Abstract
Budgerigar fledgling disease virus (BFDV) is the causative polyomavirus of budgerigar fledgling disease, an important avian immunosuppressive disease in budgerigars (Melopsittacus undulatus). In the current study, we explored the etiological role and molecular characteristics of BFDV. We identified a novel BFDV strain, designated as SC-YB19, belonging to a unique cluster with three other domestic strains (WF-GM01, SD18, and APV-P) and closely related to Polish isolates based on complete sequences. Sequence analysis showed that SC-YB19 had an 18-nucleotide (nt) deletion in the enhancer region, corresponding to the sequence position 164–181 nt, which differed significantly from all other BFDV strains. Based on sequence alignment, three unique nucleotide substitutions were found in VP4 (position 821), VP1 (position 2,383), and T-antigen (position 3,517) of SC-YB19, compared with SD18, WF-GM01, QDJM01, HBYM02, APV7, and BFDV1. Phylogenetic analyses based on complete sequences suggested that SC-YB19, along with the domestic WF-GM01, SD18, and APV-P strains, formed a single branch and were closely related to Polish, Japanese, and American isolates. These results demonstrate that BFDV genotype variations are co-circulating in China, thus providing important insight into BFDV evolution.
Keywords: budgerigar fledgling disease virus, deletion, phylogenetic analysis, genotype variation, enhancer element
Background
Budgerigar fledgling disease virus (BFDV), also called avian polyomavirus (APV), is the causative agent of budgerigar fledgling disease, an important immunosuppressive disease in budgerigars (Melopsittacus undulatus). The disease was first reported in 1981, with typical symptoms including abdominal distention, lack of down feathers on the back and abdomen, subcutaneous hemorrhage of nestlings, and acute death (1–3). Polyomaviruses have a wide host range, and have been identified in vertebrates such as humans (4, 5), bats (6, 7), non-human primates (8), and horses (9). In China, a BFDV strain, HBYM02, was first reported and isolated in Hubei Province in 1994. Since then, sporadic infections have occurred across the country, resulting in considerable losses to the budgerigar breeding industry (10).
BFDV was identified as the first non-mammalian member of the genus polyomavirus (11, 12). BFDV is a circular, double-stranded molecule with a 4 981-nt genome, which can be divided into early and late regions. The early region codes for two non-structural proteins, i.e., large T and small t antigens. The late region contains four structural proteins, i.e., VP1, VP2, VP3, and VP4 (13). The genome also contains four regulatory elements, i.e., promoter, polyadenylation signal, DNA replication origin, and enhancer regions (14).
In the present study, we report on BFDV infection in budgerigars from Sichuan Province, China, for the first time. To better understand the molecular characteristics of the identified strain, sequencing analysis was performed and a phylogenetic tree was constructed based on its complete genome. Our results should assist in elucidating the genetic evolution of BFDV in China.
Methods
Ethics Statement
No animals were sacrificed for this study.
Clinical Cases and Virus Identification
During the spring of 2019, dozens of budgerigars died at a budgerigar breeding farm in Sichuan, China. The birds showed rapid weight loss and exhibited liver and lung congestion, splenomegaly, swollen kidneys, and liver hemorrhage. Heart, liver, lung, and fecal samples were collected from the dead budgerigars. The samples were ground and centrifuged at 8,000 rpm for 10 min. DNA/RNA was extracted from the resulting supernatants, then identified using primers (15). Polymerase chain reaction (PCR) primers were employed to amplify the complete sequence, as described previously (16). Samples were examined by blood agglutination assay, PCR, or reverse transcription (RT) -PCR for the presence of DNA or RNA viruses, including avian reovirus (17), infectious bursal disease virus (18), reticuloendotheliosis virus (19), avian influenza virus (20), Newcastle disease virus (21), avian leukosis virus (22), and avian adenovirus 4 (23), according to previously described methods.
Sequence Alignment and Phylogenetic Analysis
Complete sequences were manually assembled using ClustalX (v1.83), Vector 10, and DNASTAR. Multiple sequence alignments were conducted using ClustalW in MEGA v6.0. Phylogenetic trees were constructed using the neighbor-joining (NJ) method in MEGA (v4.0). Bootstrap values were estimated for 1,000 replicates. The sequences obtained in this study were assembled and submitted to GenBank under accession number MT119153.
Results and Discussion
In 2019, more than 20 two-week-old budgerigars died at a breeding farm and were thus collected for laboratory investigation. Tissue samples from the liver and lungs were only positive for BFDV. No other viruses were identified in the samples (data not shown). To analyze genomic characteristics, PCR was employed to amplify and sequence the complete viral genome (termed SC-YB19). The genome was 4,963-nt long and composed of six regions, including early, late, promoter, polyadenylation signal, DNA replication origin, and enhancer regions. The positions 80–126, 131–178, 180–193, 682–693, and 706–715 are speculated to be enhancer elements (14) (Supplementary Figure 1), which affect interactions with cellular transcription and replication factors and regulate viral and cellular gene products (24–27). We identified an 18-nt deletion (position 164–182) in the enhancer section (positions 131–178 and 180–193) in SC-YB19, but not in the other BFDV isolates (Supplementary Figures 2A,B). However, whether this deletion affects the transcription, replication, and virulence of SC-YB19 is unknown, and further experiments are required to investigate its potential influence on biological effects. In addition, compared with SD18, WF-GM01, QDJM01, HBYM02, APV7, and BFDV1, nucleotide substitutions were observed at 19 loci in VP4, VP2/VP3, VP1, non-coding region, T-antigen, and T/t-antigen of SC-YB19. Three nucleotide substitutions in VP4 (position 821), VP1 (position 2,383), and T-antigen (position 3,517) were only found in SC-YB19, not in the other domestic strains (Table 1). All nucleotide substitutions in SC-YB19 were nonsense mutations (Table 1). Based on sequence alignment, position 2488, 2572, 2677, 2758, 2920, 2959, 3256, and 4139 of SC-YB19, SD18, and WF-JM01 were identified to that of BFDV and APV-7, which were isolated from the USA and Japan, respectively, while position 623, 2488, 2572, 2677, 2758, 2920, 2959, 3256, 3739, 4139, and 4986 of QDJM01 and HBYM02 were identified to the German strains (BFDV1, BFDV4, BFDV5, PLYGEN, and NC004764) (Supplementary Figures 3, 4). These results indicate that domestic strains show genetic diversity and may have different ancestors. We suppose that BFDVs have undergone evolution in China.
Table 1.
Point mutations in seven strains of BFDVs compared with SC-YB19.
| Nucleotide number | Region | SC-YB19 | Nucleotide exchange compared with SC-YB19 (amino acid substitution compared with predicted amino acid sequence of SC-YB19) | |||||
|---|---|---|---|---|---|---|---|---|
| SD18 | WF-GM01 | QDJM01 | HBYM02 | APV7 | BFDV1 | |||
| 386 | VP4(inton) | G | G | G | G | G | G | C |
| 387 | VP4(inton) | C | C | C | C | C | C | G |
| 623 | VP4(inton) | C | C | C | T | T | T | T |
| 821 | VP4 | C(123T) | T(123T) | T(123T) | T(123T) | T(123T) | T(123T) | T(123T) |
| 1652 | VP2/VP3 | G(115G) | G(115G) | G(115G) | G(115G) | G(115G) | G(115G) | T(115A) |
| 2383 | VP1 | T(157S) | A(157S) | A(157S) | A(157S) | A(157S) | A(157S) | A(157S) |
| 2488 | VP1 | C(192G) | C(192G) | C(192G) | T(192G) | T(192G) | C(192G) | T(192G) |
| 2572 | VP1 | A(220E) | A(220E) | A(220E) | G(220E) | G(220E) | A(220E) | G(220E) |
| 2677 | VP1 | C(255A) | C(255A) | C(255A) | A(255A) | A(255A) | C(255A) | A(255A) |
| 2758 | VP1 | A(282R) | A(282R) | A(282R) | G(282R) | G(282R) | A(282R) | G(282R) |
| 2920 | VP1 | T(336D) | T(336D) | T(336D) | C(336D) | C(336D) | T(336D) | C(336D) |
| 2959 | Non-coding region | A | A | A | C | C | A | C |
| 3256 | T-antigen | G(515T) | G(515T) | G(515T) | A(515T) | A(515T) | A(515T) | A(515T) |
| 3457 | T-antigen | C(448K) | C(448K) | T(448K) | T(448K) | T(448K) | T(448K) | T(448K) |
| 3517 | T-antigen | G(428R) | T(428R) | T(428R) | T(428R) | T(428R) | T(428R) | T(428R) |
| 3657 | T-antigen | T(382K) | T(382K) | G(382Q) | G(382Q) | G(382Q) | G(382Q) | G(382Q) |
| 3739 | T-antigen | G(354T) | G(354G) | G(354G) | A(354T) | A(354T) | A(354T) | A(354T) |
| 4139 | T-antigen | G(221P) | G(221P) | G(221P) | G(221P) | A(221L) | A(221L) | A(221L) |
| 4986 | T/t-antigen | G(4L) | G(4L) | G(4L) | A(4L) | G(4L) | G(4L) | A(4L) |
Highlights indicate the mutant position.
Phylogenetic analyses based on complete sequences suggested that SC-YB19, along with the domestic WF-GM01, SD18, and APV-P strains, formed a single branch and were closely related to Polish, Japanese, and American isolates (Figure 1; Table 2). QD-JM01 was clustered with the APV1, APV2, APV4, and APV5 strains isolated from the Japanese black-headed caique (Pionites melanocephalus). HBYM02 was distinct from the five other domestic strains and did not belong to any cluster (Figure 1). Thus, our data show that different BFDV genotypes are co-circulating in China.
Figure 1.
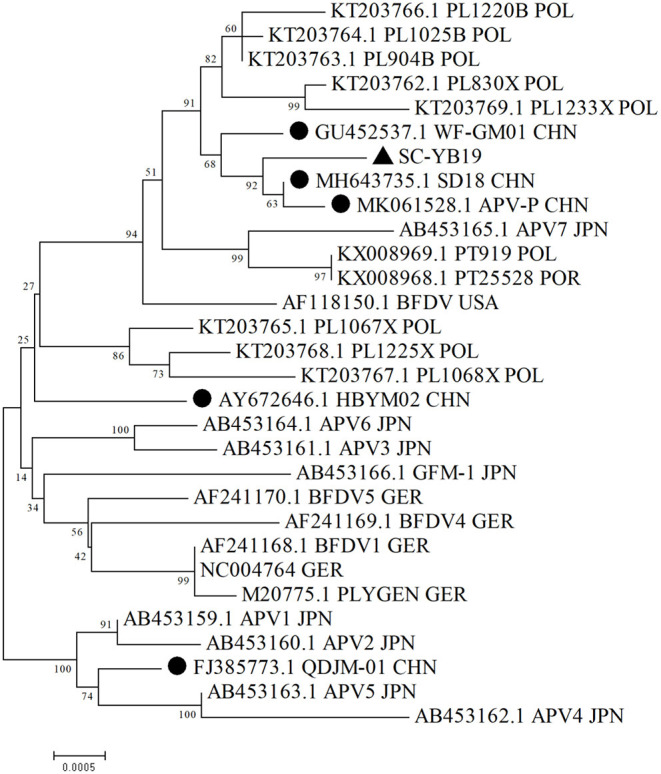
Phylogenetic analysis of complete SC-YB19 sequence and related whole-genome strains from GenBank. Neighbor-joining was used to construct a phylogenetic tree, with bootstrap values of 1,000 replicates shown on branches. Scale bar represents p-distance. • presents the domestic strains. ▴ presents the strains isolated in this study. CHN, China; GER, Germany; POL, Poland; POR, Portugal; USA, United States Of Amrica; JPN, Japan.
Table 2.
Source of Budgerigar fledgling disease polyomavirus (BFDV) sequences used in the experiment.
| Name | Genbank | Collection date | Country |
|---|---|---|---|
| SC-YB19 | MT119153 | 2019 | CHN |
| BFDV1 | AF241168 | 1984 | GER |
| BFDV4 | AF241169 | 1981 | GER |
| BFDV5 | AF241170 | 1995 | GER |
| GFM-1 | AB477106 | 1982 | JPN |
| BFDV | AF118150 | 1999 | USA |
| APV1 | AB453159 | 2003 | JPN |
| APV2 | AB453160 | 2003 | JPN |
| APV3 | AB453161 | 2004 | JPN |
| APV4 | AB453162 | 2005 | JPN |
| APV5 | AB453163 | 2005 | JPN |
| APV6 | AB453164 | 2005 | JPN |
| APV7 | AB453165 | 2006 | JPN |
| PLYGEN | M20775 | 1988 | GER |
| WF-GM01 | GU452537 | 2009 | CHN |
| SD18 | MH643735 | 2018 | CHN |
| APV-P | MK061528 | 2018 | CHN |
| QDJM-01 | FJ385773 | 2008 | CHN |
| HBYM02 | AY672646 | 1994 | CHN |
| PL830X | KT203762 | 2009 | POL |
| PL1220B | KT203766 | 2010 | POL |
| PT25528 | KX008968 | 2015 | POR |
| PT919 | KX008969 | 2016 | POL |
| PL1025B | KT203764.1 | 2010 | POL |
| PL904B | KT203763.1 | 2009 | POL |
| PL1233X | KT203769.1 | 2011 | POL |
| PL1067X | KT203765 | 2010 | POL |
| PL1225X | KT203768 | 2010 | POL |
| PL1068X | KT203767 | 2010 | POL |
| / | NC004764 | 1984 | GER |
Conclusions
We report on a novel BFDV enhancer deletion mutant (SC-YB19 strain) in China. The strain forms a unique cluster with three other domestic strains. This study improves our understanding of the genetic structure, diversity, and evolution of SC-YB19.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by Animal Ethics Committee of Yibin University.
Author Contributions
ZT and TY designed the study and revised the draft. XH, DC, SL, and ZT performed the experiments. YL, LC, GL, HP, YH, XL, LZ, and HC collected the samples. ZT, XH, and TY drafted the manuscript. All authors revised and approved the paper for publication.
Funding
This work was supported by the Doctor Launch Project of Yibin University (2019QD09 and 2019QD10) and Foundation of General Program in Civil Aviation Flight University of China (J2021-134).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- BFDV
Budgerigar fledgling disease virus
- APV
avian polyomavirus
- NJ
neighbor-joining
- nt
nucleotides
- PCR
polymerase chain reaction
- RT
reverse transcription.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.813397/full#supplementary-material
Draft enhancer element of SC-YB19 (in red box).
(A) 18-nt deletion in the enhance element at nucleotide position 164–182 of the SC-YB19; (B) Result of the ABI sequencing for SC-YB19.
Nucleotide sequences alignments between SC-YB19 and SD18, WF-GM01, QDJM01, AY672646, AF118150, APV-7, AF241168, AF241169, AF241170, M20775, and NC004764.
Multiple sequence alignment of complete sequences in BFDV strains.
References
- 1.Davis R, Bozeman L, Gaudry D, Fletcher O, Lukert P, Dykstra M. A viral disease of fledgling budgerigars. Avian Dis. (1981) 25:179–83. 10.2307/1589839 [DOI] [PubMed] [Google Scholar]
- 2.Bernier G, Morin M, Marsolais G. A generalized inclusion body disease in the budgerigar (Melopsittacus undulatus) caused by a papovavirus-like agent. Avian Dis. (1981) 25:1083–92. 10.2307/1590087 [DOI] [PubMed] [Google Scholar]
- 3.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. (2008) 319:1096–100. 10.1126/science.1152586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, et al. Identification of a third human polyomavirus. J Virol. (2007) 81:4130–6. 10.1128/JVI.00028-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, et al. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathogens. (2007) 3:e64. 10.1371/journal.ppat.0030064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao Y, Shi M, Conrardy C, Kuzmin IV, Recuenco S, Agwanda B, et al. Discovery of diverse polyomaviruses in bats and the evolutionary history of the Polyomaviridae. J Gen Virol. (2013) 94:738–748. 10.1099/vir.0.047928-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagrouch Z, Sarwari R, Lavergne A, Delaval M, De Thoisy B, Lacoste V, et al. Novel polyomaviruses in South American bats and their relationship to other members of the family Polyomaviridae. J Gen Virol. (2012) 93:2652–7. 10.1099/vir.0.044149-0 [DOI] [PubMed] [Google Scholar]
- 8.Deuzing I, Fagrouch Z, Groenewoud MJ, Niphuis H, Kondova I, Bogers W, et al. Detection and characterization of two chimpanzee polyomavirus genotypes from different subspecies. Virol J. (2010) 7:347. 10.1186/1743-422X-7-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renshaw RW, Wise AG, Maes RK, Dubovi EJ. Complete genome sequence of a polyomavirus isolated from horses. Am Soc Microbiol. (2012) 86:8903. 10.1128/JVI.01261-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia W, Feng F, Li T, Chen S, Zhao L. Identification and biochemical characteristics of a new psittacine virus in China. Virol Sinica. (1999) 14:265–272. [Google Scholar]
- 11.Bozeman L, Davis R, Gaudry D, Lukert P, Fletcher O, Dykstra M. Characterization of a papovavirus isolated from fledgling budgerigars. Avian Dis. (1981) 25:972–80. 10.2307/1590072 [DOI] [PubMed] [Google Scholar]
- 12.Lehn H, Müller H. Cloning and characterization of budgerigar fledgling disease virus, an avian polyomavirus. Virology. (1986) 151:362–70. 10.1016/0042-6822(86)90056-5 [DOI] [PubMed] [Google Scholar]
- 13.Johne R, Paul G, Enderlein D, Stahl T, Grund C, Müller H. Avian polyomavirus mutants with deletions in the VP4-encoding region show deficiencies in capsid assembly and virus release, and have reduced infectivity in chicken. J Gen Virol. (2007) 88:823–30. 10.1099/vir.0.82506-0 [DOI] [PubMed] [Google Scholar]
- 14.Rott O, Kröger M, Müller H, Hobom G. The genome of budgerigar fledgling disease virus, an avian polyomavirus. Virology. (1988) 165:74–86. 10.1016/0042-6822(88)90660-5 [DOI] [PubMed] [Google Scholar]
- 15.Zhuang Q, Chen J, Mushtaq MH, Chen J, Liu S, Hou G, et al. Prevalence and genetic characterization of avian polyomavirus and psittacine beak and feather disease virus isolated from budgerigars in Mainland China. Arch Virol. (2012) 157:53–61. 10.1007/s00705-011-1138-1 [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Wu R, Tian Y, Zhang M, Wang W, Li Y, et al. Isolation and characterization of an Aves polyomavirus 1 from diseased budgerigars in China. Vet Microbiol. (2019) 237:108397. 10.1016/j.vetmic.2019.108397 [DOI] [PubMed] [Google Scholar]
- 17.Zhong L, Gao L, Liu Y, Li K, Wang M, Qi X, et al. Genetic and pathogenic characterisation of 11 avian reovirus isolates from northern China suggests continued evolution of virulence. Sci Rep. (2016) 6:35271. 10.1038/srep35271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Z, Zhang L, Wang N, Chen Y, Gao L, Wang Y, et al. Naturally occurring reassortant infectious bursal disease virus in northern China. Virus Res. (2015) 203:92–5. 10.1016/j.virusres.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 19.Jiang L, Qi X, Gao Y, Hua Y, Li K, Deng X, et al. Molecular characterization and phylogenetic analysis of the reticuloendotheliosis virus isolated from wild birds in Northeast China. Vet Microbiol. (2013) 166:68–75. 10.1016/j.vetmic.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Li C, Liu L, Wang H, Wang C, Tian G, et al. Characterization of an avian influenza virus of subtype H7N2 isolated from chickens in northern China. Virus Genes. (2006) 33:117–22. 10.1007/s11262-005-0042-8 [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Hu S, Xu H, Liu J, Zhao Z, Wang X, et al. Characterization of virulent Newcastle disease viruses from vaccinated chicken flocks in Eastern China. BMC Vet Res. (2016) 12:113. 10.1186/s12917-016-0732-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Li X, Ji X, Wang J, Shen N, Gao Y, et al. A recombinant avian leukosis virus subgroup j for directly monitoring viral infection and the selection of neutralizing antibodies. PLoS ONE. (2014) 9:e115422. 10.1371/journal.pone.0115422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan Q, Liu L, Wang Y, Zhang Y, Qi X, Liu C, et al. The first whole genome sequence and pathogenicity characterization of a fowl adenovirus 4 isolated from ducks associated with inclusion body hepatitis and hydropericardium syndrome. Avian Pathol J WVPA. (2017) 46:571–578. 10.1080/03079457.2017.1311006 [DOI] [PubMed] [Google Scholar]
- 24.Maniatis T, Goodbourn S, Fischer JA. Regulation of inducible and tissue-specific gene expression. Science. (1987) 236:1237–45. 10.1126/science.3296191 [DOI] [PubMed] [Google Scholar]
- 25.Ondek B, Shepard A, Herr W. Discrete elements within the SV40 enhancer region display different cell-specific enhancer activities. EMBO J. (1987) 6:1017–25. 10.1002/j.1460-2075.1987.tb04854.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakraborty T, Das GC. Proteins of the nuclear factor-1 family act as an activator of the late promoter in human polyomavirus BK in vitro. J Gen Virol. (1991) 72:1935–42. 10.1099/0022-1317-72-8-1935 [DOI] [PubMed] [Google Scholar]
- 27.Prives C, Murakami Y, Kern FG, Folk W, Basilico C, Hurwitz J. DNA sequence requirements for replication of polyomavirus DNA in vivo and in vitro. Mol Cell Biol. (1987) 7:3694–704. 10.1128/mcb.7.10.3694-3704.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Draft enhancer element of SC-YB19 (in red box).
(A) 18-nt deletion in the enhance element at nucleotide position 164–182 of the SC-YB19; (B) Result of the ABI sequencing for SC-YB19.
Nucleotide sequences alignments between SC-YB19 and SD18, WF-GM01, QDJM01, AY672646, AF118150, APV-7, AF241168, AF241169, AF241170, M20775, and NC004764.
Multiple sequence alignment of complete sequences in BFDV strains.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.


