Although pediatric pneumonia has decreased substantially in the advent of improved early-life nutrition, living conditions, vaccinations, and HIV diagnosis and treatment, it remains a leading cause of preventable morbidity and mortality among children <5 years of age (1). The disparate burden of pediatric pneumonia is largely borne by those living in low- and middle-income countries (LMICs), where there is often a higher prevalence of risk factors for pneumonia in the face of fewer resources for diagnosis and treatment. Appropriate identification and timely treatment of severe pneumonia, which is often complicated by hypoxemia, is critical because of the associated high risk of morbidity and mortality (2). Treatment of severe pediatric pneumonia requires systemic access to appropriately trained staff and life-saving interventions such as antibiotics, supplemental oxygen, and in the most severe cases, ventilatory support. This remains an unmet need in many resource-poor and remote locales.
In this issue of the Journal, Simkovich and colleagues (pp. 183–197) leveraged the resources and infrastructure of the HAPIN (Household Air Pollution Intervention Network) trial to identify healthcare facilities that they defined as adequately resourced to manage severe pediatric pneumonia as part of implementing a pneumonia surveillance strategy in rural regions of four LMICs—Guatemala, Peru, Rwanda, and India (3). They defined adequately resourced healthcare facilities as those that were open daily and had overnight beds, an available physician, a pulse oximeter, supplemental oxygen, respiratory support devices, X-ray or ultrasound, and antibiotics. They surveyed administrative leaders of 350 healthcare facilities ranging from community centers and health posts to formal health centers and hospitals in the HAPIN study area, finding that only 13% of facilities had adequate resources to manage severe pneumonia, but this varied substantially across regions, from 3% in Guatemala to 42% in India. Overall, 37% of facilities had pulse oximeters and 44% had supplemental oxygen, although this also varied by country. Mean travel times to an adequately resourced facility were 31–99 minutes, with the shortest in India and the longest in Peru. Only 43–63% of the study population lived within 30 minutes of a facility that was adequately resourced to care for severe pneumonia, and 5% of the population in Peru lived outside of a two-hour travel time.
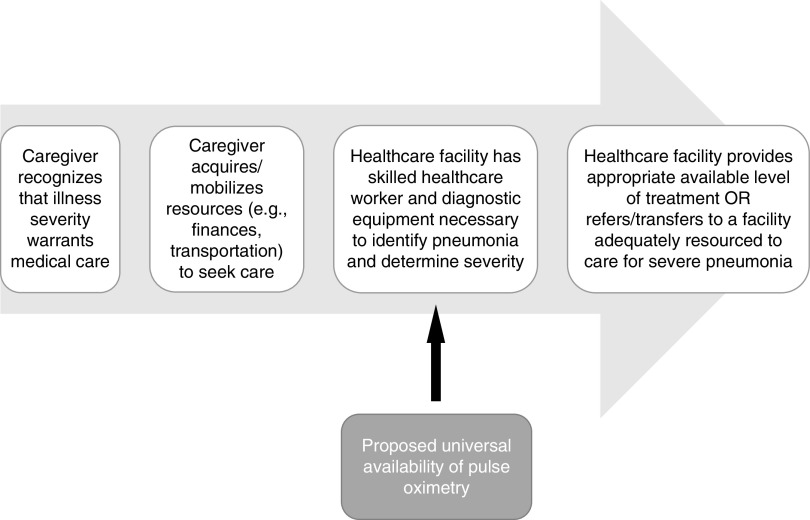
These findings bring to the forefront yet another example of the inequity of resource availability to care for highly prevalent, treatable medical problems worldwide (4, 5). We applaud the authors for positing a potential intervention to address a step in the cascade of care for pneumonia diagnosis and treatment (Figure 1). They propose that universal availability of pulse oximetry could reduce time to diagnosis of severe pneumonia based on modeling of travel time to healthcare facilities in the hypothetical situation that all facilities were supplied with pulse oximetry. Availability would theoretically reduce time to diagnosis, and, as a result, time to referral, by 3 minutes in India and up to 19 minutes in Peru. It is not clear from these data, however, how this might translate to improved access or reduced time to receipt of appropriate care for severe pneumonia.
Figure 1.
Cascade of care for pediatric pneumonia diagnosis and treatment in low- and middle-income countries. The cascade of care for pediatric pneumonia diagnosis and treatment in low- and middle-income countries encompasses key steps that begin with caregiver recognition of illness and care seeking and proceed to identification of and receiving care at healthcare facilities with the appropriate resources to treat pneumonia based on its severity. Drop-offs to access to care can occur at any step because of barriers and limitations such as lack of appropriate classification of illness severity by caregivers or healthcare providers; unavailable, inappropriately sized, or poorly functional equipment for diagnosis (e.g., imaging and pulse oximetry) and/or treatment (e.g., supplies for supplemental oxygen and intravenous fluid administration, advanced respiratory support devices, and antibiotics); lack of availability of adequately trained healthcare providers; and other systemic geographic, transportation-related, and financial barriers. Simkovich and colleagues propose universal availability of pulse oximetry at all healthcare facilities as a potential intervention to improve time to diagnosis of severe pediatric pneumonia.
Although pulse oximetry is an easy-to-use, low-cost tool, is its universal availability sufficient to impact reduction of the morbidity and mortality of childhood pneumonia on a population level in diverse settings? Pulse oximetry improved outpatient diagnosis of pediatric pneumonia that would otherwise have been missed based on World Health Organization referral guidelines in a Malawian study (6), but in a Nigerian study, only 19% of hospitalized children with hypoxemia received supplemental oxygen (7). A large multihospital study in Kenya demonstrated that adoption of pulse oximetry to identify hypoxemia increased over time but varied substantially by patient- and hospital-level factors and did not routinely guide administration of supplemental oxygen (8, 9). For universal availability of pulse oximetry to improve equitable access to the sparse resources required to manage severe pneumonia in LMICs, it must be accompanied by thoughtful implementation of additional components, including healthcare worker education, support in procuring and maintaining oximeters that do not rely on electricity and are appropriate for children, and necessary allocation of funds (9–11). Interventions should take a holistic approach tailored to the unique challenges of each setting to achieve an equitable and sustainable use of limited resources.
A notable strength of the study is that the authors present disaggregated data, highlighting the degree of heterogeneity in resource and geographic accessibility to pneumonia care across four regions in diverse LMICs. Another important strength is the comprehensive georeferencing and road network analysis used to estimate travel times to facilities with services for the diagnosis and/or treatment of pneumonia in each region. However, these facilities represent a convenience sample from areas selected for another purpose and do not reflect an exhaustive set of healthcare facilities in the specified regions. Furthermore, in defining facilities with adequate resources to care for patients with severe pneumonia, only those with physicians met inclusion criteria. In LMICs where other healthcare providers often function as physician equivalents, this may underestimate the number of facilities with available human resources to treat severe pneumonia. This is especially pertinent where respiratory support devices may be unavailable, rendering the skills required for intubation and management of noninvasive and invasive mechanical ventilation irrelevant. Notably, response rates about availability of respiratory support devices were low, and access to these modalities could not be ascertained. But, even if these providers were not considered qualified to manage the most severe pneumonia cases, understanding their presence and roles in healthcare facilities would have been a valuable addition, as these providers are indispensable in improving disparate healthcare access in LMICs.
Despite these limitations, an understanding of equitable access to pulse oximetry is timely in the setting of the ongoing coronavirus disease (COVID-19) pandemic that continues to claim lives worldwide, largely due to severe pneumonia that can present with “silent” hypoxemia (12). It is foreseeable that effects due to pneumonia from COVID-19 may be similar to those of other etiologies of early-life pneumonia, which is linked to a greater risk of impaired lung function, chronic lung diseases, and lung disease–related mortality in adulthood (13, 14). Children in LMICs remain at higher risk of severe pneumonia due to risk factors such as undernutrition, poor sanitation, lower vaccination rates, air pollution exposure, and HIV (15). In our own work, we found that children with HIV who developed pneumonia in the first year of life had impaired lung function as early as school age (16). There is an urgent need to identify equitable approaches to improving prompt and accurate identification and appropriate treatment of severe pediatric pneumonia, particularly among the vulnerable populations that bear a disproportionate share of the disease burden.
Footnotes
Supported by NIH/NHLBI grant K23 HL129888 (E.F.A.).
Originally Published in Press as DOI: 10.1164/rccm.202110-2325ED on November 17, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. McAllister DA, Liu L, Shi T, Chu Y, Reed C, Burrows J, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health . 2019;7:e47–e57. doi: 10.1016/S2214-109X(18)30408-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Subhi R, Adamson M, Campbell H, Weber M, Smith K, Duke T, Hypoxaemia in Developing Countries Study Group The prevalence of hypoxaemia among ill children in developing countries: a systematic review. Lancet Infect Dis . 2009;9:219–227. doi: 10.1016/S1473-3099(09)70071-4. [DOI] [PubMed] [Google Scholar]
- 3. Simkovich SM, Underhill LJ, Kirby MA, Crocker ME, Goodman D, McCracken JP, et al. HAPIN Investigators Resources and geographic access to care for severe pediatric pneumonia in four resource-limited settings. Am J Respir Crit Care Med . 2022;205:183–197. doi: 10.1164/rccm.202104-1013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hosseinpoor AR, Bergen N, Schlotheuber A. Promoting health equity: WHO health inequality monitoring at global and national levels. Glob Health Action . 2015;8:29034. doi: 10.3402/gha.v8.29034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley SEK, Rosapep L, Shiras T. Where do caregivers take their sick children for care? An analysis of care seeking and equity in 24 USAID priority countries. Glob Health Sci Pract. 2020;8:518–533. doi: 10.9745/GHSP-D-20-00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colbourn T, King C, Beard J, Phiri T, Mdala M, Zadutsa B, et al. Predictive value of pulse oximetry for mortality in infants and children presenting to primary care with clinical pneumonia in rural Malawi: a data linkage study. PLoS Med . 2020;17:e1003300. doi: 10.1371/journal.pmed.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bakare AA, Graham H, Ayede AI, Peel D, Olatinwo O, Oyewole OB, et al. Providing oxygen to children and newborns: a multi-faceted technical and clinical assessment of oxygen access and oxygen use in secondary-level hospitals in southwest Nigeria. Int Health . 2020;12:60–68. doi: 10.1093/inthealth/ihz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Enoch AJ, English M, McGivern G, Shepperd S, Clinical Information Network Variability in the use of pulse oximeters with children in Kenyan hospitals: A mixed-methods analysis. PLoS Med . 2019;16:e1002987. doi: 10.1371/journal.pmed.1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tuti T, Aluvaala J, Akech S, Agweyu A, Irimu G, English M, Clinical Information Network Author Group Pulse oximetry adoption and oxygen orders at paediatric admission over 7 years in Kenya: a multihospital retrospective cohort study. BMJ Open . 2021;11:e050995. doi: 10.1136/bmjopen-2021-050995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ciapponi A, Lewin S, Herrera CA, Opiyo N, Pantoja T, Paulsen E, et al. Delivery arrangements for health systems in low-income countries: an overview of systematic reviews. Cochrane Database Syst Rev . 2017;9:CD011083. doi: 10.1002/14651858.CD011083.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graham H, Tosif S, Gray A, Qazi S, Campbell H, Peel D, et al. Providing oxygen to children in hospitals: a realist review. Bull World Health Organ . 2017;95:288–302. doi: 10.2471/BLT.16.186676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh A, Kataria S, Das P, Sharma A. A proposal to make the pulse oximetry as omnipresent as thermometry in public health care systems. J Glob Health . 2020;10 doi: 10.7189/jogh.10.0203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med . 2016;375:871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 14. Galobardes B, McCarron P, Jeffreys M, Davey Smith G. Association between early life history of respiratory disease and morbidity and mortality in adulthood. Thorax . 2008;63:423–429. doi: 10.1136/thx.2007.086744. [DOI] [PubMed] [Google Scholar]
- 15. Marangu D, Zar HJ. Childhood pneumonia in low-and-middle-income countries: An update. Paediatr Respir Rev . 2019;32:3–9. doi: 10.1016/j.prrv.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Attia EF. Moraa H. Maleche-Obimbo E. Wamalwa D. Gómez LA. Rylance S. et al. Most early-treated children with perinatally-acquired HIV have preserved lung function at school age. J Acquir Immune Defic Syndr . 2022;89:69–76. doi: 10.1097/QAI.0000000000002823. [DOI] [PMC free article] [PubMed] [Google Scholar]