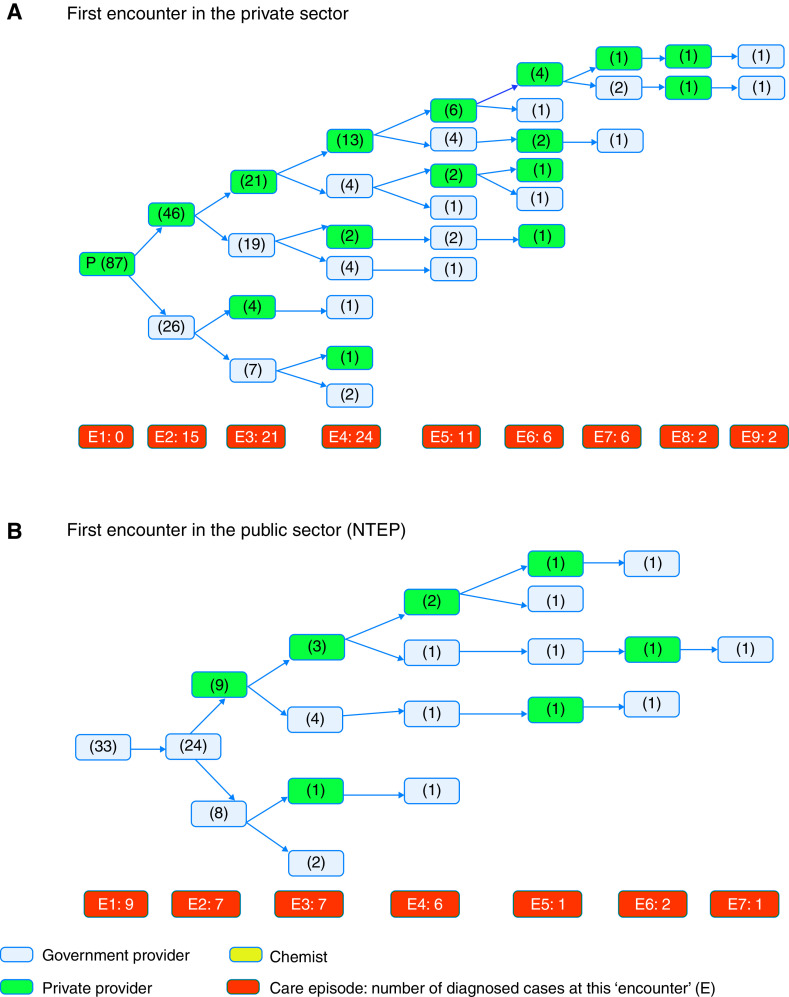
Patients with MDR-TB had their first health encounter in the private sector in 68% (87 of 128) of cases, followed by the NTEP in 26% (33 of 128) and in a pharmacy/chemist in 6% (8 of 128) of cases. Patient-reported reasons for seeing a private sector care provider included an established relationship with a family physician, confidentiality, easy access, or prior good experience. The majority of MDR patients (75%) reported that they had to shop around for care (⩾3 visits before TB diagnosis, overall distribution in Figure 1). Of the 57 patients who left the private sector in favor of the NTEP, 44 (77%) reported reasons of no symptomatic relief, and 12 (21%) reported that treatment was not affordable in the private sector. Twelve of 128 patients (9%) reported that they were admitted for “saline therapy” in the private sector. Of the 24 (19%) patients who returned to the private sector after ⩾1 encounter in the NTEP, 18 (75%) reported that they had no symptomatic relief and hence there was a need for a second opinion from their established private provider; four (17%) were unable to provide records on prior TB treatment requested by NTEP staff and their treatment initiation was delayed (by 2 wk or more), prompting their return to the private sector; two patients (8%) needed the private sector for drug susceptibility testing that was not available at the time in the government facility.
Figure 1.
Pathways to diagnosis and care among patients with multidrug-resistant tuberculosis. E = episode of care or encounter number. E1, E2, and so on indicate the first episode, second episode, and so on. The total number of patients who received diagnoses and were started on treatment at each care episode is given in the orange box. The total number of patients observed at each care episode in the respective care sector is given in parentheses within each blue or green box. Green designates the private sector. Blue designates the public sector/NTEP. (A) First encounter in the private sector. (B) First encounter in the public sector (NTEP). NTEP = National TB Elimination Program; TB = tuberculosis.
The median number of providers visited by patients with MDR-TB was 4 (IQR, 3–5). Only 9 of 128 patients (7%) received their MDR-TB diagnosis at the first healthcare encounter; all of these patients first accessed care in the NTEP. Patients continuing in the private sector, that is, not referred to the NTEP by the second encounter (n = 61), visited an additional median number of 2 providers (IQR, 2–4) before receiving their diagnosis. This was significantly higher than for patients referred to/seeking care in the NTEP at the second encounter (n = 57, median 1 additional encounter before diagnosis [IQR, 0–1]; Wilcoxon P < 0.0001). Only 11 (9%) patients received their MDR diagnosis in the private sector; the remaining received diagnoses at the NTEP.
Pathways to non–MDR-TB care
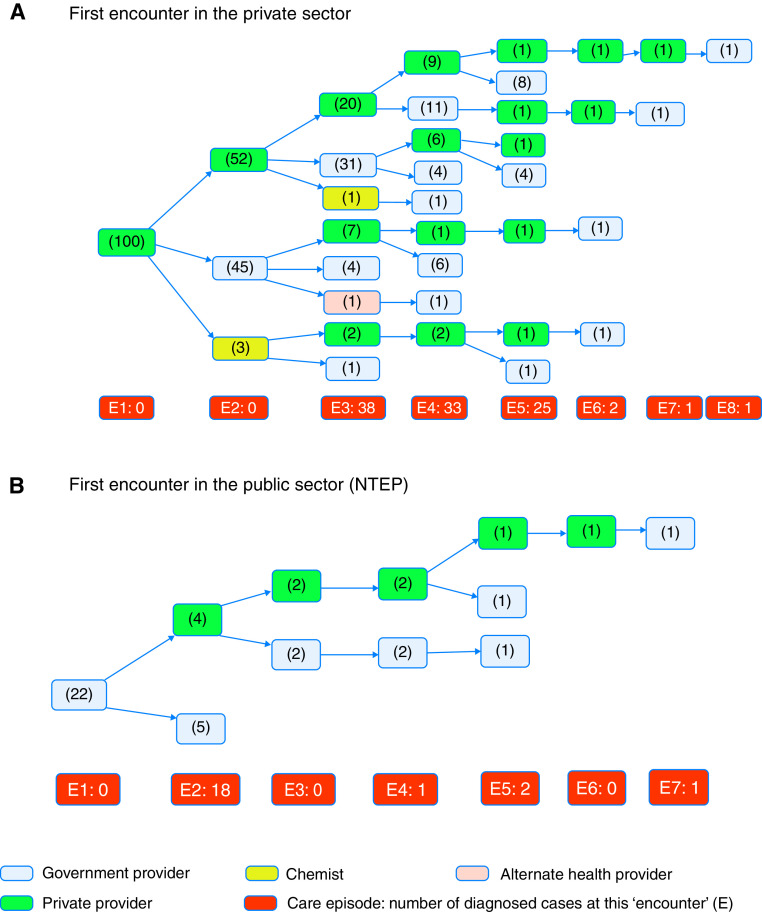
Similar to MDR patients, the majority 100/139 (72%) of patients with non–MDR-TB had their first encounter in the private sector (Figure 2) and for similar reasons. Fourteen patients (10%) first accessed care from a chemist, and the remaining from the NTEP (Annex E5). Pathways to care were also complex, albeit it lesser so than for MDR patients. The median number of providers visited by patients with non–MDR-TB was 3 (IQR, 2–4; Wilcoxon vs. MDR P < 0.001; Figure 2), and none of the 139 patients with TB received diagnoses at the first encounter. Eighteen patients (13%) left the NTEP for the private sector after ⩾1 NTEP encounters because of the lack of symptomatic relief. Thirty-six (26%) patients received diagnoses in the private sector.
Figure 2.
Pathways to diagnosis and care among patients with non–multidrug-resistant tuberculosis. E = episode of care or encounter number. E1, E2, and so on indicate the first episode, second episode, and so on. The total number of patients who received diagnoses and were started on treatment at each care episode is given in the orange box. The total number of patients observed at each care episode in the respective care sector is given in brackets within each blue or green box. Green designates the private sector. Blue designates the public sector/NTEP. (A) First encounter in the private sector. (B) First encounter in the public sector (NTEP). NTEP = National TB Elimination Program; TB = tuberculosis.
Patients with prior TB
We assessed if patients with prior TB were more likely to access care in the NTEP owing to confidence in the quality of care or familiarity. Although the majority of MDR patients with a first encounter in the NTEP had a history of TB, 26/33 (79%), the reverse was not true as less than half of MDR patients with a history of TB initially accessed care in the NTEP, 26/60 (43%). MDR patients with a prior history of TB were nevertheless more likely to access care in the NTEP than in the private sector (odds ratio, 7.9; 95% CI, 3.0–23.5; chi-square P < 0.00001). We observed a similar pattern among patients with non–MDR-TB.
Healthcare expenditure in the private sector
We asked patients to approximate out-of-pocket health expenditure in the private sector before approaching the NTEP (TB care is free in the NTEP). Sixty-two of 128 (49%) patients with MDR-TB provided estimates of expenditure, with the median expenditure being 10,000 Indian rupees (IQR, 3,000–27,000), and 14 (23%) reported catastrophic expenditure >20% of household annual income. For patients with non–MDR-TB, median private sector expenditure was significantly lower at 2,050 Indian rupees (IQR, 975–6,000) (Wilcoxon P = 0.001, data available for 110 of 139 patients), and 8% reported catastrophic expenditure (7/84 with data on household annual income).
Discussion
We studied risk factors associated with MDR-TB and quantified delays in care with analysis of patients’ pathways and perceptions. We confirm the known strong association between previous episodes of TB and MDR (22). This association is commonly believed to result from recent acquisition of de novo antibiotic resistance; however, it can also result from failure of inappropriate first-line treatment in patients with unrecognized transmitted MDR. We made several observations that support a high proportion of MDR-TB resulting from recent transmission. First, we observe a strong association between continuous residence in a crowded locality and MDR-TB, a result suggestive of MDR transmission in these localities. We observe that younger patients are more likely to have MDR-TB. An association of MDR with young age in other high-prevalence settings has been interpreted to be consistent with MDR community transmission, as TB reactivation is more common among older patients (2). In univariate analysis, we found women to be overrepresented among MDR cases. Although active TB disease has a higher incidence in males, MDR-TB was associated previously with female sex in India and several former Soviet Union countries including Estonia (23, 24). This association is yet to be explained but has been postulated to relate to higher MDR transmission to women (25). Our multivariable model supports this possibility, as sex’s effect on MDR appears to be confounded by continuous residence in crowded localities.
We observe low molecular diversity in Xpert rpoB probe binding patterns with 72.5% of MDR isolates having a pattern consistent with the S531L, S531W, or L533P rpoB mutation. In a recent study with sampling enriched for MDR-TB in Peru, a setting known for ongoing MDR-TB transmission, this proportion was lower at 62% (26). The proportion in Pune was similar to that in the North-West region of Pakistan (77%), a region recently identified to have MDR-TB transmission by whole genome sequencing (27). In contrast, in China, reported to have a restrained MDR-TB epidemic (28), this proportion was lower and estimated at 41% in clinical isolates (29). Overall, the observed low diversity is consistent with a sizable proportion of MDR-TB disease resulting from recent transmission in Pune.
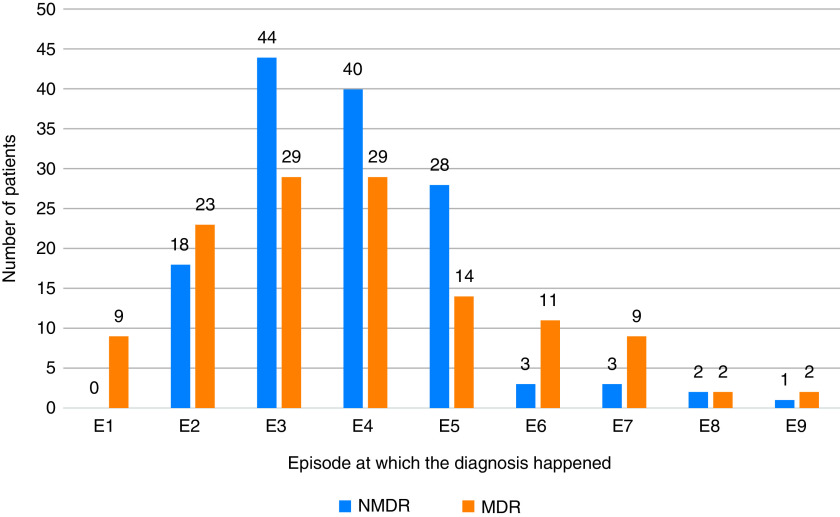
Delays in diagnosis and the subsequent initiation of effective therapy may be important drivers of MDR-TB transmission (30). A recent study of patients with drug-susceptible TB in the United States demonstrated the risk of TB infection among household members to be 5% higher with every day increase in care delay (31). The same study also demonstrated a higher rate of TB complications including hemoptysis, aspergillosis, bronchiectasis, and pneumothorax with longer delays. We observed long delays between the first healthcare encounter and diagnosis for both patients with TB and those with MDR-TB. Targeting the long delays in this portion of the care cascade is likely to have a major impact on overall delays. In more detail, pathways to care analysis revealed the private sector to be the point of first contact for most patients, even when they previously have had TB. This is despite the NTEP being the nationally endorsed provider of TB and MDR-TB care. Reasons underlying this preference most notably include a trust or established relationship with a private practitioner that can even result in “bounce back” to the private sector after referral to the NTEP in 13–19% of patients. Pathway analysis also revealed that 23% of patients with MDR-TB had catastrophic expenditure before reaching the NTEP and the provision of questionable therapies (e.g., saline) in the private sector. This is consistent with findings from prior studies in Maharashtra that identified poor knowledge of MDR-TB and its treatment among private practitioners (32, 33). Overall, MDR patients report not only a prolonged path to diagnosis but also a complex one with multiple encounters often across both private and public sectors (Figure 3).
Figure 3.
MDR tuberculosis and NMDR tuberculosis cases diagnosed at sequential healthcare encounters. MDR = multidrug-resistant; NMDR = non-MDR.
Our study was not without limitations. We enrolled patients in Pune City and the surrounding industrial belt. Although likely representative of other urban and suburban industrial settings in India, our findings may not be generalizable to all contexts in India (e.g., rural settings where TB is prevalent). We relied on patient reports, and there might have been recall bias and associated over- or underestimation of delays in care. Sampling or survivor’s bias is another concern, and the effect of this on the risk factor analysis is difficult to assess. The sex and age distribution of our patients with TB is consistent with national and statewide data for TB (34). To minimize sampling and survivor’s bias, we tracked the age of patients with MDR-TB we approached for study enrollment but who had died, were unreachable, or otherwise declined to participate. We found that our sample had a similar age distribution to patients not enrolled. The majority of deaths before enrollment (75%) were among adolescents to young adults aged 15–35 years, arguing against the association of MDR with age being due to a survivor’s bias and providing further reason for concern about MDR-TB disease in young adults (Annex E3). Finally, our assessment of delays and pathways only applies to patients eventually treated in the public sector. Based on the Indian TB Report 2020, 14% of MDR patients are notified and treated in the private sector in Maharashtra, compared with 86% in the NTEP (16). We attempted to reach patients treated in the private sector during our study period, but we were not able to enroll them, as the majority voiced fear of disclosure of their TB status.
In conclusion, the accumulation of evidence indicating transmission of MDR-TB in Pune emphasizes the need to shorten patients’ infectious period by tackling the observed long delays in care. The observed reduction of delays with expanded Xpert use suggests the need for routine molecular testing, especially among presumptive TB cases with identified risk factors for MDR. We characterize young age (<35 yr) and continuous residence in crowded localities to be new risk factors for MDR over non–MDR-TB, and these populations should be targeted. Our data support the need for outreach efforts like the Joint Effort for Elimination of Tuberculosis designed to incentivize and boost referral from the private sector to the NTEP (18). They also emphasize the need for direct private practitioner education and support, as private practitioners are and will likely continue to be established providers for a large section of the population. In addition to boosting referral, investment in patient retention efforts in the NTEP is needed, including a more patient-centered approach attentive to persistent symptoms. Reassessing NTEP processes of care to decrease patient burden may also be helpful (e.g., requiring that the private practitioners transfer care records upon referral rather than turn patients away when they cannot provide these records). Given the pressing need, the direct empowerment of patients via either in-person or virtual support communities or alternatively through the assignment of care navigators may be the best short-term solution until system reforms are implemented. As effective treatment is the only path to interrupting MDR-TB transmission, these system interventions should be prioritized within TB control efforts in India.
Acknowledgments
Acknowledgment
The authors are thankful to the Indian Council of Medical Research for providing permissions to undertake this study. Thanks are due also to the local NTEP staff and officials for their cooperation and support. The authors are grateful to Harvard Medical School and Dr. D. Y. Patil Medical College and Vidyapeeth staff (specially Drs. A. N. Suryakar, M. S. Barthwal, and A. L. Kakrani) for their support. They also thank the Boston Children’s Hospital Global Health Program for travel support to A.D. Special thanks are due to Dr. D. S. Chakor, City TB Office, Pune, for providing Xpert data. Lastly, the authors thank all the study participants, without whose cooperation the study would not have been possible.
Footnotes
Supported by Harvard University (Harvard-Dubai Centre for Global Health Delivery) grant 027562-746846-0304 (M.R.F. and S.R.A.) and Boston Children’s Hospital Global Health Program (A.D.). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author Contributions: S.R.A.: study conceptualization, tool development, data collection, data analysis, and manuscript writing. J.D.J., M.I.F., and Y.K.D.: data collection, data processing, analysis, and input in writing of the manuscript. T.U.S. and S.L.A.: data analysis and input in manuscript writing. J.S.B. and S.K.B.: help in data analysis and comments and input for revisions. P.K.J., R.S.A., B.P.H., V.J., and N.D.M.: help in National TB Elimination Program data provision and comments and input for revisions. J.E.G.: data analysis and input for revisions. A.D.: study conceptualization, tool development, data analysis, and input for revisions. M.R.F.: study conceptualization, tool development, data analysis, and manuscript writing and revisions. All authors approved the final draft of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202012-4333OC on October 27, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1.
- 2. Dheda K, Gumbo T, Maartens G, Dooley KE, McNerney R, Murray M, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med . 2017;5:291–360. doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 3.Government of India, Ministry of Health and Family Welfare. https://tbcindia.gov.in/showfile.php?lid=3315
- 4.Institute of Medicine (US) Facing the Reality of Drug-Resistant Tuberculosis in India: Challenges and Potential Solutions: Summary of a Joint Workshop by the Institute of Medicine, the Indian National Science Academy, and the Indian Council of Medical Research. Washington, DC: National Academies Press (US); 2012. [PubMed] [Google Scholar]
- 5. Ajbani K, Nikam C, Shetty A, Soman R, Rodrigues C. Multidrug-resistant tuberculosis with fluoroquinolone resistance: sinister association with other drugs and ominous implications for treatment. Clin Infect Dis . 2014;59:138–139. doi: 10.1093/cid/ciu232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D’souza DT, Mistry NF, Vira TS, Dholakia Y, Hoffner S, Pasvol G, et al. High levels of multidrug resistant tuberculosis in new and treatment-failure patients from the Revised National Tuberculosis Control Programme in an urban metropolis (Mumbai) in Western India. BMC Public Health . 2009;9:211. doi: 10.1186/1471-2458-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dalal A, Pawaskar A, Das M, Desai R, Prabhudesai P, Chhajed P, et al. Resistance patterns among multidrug-resistant tuberculosis patients in greater metropolitan Mumbai: trends over time. PLoS One . 2015;10:e0116798. doi: 10.1371/journal.pone.0116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maurya AK, Kant S, Nag VL, Kushwaha RA, Dhole TN. Trends of anti-tuberculosis drug resistance pattern in new cases and previously treated cases of extrapulmonary tuberculosis cases in referral hospitals in northern India. J Postgrad Med . 2012;58:185–189. doi: 10.4103/0022-3859.101379. [DOI] [PubMed] [Google Scholar]
- 9. Purohit MR, Purohit R, Mustafa T. Patient health seeking and diagnostic delay in extrapulmonary tuberculosis: a hospital based study from central India. Tuberc Res Treat . 2019;2019:4840561. doi: 10.1155/2019/4840561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paramasivam S, Thomas B, Chandran P, Thayyil J, George B, Sivakumar CP. Diagnostic delay and associated factors among patients with pulmonary tuberculosis in Kerala. J Family Med Prim Care . 2017;6:643–648. doi: 10.4103/2249-4863.222052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mistry N, Lobo E, Shah S, Rangan S, Dholakia Y. Pulmonary tuberculosis in Patna, India: Durations, delays, and health care seeking behaviour among patients identified through household surveys. J Epidemiol Glob Health . 2017;7:241–248. doi: 10.1016/j.jegh.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sreeramareddy CT, Qin ZZ, Satyanarayana S, Subbaraman R, Pai M. Delays in diagnosis and treatment of pulmonary tuberculosis in India: a systematic review. Int J Tuberc Lung Dis . 2014;18:255–266. doi: 10.5588/ijtld.13.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Subbaraman R, Nathavitharana RR, Satyanarayana S, Pai M, Thomas BE, Chadha VK, et al. The tuberculosis cascade of care in India’s public sector: a systematic review and meta-analysis. PLoS Med . 2016;13:e1002149. doi: 10.1371/journal.pmed.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raizada N, Sachdeva KS, Sreenivas A, Vadera B, Gupta RS, Parmar M, et al. Feasibility of decentralised deployment of Xpert MTB/RIF test at lower level of health system in India. PLoS One . 2014;9:e89301. doi: 10.1371/journal.pone.0089301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sachdeva KS, Raizada N, Sreenivas A, Van’t Hoog AH, van den Hof S, Dewan PK, et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLoS One . 2015;10:e0126065. doi: 10.1371/journal.pone.0126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministry of Health and Family Welfare. https://tbcindia.gov.in/showfile.php?lid=3538
- 17. Atre S. Tuberculosis burden in India’s private sector. Lancet Infect Dis . 2016;16:1328–1329. doi: 10.1016/S1473-3099(16)30470-4. [DOI] [PubMed] [Google Scholar]
- 18.Joint Effort for Elimination of Tuberculosis (JEET) https://projectjeet.in/wp-content/uploads/2019/08/JEET-Annual-Report-2018_v4.0_10MAY19.pdf
- 19.India Population 2020. https://indiapopulation2020.in/population-of-pune-2020.html
- 20.Pune Municipal Corporation. https://www.pmc.gov.in/en/total-slums
- 21. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods . 2006;18:59–82. [Google Scholar]
- 22. Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax . 2006;61:158–163. doi: 10.1136/thx.2005.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Atre SR, D’Souza DT, Vira TS, Chatterjee A, Mistry NF. Risk factors associated with MDR-TB at the onset of therapy among new cases registered with the RNTCP in Mumbai, India. Indian J Public Health . 2011;55:14–21. doi: 10.4103/0019-557X.82536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lockman S, Kruuner A, Binkin N, Levina K, Wang Y, Danilovitsh M, et al. Clinical outcomes of Estonian patients with primary multidrug-resistant versus drug-susceptible tuberculosis. Clin Infect Dis . 2001;32:373–380. doi: 10.1086/318489. [DOI] [PubMed] [Google Scholar]
- 25. Lomtadze N, Aspindzelashvili R, Janjgava M, Mirtskhulava V, Wright A, Blumberg HM, et al. Prevalence and risk factors for multidrug-resistant tuberculosis in the Republic of Georgia: a population-based study. Int J Tuberc Lung Dis . 2009;13:68–73. [PMC free article] [PubMed] [Google Scholar]
- 26. Farhat MR, Sixsmith J, Calderon R, Hicks ND, Fortune SM, Murray M. Rifampicin and rifabutin resistance in 1003 Mycobacterium tuberculosis clinical isolates. J Antimicrob Chemother . 2019;74:1477–1483. doi: 10.1093/jac/dkz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ullah I, Shah AA, Basit A, Ali M, Khan A, Ullah U, et al. Rifampicin resistance mutations in the 81 bp RRDR of rpoB gene in Mycobacterium tuberculosis clinical isolates using Xpert MTB/RIF in Khyber Pakhtunkhwa, Pakistan: a retrospective study. BMC Infect Dis . 2016;16:413. doi: 10.1186/s12879-016-1745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang H, Ding N, Yang T, Li C, Jia X, Wang G, et al. Cross-sectional whole-genome sequencing and epidemiological study of multidrug-resistant Mycobacterium tuberculosis in China. Clin Infect Dis . 2019;69:405–413. doi: 10.1093/cid/ciy883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yue J, Shi W, Xie J, Li Y, Zeng E, Wang H. Mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis isolates from China. J Clin Microbiol . 2003;41:2209–2212. doi: 10.1128/JCM.41.5.2209-2212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dheda K, Limberis JD, Pietersen E, Phelan J, Esmail A, Lesosky M, et al. Outcomes, infectiousness, and transmission dynamics of patients with extensively drug-resistant tuberculosis and home-discharged patients with programmatically incurable tuberculosis: a prospective cohort study. Lancet Respir Med . 2017;5:269–281. doi: 10.1016/S2213-2600(16)30433-7. [DOI] [PubMed] [Google Scholar]
- 31. El Halabi J, Palmer N, McDuffie M, Golub JJ, Fox K, Kohane I, et al. Measuring health-care delays among privately insured patients with tuberculosis in the USA: an observational cohort study. Lancet Infect Dis . 2021;21:1175–1183. doi: 10.1016/S1473-3099(20)30732-5. [DOI] [PubMed] [Google Scholar]
- 32. Udwadia ZF, Pinto LM, Uplekar MW. Tuberculosis management by private practitioners in Mumbai, India: has anything changed in two decades? PLoS One . 2010;5:e12023. doi: 10.1371/journal.pone.0012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yadav A, Garg SK, Chopra H, Bajpai SK, Bano T, Jain S, et al. Treatment practices in pulmonary tuberculosis by private sector physicians of Meerut, Uttar Pradesh. Indian J Chest Dis Allied Sci . 2012;54:161–163. [PubMed] [Google Scholar]
- 34.India TB Report 2018. https://tbcindia.gov.in/showfile.php?lid=3314