Abstract
Introduction:
Echocardiographic measurement of the right ventricular systolic pressure (RVSP) is commonly used for estimating systolic pulmonary artery pressure (PASP) measured during right heart catheterization (RHC) in patients suspected for pulmonary hypertension (PH). Generally, there seems to be a strong correlation. However, this has been reported as less robust in sarcoidosis. We aim to investigate the correlation between RVSP and RHC measurements using real world data and analyzed factors influencing the relationship between RVSP and PASP in sarcoidosis.
Methods & Results:
Data of patients with and without sarcoidosis associated PH who had both a measurable echocardiographic RVSP and invasive PASP were collected from the RESAPH registry, PULSAR study and Cincinnati Sarcoidosis Clinic database (n=173, 60.1% female, mean age 56.0±9.5 years). Among them, 124 had PH confirmed by RHC. There was a strong correlation between RVSP and PASP (r=0.640). This correlation was significant in both male and female, white or non-white, forced vital capacity (FVC) >60%, and presence of fibrosis (p<0.001). However, it was less robust in patients with FVC of 50% or less. RVSP was considered inaccurate if the difference with PASP was > 10mmHg. Inaccurate echocardiographic estimation of the invasive PASP occurred in 50.8%, with overestimation mostly in patients without PH, and underestimation in patients with severe PH. An RVSP>50mmHg was associated with worse survival.
Conclusions:
In this real world multicenter cohort of sarcoidosis patients, we found a significant correlation between RVSP as determined by echocardiography and invasive PASP. Over- or underestimation of PASP occurred frequently. Therefore, echocardiographic RVSP measurement alone to screen for PH in sarcoidosis should be used with caution.
Keywords: sarcoidosis, pulmonary hypertension, echocardiography
Introduction
Pulmonary hypertension (PH) is a well-known complication of sarcoidosis, and is related to a significant increase in mortality and morbidity (1,2). The diagnosis PH can be established by the gold standard right heart catheterization (RHC), defined by a mean pulmonary artery pressure (mPAP) of at least 25mmHg, as defined by the 2015 ESC/ERS guideline (3). However, RHC is invasive, costly and resource limited. A good non-invasive estimation of the pulmonary artery pressure might lower the burden of RHC. Transthoracic echocardiography is a commonly used method for non-invasive estimation of the pulmonary artery pressure. By using the simplified Bernoulli equation on the tricuspid regurgitation maximal velocity (TRV max) added by the right atrial pressure (RAP), the right ventricular systolic pressure (RVSP) can be calculated. Furthermore, echocardiography provides additional information about the heart dimension and function (4).
In pulmonary arterial hypertension (WHO group 1) there is generally a strong correlation between echocardiographic RVSP and systolic pulmonary artery pressure (PASP) as determined by RHC, especially under controlled study conditions (5-7). However, the relationship has been reported as much less robust in sarcoidosis patients and other interstitial lung diseases. In clinical practice, differences between RVSP and PASP of 10mmHg or more are frequently encountered (8-11).
In the present study, we aimed to investigate the correlation between RVSP and RHC measurements in using real world data and analyzed factors that may influence this relationship. We recruited patients with sarcoidosis associated PH (SAPH) from the ReSAPH database, which reports on data from many different sites all over the world. Besides these patients, we retrieved data on sarcoidosis patients without PH but with both echocardiographic as invasive haemodynamic data from the PULSAR study and the Cincinnati Sarcoidosis database.
Materials and Methods
Study design and population
This is a retrospective multinational study with real world data investigating the association and factors which may influence the echocardiographic estimate of RVSP compared to PASP, including parenchymal lung disease. Data of patients with sarcoidosis associated PH were collected from the REgistry for SArcoidosis associated Pulmonary Hypertension (RESAPH), a large international multicenter registry, aiming to enroll patients with SAPH from different regions of the world. Reports on baseline characteristics were published previously (12). The ReSAPH registry is registered as NCT01467791 (https://clinicaltrials.gov). Databases of the PULSAR study (NTR5295 www.trialregister.nl), a large prospective study investigating the presence of PH in sarcoidosis patients in a Dutch tertiary centre(13), and Cincinnati Saroidosis Clinic (an in-hospital registry for heamodynamic data collected in sarcoidosis patients) were examined in order to add additional sarcoidosis patients without PH as confirmed by RHC (Figure 1).
Figure 1.
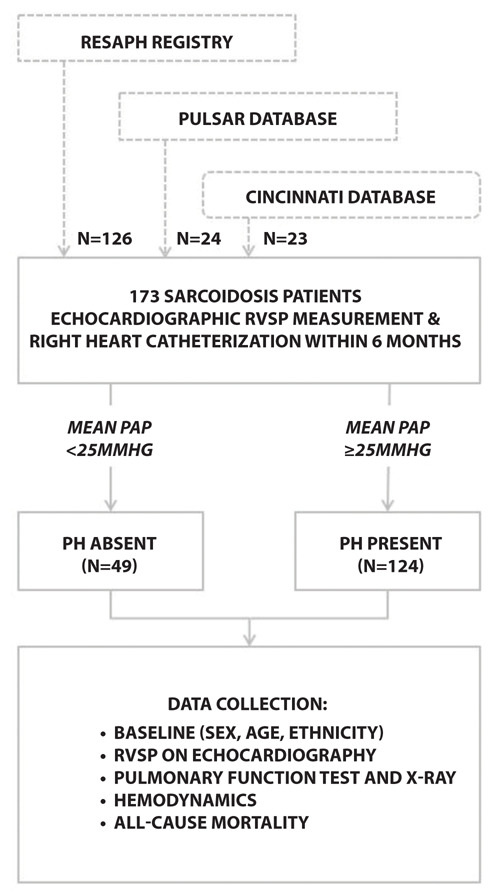
Flowchart of patient selection and data collection.
Patients were included if they had undergone both RHC and echocardiography with RVSP estimation within a timeframe of 6 months. Furthermore, we collected data on sex, age, ethnicity, Scadding stage (14), forced vital capacity percent predicted (FVC%), and presence of fibrosis on chest imaging within this timeframe. Not all of the patients had recorded data on spirometry or imaging. Data on survival was collected and scored as alive or the composite endpoint dead/transplanted at the last day of visit.
Haemodynamics
RHC data on RAP, systolic, mean and diastolic PAP, pulmonary capillary wedge pressure, cardiac output and pulmonary vascular resistance were collected. PH was defined as a mPAP ≥25mmHg. A mPAP between 25mmHg and 35mmHg was considered as mild to moderate PH, whereas a mPAP ≥35mmHg was considered severe PH (15).
As for transthoracic echocardiography, measurement of the TRV max was recorded. As shown in figure 2, RVSP was calculated using the simplified Bernoulli equation (PASP = 4 X TRV max2 + RAP), with RAP estimated based on the size and collapse of the inferior vena cava (4). No data was captured on the use of contrast methods during echocardiography nor other contributing factors such as volume status for the measurement of the TRV.
Figure 2.
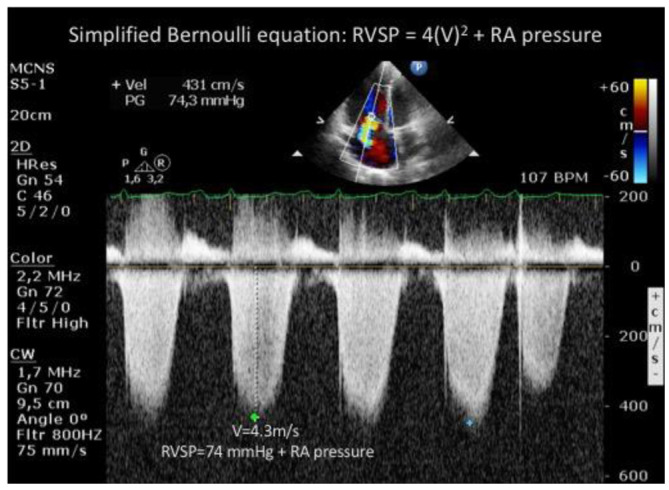
Echocardiographic measurement of the tricuspid regurgitation velocity and calculation of RVSP using the simplified Bernoulli equation.
Statistics
Comparison between groups was made using Student T-test, Mann Whitney U test and Chi-squared test where appropriate. Pearson correlations were determined for the overall group and for different subgroups. Bland-Altman analyses were performed to evaluate the agreement between echocardiography derived RVSP and PASP on RHC. Survival was compared between groups using Kaplan-Meier curves including log rank test. Hazard ratio’s (HR) including 95% confidence interval (CI) were calculated using Cox regression analysis. Regardless the total time of follow up, patients who were still alive at time of the analysis were censored as alive for further analysis. Statistics were calculated using IBM SPSS Statistics 24.
Results
Baseline characteristics
A total of 274 patients with sarcoidosis were identified from the three databases who had both RVSP measurements on echocardiography and invasive RHC measurements between June 2002 and September 2018. In 101 patients, the date difference between echocardiography and RHC exceeded our pre-determined maximum time interval of six months. Of the remaining 173 patients (mean time interval between echo and RHC of 52 days, ranging from 0 to 183 days), 124 had confirmed PH. The other 49 patients had no PH. After recalculation of the mPAP in all patients, two of the RESAPH patients turned out to have a mPAP <25mmHg. Baseline characteristics of patients are presented in Table 1.
Table 1.
Baseline characteristics
| Baseline parameters | All patients (n=173) | PH present (mPAP≥25mmHg) (n=124) | PH absent (mPAP<25mmHg) (n=49) | p-value PH present vs PH absent | |
| Sex (% female) | 60.1 | 62.9 | 53.1 | 0.234 | |
| Age (years) | 56.0±9.5 | 56.4±9.2 | 55.2±10.3 | 0.524 | |
| Ethnicity (%) | white | 43.4 | 35.5 | 63.3 | 0.003 |
| black | 40.5 | 47.6 | 22.4 | ||
| other | 16.2 | 16.9 | 14.3 | ||
| Scadding stage (%) |
0
I II III IV |
4.2 | 2.5 | 8.3 | 0.009 |
| 8.3 | 5.0 | 16.7 | |||
| 12.5 | 11.7 | 14.6 | |||
| 14.9 | 13.3 | 18.8 | |||
| 60.1 | 67.5 | 41.7 | |||
| Fibrosis on chest CT >20% (%) | 60.3 | 64.2 | 51.1 | 0.123 | |
| FVC (% predicted) | 66.6±21.4 (n=151) | 61.2±18.5 | 79.9±22.6 | 0.067 | |
| DLCO (% predicted) | 50.7±51.4 (n=123) | 45.9±19.9 | 48.5±26.4 | 0.034 | |
| RVSP (mmHg) | 52.0±18.7 | 57.5±18.4 | 38.2±10.3 | <0.001 | |
| MPAP (mmHg) | 32.5±12.1 | 37.9±10.3 | 19.3±4.1 | <0.001 | |
| PASP (mmHg) | 53.2±19.3 | 61.8±16.3 | 32.0±7.3 | <0.001 | |
| Cardiac output (L/min) | 5.7±1.7 (n=172) | 5.5±1.6 | 6.1±1.7 | 0.442 | |
| PCWP (mmHg) | 11.7±6.7 (n=172) | 12.7±7.2 | 9.0±3.9 | <0.001 | |
| PVR (wood units) | 4.3±3.2 (n=172) | 5.2±3.3 | 1.9±1.2 | <0.001 | |
Data is expressed a percentage and as percentage or mean±standard deviation. DLCO= diffusion lung capacity for carbon monoxide; FEV1= forced expiratory volume in 1 second; FVC= forced vital capacity; PASP= systolic pulmonary artery pressure; PCWP= pulmonary capillary wedge pressure; PVR= pulmonary vascular resistance
Hemodynamic assessment
Hemodynamic characteristics are listed in table 1. Among patients with PH, 32 had a pulmonary capillary wedge pressure >15mmHg. As shown in figure 3, there was only a moderate correlation between RVSP versus PASP and mPAP (r=0.640 and 0.626 respectively, p<0.0001).
Figure 3.
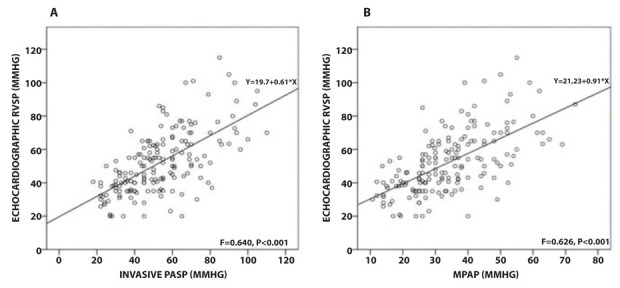
Scatter plot RVSP versus PASP (A) and mPAP (B), showing a moderate correlation between measurements.
Correlation between RVSP and PASP
We compared the RVSP and PASP including correlation based on sex, white versus non-white, FVC% greater or less than 60, 50 or 40, and presence or absence of fibrosis. Table 2 summarizes the correlation within these subgroups. While almost all correlations were significant (p<0.001), for the small sample size of 15 patients with a FVC% of less than 40%, there was no longer a significant correlation between RVSP and PASP.
Table 2.
Pearson correlations for RVSP and PASP within specific subgroups
| Female | White | FVC≤60% | FVC≤50% | FVC≤40% | Fibrosis>20% | |
| Yes | n=104 (r=0.541) |
n=75 (r=0.634) |
n=59 (r=0.544) |
n=34 (r=0.520)† |
n=15 (r=0.404)†† |
n=94 (r=0.560) |
| No | n=69 (r=0.692) |
n=98 (r=0.559) |
n=98 (r=0.559) |
n=115 (r=0.607) |
n=134 (r=0.616) |
n=62 (r=0.569) |
FVC=forced vital capacity; RVSP = right ventricular systolic pressure
p<0.001 for all, except for †p=0.02; ††p=0.135
The difference between echocardiographic RVSP and invasive PASP reflects the accuracy of echocardiography for assessing invasively measured PASP. As an absolute value, the mean difference for both over- and underestimation of RVSP compared to PASP in this cohort was 12.9 ± 1.0 mmHg. This difference did not correlate with the number of days between echocardiography and RHC (r=-0.057, p=0.455).
There was a significant correlation for the difference between RVSP and PASP compared to both PASP and mPAP with a Pearson correlation coefficient of r=-0.475 and r=-0.462 respectively (p<0.001). Figure 4 shows the difference between RVSP and PASP values compared to invasively measured PASP. The green line reflects the acceptable variance of 10 mmHg between measurements (8,10,11). Taking this into account, echocardiographic over- and underestimation of PASP occurred in 23.7% and 27.2% respectively. Therefore, within a 10 mmHg variance limit, RVSP on echocardiography correctly reflects the invasive PASP in only 49.1% of the patients. Overestimation tended to occur mostly in lower PASP values, whereas underestimation mainly occurred in higher PASP values. FVC% predicted or presence of >20% fibrosis on chest CT did not correlate significantly with the difference between RVSP and PASP (figure 3B and C).
Figure 4.
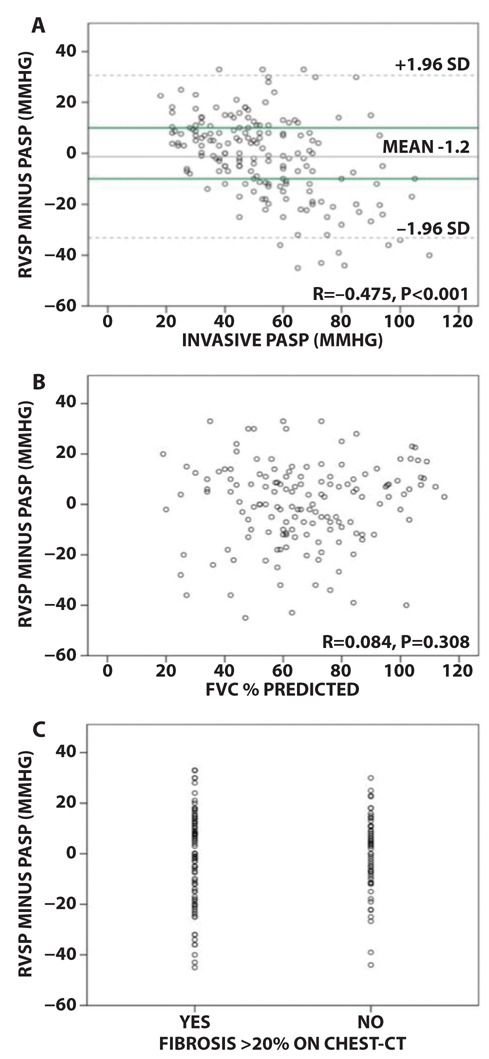
A. Bland Altman analysis for the difference in RVSP versus PASP compared to the PASP (r= -0.475, p<0.001). Grey lines: mean ±1.96 SD. Green lines: reference line for acceptable variance of 10mmHg; B. Scatterplot for the difference between RVSP and PASP compared to FVC% predicted. There is no significant correlation; C. Plot for difference between RVSP and PASP related to presence of significant fibrosis on chest-CT with a cut-off value of 20%. This figure shows no significant difference.
Figure 5 shows the number of patients expected to have PH based on different cut-off values for RVSP in each of the subgroups ‘no PH (n=49), ‘mild to moderate PH’ (n=56) and ‘severe PH’ (n=68). The figure shows that, for example using an RVSP cut-off value of 40mmHg (which is frequently used in literature), it tremendously overestimates the presence of PH in group A (no PH with mPAP <25mmHg), whereas a cut off value of 60mmHg significantly underestimates the presence of PH in group C (severe PH >35mmHg). Based on these results, there is no clear cut off value for RVSP to predict the presence of PH and therefore it cannot be used as a diagnostic tool.
Figure 5.
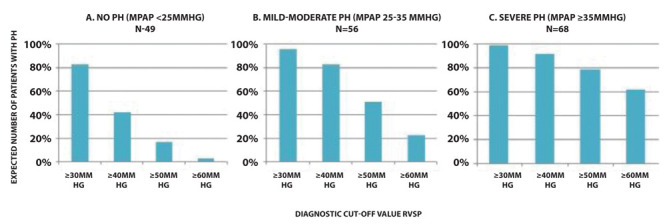
Overview of the number of patients expected to have PH (Y-axis) based on different cut-off values for RVSP (X-axis) within the subgroups no PH (A), mild to moderate PH (B) and severe PH (C). This This figure shows that echocardiographic RVSP overestimates the presence of PH in patients with no PH (group A), whereas it underestimates the presence of PH in patients with severe PH (group C).
Survival
Data on survival, defined as alive or dead/transplanted on the last day of visit, was available for 167 patients, with a mean follow up of 3.5±3.2 years. Figure 6A displays the overall survival of all patients up to a maximum follow up of ten years. Patients with PH had significantly worse outcomes (p=0.009) with a 1, 2, 5 and 10 year survival of 87.5%, 71.4%, 47.8% and 32.4% for patients with PH. Figure 6B further divides the group into no PH (mPAP <25mmHg), mild to moderate PH (mPAP ≥25 and <35mmHg), and severe PH (mPAP ≥35mmHg), with a significant difference in survival between these groups (P<0.001). Survival was comparable between patients without PH and mild to moderate PH. However, for those with severe PH, survival was significantly worse (HR 3.1; 95% CI 1.835-5.115; p<0.001). There was no significant difference in survival based on sex, age or ethnicity. A significant difference in survival using Cox Regression analysis was found for mPAP (HR per percent 1.04; 95% CI 1.02-1.06; p<0.001), FVC% (HR per percent 1.02; 95% CI 1.01-1.04; p=0.002), DLCO% predicted (HR per percent 1.011, 95% CI 1.003-1.019, p=0.004) and RVSP (HR per percent 1.02; 95% CI 1.004-1.03; p=0.11). An RVSP>40mmHg was not significantly associated with worse mortality (HR 1.75; 95% CI 0,98-3.07; p=0.06) (figure 6C). For an RVSP of >50mmHg, a significant difference in mortality was found (HR 2.2; 95% CI 1.33-3.74, p=0.002).
Figure 6.
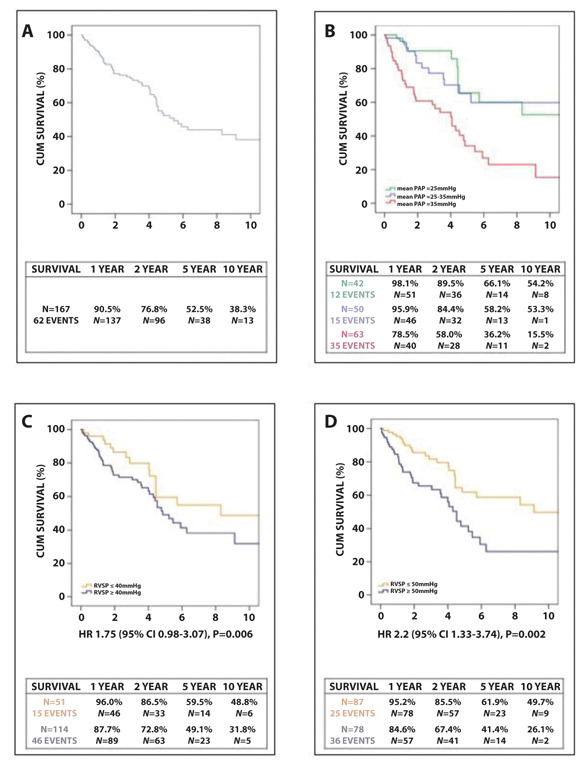
Kaplan Meier survival curves with comparison between different subgroups. A. All patients; B. Divided by severity of PH (no PH, mild-moderate PH and severe PH), with a significant decrease in survival for severe PH ; C. RVSP>40mmHg (no significant difference in survival); D. RVSP>50mmHg (significant difference in survival).
Discussion
PH in sarcoidosis is associated with increased mortality and morbidity. RVSP measurement on echocardiography is widely used for non-invasive estimation of the PASP. In this study, we focused on the relationship between echocardiographic RVSP and invasive PASP using real world data, including possible factors by which this relationship might be influenced. As a result, the correlation between RVSP and PASP in our cohort of 173 sarcoidosis patients was moderate (r=0.640; p<0.001). Over- or underestimation by echocardiography occurred in the majority of patients. Survival was significantly worse in sarcoidosis patients with an invasive mean PAP of ≥ 35 mmHg. An RVSP of 40 mmHg or higher tended to be associated with worse survival.
Under controlled study circumstances, a strong correlation of RVSP with PASP has been described (5-7). A meta-analysis conducted in 2011 (16) demonstrated an overall strong correlation in PH patients (r=0.70, 95% CI 0.67-0.73). In line with our findings, this meta-analysis also showed that the correlation decreased in patients with high PASP and different lung diseases, varying between r=0.48 to r=0.69, depending on the type and stage of lung disease (8,17,19). Part of the inaccuracy might be explained by incorrect measurement of the tricuspid regurgitation velocity signal, where measurement of the “chin” or “beard” of the tricuspid regurgitation signal can make a significant difference (18). Amsallem et al (20) re-measured all TRV signals in a standardized manner (21), resulting in a very strong correlation of r=0.88 (p<0.0001) in a cohort of 192 patients with advanced lung disease. In our cohort of sarcoidosis patients, the correlation weakened if FVC% predicted decreased. In a small cohort of patients with FVC% predicted <40%, the correlation was no longer significant.
The ability of RVSP to accurately correlate with the PASP is disputable. Differences up to 10 mmHg between echocardiographic RVSP and invasive PASP have been marked as an acceptable range between both measurements in previous studies (8,10,11). Following this cut-off value in our cohort, over- and underestimation using RVSP on echocardiography occurred in 23.7% and 27.2% of the patients respectively. This is in line with other studies, describing differences of 10 mmHg or more in up to 50% of patients with or without interstitial lung disease (8,10,11,19). In our cohort, a difference of more than 10 mmHg was more likely in patients with higher PASP values, mostly due to underestimation. This finding is in line with a study of Rich et al. (11) and could be due to equalization of right ventricular and right atrial pressures due to severe tricuspid regurgitation (22). On the other hand, overestimation of PASP values in patients without PH may lead to unnecessary invasive diagnostic investigations.
Another limitation of echocardiography is that RVSP measurements frequently cannot be obtained, especially in patients with lung disease. The present study did not capture this data, however several studies have reported that Doppler tricuspid regurgitation signal for measuring RVSP is interpretable in only 30-50% of patients with advanced lung disease (8,10,20), compared to 80-90% in those without advanced lung disease (20). If an adequate RVSP measurement is possible, no clear cut-off value has been established for diagnosing PH. A cut-off value of 40 mmHg is frequently used, with varying but insufficient data on accuracy in patients with lung disease (8,10). A meta-analysis evaluating the diagnostic accuracy of echocardiography for RHC concluded that this cut-off value performed only modestly in predicting PH. Other cut-off values, ranging from 32 to 50 mmHg, were not able to improve diagnostic accuracy (16). International guidelines use other echocardiographic signs for right ventricular overload and pulmonary vascular resistance to improve the diagnostic accuracy (3).
As for long-term outcome in SAPH, previous studies have shown SAPH to be associated with significant increase in mortality, especially in patients with severe PH (1,23). Our study confirms this association. There was a trend to worse outcome in patients with an RVSP >40 mmHg on echocardiography (HR 1.73; 95% CI 0,98-3.07; p=0.06), while an RVSP >50 mmHg was significantly associated with lung transplantation-free survival (HR 2.2; 95%CI 1.33-3.74), p=0.002).
Concluding this manuscript considering previous literature, echocardiography is an affordable and non-invasive method to screen for PH in sarcoidosis, and might be helpful in estimating prognosis in patients with SAPH. However, measurements by echocardiography have significant limitations and cannot be used as a one-on-one replacement for RHC. RVSP on echocardiography only approximates PASP. As the pulmonary disease progresses and the FVC% decreases, while simultaneously the likelihood of PH increases, RVSP measurements showed less correlation with PASP in sarcoidosis patients. Underestimation frequently occurred. Therefore, echocardiography derived RVSP should not be used as the only diagnostic tool. On the other hand, absence of an RVSP signal or a low RVSP measurement with high clinical suspicion should not refrain a clinician from further diagnostics since overestimation also occurs frequently. Furthermore, echocardiography is unable to quantitatively assess pulmonary capillary wedge pressure (PCWP) and pulmonary vascular resistance, which are important parameters in the classification of PH. However, echocardiography is able to detect left ventricular heart disease as a potential cause of an increased PCWP. Therefore, in sarcoidosis patients with a clinical suspicion for PH, RVSP measurement on echocardiography should not be the only factor to decide if further diagnostic evaluation is required.
This study has several limitations, inherent to the retrospective and multicenter registry design. First, we only included patients with a measurable RVSP. Second, since this study reflects real world data representative for the day to day practice of a health care provider with only a report of the echocardiogram available, the RVSP measurements were not standarized and based on the local protocols. It was not re-measured in a standardized manner throughout all participating centers and no data was captured for the use of contrast methods or other contributing factors such as volume status. Supposedly, there is a lot of variability in this measurement in both measurement technique and physiological circumstances influencing the correlation. Furthermore, we choose a maximum of six months’ time interval between echocardiography and RHC. This is a relatively large time interval, which is also known to influence the correlation (16,21). Also, there is no data on treatment within this time interval.
Conclusion
In this multicenter cohort of sarcoidosis patients, we found a significant correlation between RVSP as determined by echocardiography and PASP measured by RHC. Over- or underestimation was encountered in more than half of the patients. Therefore, using echocardiographic RVSP measurement alone to screen for PH in sarcoidosis should be used with caution, especially in those with severe lung disease.
Acknowledgements:
The statistical analysis was performed in collaboration with Hans Kelder (epidemiologist and statistician).
Funding sources:
This work was supported by Gilead Pharmaceuticals (NIH 1UL1TR001425-01) and ZonMW topzorg (project number 842001006)
References
- 1.Nunes H, Humbert M, Capron F, Brauner M, Sitbon O, Battesti JP, Simonneau G, Valeyre D. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61:68–74. doi: 10.1136/thx.2005.042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shlobin OA, Baughman RP. Sarcoidosis-Associated Pulmonary Hypertension. Semin Respir Crit Care Med. 2017;38:450–462. doi: 10.1055/s-0037-1603767. [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G Guidelines ESCCfP. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 4.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 5.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 6.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6:359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 7.Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 8.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Resp Crit Care Med. 2003;167:735–40. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 9.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, Corretti MC, Hassoun PM. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Resp Crit Care Med. 2009;179:615–21. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan SD. Pulmonary hypertension in interstitial lung disease. Int J Clin Prac. 2008:21–8. doi: 10.1111/j.1742-1241.2008.01624.x. [DOI] [PubMed] [Google Scholar]
- 11.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988–993. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 12.Baughman RP, Shlobin OA, Wells AU, Alhamad EH, Culver DA, Barney J, Cordova FC, Carmona EM, Scholand MB, Wijsenbeek M, Ganesh S, Birring SS, Kouranos V, O’Hare L, Baran JM, Cal JG, Lower EE, Engel PJ, Nathan SD. Clinical features of sarcoidosis associated pulmonary hypertension: Results of a multi-national registry. Respir Med. 2018;139:72–78. doi: 10.1016/j.rmed.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Huitema M, Bakker A, Mager J, Rensing B, Smits F, Grutters J, Post M. Prevalence of pulmonary hypertension in pulmonary sarcoidosis; the first large European prospective study. Eur Resp Journal. 2019 doi: 10.1183/13993003.00897-2019. [DOI] [PubMed] [Google Scholar]
- 14.Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years' observation. Br Med J. 1961;2:1165–1172. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015 doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 16.Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612–622. doi: 10.1136/hrt.2010.212084. [DOI] [PubMed] [Google Scholar]
- 17.Keir GJ, Wort SJ, Kokosi M, George PM, Walsh SLF, Jacob J, Price L, Bax S, Renzoni EA, Maher TM, MacDonald P, Hansell DM, Wells AU. Pulmonary hypertension in interstitial lung disease: Limitations of echocardiography compared to cardiac catheterization. Respirology. 2018;23:687–694. doi: 10.1111/resp.13250. [DOI] [PubMed] [Google Scholar]
- 18.Kyranis SJ, Latona J, Platts D, Kelly N, Savage M, Brown M, Hamilton-Craig C, Scalia GM, Burstow D. Improving the echocardiographic assessment of pulmonary pressure using the tricuspid gegurgitant signal - The "chin" vs the "beard. Echocardiography. 2018;35:1085–1096. doi: 10.1111/echo.13893. [DOI] [PubMed] [Google Scholar]
- 19.Swanson KL, Utz JP, Krowka MJ. Doppler echocardiography-right heart catheterization relationships in patients with idiopathic pulmonary fibrosis and suspected pulmonary hypertension. Med Sci Monit. 2008;14:CR177–82. [PubMed] [Google Scholar]
- 20.Amsallem M, Boulate D, Kooreman Z, Zamanian RT, Fadel G, Schnittger I, Fadel E, McConnell MV, Dhillon G, Mercier O, Haddad F. Investigating the value of right heart echocardiographic metrics for detection of pulmonary hypertension in patients with advanced lung disease. Int J Cardiovasc Imaging. 2017;33:825–835. doi: 10.1007/s10554-017-1069-3. [DOI] [PubMed] [Google Scholar]
- 21.Amsallem M, Sternbach JM, Adigopula S, Kobayashi Y, Vu TA, Zamanian R, Liang D, Dhillon G, Schnittger I, McConnell MV, Haddad F. Addressing the Controversy of Estimating Pulmonary Arterial Pressure by Echocardiography. J Am Soc Echocardiogr. 2016;29:93–102. doi: 10.1016/j.echo.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 22.D'Alto M, Bossone E, Opotowsky AR, Ghio S, Rudski LG, Naeije R. Strengths and weaknesses of echocardiography for the diagnosis of pulmonary hypertension. Int J Cardiol. 2018;263:177–183. doi: 10.1016/j.ijcard.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Boucly A, Cottin V, Nunes H, Jais X, Tazi A, Prevot G, Reynaud-Gaubert M, Dromer C, Viacroze C, Horeau-Langlard D, Pison C, Bergot E, Traclet J, Weatherald J, Simonneau G, Valeyre D, Montani D, Humbert M, Sitbon O, Savale L. Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J. 2017;50 doi: 10.1183/13993003.00465-2017. 10.1183/13993003.00465-2017. Print 2017 Oct. [DOI] [PubMed] [Google Scholar]


