Abstract
Wound healing is one of the most complex physiological regulation mechanisms of the human body. Stem cell technology has had a significant impact on regenerative medicine. Adipose stem cells (ASCs) have many advantages, including their ease of harvesting and high yield, rich content of cell components and cytokines, and strong practicability. They have rapidly become a favored tool in regenerative medicine. Here, we summarize the mechanism and clinical therapeutic potential of ASCs in wound repair.
Keywords: adipose stem cells, wound healing, regenerative medicine, skin regeneration, inflammation
Introduction
The skin is the largest organ of the body. It is a key structure that protects internal tissues from mechanical damage, microbial infection, ultraviolet radiation, and extreme temperatures (Falanga, 2005; Ren et al., 2019; Rodrigues et al., 2019; Yang et al., 2020). In the United States, the annual medical cost of adverse wounds, including surgical incisions and scars, is $12 billion (Fife and Carter, 2012; Leavitt et al., 2016). Wound healing is a highly complex physiological regulation mechanism (Rodrigues et al., 2019) and a sophisticated multicellular process involving the coordination of various cell types and cytokines (Ho Jeong, 2010). Interactions involving epidermal and dermal cells, extracellular matrix (ECM), cytokines, and growth factors coordinate the entire repair process, which can be roughly divided into three stages: inflammation, new tissue formation, and reconstruction (Heublein et al., 2015; Rodrigues et al., 2019). The inflammatory stage includes neutrophil and monocyte recruitment and macrophage activation (Park and Barbul, 2004; Larouche et al., 2018). New tissue formation mainly refers to the proliferation, migration, and recombination of endothelial cells to form new blood vessels. When new blood vessels are formed, resident fibroblasts proliferate and invade fibrin clots to form contractile granulation tissue and produce collagen (Heng, 2011; Ansell and Izeta, 2015; Morikawa et al., 2019). This is followed by the proliferation of epidermal stem cells to rebuild the epidermis and stem cells from sebaceous glands, sweat glands, and hair follicles to form epidermal attachments.
Routine Treatment of Wounds
In view of the complex, multi-stage, physiological and pathological processes of acute and chronic skin wound healing, efficient targeted wound healing treatment methods have been studied and applied. Thorough surgical debridement, prevention of infection, and elimination of dead spaces can minimize the risk of poor wound healing. Emerging technologies, such as those based on growth factors, bioactive molecules, and gene modification, can also overcome the limitations of wound healing technology to some extent and serve as personalized therapeutic strategies (Tottoli et al., 2020).
However, despite these efforts, existing interventions for wound healing have not been sufficiently effective. While there are several treatments available for both acute and chronic wounds, traditional approaches have had limited success. Due to the limitations of traditional methods, such as drug-based therapy, more effective treatments are needed. Skin regeneration therapy strategies and experimental techniques for cell and tissue engineering have also emerged. Stem cell-based therapy has opened a new door for wound repair and has attracted extensive attention in the field of regenerative medicine.
Stem Cells
There are thousands of cells undergoing constant daily dynamic changes, such as loss and self-renewal, to maintain tissue homeostasis. Self-renewal is mainly driven by stem cells. Stimulation from regeneration signals, such as the accumulation of crosstalk with niche factors or environmental changes at the time of injury, can disrupt tissue homeostasis, change stem cell behavior, induce self-renewal, and promote tissue growth (Hsu et al., 2011; Cosgrove et al., 2014; Porpiglia et al., 2017). When homeostasis is restored, differentiated progeny can return to their niche, preventing further proliferation and tissue regeneration, and this process is regulated by a careful balance of time-coordinated cell interactions and molecular feedback loops (Fuchs and Blau, 2020).
Stem cells can be divided into embryonic and adult stem cells according to their developmental stage. Embryonic stem cells refer to cells derived from the embryonic inner cell mass or primordial germ cells in vitro. Embryonic stem cells have developmental totipotency and can differentiate into any type of cell. Embryonic stem cells can be extensively amplified, screened, frozen, and resuspended in vitro without them losing their original characteristics (VanOudenhove et al., 2017; Wang et al., 2019; Sun et al., 2021). Adult stem cells, which are found in various tissues and organs of the body, are undifferentiated cells in a differentiated tissue that can self-renew and differentiate into the specialized cells composing that tissue. These stem cells include hematopoietic stem cells, bone marrow mesenchymal stem cells, neural stem cells, muscle satellite cells, epidermal stem cells, and adipose stem cells (ASCs) (Cinat et al., 2021; Menche and Farin, 2021). In this review, we focus on ASCs.
Sources and Applications of ASCs
Adipose tissue is a multifunctional tissue that contains a variety of cell types, such as the stromal vascular fraction and mature adipose cells. Stromal vascular fragments (SVFs) are a rich source of ASCs that can be easily isolated from human fat (Whiteside, 2008; O’Halloran et al., 2017). The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT MSC) proposes minimal criteria to define human MSC follows: First, MSC must be plastic-adherent when maintained in standard culture conditions. Second, MSC must express CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79alpha or CD19 and HLA-DR surface molecules. Third, MSC must differentiate to osteoblasts, adipocytes and chondroblasts in vitro. ASCs conform to most of the mesenchymal criteria of ISCT MSC, defined as CD45−CD235a−CD31−CD34+. The phenotype of cultured ASCs is CD13+CD73+CD90+CD105+CD31−CD45−CD235a− (Dominici et al., 2006; Bourin et al., 2013).
ASCs have many advantages. They can be directly extracted from the adipose layer of a patient. Adipose tissue has a high frequency of stem cells, and ASCs can be used immediately with primary cells without the need for culture amplification. In addition, ASCs provide not only cellular components, but also a large number of cytokines. Currently, ASCs have various clinical applications, including in scar reshaping and tissue repair, regeneration, and reconstruction, which are treatments often associated with cancer and metabolic diseases (Brayfield et al., 2010; Gir et al., 2012; Rodrigues et al., 2014; Strong et al., 2015; Clevenger et al., 2016; Gentile and Garcovich, 2019; Sabol et al., 2019; Qin et al., 2020). Skin repair/regeneration is one of the most common clinical applications of ASCs, which has a positive therapeutic effect when used to treat skin wounds in patients with diabetes, vascular dysfunction, radiation history, or burn history.
Mechanism of ASCs in Wound Healing
Factors Secreted by ASCs
The mechanisms of wound healing by ASCs are complex and diverse. ASCs are involved throughout the entire process of wound healing, including inflammation, proliferation, and remodeling (Hyldig et al., 2017). During inflammation, ASCs may induce the transformation of the macrophage phenotype from pro-inflammatory M1 to anti-inflammatory M2 to regulate inflammation (Lo Sicco et al., 2017). During proliferation and remodeling, ASCs secrete biological factors such as VEGF, HGF, IGF, PDGF, and TGF-β, which promote the proliferation and migration of fibroblasts, the growth of new blood vessels, and the synthesis of collagen and other ECM proteins, which have beneficial effects on the skin (Rehman et al., 2004; Ho Jeong, 2010; Rodrigues et al., 2014; Na et al., 2017). For example, radiation damage to the skin can cause progressive occlusive endarteritis in local tissues, leading to severe tissue ischemia. Mesenchymal stem cells can be used to repair cellular damage and regenerate new blood vessels in ischemic tissues in patients with radioactive skin injury (Bensidhoum et al., 2005; François et al., 2006). ASC replacement after radiotherapy may reduce the incidence of radiation-related skin complications and is used for the prevention and treatment of skin injury related to tumor radiotherapy (Rigotti et al., 2007).
In addition, ASCs inhibit ECM degradation by increasing the binding of matrix metalloproteinases and secreting tissue metalloproteinase inhibitors (Lozito et al., 2014). Proteins in the ECM, in turn, protect against degradation of growth factors and cytokines produced by activated platelets and macrophages, such as PDGF and TGF-β (Barrientos et al., 2008). Finally, in vitro studies have confirmed that ASCs may promote re-epithelialization by regulating keratinocyte proliferation and migration (Riis et al., 2017). In summary, ASCs can promote wound healing by reducing inflammation, inducing angiogenesis, promoting the growth of fibroblasts and keratinocytes, and generating ECM.
ASC-Derived Extracellular Vesicles
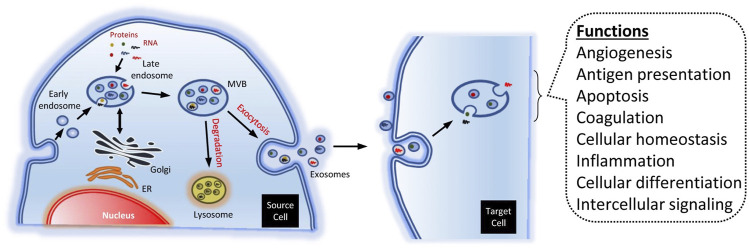
Recent studies have shown that paracrine factors significantly promote the effect of stem cells during tissue repair and that extracellular vesicles may play an important role. Extracellular vesicles include exosomes and microvesicles, which play an important role in and are considered mediators of intercellular communication (Shao et al., 2018; Théry et al., 2018). The differences between exosomes and microvesicles in terms of physical function are yet to be clarified. Microvesicles are large vesicles (50–1000 nm in diameter) that germinate outward from the plasma membrane, whereas exosomes are small vesicles (50–150 nm in diameter), and their secretion requires the fusion of multiple vesicles with the plasma membrane.
In recent years, there has been extensive research on different types of cells, such as fibroblasts, endothelial progenitor cells, and human umbilical cord mesenchymal stem cells, that are involved in tissue repair by regulating cell function and promoting angiogenesis and wound healing (Zhang et al., 2015a; Li et al., 2016; Geiger et al., 2015; Zhang et al., 2015b). ASC-derived exosomes have also been shown to accelerate wound healing by optimizing fibroblast function (Figure 1) (Ren et al., 2019; Casado-Díaz et al., 2020). Studies have found that ASC-derived microvesicles (ASC-MVs) are easily internalized by human umbilical vein endothelial cells (HUVECs), HaCaTs, and fibroblasts, suggesting that ASC-MVs can serve as a suitable vector for delivering a variety of biomolecules and signals to these targeted cells. ASC-MVs can enhance the migration and proliferation of HUVECs, HaCaTs, and fibroblasts through internalization (Zhang et al., 2018; Bi et al., 2019; Ren et al., 2019). Cell cycle progression can be accelerated in a variety of ways, including by increasing the expression of genes related to cyclin D1, cyclin D2, cyclin A1, and cyclin A2, ultimately promoting wound healing (Bretones et al., 2015).
FIGURE 1.
Biogenesis and function of exosomes (Casado-Díaz et al., 2020).
The migration of HUVECs and angiogenesis play an important role in promoting wound healing. ASC-MVs can significantly upregulate the gene expression of integrin β1 and CXCL16 and regulate migration of HUVECs (Hattermann et al., 2008; Tang et al., 2017). ASC-MVs can also accelerate the wound healing process by promoting angiogenesis (Zhang et al., 2018).
ASCs Serve as Effective Immunomodulators in Inflammatory Environments to Promote Wound Healing and Regeneration
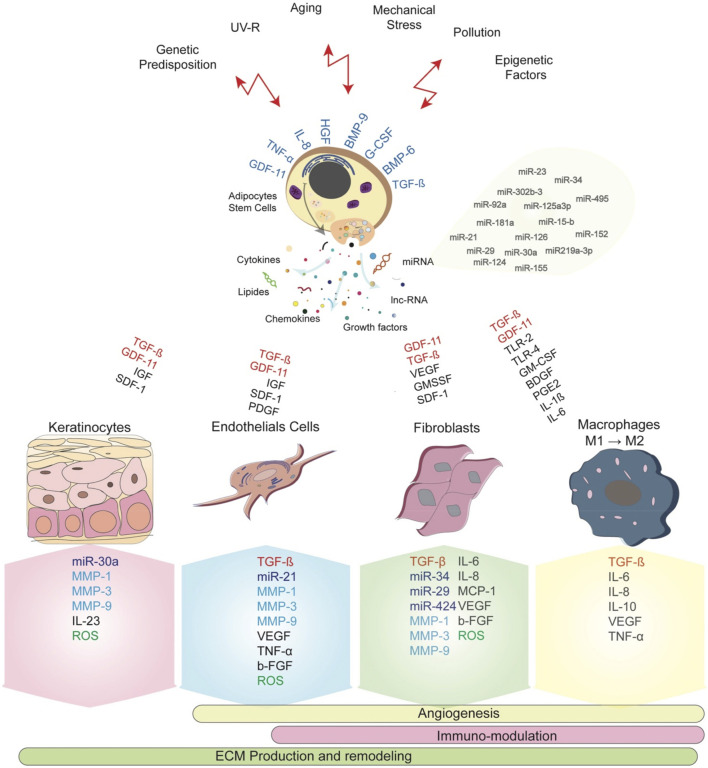
Adipose tissue has an immune function because it contains many immune cells and immunomodulatory cells, including ASCs. ASCs regulate mechanisms related to cell differentiation, proliferation, and migration through exosomes by upregulating genes involved in different functions, including skin barrier, immune regulation, cell proliferation, and epidermal regeneration (58). In addition, there are several populations of stromal and immune cells in heterogeneous products obtained after the digestion of adipose tissue, including SVFs. These properties make ASCs effective immune modulators in inflammatory environments (DelaRosa et al., 2012; Gardin et al., 2018; Li and Guo, 2018).
ADSCs directly interact with their microenvironment and specifically the immune cells, including macrophages, NK cell, T cells and B cells, resulting in differential inflammatory and anti-inflammatory effect (Figure 2) (Mazini et al., 2021). The immune regulatory function of ASCs is manifested as regulation of the Th1/Th2 balance and promotion of Tregs to restore immune tolerance. ASCs secrete the anti-inflammatory cytokine interleukin-10 (IL-10), which enhances Treg activity, and Tregs respond by further secreting IL-10 and amplifying IL-10 signaling (Chaudhry et al., 2011). Tregs and IL-10 attenuate Th1 and Th17 activity, thereby reducing the aggregation of additional pro-inflammatory immune cells at pathological sites (Skapenko et al., 2005; Chaudhry et al., 2011). Additionally, the low expression of NK-activated receptor ligands increases human ASC resistance to NK-mediated recognition, which enables them to remain in the host for longer period. Furthermore, the mechanism by which human ASCs develop NK cell tolerance may be mediated by soluble factors (Spaggiari et al., 2008; DelaRosa et al., 2012). The role of these anti-inflammatory and immunomodulatory effects of ASCs in wound healing needs to be further confirmed.
FIGURE 2.
ASCs serve as effective immunomodulators in inflammatory environments to promote wound healing and regeneration (Mazini et al., 2021).
Discussion
Although ASCs are fundamental to the tissue regeneration process, the clinical transformation of ASC-based therapies remains problematic. Due to the variation in donor age, sex, body mass index, clinical condition, and cell sampling location, ASCs are heterogeneous. Transplanted cells in severe trauma cases have only a limited ability to survive, which can affect their phenotypic features and functions, including proliferation, differentiation potential, immune phenotype, and paracrine activity (Prieto González, 2019). Therefore, future studies on the role of ASCs in regenerative medicine, especially dermatology, are still needed. Nevertheless, ASCs have promising applications in regenerative medicine, including the development of lipogenic potential and the construction of artificial skin by replacing dermal fibroblasts (Trottier et al., 2008; Tartarini and Mele, 2015), which will be the direction of our future research.
Author Contributions
All authors contributed to the design of the study and writing of the manuscript. NZ and HC undertook the research, YW and HC wrote the main manuscript text and prepared figures. ZL revised the article critically for important intellectual content and final approval of the version to be submitted. All authors reviewed the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ASCs, adipose stem cells; ECM, extracellular matrix; IGF, insulin-like growth factor; IL-10, interleukin-10; MVs, microvesicles; PDGF, platelet derived growth factor; SVFs, stromal vascular fragments; TGF-β, transforming growth factor-β.
References
- Ansell D. M., Izeta A. (2015). Pericytes in Wound Healing: Friend or Foe? Exp. Dermatol. 24 (11), 833–834. 10.1111/exd.12782 [DOI] [PubMed] [Google Scholar]
- Barrientos S., Stojadinovic O., Golinko M. S., Brem H., Tomic-Canic M. (2008). PERSPECTIVE ARTICLE: Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 16 (5), 585–601. 10.1111/j.1524-475X.2008.00410.x [DOI] [PubMed] [Google Scholar]
- Bensidhoum M., Gobin S., Chapel A., Lemaitre G., Bouet S., Waksman G., et al. (2005). Potentiel thérapeutique des cellules souches mésenchymateuses humaines dans les lésions cutanées radioinduites. J. Soc. Biol. 199 (4), 337–341. 10.1051/jbio:2005035 [DOI] [PubMed] [Google Scholar]
- Bi H., Li H., Zhang C., Mao Y., Nie F., Xing Y., et al. (2019). Stromal Vascular Fraction Promotes Migration of Fibroblasts and Angiogenesis Through Regulation of Extracellular Matrix in the Skin Wound Healing Process. Stem Cel Res Ther 10 (1), 302. 10.1186/s13287-019-1415-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin P., Bunnell B. A., Casteilla L., Dominici M., Katz A. J., March K. L., et al. (2013). Stromal Cells from the Adipose Tissue-Derived Stromal Vascular Fraction and Culture Expanded Adipose Tissue-Derived Stromal/stem Cells: A Joint Statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15 (6), 641–648. 10.1016/j.jcyt.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayfield C., Marra K., Rubin J. P. (2010). Adipose Stem Cells for Soft Tissue Regeneration. Handchir Mikrochir Plast. Chir 42 (2), 124–128. 10.1055/s-0030-1248269 [DOI] [PubMed] [Google Scholar]
- Bretones G., Delgado M. D., León J. (2015). Myc and Cell Cycle Control. Biochim. Biophys. Acta (Bba) - Gene Regul. Mech. 1849 (5), 506–516. 10.1016/j.bbagrm.2014.03.013 [DOI] [PubMed] [Google Scholar]
- Casado-Díaz A., Quesada-Gómez J. M., Dorado G. (2020). Extracellular Vesicles Derived from Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 8, 146. 10.3389/fbioe.2020.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A., Samstein R. M., Treuting P., Liang Y., Pils M. C., Heinrich J.-M., et al. (2011). Interleukin-10 Signaling in Regulatory T Cells Is Required for Suppression of Th17 Cell-Mediated Inflammation. Immunity 34 (4), 566–578. 10.1016/j.immuni.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinat D., Coppes R. P., Barazzuol L. (2021). DNA Damage-Induced Inflammatory Microenvironment and Adult Stem Cell Response. Front. Cel Dev. Biol. 9, 729136. 10.3389/fcell.2021.729136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger T. N., Luna G., Fisher S. K., Clegg D. O. (2016). Strategies for Bioengineered Scaffolds That Support Adipose Stem Cells in Regenerative Therapies. Regenerative Med. 11 (6), 589–599. 10.2217/rme-2016-0064 [DOI] [PubMed] [Google Scholar]
- Cosgrove B. D., Gilbert P. M., Porpiglia E., Mourkioti F., Lee S. P., Corbel S. Y., et al. (2014). Rejuvenation of the Muscle Stem Cell Population Restores Strength to Injured Aged Muscles. Nat. Med. 20 (3), 255–264. 10.1038/nm.3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelaRosa O., Sánchez-Correa B., Morgado S., Ramírez C., del Río B., Menta R., et al. (2012). Human Adipose-Derived Stem Cells Impair Natural Killer Cell Function and Exhibit Low Susceptibility to Natural Killer-Mediated Lysis. Stem Cell Dev. 21 (8), 1333–1343. 10.1089/scd.2011.0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F. C., Krause D. S., et al. (2006). Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 8 (4), 315–317. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- Falanga V. (2005). Wound Healing and its Impairment in the Diabetic Foot. The Lancet 366 (9498), 1736–1743. 10.1016/S0140-6736(05)67700-8 [DOI] [PubMed] [Google Scholar]
- Fife C. E., Carter M. J. (2012). Wound Care Outcomes and Associated Cost Among Patients Treated in US Outpatient Wound Centers: Data from the US Wound Registry. Wounds 24 (1), 10–17. [PubMed] [Google Scholar]
- François S., Mouiseddine M., Mathieu N., Semont A., Monti P., Dudoignon N., et al. (2006). Human Mesenchymal Stem Cells Favour Healing of the Cutaneous Radiation Syndrome in a Xenogenic Transplant Model. Ann. Hematol. 86 (1), 1–8. 10.1007/s00277-006-0166-5 [DOI] [PubMed] [Google Scholar]
- Fuchs E., Blau H. M. (2020). Tissue Stem Cells: Architects of Their Niches. Cell Stem Cell 27 (4), 532–556. 10.1016/j.stem.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardin C., Ferroni L., Bellin G., Rubini G., Barosio S., Zavan B. (2018). Therapeutic Potential of Autologous Adipose-Derived Stem Cells for the Treatment of Liver Disease. Int. J. Mol. Sci. 19 (12), 4064. 10.3390/ijms19124064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger A., Walker A., Nissen E. (2015). Human Fibrocyte-Derived Exosomes Accelerate Wound Healing in Genetically Diabetic Mice. Biochem. Biophysical Res. Commun. 467 (2), 303–309. 10.1016/j.bbrc.2015.09.166 [DOI] [PubMed] [Google Scholar]
- Gentile P., Garcovich S. (2019). Concise Review: Adipose-Derived Stem Cells (ASCs) and Adipocyte-Secreted Exosomal microRNA (A-SE-miR) Modulate Cancer Growth and proMote Wound Repair. J. Clin. Med. 8 (6), 855. 10.3390/jcm8060855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gir P., Oni G., Brown S. A., Mojallal A., Rohrich R. J. (2012). Human Adipose Stem Cells. Plast. Reconstr. Surg. 129 (6), 1277–1290. 10.1097/PRS.0b013e31824ecae6 [DOI] [PubMed] [Google Scholar]
- Hattermann K., Ludwig A., Gieselmann V., Held-Feindt J., Mentlein R. (2008). The Chemokine CXCL16 Induces Migration and Invasion of Glial Precursor Cells via its Receptor CXCR6. Mol. Cell Neurosci. 39 (1), 133–141. 10.1016/j.mcn.2008.03.009 [DOI] [PubMed] [Google Scholar]
- Heng M. C. Y. (2011). Wound Healing in Adult Skin: Aiming for Perfect Regeneration. Int. J. Dermatol. 50 (9), 1058–1066. 10.1111/j.1365-4632.2011.04940.x [DOI] [PubMed] [Google Scholar]
- Heublein H., Bader A., Giri S. (2015). Preclinical and Clinical Evidence for Stem Cell Therapies as Treatment for Diabetic Wounds. Drug Discov. Today 20 (6), 703–717. 10.1016/j.drudis.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Ho Jeong J. (2010). Adipose Stem Cells and Skin Repair. Curr. Stem Cel Res. 5 (2), 137–140. 10.2174/157488810791268690 [DOI] [PubMed] [Google Scholar]
- Hsu Y.-C., Pasolli H. A., Fuchs E. (2011). Dynamics Between Stem Cells, Niche, and Progeny in the Hair Follicle. Cell 144 (1), 92–105. 10.1016/j.cell.2010.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyldig K., Riis S., Pennisi C., Zachar V., Fink T. (2017). Implications of Extracellular Matrix Production by Adipose Tissue-Derived Stem Cells for Development of Wound Healing Therapies. Int. J. Mol. Sci. 18 (6), 1167. 10.3390/ijms18061167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larouche J., Sheoran S., Maruyama K., Martino M. M. (2018). Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 7 (7), 209–231. 10.1089/wound.2017.0761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt T., Hu M. S., Marshall C. D., Barnes L. A., Lorenz H. P., Longaker M. T. (2016). Scarless Wound Healing: Finding the Right Cells and Signals. Cell Tissue Res 365 (3), 483–493. 10.1007/s00441-016-2424-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Guo X. (2018). A Review: Therapeutic Potential of Adipose-Derived Stem Cells in Cutaneous Wound Healing and Regeneration. Stem Cel Res Ther 9 (1), 302. 10.1186/s13287-018-1044-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Jiang C., Zhao J. (2016). Human Endothelial Progenitor Cells-Derived Exosomes Accelerate Cutaneous Wound Healing in Diabetic Rats by Promoting Endothelial Function. J. Diabetes its Complications 30 (6), 986–992. 10.1016/j.jdiacomp.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Lo Sicco C., Reverberi D., Balbi C., Ulivi V., Principi E., Pascucci L., et al. (2017). Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-inflammatory Effects: Endorsement of Macrophage Polarization. STEM CELLS Translational Med. 6 (3), 1018–1028. 10.1002/sctm.16-0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozito T. P., Jackson W. M., Nesti L. J., Tuan R. S. (2014). Human Mesenchymal Stem Cells Generate a Distinct Pericellular Zone of MMP Activities via Binding of MMPs and Secretion of High Levels of TIMPs. Matrix Biol. 34, 132–143. 10.1016/j.matbio.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Mazini L., Rochette L., Hamdan Y., Malka G. (2021). Skin Immunomodulation During Regeneration: Emerging New Targets. J. Personalized Med. 11 (2), 85. 10.3390/jpm11020085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menche C., Farin H. F. (2021). Strategies for Genetic Manipulation of Adult Stem Cell-Derived Organoids. Exp. Mol. Med. 53 (10), 1483–1494. 10.1038/s12276-021-00609-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S., Iribar H., Gutiérrez-Rivera A., Ezaki T., Izeta A. (2019). Pericytes in Cutaneous Wound Healing. Adv. Exp. Med. Biol. 1147, 1–63. 10.1007/978-3-030-16908-4_1 [DOI] [PubMed] [Google Scholar]
- Na Y. K., Ban J.-J., Lee M., Im W., Kim M. (2017). Wound Healing Potential of Adipose Tissue Stem Cell Extract. Biochem. Biophysical Res. Commun. 485 (1), 30–34. 10.1016/j.bbrc.2017.01.103 [DOI] [PubMed] [Google Scholar]
- O’Halloran N., Courtney D., Kerin M. J., Lowery A. J. (2017). Adipose-Derived Stem Cells in Novel Approaches to Breast Reconstruction: Their Suitability for Tissue Engineering and Oncological Safety. Breast Cancer (Auckl) 11, 117822341772677. 10.1177/1178223417726777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. E., Barbul A. (2004). Understanding the Role of Immune Regulation in Wound Healing. Am. J. Surg. 187 (5A), 11S–16S. 10.1016/S0002-9610(03)00296-4 [DOI] [PubMed] [Google Scholar]
- Porpiglia E., Samusik N., Ho A. T. V., Cosgrove B. D., Mai T., Davis K. L., et al. (2017). High-resolution Myogenic Lineage Mapping by Single-Cell Mass Cytometry. Nat. Cel Biol 19 (5), 558–567. 10.1038/ncb3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto González E. A. (2019). Heterogeneity in Adipose Stem Cells. Adv. Exp. Med. Biol. 1123, 119–150. 10.1007/978-3-030-11096-3_8 [DOI] [PubMed] [Google Scholar]
- Qin F., Huang J., Zhang W., Zhang M., Li Z., Si L., et al. (2020). The Paracrine Effect of Adipose-Derived Stem Cells Orchestrates Competition Between Different Damaged Dermal Fibroblasts to Repair UVB-Induced Skin Aging. Stem Cell Int. 2020, 1–19. 10.1155/2020/8878370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C. J., Bovenkerk J. E., et al. (2004). Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 109 (10), 1292–1298. 10.1161/01.CIR.0000121425.42966.F1 [DOI] [PubMed] [Google Scholar]
- Ren S., Chen J., Duscher D., Liu Y., Guo G., Kang Y., et al. (2019). Microvesicles from Human Adipose Stem Cells Promote Wound Healing by Optimizing Cellular Functions via AKT and ERK Signaling Pathways. Stem Cel Res Ther 10 (1), 47. 10.1186/s13287-019-1152-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti G., Marchi A., Gali M., Baroni G., Benati D., Krampera M., et al. (2007). Clinical Treatment of Radiotherapy Tissue Damage by Lipoaspirate Transplant: A Healing Process Mediated by Adipose-Derived Adult Stem Cells. Plast. Reconstr. Surg. 119 (5), 1409–1422. 10.1097/01.prs.0000256047.47909.71 [DOI] [PubMed] [Google Scholar]
- Riis S., Newman R., Ipek H., Andersen J. I., Kuninger D., Boucher S., et al. (2017). Hypoxia Enhances the Wound-Healing Potential of Adipose-Derived Stem Cells in a Novel Human Primary Keratinocyte-Based Scratch Assay. Int. J. Mol. Med. 39 (3), 587–594. 10.3892/ijmm.2017.2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues C., de Assis A. M., Moura D. J., Halmenschlager G., Saffi J., Xavier L. L., et al. (2014). New Therapy of Skin Repair Combining Adipose-Derived Mesenchymal Stem Cells with Sodium Carboxymethylcellulose Scaffold in a Pre-clinical Rat Model. PLoS One 9 (5), e96241. 10.1371/journal.pone.0096241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M., Kosaric N., Bonham C. A., Gurtner G. C. (2019). Wound Healing: A Cellular Perspective. Physiol. Rev. 99 (1), 665–706. 10.1152/physrev.00067.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol R. A., Giacomelli P., Beighley A., Bunnell B. A. (2019). Adipose Stem Cells and Cancer: Concise Review. Stem Cells 37 (10), 1261–1266. 10.1002/stem.3050 [DOI] [PubMed] [Google Scholar]
- Shao H., Im H., Castro C. M., Breakefield X., Weissleder R., Lee H. (2018). New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 118 (4), 1917–1950. 10.1021/acs.chemrev.7b00534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapenko A., Leipe J., Lipsky P. E., Schulze-Koops H. (2005). The Role of the T Cell in Autoimmune Inflammation. Arthritis Res. Ther. 7 (Suppl. 2), S4–S14. 10.1186/ar1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaggiari G. M., Capobianco A., Abdelrazik H., Becchetti F., Mingari M. C., Moretta L. (2008). Mesenchymal Stem Cells Inhibit Natural Killer-Cell Proliferation, Cytotoxicity, and Cytokine Production: Role of Indoleamine 2,3-dioxygenase and Prostaglandin E2. Blood 111 (3), 1327–1333. 10.1182/blood-2007-02-074997 [DOI] [PubMed] [Google Scholar]
- Strong A. L., Burow M. E., Gimble J. M., Bunnell B. A. (2015). Concise Review: The Obesity Cancer Paradigm: Exploration of the Interactions and Crosstalk with Adipose Stem Cells. Stem Cells 33 (2), 318–326. 10.1002/stem.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Fu X., Ma G., Hutchins A. P. (2021). Chromatin and Epigenetic Rearrangements in Embryonic Stem Cell Fate Transitions. Front. Cel Dev. Biol. 9, 637309. 10.3389/fcell.2021.637309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Yan T., Zhang J., Jiang X., Zhang D., Huang Y. (2017). Notch1 Signaling Contributes to Hypoxia-Induced High Expression of Integrin β1 in Keratinocyte Migration. Sci. Rep. 7, 43926. 10.1038/srep43926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartarini D., Mele E. (2015). Adult Stem Cell Therapies for Wound Healing: Biomaterials and Computational Models. Front. Bioeng. Biotechnol. 3, 206. 10.3389/fbioe.2015.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C., Witwer K. W., Aikawa E., Alcaraz M. J., Anderson J. D., Andriantsitohaina R., et al. (2018). Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell Vesicles 7 (1), 1535750. 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottoli E. M., Dorati R., Genta I., Chiesa E., Pisani S., Conti B. (2020). Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 12 (8), 735. 10.3390/pharmaceutics12080735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier V., Marceau‐Fortier G., Germain L., Vincent C., Fradette J. (2008). IFATS Collection: Using Human Adipose‐Derived Stem/Stromal Cells for the Production of New Skin Substitutes. Stem Cells 26 (10), 2713–2723. 10.1634/stemcells.2008-0031 [DOI] [PubMed] [Google Scholar]
- VanOudenhove J. J., Grandy R. A., Ghule P. N., Lian J. B., Stein J. L., Zaidi S. K., et al. (2017). Unique Regulatory Mechanisms for the Human Embryonic Stem Cell Cycle. J. Cel. Physiol. 232 (6), 1254–1257. 10.1002/jcp.25567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Bu F., Zhang W. (2019). The Role of Ubiquitination in Regulating Embryonic Stem Cell Maintenance and Cancer Development. Int. J. Mol. Sci. 20 (11), 2667. 10.3390/ijms20112667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside T. L. (2008). The Tumor Microenvironment and its Role in Promoting Tumor Growth. Oncogene 27 (45), 5904–5912. 10.1038/onc.2008.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Gu Z., Lu C., Zhang T., Guo X., Xue G., et al. (2020). Neutrophil Extracellular Traps Are Markers of Wound Healing Impairment in Patients with Diabetic Foot Ulcers Treated in a Multidisciplinary Setting. Adv. Wound Care 9 (1), 16–27. 10.1089/wound.2019.0943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Wang M., Gong A., Zhang X., Wu X., Zhu Y., et al. (2015). HucMSC‐Exosome Mediated‐Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 33 (7), 2158–2168. 10.1002/stem.1771 [DOI] [PubMed] [Google Scholar]
- Zhang J., Guan J., Niu X., Hu G., Guo S., Li Q., et al. (2015). Exosomes Released from Human Induced Pluripotent Stem Cells-Derived MSCs Facilitate Cutaneous Wound Healing by Promoting Collagen Synthesis and Angiogenesis. J. Transl Med. 13, 49. 10.1186/s12967-015-0417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Bai X., Zhao B., Li Y., Zhang Y., Li Z., et al. (2018). Cell-free Therapy Based on Adipose Tissue Stem Cell-Derived Exosomes Promotes Wound Healing via the PI3K/Akt Signaling Pathway. Exp. Cel Res. 370 (2), 333–342. 10.1016/j.yexcr.2018.06.035 [DOI] [PubMed] [Google Scholar]