Abstract
Background:
Granulomatous inflammation is found in a wide range of diseases, and most commonly associated with sarcoidosis and tuberculosis. Granulomas are pathologically classified into two main groups; necrotic and non-necrotic.
Objectives:
The aim of this study was to evaluate the radiological, laboratory, and pathological findings of a large patient population with granuloma in biopsy samples, to determine the final diagnostic distribution.
Methods:
This study was designed as a retrospective, descriptive, observational, cross-sectional study. It was conducted in patients with granulomatous inflammation detected in lung, pleural, mediastinal, hilar, and/or peripheral lymph node biopsies. Demographic information, radiological, microbiological, and laboratory results of the patients were obtained via the information processing system of the hospital. The diagnoses recorded were re-evaluated by at least two experienced clinicians and the final diagnosis distributions were made.
Results:
A total of 392 patients were included in the study. Non-necrotizing inflammation was detected in 268 patients, and necrotizing granulomatous inflammation was found in 124 patients. The most common cause of non-necrotizing inflammation was sarcoidosis, and tuberculosis in the case of necrotizing inflammation. A total of 77.2% of sarcoidosis patients had non-necrotizing inflammation and 54.3% of the tuberculosis patients had necrotizing inflammation. In the diagnosis distribution of granulomatous inflammation sarcoidosis, mycobacterium infections (especially tuberculosis), sarcoid reaction due to malignancy, pneumoconiosis, granulomatosis with polyangiitis and hypersensitivity pneumonitis were detected, respectively. A total of 392 patients were diagnosed with 13 different diseases. In 15 patients (3.8%) no specific diagnosis could be made.
Conclusions:
The diagnosis of granulomatous inflammation detected in biopsy samples is common for clinicians and a differential diagnosis is difficult in many cases. A patient’s clinical findings, laboratory results, and radiological appearance, should be evaluated in detail and a final diagnosis only made following a multidisciplinary discussion. The presence of necrosis in tissue samples alone is not a reliable finding for a final diagnosis.
Keywords: granuloma, diagnosis, necrosis
Introduction
Granulomatous lung diseases are characterized by focal aggregation of inflammatory cells, such as histologically activated macrophages (epithelioid histiocytes), Langhans-type giant cells, and lymphocytes (1). In general, granulomas are classified as either necrotizing or non-necrotizing. Necrotizing lung disease is most commonly caused by infectious agents, especially tuberculosis (TB), and non-necrotizing disease is caused by non-infectious conditions, particularly sarcoidosis (2).
The diagnosis distribution of granulomatous disease is very heterogeneous. Detailed anamnesis, clinical, laboratory, radiological, and pathological evaluations help to make a differential diagnosis, but it is not always easy to differentiate, or diagnose, the cause of granulomatous diseases. In some cases, a final diagnosis cannot be made, even with all detailed evaluations (3,4). There have been a few studies that have investigated the diagnosis distribution of granulomatous diseases (4,5). In most studies, the number of cases is limited (2,6).
An accurate diagnosis of granulomatous diseases is very important as it changes the treatment strategy. The aim of this study was to evaluate the radiological, laboratory, and pathological findings of a large patient population with granuloma in lung, lymph node, or pleural tissue biopsy samples, to determine the final diagnostic distribution.
Materials and Methods
Our hospital is a key pulmonary disease and thoracic surgery hospital in Turkey. In this center, sarcoidosis and interstitial lung disease outpatient services are provided, regular multidisciplinary council meetings (chest diseases, thoracic surgery, radiology, pathology) are held, and final diagnoses are made by considering the clinical, radiological, and pathological characteristics of the patients. The study protocol was approved by the hospital’s ethics committee in accordance with the Helsinki Declaration (date: 03.01.2019, protocol number 073). It was designed as a retrospective, descriptive, observational, cross-sectional study. The name, surname, and identification numbers of all patients with the words ‘granuloma’ or ‘granulomatous inflammation’ in their pathology report between January 1, 2016 and December 31, 2018 were identified via the data processing system of the hospital.
Patient Selection:
Inclusion Criteria:
1- Patients with granuloma or granulomatous inflammation in lung parenchyma, pleura, mediastinal, hilar, or peripheral lymph node biopsy samples (Figure 1 and 2).
Figure 1.
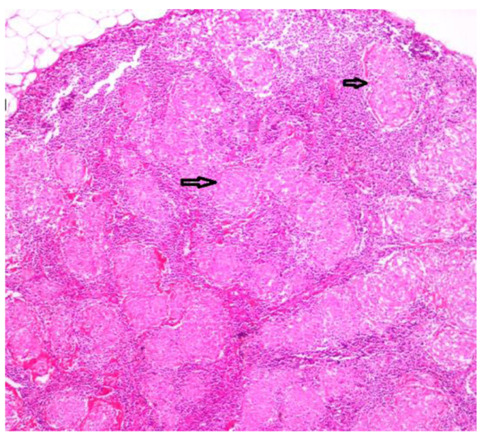
Confluent non-necrotizing granuloma structures filling the lymph node structure (H&E x4).
Figure 2.
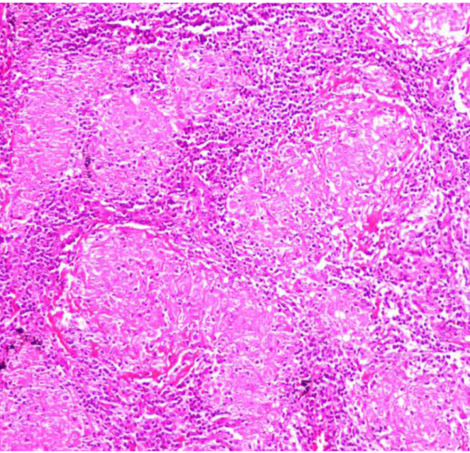
A closer view of the same granuloma structures (H&E x10).
2- Adult patients over the age of 18 years.
Exclusion Criteria:
1- Patients with no evidence of granuloma and/or granulomatous inflammation in their pathology report.
2- Patients who have undergone organ biopsy other than lung, pleura and lymph nodes.
3- Patients who were not examined in our hospital but whose pathology preparations were evaluated for pathological consultation.
4- Patients who’s radiological and laboratory data were not available from the hospital information processing system.
The patient data recorded in the hospital system was examined and demographic, radiological (chest X-ray and thorax computed tomography), laboratory (angiotensin converting enzyme [ACE], C-reactive protein [CRP], calcium, and albumin), and microbiological findings (lavage/sputum acid fast bacilli [AFB], culture, and tissue AFB culture results), diagnostic methods (fiberoptic bronchoscopy [FOB], endobronchial ultrasound [EBUS], mediastinoscopy [MDx] or video-assisted thoracoscopic surgery [VATS], etc.), and final diagnoses, were recorded. Laboratory, radiological, and pathological results were examined and the diagnoses were re-evaluated by at least two experienced chest diseases specialist clinicians. Controversial cases were contacted by phone to obtaining formation from the patient and/or their relatives’ regarding their current status. Data regarding whether or not treatment had been received for TB was obtained from the National TB Surveillance System.
Main Diagnoses
In the presence of clinical and pathological findings compatible with infectious agents (TB, NTM, fungal, etc.), when microorganisms are seen in tissue or sputum/bronchial lavage fluid and/or microbial growth in culture results or serology and antigen positivity are detected, the diagnosis of infection was made.
Sarcoidosis was diagnosed in patients who had clinical, radiological, laboratory, and pathological findings consistent with sarcoidosis, and infectious agents were not detected in the direct examination or culture results.
Those patients who had no pathology other than malignancy that could cause granulomatous disease, had no environmental and/or occupational exposure, and no drug use, were diagnosed as malignant sarcoid reaction.
Patients whose clinical, radiological, and pathological properties, and serological results were not consistent with any other pathology capable of causing granuloma and who meet national/international diagnostic criteria for rheumatic diseases were diagnosed as collagen tissue disease or vasculitis by a rheumatologist.
The diagnosis of pneumoconiosis was made in the presence of appropriate occupational and/or environmental exposure history, and compatible radiological, clinical, and pathological findings.
A diagnosis of hypersensitivity pneumonitis was made with compatible occupational and/or environmental exposure, as well as compatible clinical, radiological, laboratory, and pathological findings.
Cryptogenic organizing pneumonia was diagnosed when organizing pneumonia was detected in tissue biopsies and there was no other disease that would cause granulomatous inflammation.
Patients who could not be diagnosed by clinical, radiological, bacteriological and laboratory evaluation, medication/occupational history and rheumatological evaluation were classified as unknown.
Statistical Analysis
The SPSS 16.0 (SPSS for Windows, version 16.0; SPSS Inc.; Chicago, IL, ABD) package program was used for statistical evaluation. Data were given as the mean ± standard deviation, or the median (minimum-maximum). The student’s t-test or Mann-Whitney U test was used to compare the mean of two independent groups. The relationship between categorical variables was determined using the Pearson chi-square test. A p value <0.05 was considered significant.
Results
There were a total of 425 patients screened who had the word ‘granuloma’ and ‘granulomatous inflammation’ in their pathology report between January 1, 2016 and December 31, 2018. After a total of 33 patients were excluded, 392 patients were included in the study (Figure 3).
Figure 3.
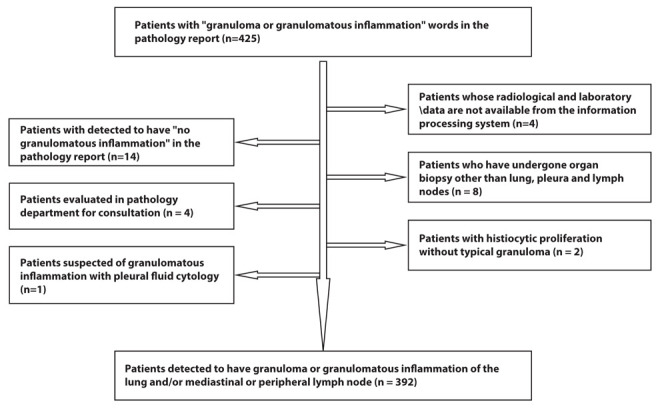
Flow chart showing study enrollment of patients with granulomatous inflammation.
The mean age of the patients was 46 ± 14 (19–88) years. A total of 56.6% of the patients were female (n=222), and 43.4% were male (n=170).
The most common radiological findings were mediastinal or hilar lymphadenopathy (LAP) (n=151, 38.5%), followed by LAP and parenchymal nodular infiltrations (n=104, 26.5%), and LAP and parenchymal consolidations and/or reticular opicities (n=47, 12%), respectively (Table 1).
Table 1.
Thoracic HRCT findings
| n | % | |
| LAP | 151 | 38.5 |
| LAP + nodular infiltrates | 104 | 26.5 |
| LAP + consolidations and reticular opacities | 47 | 12 |
| Pleural effusion | 29 | 7.4 |
| Parenchymal consolidations and reticular opacities | 15 | 3.8 |
| Parenchymal nodular infiltrates | 12 | 3.1 |
| LAP + atelectasis | 8 | 2 |
| LAP + pleural effusion | 7 | 1.8 |
| Normal | 7 | 1.8 |
| Nodular infiltrates + pleural effusion | 6 | 1.5 |
| Reticulonodular densities + honeycomb pattern | 3 | 0.8 |
| LAP + miliary nodules | 3 | 0.8 |
LAP: lymphadenopathy
The most commonly used diagnostic biopsy methods were, endobronchial ultrasound (EBUS) in 155 patients (39.5%), mediastinoscopy (MDx) in 128 patients (32.6%), surgical biopsy (video-assisted thoracoscopic surgery [VATS] wedge biopsy, lobectomy) in 77 patients (19.6%), video-assisted thoracoscopic surgery (VATS) pleural biopsy in 29 patients (7.4%), and bronchoscopic mucosal biopsy in 18 patients (4.6%) (Table 2). Granulomatous inflammation was detected by more than one diagnostic method in 41 patients. Serological and bacteriological methods were used for the diagnosis of 25 patients (6.4%) (Table 2). In 22 patients, the diagnosis of tuberculosis was confirmed by AFB positivity in sputum and/or bronchial lavage culture. In two patients, the diagnosis of “granulomatosis with polyangiitis” was confirmed by c-ANCA positivity, and in one patient the diagnosis of Rheumatoid Arthritis by rheumatoid factor and anti-CCP positivity.
Table 2.
Diagnosis methods
| n | % | |
| EBUS | 155 | 39.5 |
| Mediastinoscopy | 128 | 32.6 |
| Surgical biopsy | 77 | 19.6 |
| VATS pleural biopsy | 29 | 7.4 |
| FOB mucosa biopsy | 18 | 4.6 |
| Peripheral lymph node biopsy | 17 | 4.3 |
| FOB TBB | 6 | 1.5 |
| Transthoracic biopsy | 3 | 0.8 |
EBUS: Endobronchial ultrasound, VATS: video-assisted thoracoscopic surgery, FOB: fiberoptic bronchoscopy, TBB: transbronchial biopsy
A total of 392 patients were diagnosed with 13 different diseases. The most common causes of granulomatous disease were sarcoidosis (n=246, 62.8%), TB (n=88, 22.4%), and sarcoid reaction due to malignancy (n=23, 5.9%) (Table 3). In 15 (3.8%) patients, a diagnosis could not be made despite a detailed history and further investigations (Table 3). The diagnosis of TB was made by sputum or bronchial lavage culture and biopsy results in 22 (25%) of 88 patients, and clinical-radiological evaluation and biopsy results in 66 patients (75%)(Table 3). The distribution of malignancy types were as follows: squamous cell lung cancer (ca) in 7 patients, lung adeno ca in 4 patients, lymphoma in three patients, carcinoid tumor in one patient, large cell lung ca in one patient, thymoma in two patients, renal clear cell ca metastasis in one patient, ovarian ca in one patient, cervix ca in one patient, and sarcoid reaction due to gastrointestinal stromal tumor in one patient. In one patient, non-small cell lung ca was diagnosed but type discrimination could not be made accurately.
Table 3.
Diagnosis distributions
| n | % | |
| Sarcoidosis | 246 | 62.8 |
| Tuberculosis | 88 | 22.4 |
| Malignancy | 23 | 5.9 |
| Unknown | 15 | 3.8 |
| Pneumoconiosis | 5 | 1.3 |
| Non-tuberculous mycobacteria | 4 | 1.0 |
| Granulomatosis with polyangiitis | 2 | 0.5 |
| Hypersensitivity pneumonitis | 2 | 0.5 |
| Cryptogenic organized pneumonia | 1 | 0.25 |
| Fungal infection | 1 | 0.25 |
| Benign fibrous histiocytoma | 1 | 0.25 |
| Rheumatoid arthritis | 1 | 0.25 |
| Foreign body | 1 | 0.25 |
| Cyst hydatid | 1 | 0.25 |
| Sequela of TB + sarcoidosis | 1 | 0.25 |
| Total | 392 | 100 |
Non-necrotizing granulomatous inflammation was found in 268 (68.4%) patients, and necrotizing granulomatous inflammation was found in 124 (31.6%) patients (Table 4). We investigated any associations between the demographic data and laboratory values in the necrotizing and non-necrotizing groups (Table 5). No statistically significant differences were detected in age, gender, serum ACE, calcium, and CRP values between the necrotizing and non-necrotizing groups (Table 5). When we compared the radiological findings we found that 92.5% (n=248) of patients with non-necrotizing granuloma had hilar and/or mediastinal LAP on thorax CT, compared with 58.1% (n=72) with necrotizing granuloma, and the difference was statistically significant. (p<0.001) (Table 5). The rates of necrotizing and non-necrotizing granulomas detected in sarcoidosis and TB patients were compared. TB and non-TB mycobacteria (NTM) infections were evaluated together and the total number of patients with TB was recorded as 92. Non-necrotizing granuloma was detected in 190 sarcoidosis patients (77.2%), and necrotizing granuloma was found in 56 (22.8%) patients. Necrotizing granulomatous inflammation was detected in 50 TB patients (54.3%) and, 42 (45.7%) had non-necrotizing granuloma. The difference between the two groups was statistically significant. (p<0.001) (Table 5).
Table 4.
Diagnostic distributions according to necrosis status
| Non-necrotizing n (%) | Necrotizing n (%) | |
| Sarcoidosis | 190 (48.5%) | 56 (14.3%) |
| Tuberculosis | 39 (9.9%) | 49 (12.5%) |
| Malignancy | 13 (3.3%) | 10 (2.5%) |
| Unknown | 14 (3.6%) | 1 (0.25%) |
| Pneumoconiosis | 4 (1.0%) | 1 (0.25%) |
| Non-tuberculous mycobacteria | 3 (0.8%) | 1 (0.25%) |
| Granulomatosis with polyangiitis | 1 (0.25%) | 1 (0.25%) |
| Hypersensitivity pneumonitis | 1 (0.25%) | 1 (0.25%) |
| Cryptogenic organizing pneumonia | 0 | 1 (0.25%) |
| Fungal infection | 0 | 1 (0.25%) |
| Foreign body | 1 (0.25%) | 0 |
| Rheumatoid arthritis | 0 | 1 (0.25%) |
| Cyst hydatid | 1 (0.25%) | 0 |
| Benign fibrous histiocytoma | 1 (0.25%) | 0 |
| Sequela of TB + sarcoidosis | 0 | 1 (0.25%) |
| Total | 268 (68.4%) | 124 (31.6%) |
Table 5.
Demographic data and laboratory characteristics of necrotizing and non-necrotizing groups
| Non-necrotizing | Necrotizing | P value | |
| Age (years) | 45±13 | 48±14 | 0.76 |
| C-reactive protein (ng/ml) | 21.9±34.6 | 22.7±39.1 | 0.7 |
| Angiotensin converting enzyme (Units/L) | 57.8±50.2 | 56.8±33.7 | 0.23 |
| Calcium (mg/dL) | 9.5±0.9 | 9.5±0.8 | 0.66 |
| Albumin (g/dL) | 4.09±0.8 | 4.13±0.4 | 0.09 |
| Gender Female n (%) Male n (%) |
155 (69.8%) 113 (66.5%) |
67 (30.2%) 57 (33.5%) |
0.49 |
| Hilar/mediastinal LAP n (%) Yes No |
248 (77.5%) 20 (27.8%) |
72 (22.5%) 52 (72.2%) |
<0.001 |
| Sarcoidosis n (%) Tuberculosis n (%) |
190 (77.2%) 42 (45.7%) |
56 (22.8%) 50 (54.3%) |
<0.001 |
Discussion
According to our knowledge, this is a most comprehensive study investigating the distribution of granulomatous inflammation in a total of 392 cases in a single center, tertiary level chest diseases and thoracic surgery hospital. We determined the most common causes of granulomatous inflammation to be sarcoidosis, TB, and sarcoid-like reaction due to malignancy, respectively. Non-necrotizing inflammation was most common in patients with sarcoidosis, and necrotizing granulomatous inflammation was most common in patients with TB. In addition, we observed that the diagnostic distribution of disease with granulomatous inflammation covered a wide range of diseases. A total of 392 patients were diagnosed with 13 different diseases. The percentage of patients who could not be diagnosed was found to be relatively low (3.8 %).
In a multi-center study conducted in the USA, with 500 patients from seven countries, Mukophadhyay et al. reported the causes of granulomatous inflammation to be 31% sarcoidosis and 28% infectious causes. The most common infectious agents in the USA are fungal, and NTM and TB in countries other than the USA (4). As far as we can see, this study is a multicenter study investigating the etiology of granulomatous inflammation with the largest number of patients (4). Woodard et al. reported in their single-center study of 303 patients that sarcoidosis and tuberculosis were the most common causes of granulomatous inflammation (5). These studies have been performed by pathologists (4,5). Although our country is among the list of developing countries, it has made significant progress in the fight against TB. The current TB incidence is 14.6 per 100,000. This may explain the lower frequency of TB compared with sarcoidosis in our study.
In our study, non-necrotizing inflammation was observed in 77.2% of sarcoidosis patients, and necrotizing inflammation was observed in 22.8%. In other studies, the frequency of necrosis in sarcoidosis reportedly ranges between 6–35% (1). Our results are consistent with the literature. In our study, non-necrotizing inflammation was found in 45.7% of TB patients, and necrotizing inflammation was found in 54.3%. While typical necrotizing granuloma development is expected in TB, non-necrotizing granulomas is also reported to occur in some TB patients (6). In a study conducted with EBUS in patients with granulomatous inflammation in the lymph node, necrotizing granulomatous inflammation was found in 26.1% of TB patients (7). These results suggest that the presence of necrosis in the biopsy specimen may not indicate TB, and the absence of necrosis may not indicate sarcoidosis.
In our study, NTM infection was detected in 4 patients (1.02%) as the cause of granulomatous inflammation, and fungal infection was found in one patient. This may be due to the low frequency of NTM and fungal infections in our country. In addition, although our hospital is one of the largest chest diseases hospital in our country, it does not include other clinical departments, and immunosuppressed patients with solid organ or hematological stem cell transplantation are not admitted, and there is no inpatient oncology service. The prevalence of NTM has been reported between 1.26% and 1.6% in countries other than the USA (2,4). This rate was reported to be as high as 12.1% in a study conducted in Texas, USA (3). In some studies, the etiological cause of granulomatous inflammation has been found to be primarily infectious agents, and then sarcoidosis. This may be due to the higher prevalence of tuberculosis in some countries, and NTM and endemic fungal infections in others (2,3). Mycobacteria are detected more frequently in countries with a high TB incidence and HIV/TB coexistence, or NTM infections. On the other hand, sarcoidosis diagnoses have reportedly increased significantly in countries where TB infections are common, together with an increase in awareness, diagnostic possibilities, and the ability of physicians to distinguish sarcoidosis from TB (8).
In our study, the third most common cause of granulomatous inflammation was sarcoid reaction due to malignancy. Similar results have been reported in other studies (7). Detection of sarcoidosis-like epithelioid granulomas in regional lymph nodes due to the underlying disease, without systemic sarcoidosis findings, is called a sarcoid-like reaction (9). Sarcoid reaction is the most common non-malignant disease associated with hematological malignancies, and solid organ tumors such as cervix, liver, lung, testis, and uterine cancer (10, 11, 12). The most common causes of sarcoid reaction in our study were lung ca, lymphoma, renal cell ca, ovarian ca, cervix ca, thymoma, and gastrointestinal stromal tumor. To our knowledge, no sarcoid reaction related to thymoma has been reported, although there are case reports of granulomatous thymoma or Hodgkin’s disease of the thymus (13). In a study similar to that reported here, sarcoid reaction related to lung cancer, lymphoma, breast, stomach, urological, larynx, and gynecological cancers were found (14). Case reports of renal cell ca, stomach ca, and sarcoid reaction related to breast ca, have also been reported (15–18).
Our study differs from others in that we report a small number of patients with cyst hydatid, benign fibrous histiocytoma, and cryptogenic organizing pneumonia as the causes of granulomatous inflammation. There is a case report of epithelioid sarcoma resembling benign fibrous histiocytoma causing granuloma (19), and case reports of granulomatous inflammation due to hydatid cyst infection (20,21). Granulomatous infiltration foci may rarely accompany cryptogenic organizing pneumonia (22). The frequency of granulomatosis with polyangiitis, rheumatoid arthritis, hypersensitivity pneumonitis, and granulomatous inflammation due to foreign body aspiration in our study was similar to that described in the literature (2,3).
The proportion of patients who could not be diagnosed in our study was 3.8% (n=15). In general, the reported proportion of undiagnosed patients is higher. It is important to evaluate the clinical, radiological, microbiological, and pathological findings together for a definitive diagnosis in patients with granulomatous inflammation, but despite all of these findings, some cases cannot be diagnosed. The proportion of undiagnosed patients was 42% in the study of Mukopadhyay et al, and 18% in the study of Nazurallah et al (3,4). In two other studies, lack of diagnosis was reported at a proportion of 16% and 10.3% (2,23). In our hospital, a multidisciplinary council convenes every week to evaluate patients with granuloma or suspected interstitial lung disease to ensure a correct diagnosis, and these cases are discussed by experienced physicians. As such, we believe our specific diagnosis proportion would be relatively higher than other studies.
The limitations of our study are that it is a single center and retrospective study. In addition, our hospital does not have an inpatient service for other clinical units, other than chest diseases and thoracic surgery. Only patients in whose lung, pleura and lymph node biopsies granuloma was detected were included in our study. For these reasons, alternative etiologies may have been missed. Nonetheless, due to the fact that it is a branch hospital with a high patient circulation and number, factors only previously described in case reports were determined as the cause of granulomatous inflammation in our study.
Conclusion
The diagnosis of granulomatous inflammation detected in biopsy specimens is common for clinicians, but in many cases a differential diagnosis is difficult. We present a comprehensive study investigating the distribution of granulomatous inflammation in which all cases were evaluated clinically, radiologically and pathologically, and diagnosed. Diagnostic distributions were assessed in detail and we also took into account conditions specific to the studied patient population in reference to diagnosis distribution. The presence of necrosis in tissue samples alone was not a reliable finding for a definitive diagnosis. A patient’s clinical findings, laboratory results, and radiological appearance, should be evaluated in detail and a final diagnosis only made following a multidisciplinary discussion.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls. Eur Respir Rev. 2017;26:170012. doi: 10.1183/16000617.0012-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Harbi A, Al-Otaibi S, Abdulrahman A, et al. Lung granuloma: A clinicopathologic study of 158 cases. Ann Thorac Med. 2017;12(4):278–81. doi: 10.4103/atm.ATM_1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nazarullah A, Nilson R, Jose Maselli D, Jagirdar J. Incidence and a etiologies of pulmonary granulomatous inflammation: A decade of experience. Respirology. 2015;20:115–21. doi: 10.1111/resp.12410. [DOI] [PubMed] [Google Scholar]
- 4.Mukhopadhyay S, Farver CF, Vaszar LT, et al. Causes of pulmonary granulomas: A retrospective study of 500 cases from seven countries. J Clin Pathol. 2012;65:51–7. doi: 10.1136/jclinpath-2011-200336. [DOI] [PubMed] [Google Scholar]
- 5.Woodard BH, Rosenberg SI, Farnham R, Adams DO. Incidence and nature of primary granulomatous inflammation in surgically removed material. Am J Surg Pathol. 1982;6(2):119–29. doi: 10.1097/00000478-198203000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyay S, Gal AA. Granulomatous Lung Disease an Approach to the Differential Diagnosis. Archives of Pathology & Laboratory Medicine. 2010;134(5):667–90. doi: 10.5858/134.5.667. [DOI] [PubMed] [Google Scholar]
- 7.Dhooria S, Agarwal R, Aggarwal AN, Bal A, Gupta N, Gupta D. Differentiating tuberculosis from sarcoidosis by sonographic characteristics of lymph nodes on endobronchial ultrasonography: A study of 165 patients. J Thorac Cardiovasc Surg. 2014;148:662–7. doi: 10.1016/j.jtcvs.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Guleria R, Mahashur A, Ghoshal AG, Thomas PK, Raghu G, Baughman RP. Challenges in diagnosing sarcoidosis in tuberculosis endemic regions: Clinical scenario in India. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33(4):381–4. [PubMed] [Google Scholar]
- 9.Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev. 1986;13(3):147–56. doi: 10.1016/0305-7372(86)90002-2. [DOI] [PubMed] [Google Scholar]
- 10.Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clinics in Dermatology. 2007;25(3):326–33. doi: 10.1016/j.clindermatol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Butt S, Alzebdeh R, Kable TD, Soubani AO. Non-caseating granulomas in patients after the diagnosis of cancer: clinical characteristics and outcome. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:44–49. [PubMed] [Google Scholar]
- 12.Kiess AP, Wang H, Travis WD, Yahalom J. Sarcoid in cancer patients: clinical characteristics and associated disease status. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32(3):200–7. [PubMed] [Google Scholar]
- 13.Katz A, Lattes R. Granulomatous Thymoma or Hodgkin’s disease of Thymus? A clinical and histologic study and a re-evaluation. Cancer. 1969;23(1):1–15. doi: 10.1002/1097-0142(196901)23:1<1::aid-cncr2820230101>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Ravaglia C, Gurioli C, Casoni GL, et al. Sarcoid-like lesion is a frequent benign cause of lymphadenopathy in neoplastic patients. European Respiratory Journal. 2013;41:754–5. doi: 10.1183/09031936.00141212. [DOI] [PubMed] [Google Scholar]
- 15.Singer AJ. Sarcoidosis and renal cell carcinoma. Infect Urol. 2002;15:4. [Google Scholar]
- 16.Iftikhar A, Cheema MAI, Ramachandran P, Sahnic S. Sarcoid-like reaction associated with renal cell carcinoma – A case report. Respir Med Case Rep. 2019;27:100847. doi: 10.1016/j.rmcr.2019.100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima M, Nakamura S, Fujisaki M, et al. Sarcoid-like reaction in the regional lymph nodes and spleen in gastric carcinoma: a clinicopathologic study of five cases. Gen Diagn Pathol. 1997;142(5-6):347–52. [PubMed] [Google Scholar]
- 18.Bässler R, Birke F. Histopathology of tumour associated sarcoid-like stromal reaction in breast cancer. An analysis of 5 cases with immunohistochemical investigations. Virchows Arch A Pathol Anat Histopathol. 1988;412(3):231–9. doi: 10.1007/BF00737147. [DOI] [PubMed] [Google Scholar]
- 19.Lynch MC, Graber EM, Johnson TS. Epithelioid Sarcoma Resembling Benign Fibrous Histiosytoma. Cutis. 2015;95(2):83–6. [PubMed] [Google Scholar]
- 20.Almadani N, Almutairi B, Alassiri AH. Primary Subcutaneous Hydatid Cyst with Palisading Granulomatous Reaction. Case Reports in Pathology. 2013:126541. doi: 10.1155/2013/126541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deonarain J, Sing Y, Calonje E, Singh B. Subcutaneous palisading granulomatous pseudocysts of Echinococcus granulosus origin. Journal of Cutaneous Pathology. 2009;36(2):240–5. doi: 10.1111/j.1600-0560.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 22.Feinstein MB, DeSouza SA, Moreira AL, et al. A comparison of the pathological, clinical and radiographical, features of cryptogenic organising pneumonia, acute fibrinous and organising pneumonia and granulomatous organising pneumonia. J Clin Pathol. 2015;68:441–7. doi: 10.1136/jclinpath-2014-202626. [DOI] [PubMed] [Google Scholar]
- 23.Erbay M, Ozsu S, Ayaydın Mürtezaoglu ES, et al. Mediastinal/hiler granulomatoz lenfadenit etyolojisi. Tuberk Toraks. 2018;66(3):212–6. doi: 10.5578/tt.67018. [DOI] [PubMed] [Google Scholar]


