Abstract
One hundred twenty Salmonella enterica serotype Typhimurium strains, including 103 isolates from cattle gathered between 1977 and 1999 in the prefecture located on the northern-most island of Japan, were analyzed by using fluorescent amplified-fragment length polymorphism (FAFLP) and pulsed-field gel electrophoresis (PFGE) to examine the genotypic basis of the epidemic. Among these strains, there were 17 FAFLP profiles that formed four distinct clusters (A, B, C, and D). Isolates that belonged to cluster A have become increasingly common since 1992 with the increase of bovine salmonellosis caused by serotype Typhimurium. PFGE resolved 25 banding patterns that formed three distinct clusters (I, II, and III). All the isolates that belonged to FAFLP cluster A, in which all the strains of definitive phage type 104 examined were included, were grouped into PFGE cluster I. Taken together, these results indicate that clonal exchange of serotype Typhimurium has taken place since 1992, and they show a remarkable degree of homogeneity at a molecular level among contemporary isolates from cattle in this region. Moreover, we have sequenced two kinds of FAFLP markers, 142-bp and 132-bp fragments, which were identified as a polymorphic marker of strains that belonged to clusters A and C, respectively. The sequence of the 142-bp fragment shows homology with a segment of P22 phage, and that of the 132-bp fragment shows homology with a segment of traG, which is an F plasmid conjugation gene. FAFLP is apparently as well suited for epidemiological typing of serotype Typhimurium as is PFGE, and FAFLP can provide a source of molecular markers useful for studies of genetic variation in natural populations of serotype Typhimurium.
Salmonella infections in livestock have been a concern for both animal and human health. In particular, a common serotype causing salmonellosis in humans is Salmonella enterica serotype Typhimurium, a globally distributed zoonotic serotype that is common in both cattle and poultry. In order to study the epidemiology of its outbreaks and determine the source of contamination so that a recurrence can be avoided, detailed characterization is necessary. Although the majority of outbreaks in livestock are caused by a select number of serotypes, serotyping is not an adequate method for determination of the source of contamination during an outbreak. One subtyping method for epidemiological investigations of human and animal salmonellosis outbreaks is phage typing (3), which discriminates phenotypically at the intraserotype level. However, phage typing requires access to special reagents and a specialized laboratory and fails to reflect evolutionary relationships of bacterial strains. In the last decade, with the development of new techniques in molecular biology techniques, new approaches have become available. Widely used are plasmid analysis (29, 39), chromosomal fingerprinting by Southern hybridization (12, 16, 31, 36, 37), and macrorestriction analysis of chromosomal DNA by pulsed-field gel electrophoresis (PFGE) (4). PFGE is currently the method of choice to discriminate between strains on the DNA level (4, 31, 38). However, this technique is difficult to standardize among laboratories.
Recently, a novel high-resolution technique has been introduced for whole-genome analysis: amplified-fragment length polymorphism (AFLP) (21, 34). This technique is based on the selective amplification of genomic restriction fragments by PCR in order to generate fingerprinting patterns consisting of large numbers of bands. As originally proposed, AFLP used radioactively labeled primers for the PCR amplification of small genomic fragments defined by known restriction sites and adapters. Several bacterial genera have been studied by using radioactive AFLP (18, 23, 25). In the case of Salmonella, in 1998 Aarts and colleagues analyzed 78 Salmonella strains comprising 62 different serotypes by using AFLP analysis and showed that the patterns were specific for serotypes and in some cases even for strains (1). Recently, AFLP analyses with fluorescently labeled primer (FAFLP) have been reported for the molecular epidemiological investigation of Streptococcus pyogenes (7, 8), Escherichia coli (5, 6, 19), Listeria monocytogenes (2), Mycoplasma species (24), Staphylococcus aureus (15, 17), and Mycobacterium tuberculosis (14). An automated sequencer using a genetic analysis system along with in-lane size standards can automatically analyze the fragments generated by FAFLP. This enables the standardization of fragment sizes and facilitates the identification of polymorphic bands. In 2000, Lindstedt and coworkers performed FAFLP with Salmonella comprising seven different serotypes and reported that the FAFLP method showed a discriminatory power equal to that of PFGE (26).
In the present study, we have used FAFLP analysis for the molecular epidemiological investigation of serotype Typhimurium isolated from cattle and compared the results with those obtained using PFGE. Surveillance program data show that the incidence of salmonellosis in cattle in the prefecture located on the northernmost island of Japan has increased continuously since 1992, with cases stabilizing and declining after 1995. Both FAFLP and PFGE analyses in this study showed clonal propagation of serotype Typhimurium isolates from cattle after the epidemic of 1992. Furthermore, we determined the nucleotide sequence of the polymorphic fragment that is an FAFLP marker specific for the epidemic strain.
MATERIALS AND METHODS
Bacterial strains.
The 120 serotype Typhimurium strains used in this study are listed in Table 1. The majority of the collected strains reported here were isolated from diseased cattle at local livestock Animal Hygiene Centers by local public employees in the period 1977 to 1999 in the prefecture located in the northern-most island of Japan. This study also included 12 serotype Typhimurium definitive phage type 104 (DT104) strains (33) of animal origin (9 from cattle, 1 from pigeon, 1 from chicken, and 1 from crow) and five laboratory strains (NCTC73, NCTC9324, LT2, L719, and L767).
TABLE 1.
Origin and characterization of serotype Typhimurium strains used in this study
| Strain | Animal | Sourcea | Year | Drug(s) to which resistance was shown | FAFLP profile | PFGE profile |
|---|---|---|---|---|---|---|
| Field isolates | ||||||
| 478 | Cattle | UN | 1977 | SUL, STR, TET | B4 | IIh |
| NET19 | Cattle | UN | 1980 | SUL | B2 | IIe |
| NET20 | Cattle | UN | 1981 | AMP | B2 | IIc |
| KT1 | Cattle | UN | 1982 | STR, TET | B2 | IIf |
| #2 | Cattle | UN | 1983 | AMP, SUL, STR, TET | D2 | IIe |
| N48 | Cattle | Intestine | 1987 | AMP, SUL, STR, TET, CHL | C5 | IIIc |
| N49 | Cattle | Feces | 1987 | B3 | IIIc | |
| N50 | Cattle | Feces | 1987 | AMP, SUL, TET, CHL | C4 | IIIc |
| N54 | Cattle | Feces | 1988 | AMP, SUL, TET, CHL | C3 | IIId |
| NET21 | Cattle | Feces | 1988 | AMP, TET, CHL | C2 | IIIc |
| N57 | Cattle | Feces | 1989 | AMP, SUL, TET, CHL | C4 | IIIc |
| N59 | Cattle | Feces | 1989 | SUL, STR, TET | B5 | IIm |
| N60 | Cattle | Feces | 1989 | B3 | IIIc | |
| N61 | Cattle | Feces | 1989 | B3 | IIIc | |
| N62 | Cattle | Feces | 1989 | AMP, SUL | B3 | IIIc |
| NET25 | Cattle | Feces | 1989 | STR, TET | B1 | IIi |
| NET26 | Cattle | Feces | 1989 | STR, TET | B1 | IIi |
| NET29 | Cattle | Feces | 1989 | AMP, SUL, TET, CHL | C4 | IIIc |
| NET30 | Cattle | Feces | 1989 | AMP, TET, CHL | C1 | IIIa |
| NET31 | Cattle | Feces | 1989 | B2 | IIl | |
| KT2 | Cattle | Intestine | 1990 | SUL, CHL | B2 | IIn |
| N68 | Cattle | Feces | 1990 | AMP, SUL, TET, CHL | C1 | IIIf |
| NET32 | Cattle | Feces | 1990 | AMP, SUL, TET, CHL | C4 | IIIc |
| NET33 | Cattle | Feces | 1990 | AMP, TET, CHL | C2 | IIIf |
| NET35 | Cattle | Feces | 1990 | AMP, SUL, TET, CHL | C4 | IIIc |
| NET36 | Cattle | Feces | 1990 | AMP, SUL, TET, CHL | C4 | IIIc |
| KT3 | Cattle | Intestine | 1991 | AMP, SUL, STR, TET, CHL | C1 | IIIg |
| KT4 | Cattle | Feces | 1991 | AMP, SUL, STR, TET, CHL | C1 | IIIf |
| KT6 | Cattle | Feces | 1991 | AMP, SUL, STR, TET, CHL | C1 | IIIe |
| NET37 | Cattle | Feces | 1991 | AMP, SUL, STR, TET, CHL | B5 | IIn |
| NET38 | Cattle | Feces | 1991 | AMP, SUL, STR, TET, CHL | B5 | IIn |
| NET39 | Cattle | Feces | 1991 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| NET40 | Cattle | Intestine | 1991 | AMP, SUL, TET, CHL | C1 | IIIb |
| KT7 | Cattle | Feces | 1992 | AMP, SUL, TET, CHL | C1 | IIIf |
| N30 | Cattle | Feces | 1992 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| N34 | Cattle | Feces | 1992 | AMP, SUL, STR, TET, CHL | B5 | IIn |
| N36 | Cattle | Feces | 1992 | AMP, SUL, STR, TET, CHL | A1 | Ic |
| N77 | Cattle | Feces | 1992 | TET | B2 | IIa |
| N78 | Cattle | Feces | 1992 | B2 | IIo | |
| N79 | Cattle | Feces | 1992 | AMP, SUL, STR, TET, CHL | B5 | IIo |
| N81 | Cattle | Feces | 1992 | AMP, SUL, STR, TET, CHL | B5 | IIn |
| N82 | Cattle | Feces | 1992 | B2 | IIo | |
| NET48 | Cattle | Feces | 1992 | AMP, SUL, STR, TET, CHL | B5 | IIn |
| NET49 | Cattle | Feces | 1992 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| H6 | Cattle | Feces | 1993 | AMP, SUL, STR, TET, CHL | A4 | Ib |
| KT8 | Cattle | Feces | 1993 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT9 | Cattle | Feces | 1993 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET50 | Cattle | Feces | 1993 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| NET51 | Cattle | Feces | 1993 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| NET52 | Cattle | Feces | 1993 | B6 | IIo | |
| NET53 | Cattle | Feces | 1993 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| NET55 | Cattle | Feces | 1993 | B2 | IIk | |
| KT10 | Cattle | Feces | 1994 | SUL, STR | A2 | Ib |
| KT11 | Cattle | Feces | 1994 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT13 | Cattle | Feces | 1994 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT14 | Cattle | Feces | 1994 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET56 | Cattle | Feces | 1994 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET57 | Cattle | Feces | 1994 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| NET58 | Cattle | Feces | 1994 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| NET59 | Cattle | Feces | 1994 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET60 | Cattle | Feces | 1994 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET61 | Cattle | Feces | 1994 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET63 | Cattle | Feces | 1994 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT16 | Cattle | Feces | 1995 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT17 | Cattle | Feces | 1995 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT19 | Cattle | Feces | 1995 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET16 | Cattle | Feces | 1995 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| KT21 | Cattle | Feces | 1996 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT22 | Cattle | Feces | 1996 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT24 | Cattle | Feces | 1996 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET14 | Cattle | Feces | 1996 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT25 | Cattle | Feces | 1997 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT26 | Cattle | Feces | 1997 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT28 | Cattle | Feces | 1997 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET10 | Cattle | Feces | 1997 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET12 | Cattle | Feces | 1997 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET9 | Cattle | Feces | 1997 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT29 | Cattle | Feces | 1998 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT30 | Cattle | Feces | 1998 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT31 | Cattle | Feces | 1998 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| KT32 | Cattle | Feces | 1998 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| KT33 | Cattle | Feces | 1998 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET5 | Cattle | Feces | 1998 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| NET6 | Cattle | Feces | 1998 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| NET7 | Cattle | Feces | 1998 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET8 | Cattle | Feces | 1998 | AMP, SUL, STR, TET, CHL | A3 | Ib |
| KT34 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT35 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT36 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT37 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT38 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT39 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT40 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT41 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT42 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT43 | Cattle | Feces | 1999 | STR | A2 | Ia |
| KT44 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT45 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT46 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| KT47 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET2 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET3 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| NET64 | Cattle | Feces | 1999 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| DT104 strains | ||||||
| U1 | Cattle | UN | 1992 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| U2 | Cattle | UN | 1993 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| U3 | Cattle | UN | 1994 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| U4 | Cattle | UN | 1995 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| U5 | Cattle | UN | 1994 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| U6 | Cattle | UN | 1996 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| U7 | Cattle | UN | 1997 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| U8 | Cattle | UN | 1997 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| U9 | Cattle | UN | 1998 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| U17 | Pigeon | UN | 1996 | AMP, SUL, STR, TET, CHL | A2 | Ib |
| U18 | Chicken | UN | 1990 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| U20 | Crow | UN | 1998 | AMP, SUL, STR, TET, CHL | A1 | Ib |
| Other strains | ||||||
| NCTC73 | Human | UN | 1917 | SUL | B2 | IIg |
| NCTC9324 | UNa | UN | 1949 | B2 | IIg | |
| LT2 | UN | UN | UNa | B2 | IIj | |
| L719 | Cattle | UN | 1983 | AMP, SUL, STR, TET, CHL | B5 | IIb |
| L767 | Cattle | UN | 1983 | AMP, SUL, STR, TET | D1 | IId |
UN, unknown.
DNA isolation.
Strains were grown aerobically at 37°C in 5 ml of LB broth with shaking for 18 h. The genomic DNAs of the Salmonella strains were extracted from these cultures using an ISOPLANT kit (Nippon Gene Corp., Tokyo, Japan) according to the method recommended by the manufacturer. Plasmid DNA was isolated by the method described by Kado and Liu (22). The approximate molecular weight of the plasmid was determined in terms of mobility to plasmid, using known-molecular-weight plasmids from E. coli V517 (27).
Antimicrobial susceptibility testing.
The susceptibility of the isolates to antimicrobial agents was determined by disk diffusion tests on Mueller-Hinton agar (Difco, Detroit, Mich.). The following antibiotics were used: ampicillin (AMP), 10 μg/disk; chloramphenicol (CHL), 30 μg/disk; tetracycline (TET), 30 μg/disk; streptomycin (STR), 10 μg/disk; and sulfisoxazole (SUL), 250 μg/disk.
FAFLP.
FAFLP was carried out using an AFLP kit (PE Biosystems, Foster City, Calif.) according to the manufacturer's instructions. The enzymes EcoRI (New England Biolabs, Hertfordshire, United Kingdom [NEB]) and MseI (NEB) were used to digest approximately 10 ng of genomic DNA from each isolate and were ligated with EcoRI and MseI adapter using T4 DNA ligase (NEB). The ligated fragments were then diluted 20-fold and amplified by preselective primers, EcoRI primer (5′-GACTGCGTACCAATTC-3′) and MseI primer (5′-GATGAGTCCTGAGTAA-3′). Preselective PCR was performed as follows: 2 min at 72°C, followed by 20 cycles of denaturation at 94°C for 18 s, a 27-s annealing step at 56°C, and a 108-s extension step at 72°C. PCR was performed in a PE-2400 thermocycler (Perkin-Elmer Corp., Norwalk, Conn.).
The resulting preselective mixture was again diluted 20-fold and used as a template for selective amplification with EcoRI primer (EcoRI plus A) labeled with a blue fluorescent dye, 5-carboxyfluorescein and MseI primer (MseI plus A). Touchdown PCR cycling was used for amplifying the fragment with the following conditions: a 2-min denaturation step at 94°C (one cycle), followed by 30 cycles of denaturation at 94°C for 18 s, a 27-s annealing step, and a 108-s extension step at 72°C. The annealing temperature for the first cycle was 66°C; for the next nine cycles, the temperature was decreased by one degree at each cycle. The annealing temperature for the remaining 20 cycles was 56°C. This was followed by a final extension at 60°C. The amplification products were stored at −20°C.
FAFLP fragments were separated in 5% denaturing polyacrylamide gels (LongRanger; FMC Bioproducts, Rockland, Maine) on an ABI Prism 377 automated DNA sequencer (Perkin-Elmer Corp.). The sample (1.0 μl) was added to 2.0 μl of loading dye, which was a mixture containing 1.25 μl of formamide, 0.25 μl of loading solution (dextran blue in 50 mM EDTA), and 0.5 μl of the internal lane standard, GeneScan 500, labeled with the red fluorescent dye 6-carboxy-χ-rhodamine (PE Biosystems). The sample mixture was heated at 95°C for 2 min, cooled on ice, and immediately loaded onto the gel. Running buffer was 1× TBE (89 mM Tris, 89 mM boric acid, 2 mM EDTA), and electrophoresis conditions were 1.68 kV and 51°C for 8 h.
GeneScan collection software (PE Biosystems) was used to automatically size and quantify individual fragments by using the internal lane standards. Results were viewed in the form of gel image, electrophorogram, and tabular data, or a combination of all three. For the purpose of numerical analysis, background level was subtracted from the GeneScan-derived data using Genotyper software (PE Biosystems). The presence or absence of precisely sized fragments was ascertained, and these digital data were transferred to spreadsheets for further analysis. Pairwise comparisons were made between all strains in terms of the Dice coefficient (30) using in-house software. The distance matrix thus generated was used as input for the UPGMA (NEIGHBOR program of PHYLIP [9]).
PFGE.
PFGE was performed by clamped homogeneous electric field electrophoresis using a CHEFF DRII apparatus (Bio-Rad Laboratories, Hercules, Calif.). Genomic DNA was prepared as previously described elsewhere (31). Each strain was grown overnight at 37°C in LB broth. Cells were harvested by centrifugation for 10 min at 3,600 × g and resuspended in 0.5 ml of NT buffer (10 mM Tris-HCl [pH 7.5], 1 M NaCl). An aliquot (0.3 ml) of the suspension was transferred to a microcentrifuge tube, and cells were pelleted at 12,000 × g and washed twice in NT buffer. The cell suspension was mixed with an equal volume of 1.5% low-melting-point agarose (FMC Bioproducts) and allowed to solidify in a 100-μl plug mold (Bio-Rad Laboratories). The agarose plugs were incubated overnight at 55°C in 1 ml of lysis buffer (60 mM Tris-HCl [pH 7.5], 50 mM EDTA, 1.0% sodium-lauryl sarcosine, lysozyme [1 mg/ml], RNase [1 μg/ml], proteinase K [1 mg/ml]), washed twice for 30 min with TE buffer (10 mM Tris-HCl [pH 7.5], 0.1 mM EDTA) containing phenylmethylsulfonyl fluoride (1 mM), and then washed four times for 30 min in 1 ml of TE buffer. A slice of each plug was cut and incubated in 400 μl of restriction buffer containing 50 U of XbaI at 37°C for 2 h. The restricted DNA fragments were separated on pulsed-field-certified agarose (Bio-Rad Laboratories). Electrophoresis was done for 25 h at 14°C at 6V/cm in twofold-diluted TBE buffer with pulse times of 5 to 80 s. Lambda PFG DNA markers (NEB) were used as DNA size markers. The PFGE profiles were scanned and analyzed using the Taxotron package according to the instructions of the user's manual (Patrick A. D. Grimont, Institute Pasteur, Paris, France). This package is composed of the RestrictoScan, RestrictoTyper, Anderson, and Dendrograph programs. Lines and bands were detected with the RestrictoScan program. Fragment lengths were interpolated using the Spline algorithm (implemented with the RestrictoTyper software). The similarity index was calculated using the RestrictoTyper program with the fragment length error tolerance set at 4%. The single linkage was computed with the Anderson program, and a dendrogram was drawn using the Dendrograf program.
Sequencing.
FAFLP products of the 132-bp and 142-bp fragments yielded by the strains NET30 and H6, respectively, were eluted from a 5% acrylamide gel using the method described elsewhere (28). Eluted fragments were amplified again by PCR, using the selective primer pair as described above. The resulting PCR product was cloned to pCRII to give pCR132 and pCR142, respectively, using a TA cloning kit (Invitrogen Corp., San Diego, Calif.) by the method recommended by the manufacturer. The cloned PCR product was sequenced on an ABI 377 automatic sequencer using a BigDye Terminator kit (PE Biosystems) according to the manufacturer's instruction.
Hybridization experiments.
FAFLP products of the 132- and 142-bp fragments were amplified by PCR as described above and purified using a PCR product purification kit (Qiagen, Hilden, Germany). The purified fragments were labeled with digoxigenin (DIG)-11-dUTP by random priming using a DIG High Prime Labelling Kit (Boehringer GmbH, Mannheim, Germany) as described by the manufacturer. For Southern hybridization, plasmid DNA or cleaved genomic DNA was separated on a 0.8% agarose gel and transferred to a positive membrane (Boehringer) with a vacuum blotter (LKB Vac Gene; Pharmacia LKB Biotechnology, Uppsala, Sweden). Prehybridizations (>30 min) and hybridizations (>16 h) using Easy Hyb solution (Boehringer) under high-stringent conditions and detection of hybrids by means of enhanced chemiluminescence with anti-DIG-alkaline phosphatase and CSPD were carried out using a DIG Luminescent Detection kit (Boehringer), following the manufacturer's instructions. DIG-labeled marker III (Boehringer) was used as a DNA size marker. Hyper MP (Amersham International, Little Chalfont, United Kingdom) was exposed to membranes for 1 to 10 min at room temperature and developed in a Kodak X-Omat processor.
Nucleotide sequence accession numbers.
The nucleotide sequences of the 132- and 142-bp fragments determined in this study (see Fig. 4) are deposited with DDBJ under accession numbers AB047311 and AB047310, respectively.
FIG. 4.
Sequence analysis of cluster-specific fragments. (A) Sequence similarity between the 132-bp fragment (bp 14 to 120) and traG (bp 3981 to 4087; GenBank accession no. M5976). (B) Sequence similarity between the 142-bp fragment (bp 14 to 130) and P22 phage (bp 4868 to 4984; GenBank accession no. L06296). Asterisks represent identity to the corresponding nucleotides, and dashes represent missing nucleotides.
RESULTS
FAFLP analysis of serotype Typhimurium strains.
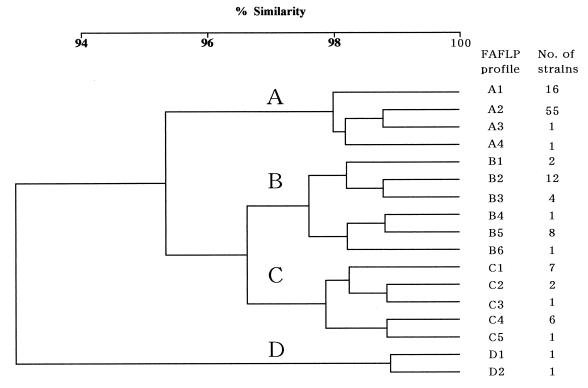
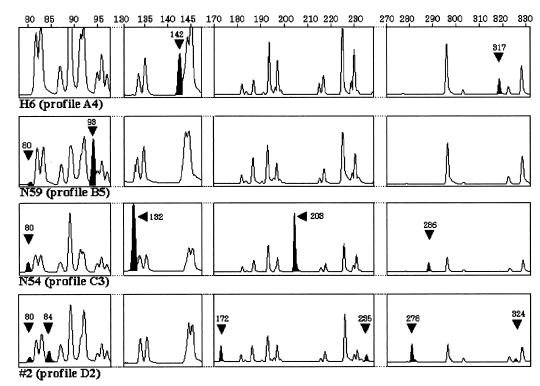
A total of 120 serotype Typhimurium strains were analyzed by the EcoRI plus A and MseI plus A FAFLP primer combination. FAFLP analysis generated 45 to 50 amplified fragments ranging in size from 80 to 430 bp and exhibited 12 polymorphic amplified fragments among them (Table 2). Seventeen profiles were detected among the 120 strains (Fig. 1). At a cutoff value of 96.5%, cluster analysis identified four clusters: cluster A (73 isolates), cluster B (28 isolates), cluster C (17 isolates), and cluster D (2 isolates). Within these clusters, four (A1 to A4), six (B1 to B6), five (C1 to C5) and two (D1 and D2) different profiles were detected (Fig. 1). Eight strains had unique FAFLP profiles (Fig. 1). The other 112 strains were assigned to nine profiles: 55 were assigned to profile A2, 16 were assigned to profile A1, 12 were assigned to profile B2, 8 were assigned profile B5, 7 were assigned to profile C1, 6 were assigned to profile C4, 4 were assigned to profile B3, and 2 each were assigned to profiles B1 and C2 (Fig. 1). Examples of the areas of polymorphism within FAFLP profiles derived by GeneScan analysis for four EcoRI plus A and MseI plus A amplifications are shown in Fig. 2. All four profiles in FAFLP cluster A contained a characteristic fragment of 142 bp and lacked the 80-bp fragment found in other FAFLP profiles (Table 2; Fig. 2). A fragment of 142 bp was also found in the profile B1 that contained a fragment of 80 bp, whereas the 142-bp fragment was absent in the other profiles in cluster B (Table 2; Fig. 2). Five profiles in cluster C contained the 132-bp fragment, which was absent from the profiles in the other clusters (Table 2; Fig. 2). Four fragments of 172, 235, 278, and 324 bp in profiles D1 and D2 are absent from the other profiles (Table 2; Fig. 2).
TABLE 2.
Polymorphisms of FAFLP profiles exhibited by serotype Typhimurium strains
| FAFLP profile | Presence of polymorphic fragments of size (bp)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 80 | 84 | 93 | 132 | 142 | 172 | 203 | 235 | 278 | 286 | 317 | 324 | |
| A1 | − | + | − | − | + | − | − | − | − | − | − | − |
| A2 | − | − | − | − | + | − | − | − | − | − | − | − |
| A3 | − | − | − | − | + | − | + | − | − | − | − | − |
| A4 | − | − | − | − | + | − | − | − | − | − | + | − |
| B1 | + | − | − | − | + | − | − | − | − | − | − | − |
| B2 | + | − | − | − | − | − | − | − | − | − | − | − |
| B3 | + | − | − | − | − | − | − | − | − | + | − | − |
| B4 | + | + | + | − | − | − | − | − | − | − | − | − |
| B5 | + | − | + | − | − | − | − | − | − | − | − | − |
| B6 | + | + | − | − | − | − | − | − | − | − | − | − |
| C1 | + | − | − | + | − | − | − | − | − | − | − | − |
| C2 | + | − | − | + | − | − | + | − | − | − | − | − |
| C3 | + | − | − | + | − | − | + | − | − | + | − | − |
| C4 | + | − | − | + | − | − | − | − | − | + | − | − |
| C5 | + | − | + | + | − | − | − | − | − | + | − | − |
| D1 | + | − | − | − | − | + | − | + | + | − | − | + |
| D2 | + | + | − | − | − | + | − | + | + | − | − | + |
The presence or absence of differential fragments is shown. +, fragment is characteristically present in this FAFLP profile; −, fragment is characteristically absent from this FAFLP profile.
FIG. 1.
Dendrogram showing the results of cluster analysis on the basis of FAFLP fingerprintings of 120 serotype Typhimurium strains. The dendrogram was constructed by using UPGMA clustering on a matrix based on the Dice coefficient.
FIG. 2.
GeneScan 2.1 software-derived electropherograms showing examples of areas of polymorphism within FAFLP profiles for EcoRI plus A and MseI plus T amplifications of serotype Typhimurium genomes. Segments of FAFLP profiles obtained from serotype Typhimurium H6 (profile A4), N59 (profile B5), N54 (profile C3), and #2 (profile D2) are represented. The fragment size scale (base pairs) is indicated above each segment. The solid arrowheads and peaks indicate a fragment characteristic of that profile (sizes are indicated in base pairs).
Genetic diversity of serotype Typhimurium strains as defined by PFGE.
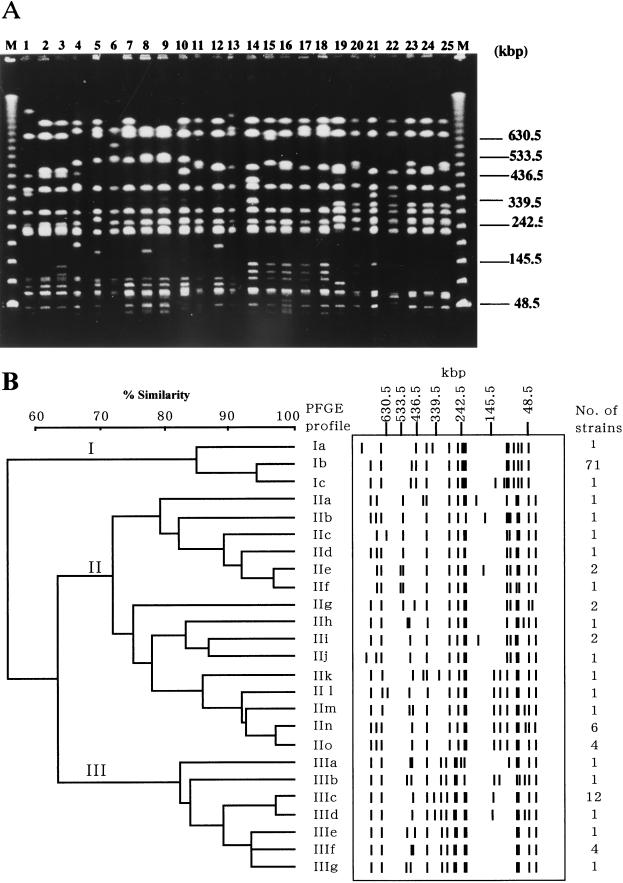
All the strains were analyzed by PFGE, and the results obtained were compared with those of FAFLP. Digestion of serotype Typhimurium with XbaI gave 13 to 17 fragments with sizes between 40 and 800 kbp (Fig. 3). Twenty-five PFGE profiles after digestion of DNA with XbaI were observed among the 120 strains and with a 72% level of similarity; three clusters (I, II, and III) were found (Fig. 3). Comparative data for PFGE and FAFLP analyses for the 120 strains are presented in Table 1. All of the 73 strains which belonged to FAFLP cluster A could be grouped into PFGE cluster I. Except for four strains (N49, N60, N61, and N62) yielding FAFLP profile B3, all the strains belonging to FAFLP cluster B fell into PFGE cluster II. Four strains yielding FAFLP B3 profile, together with all 17 strains grouped into FAFLP cluster C, were classified into PFGE cluster III. The four strains yielding FAFLP B3 profile lacked plasmid, while the other isolates belonging to PFGE cluster III had a large plasmid (data not shown). Two strains belonging to FAFLP cluster D had PFGE profiles IId and IIe.
FIG. 3.
(A) PFGE analysis of XbaI-digested genomic DNA from serotype Typhimurium strains. Lanes (with designated PFGE profiles in parentheses [Table 1]): M, lambda 48.5-kbp ladder; 1, KT43 (Ia); 2, NET2 (Ib); 3, N36 (Ic); 4, N77 (IIa); 5, L719 (IIb); 6, NET20 (IIc); 7, L767 (IId); 8, #2 (IIe); 9, KT1 (IIf); 10, NCTC9324 (IIg); 11, 478 (IIh); 12, NET25 (IIi); 13, LT2 (IIj); 14, NET55 (IIk); 15, NET31 (III); 16, N59 (IIm); 17, NET37 (IIn); 18, N78 (IIo); 19, NET30 (IIIa); 20, NET40 (IIIb); 21, N50 (IIIc); 22, N54 (IIId); 23, KT6 (IIIe); 24, N68 (IIIf); 25, KT3 (IIIg). (B) Dendrogram and schematic representation of PFGE fragments following XbaI macrorestriction of serotype Typhimurium genomic DNA. Similarity analysis was performed using the Dice coefficient, and clustering was by UPGMA.
Antimicrobial resistance.
The antibiotic resistance profiles of 120 serotype Typhimurium strains are listed in Table 1. Overall, 91.7% of 120 strains were resistant to at least one antibiotic. The antibiotic to which resistance was most frequently detected was TET (85.8%), followed by SUL (84.2%), AMP (82.5%), CHL (80.0%), and STR (75.8%). Most of the strains belonging to FAFLP cluster A, including DT104 strains, showed antibiotic resistance to AMP, SUL, STR, TET, and CHL, except for KT10 (SUL and STR) and KT43 (STR).
Comparison of isolates associated with an epidemic.
The incidence of bovine salmonellosis caused by serotype Typhimurium increased since 1992 in the prefecture located in the northernmost island of Japan, and a prolonged epidemic continued until 1996. To compare the isolates associated with the epidemic, the FAFLP profiles of 103 strains isolated from cattle between 1977 and 1999 in this area were examined. The FAFLP results are summarized in Table 1. Although before 1992, with one exception (NET39), all the isolates were grouped into cluster B, C, or D, the number of isolates that belong to cluster A has increased dramatically since 1992. This genotype has become common since 1994. Furthermore, all the DT104 strains examined belonged to FAFLP cluster A, which corresponds to PFGE cluster I (Table 1).
Sequence analysis of 132- and 142-bp fragments.
In order to analyze cluster-specific FAFLP fragments of 132 and 142 bp, we decided to clone them from strain NET30 and H6, respectively, and sequence the cloned fragments. A search for homologies to the sequence of the 132-bp fragment in the database revealed that this sequence was 97% identical to a 107-bp segment of traG that is an F plasmid conjugation gene (10) (Fig. 4A). The sequence of the 142-bp fragment was 86% identical to the 117-bp segment of the P22 phage (42) (Fig. 4B).
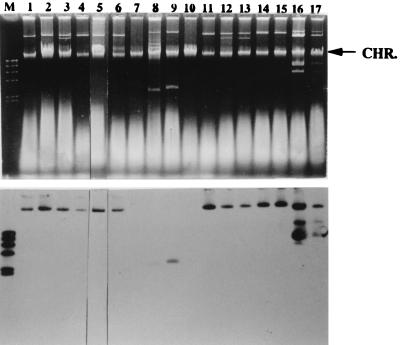
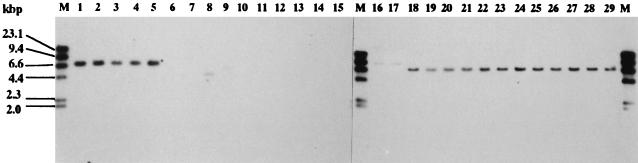
We tested the location and prevalence of a homologous sequence with that of 132-bp or 142-bp fragments in serotype Typhimurium by using Southern hybridization. For analysis of hybridization, 17 strains were selected from the four FAFLP clusters, and chromosome or plasmid DNA was hybridized with these fragments. Hybridization with the 132-bp fragment probe demonstrated that a corresponding locus was located on the plasmid, ranging from 3.5 to 100 kb (Fig. 5). This fragment hybridized to the plasmid not only from the strains containing the 132-bp fragment but also from strains lacking this fragment (Fig. 5). Hybridization signals were observed in 90-kb plasmids of all the strains of DT104 (data not shown). By contrast, since the 142-bp fragment did not hybridize to plasmid DNA, the sequence corresponding to the 142-bp fragment appears to be located on a chromosome (data not shown). As shown in Fig. 6, when the 142-bp fragment was used as a probe, HindIII-digested chromosomal DNA from all the strains of DT104, strain NET57 (profile A1), NET2 (profile A2), NET8 (profile A3), H6 (profile A4), and NET25 (profile B1), revealed hybridizing signals at 7.0 kb. In addition, weak positive signals were also obtained with a 5.5-kb HindIII fragment of strain 478 (profile B4) and a 9.0-kb HindIII fragment of both L767 (profile D1) and #2 (profile D2), but not with DNA of the other strains belonging to FAFLP cluster B or C (Fig. 6).
FIG. 5.
Hybridization of 132-bp fragment to plasmids in serotype Typhimurium. (Top) Visualization of Salmonella plasmids by agarose gel electrophoresis. Lanes (with designated FAFLP profiles in parentheses [Table 1]): M, molecular weight standards (lambda DNA digested with HindIII); 1, NET57 (A1); 2, NET2 (A2); 3, NET8 (A3); 4, H6 (A4); 5, NET25 (B1); 6, NET20 (B2); 7, N49 (B3); 8, 478 (B4); 9, N81 (B5); 10, NET52 (B6); 11, NET30 (C1); 12, NET21 (C2); 13, N54 (C3); 14, N57 (C4); 15, N48 (C5); 16, L767 (D1); 17, #2 (D2). Chromosomal DNA bands (arrow) are seen in each lane. (Bottom) Southern blot analysis of the plasmid in serotype Typhimurium, using a 132-bp fragment probe.
FIG. 6.
Hybridization of 142-bp fragment to HindIII digest of serotype Typhimurium chromosomal DNA. A Southern blot was made with HindIII and hybridized with the 142-bp fragment. Lanes (with designated FAFLP profiles in parentheses [Table 1]): M, molecular weight standards (lambda DNA digested with HindIII); 1, NET57 (A1); 2, NET2 (A2); 3, NET8 (A3); 4, H6 (A4); 5, NET25 (B1); 6, NET20 (B2); 7, N49 (B3); 8, 478 (B4); 9, N81 (B5); 10, NET52 (B6); 11, NET30 (C1); 12, NET21 (C2); 13, N54 (C3); 14, N57 (C4); 15, N48 (C5); 16, L767 (D1); 17, #2 (D2); 18, U1 (A2); 19, U2 (A2); 20, U3 (A2); 21, U4 (A2); 22, U5 (A2); 23, U6 (A1); 24, U7 (A2); 25, U8 (A2); 26, U9 (A1); 27, U17 (A2); 28, U18 (A1); 29, U20 (A1).
DISCUSSION
In 1992, we observed an apparent increase in the incidence of bovine salmonellosis caused by serotype Typhimurium in the prefecture located in the northernmost island of Japan, where dairy farming is one of the main agroindustries. Surveillance program data showed that the incidence was stable until 1991 but that the number increased during the next 3 years, with cases stabilizing and even declining after 1995. The reason for this increment is unclear, and further epidemiological investigation is needed in order to examine the genotypic basis of the epidemic.
In this study, we used a recently developed genotyping method, FAFLP, which is based on selective amplification of restriction fragments of chromosomal DNA, for the genetic typing of serotype Typhimurium strains isolated from cattle. Among 120 strains including 114 isolates from cattle, 17 FAFLP profiles and four clusters (A, B, C, and D) were identified. Before 1992, only one isolate was grouped into FAFLP cluster A, and the other strains were grouped into cluster B, C, or D. The number of strains which belonged to cluster A has increased since 1992, and all the isolates fell into a single cluster, A, since 1994. These results show that the isolates that belonged to clusters B, C, and D had been circulating in this area until at least 1993, whereas isolates that belonged to cluster A seem to have spread since 1992. Thus, isolates belonging to FAFLP cluster A seem to be responsible for prolonging the epidemic in this area. With respect to the XbaI macrorestriction profiles detected by PFGE, 25 distinct profiles were observed among the 120 strains. Three groups were identified with a similarity of 72%. Strains that were grouped into FAFLP clusters A and C belonged to PFGE clusters I and III, respectively, and with the exception of strains showing FAFLP profile B3, strains that were grouped into FAFLP cluster B or D belonged to PFGE cluster II. Therefore, overall, the data generated by FAFLP analysis gave results almost consistent with those of PFGE, and both methods were able to distinguish between a preepidemic lineage of serotype Typhimurium and the lineage of isolates which caused the epidemic.
An increase in the occurrence of antibiotic resistance in Salmonella isolated from food animals has been observed in several countries, and in some cases the emergence of resistance has been caused by the clonal spread of multiresistant strains (11, 32). Recently, multiresistant serotype Typhimurium DT104 has been reported with increasing frequency in several countries worldwide (13, 40, 41). Sameshima et al. (33) reported that DT104 strains have existed in Japanese livestock since 1990 and that 36 of 68 isolates which exhibited resistance to five or more antimicrobials were identified as DT104. These isolates are resistant to AMP, CHL, STR, SUL, and TET but have also shown a tendency to acquire resistance to additional antimicrobial agents. In this study, we showed that 76 of 78 strains belonging to FAFLP cluster A, in which most of the contemporary isolates were included, have the same antibiotic resistance pattern (AMP, SUL, STR, TET, and CHL). Furthermore, all the DT104 strains examined belonged to FAFLP cluster A. Although we did not determine the phage type of the isolates from cattle, these results indicated that clones genetically similar to DT104 have been widely spread in this area. In Japan, only four DT104-related outbreaks in humans were reported (20); however, fortunately, a human case caused by DT104 has not been reported in the northernmost island of Japan. Further surveillance of serotype Typhimurium is required to examine the relationship between human and animal origin. The FAFLP method might be a useful tool for this surveillance.
Using FAFLP, we identified a 142-bp fragment which was one of the polymorphic markers of the strains belonging to FAFLP cluster A. A Southern hybridization study revealed that the 142-bp fragment originated from a chromosome and hybridized to a common band of the 7.0-kb HindIII fragment present in all isolates which belonged to FAFLP cluster A. The sequence of the 142-bp fragment was highly similar to the segment of P22 phage (42). Recent reports have demonstrated that antimicrobial resistance genes are clustered in the genome of serotype Typhimurium DT104 and that these genes can be efficiently transduced by P22-like phages (35). The DT104 strain may carry a P22-like prophage in its genome, and such a prophage may confer horizontal transfer and further spread of resistance genes. The usefulness of the 142-bp fragment as a marker to detect DT104 strains needs to be confirmed in prospective studies. However, on the contrary, a 132-bp fragment originated from plasmid. Although the fragment of 132 bp is specific for FAFLP cluster C, the hybridization study showed that the 132-bp fragment hybridized with a plasmid not only from isolates that are grouped into FAFLP cluster C but also from isolates that are grouped into the other clusters, suggesting that a homologous sequence with that of the 132-bp fragment is conserved among plasmids in serotype Typhimurium strains. All the strains belonging to FAFLP cluster C are multidrug resistant, and plasmids of these strains were conjugative with R plasmid (data not shown). Since the sequence of the 132-bp fragment showed similarity with traG, which is an F plasmid conjugation gene (10), this fragment might be a marker of R plasmid.
In the present study, we examined whether FAFLP is applicable to the epidemiological study of serotype Typhimurium. Our results indicated that FAFLP has almost the same discriminatory ability as that of PFGE, which is now considered to be one of the more powerful tools for molecular subtyping of serotype Typhimurium. Moreover, when used with another pair of primers the combination of these results might increase the discrimination power of FAFLP. The sizing of the fragments by FAFLP with the use of an internal standard was precise, having a resolution of ±1 bp (data not shown). The internal standard also allows us to directly compare fingerprint patterns from different runs, and FAFLP profiles are suitable for rapid electronic transmission for interlaboratory comparisons. Therefore, FAFLP profiles are well suited for constructing a database for later comparisons and epidemiological analyses. Furthermore, as we were able to characterize cluster-specific fragments of 132 and 142 bp in this study, FAFLP may provide a rich source of molecular markers which are useful for studies of the epidemiology, pathogenicity, and genetic variation in natural populations of serotype Typhimurium.
ACKNOWLEDGMENTS
We thank the staff of the Hokkaido local government for kindly providing serotype Typhimurium strains isolated from cattle.
This project was funded by the Ministry of Agriculture, Forestry, and Fisheries of Japan.
REFERENCES
- 1.Aarts H J, van Lith L A, Keijer J. High-resolution genotyping of Salmonella strains by AFLP-fingerprinting. Lett Appl Microbiol. 1998;26:131–135. doi: 10.1046/j.1472-765x.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- 2.Aarts H J, Hakemulder L E, Van Hoef A M. Genomic typing of Listeria monocytogenes strains by automated laser fluorescence analysis of amplified fragment length polymorphism fingerprint patterns. Int J Food Microbiol. 1999;49:95–102. doi: 10.1016/s0168-1605(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 3.Anderson E S, Ward L R, de Saxe M J, de Sa J D. Bacteriophage-typing designations of Salmonella typhimurium. J Hyg. 1977;78:297–300. doi: 10.1017/s0022172400056187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbeit R D. Laboratory procedures for the epidemiologic analysis of microorganisms. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 190–208. [Google Scholar]
- 5.Arnold C, Metherell L, Willshaw G, Maggs A, Stanley J. Predictive fluorescent amplified-fragment length polymorphism analysis of Escherichia coli: high-resolution typing method with phylogenetic significance. J Clin Microbiol. 1999;37:1274–1279. doi: 10.1128/jcm.37.5.1274-1279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold C, Metherell L, Clewley J P, Stanley J. Predictive modelling of fluorescent AFLP: a new approach to the molecular epidemiology of E. coli. Res Microbiol. 1999;150:33–44. doi: 10.1016/s0923-2508(99)80044-8. [DOI] [PubMed] [Google Scholar]
- 7.Desai M, Efstratiou A, George R, Stanley J. High-resolution genotyping of Streptococcus pyogenes serotype M1 isolates by fluorescent amplified-fragment length polymorphism analysis. J Clin Microbiol. 1999;37:1948–1952. doi: 10.1128/jcm.37.6.1948-1952.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai M, Tanna A, Wall R, Efstratiou A, George R, Stanley J. Fluorescent amplified-fragment length polymorphism analysis of an outbreak of group A streptococcal invasive disease. J Clin Microbiol. 1998;36:3133–3137. doi: 10.1128/jcm.36.11.3133-3137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felsenstein J. PHYLIP-phylogeny inference package. Cladistics. 1989;5:164–166. [Google Scholar]
- 10.Firth N, Skurray R. Characterization of the F plasmid bifunctional conjugation gene, traG. Mol Gen Genet. 1992;232:145–153. doi: 10.1007/BF00299147. [DOI] [PubMed] [Google Scholar]
- 11.Frost J A, Rowe B, Ward L R, Threlfall E J. Characterization of resistance plasmids and carried phages in an epidemic clone of multi-resistant Salmonella typhimurium in India. J Hyg Camb. 1982;88:193–204. doi: 10.1017/s0022172400070066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibert I, Barbe J, Casadesus J. Distribution of insertion sequence IS200 in Salmonella and Shigella. J Gen Microbiol. 1990;136:2555–2560. doi: 10.1099/00221287-136-12-2555. [DOI] [PubMed] [Google Scholar]
- 13.Glynn M K, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo F J. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N Engl J Med. 1998;338:1333–1338. doi: 10.1056/NEJM199805073381901. [DOI] [PubMed] [Google Scholar]
- 14.Goulding J N, Stanley J, Saunders N, Arnold C. Genome-sequence-based fluorescent amplified-fragment length polymorphism analysis of Mycobacterium tuberculosis. J Clin Microbiol. 2000;38:1121–1126. doi: 10.1128/jcm.38.3.1121-1126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grady R, Desai M, O'Neill G, Cookson B, Stanley J. Genotyping of epidemic methicillin-resistant Staphylococcus aureus phage type 15 isolates by fluorescent amplified-fragment length polymorphism analysis. J Clin Microbiol. 1999;37:3198–3203. doi: 10.1128/jcm.37.10.3198-3203.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimont F, Grimont P A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986;137B:165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- 17.Hookey J V, Edwards V, Patel S, Richardson J F, Cookson B D. Use of fluorescent amplified fragment length polymorphism (fAELP) to characterise methicillin-resistant Staphylococcus aureus. J Microbiol Methods. 1999;37:7–15. doi: 10.1016/s0167-7012(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 18.Huys G, Coopman R, Janssen P, Kersters K. High-resolution genotypic analysis of the genus Aeromonas by AFLP fingerprinting. Int J Syst Bacteriol. 1996;46:572–580. doi: 10.1099/00207713-46-2-572. [DOI] [PubMed] [Google Scholar]
- 19.Iyoda S, Wada A, Weller J, Flood S J, Schreiber E, Tucker B, Watanabe H. Evaluation of AFLP, a high-resolution DNA fingerprinting method, as a tool for molecular subtyping of enterohemorrhagic Escherichia coli O157:H7 isolates. Microbiol Immunol. 1999;43:803–806. doi: 10.1111/j.1348-0421.1999.tb02473.x. [DOI] [PubMed] [Google Scholar]
- 20.Izumiya H, Tamura K, Terajima J, Watanabe H. Salmonella enterica serovar Typhimurium phage type DT104 and other multi-drug resistant strains in Japan. Jpn J Infect Dis. 1999;52:133. [PubMed] [Google Scholar]
- 21.Janssen P, Coopman R, Huys G, Swings J, Bleeker M, Vos P, Zabeau M, Kersters K. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology. 1996;142:1881–1893. doi: 10.1099/13500872-142-7-1881. [DOI] [PubMed] [Google Scholar]
- 22.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keim P, Kalif A, Schupp J, Hill K, Travis S E, Richmond K, Adair D M, Hugh-Jones M, Kuske C R, Jackson P. Molecular evolution and diversity in Bacillus anthracis as detected by amplified fragment length polymorphism markers. J Bacteriol. 1997;179:818–824. doi: 10.1128/jb.179.3.818-824.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokotovic B, Friis N F, Jensen J S, Ahrens P. Amplified-fragment length polymorphism fingerprinting of Mycoplasma species. J Clin Microbiol. 1999;37:3300–3307. doi: 10.1128/jcm.37.10.3300-3307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin J J, Kuo J, Ma J. A PCR-based DNA fingerprinting technique: AFLP for molecular typing of bacteria. Nucleic Acids Res. 1996;24:3649–3650. doi: 10.1093/nar/24.18.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindstedt B A, Heir E, Vardund T, Kapperud G. Fluorescent amplified-fragment length polymorphism genotyping of Salmonella enterica subsp. enterica serovars and comparison with pulsed-field gel electrophoresis typing. J Clin Microbiol. 2000;38:1623–1627. doi: 10.1128/jcm.38.4.1623-1627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macrina F L, Kopecko D K, Jones K R, Ayers D J, McCowen S M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978;1:417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- 28.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 29.Nakamura M, Sato S, Ohya T, Suzuki S, Ikeda S. Plasmid profile analysis in epidemiological studies of animal Salmonella typhimurium infection in Japan. J Clin Microbiol. 1986;23:360–365. doi: 10.1128/jcm.23.2.360-365.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nei M, Li W-H. Mathematical model for studying genetic variations in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen J E, Skov M N, Threlfall E J, Brown D J. Clonal lines of Salmonella enterica serotype Enteritidis documented by IS200-, ribo-, pulsed-field gel electrophoresis and RFLP typing. J Med Microbiol. 1994;40:15–22. doi: 10.1099/00222615-40-1-15. [DOI] [PubMed] [Google Scholar]
- 32.Rowe B, Threlfall E J, Ward L R, Ashley A S. International spread of multiresistent strains of Salmonella typhimurium phage types 204 and 193 from Britain to Europe. Vet Rec. 1979;105:468–469. doi: 10.1136/vr.105.20.468. [DOI] [PubMed] [Google Scholar]
- 33.Sameshima T, Akiba M, Izumiya H, Terajima J, Tamura K, Watanabe H, Nakazawa M. Salmonella Typhimurium DT104 from livestock in Japan. Jpn J Infect Dis. 2000;53:15–16. [PubMed] [Google Scholar]
- 34.Savelkoul P H, Aarts H J, de Haas J, Dijkshoorn L, Duim B, Otsen M, Rademaker J L, Schouls L, Lenstra J A. Amplified-fragment length polymorphism analysis: the state of an art. J Clin Microbiol. 1999;37:3083–3091. doi: 10.1128/jcm.37.10.3083-3091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmieger H, Schicklmaier P. Transduction of multiple drug resistance of Salmonella enterica serovar DT104. FEMS Microbiol Lett. 1999;170:251–256. doi: 10.1111/j.1574-6968.1999.tb13381.x. [DOI] [PubMed] [Google Scholar]
- 36.Stanley J, Baquar N, Threlfall E J. Genotypes and phylogenetic relationships of Salmonella typhimurium are defined by molecular fingerprinting of IS200 and 16S rrn loci. J Gen Microbiol. 1993;139:1133–1140. doi: 10.1099/00221287-139-6-1133. [DOI] [PubMed] [Google Scholar]
- 37.Stull T L, LiPuma J J, Edlind T D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988;157:280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- 38.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Threlfall E J, Frost J A, Ward L R, Rowe B. Plasmid profile typing can be used to subdivide phage-type 49 of Salmonella typhimurium in outbreak investigations. Epidemiol Infect. 1990;104:243–251. doi: 10.1017/s0950268800059410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Threlfall E J, Frost J A, Ward L R, Rowe B. Epidemic in cattle and humans of Salmonella typhimurium DT 104 with chromosomally integrated multiple drug resistance. Vet Rec. 1994;134:577. doi: 10.1136/vr.134.22.577. [DOI] [PubMed] [Google Scholar]
- 41.Threlfall E J, Frost J A, Ward L R, Rowe B. Increasing spectrum of resistance in multiresistant Salmonella typhimurium. Lancet. 1996;347:1053–1054. doi: 10.1016/s0140-6736(96)90199-3. [DOI] [PubMed] [Google Scholar]
- 42.Wulff D L, Ho Y S, Powers S, Rosenberg M. The int genes of bacteriophage P22 and lambda are regulated by different mechanisms. Mol Microbiol. 1993;9:261–271. doi: 10.1111/j.1365-2958.1993.tb01688.x. [DOI] [PubMed] [Google Scholar]