Abstract
The shuttling behavior and slow conversion kinetics of the intermediate lithium polysulfides are the severe obstacles for the application of lithium‐sulfur (Li‐S) batteries over a wide temperature range. Here, an engineered lamellar yolk–shell structure of In2O3@void@carbon for the Li‐S battery cathode is developed for the first time to construct a powerful barrier that effectively inhibits the shuttling of polysulfides. On the basis of the unique nanochannel‐containing morphology, the continuous kinetic transformation of sulfur and polysulfides is confined in a stable framework, which is demonstrated by using X‐ray nanotomography. The constructed Li‐S battery exhibits a high cycling capability over 1000 cycles at 1.0 C with a capacity decay rate as low as 0.038% per cycle, good rate performance, and temperature tolerance at −10, 25, and 50 °C. A nondestructive in situ monitoring method of the interfacial reaction resistance in different cycling stages is proposed, which provides a new analysis perspective for the development of emerging electrochemical energy‐storage systems.
Keywords: binding energy, cycling stability, nano‐CT, secondary batteries, shuttle effect
An engineered lamellar yolk–shell structure of In2O3@void@carbon for the Li‐S battery cathode is developed for the first time to construct a powerful barrier that effectively inhibits the shuttling of polysulfides. The continuous kinetic transformation of sulfur and polysulfides is confined in a stable framework, which is demonstrated by using X‐ray nanotomography. The battery exhibits a long cycling life over 1000 cycles with a capacity fade rate as small as 0.038% per cycle, good rate performance, and temperature tolerance.
1. Introduction
Due to the ever‐increasing demands of global energy consumption, electrochemical energy‐storage systems are developing rapidly. Since that, high energy‐density secondary batteries with long cycle lives become increasingly important. Lithium‐sulfur (Li‐S) batteries are considered to be a promisingly candidate for next‐generation battery systems due to their high theoretical energy density of 2600 Wh kg−1 and capacity of 1675 mAh g−1,[ 1 , 2 , 3 ] in addition to the high natural abundance of sulfur and low cost.[ 4 , 5 , 6 ] However, practical applications of Li‐S batteries are hampered by the poor conductivity and large volume‐change of sulfur, the shuttle effect of soluble polysulfides, and slow redox reaction kinetic of sulfur.[ 7 , 8 , 9 , 10 , 11 ]
Considering the conductivity and volume‐change issues of the sulfur cathodes, engineering a yolk–shell structure with a reserved buffering space for sulfur is an efficient strategy. For example, Lin's group used electrospinning to synthesize the interconnected yolk–shell carbon nanospheres assembled fiber network, exhibiting a capacity of 700 mAh g−1 at 1.0 C after cycling 500 times.[ 12 ] Liu and co‐workers prepared Fe2O3 nanoparticles within Mn3O4 nanosheet‐grafted hollow carbon capsules, which delivered a capacity as high as 1122 mAh g−1 after 200 cycles.[ 13 ] Novel hierarchical yolk–shell microspheres composed of 1D bamboo‐like N‐doped carbon nanotubes (CNTs) encapsulating Co nanoparticle as sulfur host were reported by Park et al., which displayed a good stability.[ 14 ] The yolk–shell structure has been considered to be able to alleviate several issues of Li‐S batteries; however, most of the studies focused on the properties at room temperature, which are quite different from the complicated temperature conditions in real applications.
Here, we report a novel lamellar yolk–shell indium oxide (In2O3)@void@carbon composite for encapsulating sulfur for the first time as a Li‐S battery cathode. The inner is assembled by dense In2O3 nanoflakes, forming numerous nanochannels, which exposes several sites to load sulfur and remain space for volume‐change; and the highly graphitized conductive carbon shell could improve the conductivity of the overall microsphere. A 3D space verified by X‐ray nanotomography (nano‐CT) proves an effective and continuous kinetic conversion of reversible capacity by limiting sulfur within the confined framework. As expected, an enhanced cycling performance is achievable at different temperatures by the accelerated redox reaction kinetics. The capacity decay behavior of the Li‐S battery at different temperatures is also demonstrated through the differential voltage (dV/dQ) analysis and a periodic galvanostatic intermittent titration technique (GITT). The adsorption models by density functional theory (DFT) calculations reveal the powerful capture ability of In2O3 toward polysulfides.
2. Results and Discussion
In2O3 microspheres were prepared by using a facile hydrothermal approach.[ 15 ] Scanning electron microscopy (SEM) displays the morphology of a flower‐like spherical structure with numerous laminated nanoflakes assembled radially with a diameter of 6–8 µm (Figure 1a,b). The formation mechanism of the structure was investigated through a series of synthesis under different conditions, as shown in Figure S1 (Supporting Information). In our study, cetyltrimethylammonium bromide (CTAB) was used as growth template, the microchannels among nanoflakes are correlated with the amount of CTAB. A higher reaction temperature makes the nanoflakes aggregate more tightly, and the overall shape tends to be spherical. Besides, both the amount of CATB and reaction temperature influence the assembly of nanoflakes on growth template. Figure S2c,d (Supporting Information) presents the SEM images of In2O3@SiO2. The In2O3@C yolk–shell structure after the carbon coating and alkali etching SiO2 process is shown in Figure 1c,d. The orange‐shaped nanochannel structure would provide several buffer spaces for the volume change of sulfur upon change–discharge. The hierarchical structure of In2O3@S@C microsphere is observed in Figure 1e,f and Figure S2e,f (Supporting Information). The zoomed‐in images of In2O3@C are shown in Figure S2h,i (Supporting Information). In addition, the high‐resolution transmission electron microscopy (HRTEM) image shows the highly crystalline nature by the clear lattice fringes with a d‐spacing of 0.292 nm, which matches the (222) plane of In2O3 (Figure 1g and inset). Elemental mappings and energy‐dispersive spectrometer (EDS) spectrum confirm the uniform distribution of In, O, C, and S in the In2O3@S@C microsphere (Figure 1h–m). The crystallographic structures of the as‐prepared In2O3 and In2O3@S@C are further identified by X‐ray diffraction (XRD) patterns (Figure 1n). All of the diffraction peaks are well‐indexed to the tetragonal In2O3 (JCPDS No. 71‐2194). For comparison, XRD pattern of the pure sulfur was also measured, which confirmed an obvious sulfur phase (JCPDS No. 78‐1889) within the In2O3@S@C composite.
Figure 1.
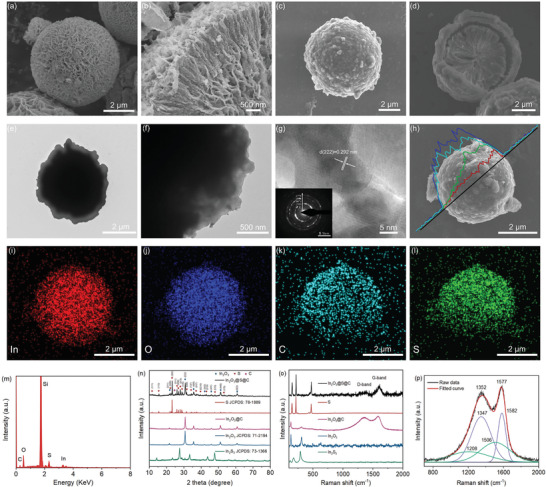
a) Low‐ and b) high‐magnification SEM images of the as‐synthesized In2O3 microsphere. c) SEM images of In2O3@C microsphere and d) sectional view of In2O3@C composites. e) TEM images of In2O3@S@C composites, f) edge of microsphere. g) HRTEM image inserted with a corresponding selected area electron diffraction pattern. h) Line‐scanning curves and i–l) elemental mapping images of the In2O3@S@C composites. m) EDS spectrum. n) XRD patterns of the In2S3, In2O3, In2O3@C, sulfur, and In2O3@S@C composites. o) Raman spectra and p) the deconvoluted Raman spectra from the Raman data in panel o) at the D and G band.
The Raman spectrum of each sample is displayed in Figure 1o. The Raman spectra show two prominent peaks, which correspond to A1g symmetry model of breathing vibration of rings (D‐band) and the E2g phonon of sp2‐bonded hybridized carbon atoms (G‐band).[ 16 ] The G and D peaks of the In2O3@C composite locate at 1577 and 1352 cm−1, respectively. The Raman spectrum was further fitted into four bands centered at 1582, 1506, 1347, and 1208 cm−1 by a Voigt model function (Figure 1p). The peaks at 1582 and 1347 cm−1 are the splitting peaks from peak G which are ascribed to the sp2 hybrid carbon atoms. The peaks at 1506 and 1208 cm−1 correspond to sp3 hybrid carbons belong to peak D.[ 17 , 18 ] The calculated peak area ratio of peak G and peak D is 1.32, indicating a high degree of graphitization and good conductivity. In addition, the high value of the ratio of D FWHM/G FWHM shows better elastic and mechanical strength properties,[ 19 ] which is beneficial for suppressing the volume change of sulfur during charge–discharge.
To compare the porous structure information between the In2O3@C and In2O3@S@C, N2 adsorption–desorption was measured (Figure S3, Supporting Information). The increase of the inner surface of the yolk–shell structure results in a high Brunauer−Emmett−Teller (BET) surface area of 81.1 m2 g−1. The BET surface area of In2O3@S@C decreases to 4.44 m2 g−1 after sulfur loading, which indicates the nearly complete filling of sulfur in the pores. The surface composition and chemical states of In2O3@S@C were characterized by using X‐ray photoelectron spectroscopy (XPS). Figure S4a (Supporting Information) displays the survey spectrum in which the S 2p3/2, S 2s, C 1s, In 3d5/2, In 3d3/2, and O 1s signals are observed.[ 20 , 21 ] The binding energies of In 3d5/2 and In 3d3/2 (Figure S4b, Supporting Information) are measured with peaks located at 444.5 and 452.0 eV, respectively, which indicate a trivalent oxidation state for In element.[ 22 , 23 ] As displayed in the O 1s spectrum (Figure S4c, Supporting Information), lattice oxygen peaks at 530.0, 531.9, and 532.5 eV correspond to —OH stems from In(OH)3, In—O, and C—O/C═O, respectively.[ 24 ] Figure S4d (Supporting Information) shows the high‐resolution XPS spectrum of the C 1s. An asymmetric peak at 284.8 eV is associated with sp2 carbon, while other peaks are assigned to C—N/C═N and O—C═O.[ 25 ] The S 2p in Figure S4e (Supporting Information) was fitted into two peaks. The peaks at 163.6 and 164.7 eV are attributed to the S 2p3/2 and S 2p1/2, respectively.[ 26 , 27 ]
The electrochemical performance of the constructed Li‐S batteries was evaluated. Samples with sulfur contents from 74.5 to 90.1 wt% were obtained based on different mass mixing and were measured by thermogravimetric analysis (TGA), as shown in Figure S5 (Supporting Information). Figure 2a displays the cyclic voltammetry (CV) studies based on cathode with 74.5 wt% sulfur content in the In2O3@S@C, the CV curves show a typical cathodic peak at 2.2–2.4 V corresponds to the reduction of S8 to soluble long chain polysulfide (Li2S n , 4 ≤ n ≤ 8).[ 28 ] The peak at 1.9–2.1 V corresponds to the process in which long Li2S n chain is reduced to short chain Li2S/Li2S2.[ 29 ] The overlapped CV curves after initial scanning demonstrate high reversibility and excellent stability of redox reaction. Figure 2b and Figure S6 (Supporting Information) show the rate performance of the cathodes based on In2O3@S and In2O3@S@C during three rounds cycling from 0.2 to 3.2 C. In2O3@S@C cathode achieves highly reversible discharge capacities of 1042.9, 871.3, 759.1, 635.5, and 392.3 mAh g−1 at rate of 0.2, 0.4, 0.8, 1.6, and 3.2 C in first round, respectively. Even after three rounds, the capacity keeps well. In contrast, In2O3@S without carbon shell has a poor rate performance, especially at a high rate.
Figure 2.
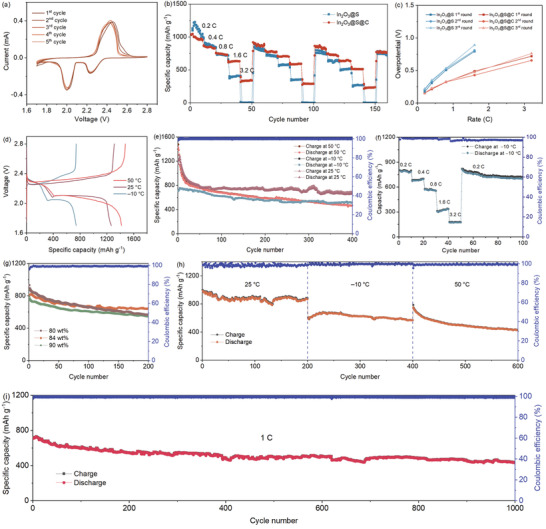
a) Initial five CV cycles of the In2O3@S@C at a scan rate of 0.1 mV s−1. b) Rate performance. c) Overpotentials of the discharge/charge potential plateaus at different rates. d,e) Galvanostatic charge–discharge profiles and cycling performance of In2O3@S@C at 0.2 C over 400 cycles under different temperatures. f) Capacity and Coulombic efficiency as the discharge rate was changed while the charge rate was kept at 0.2 C. g) Cycling capacity of the In2O3@S@C composite with various sulfur contents at 0.5 C under room temperature. h) Prolonged cycling behavior of In2O3@S@C with S content of 84 wt% at 0.2 C under different temperature gradients. i) Long‐term cycling performance of In2O3@S@C at 1.0 C.
To quantitatively evaluate the reaction kinetics, we calculated the corresponding overpotentials at different rates of each round, as shown in Figure 2c. The In2O3@S@C cathode displays low overpotentials. Reduction of polarization is attributed to the strong chemical adsorption of polysulfides by the In2O3 core, and a powerful barrier established by the yolk–shell structure further against the irreversible diffusion of lithium polysulfides, resulting in efficient inhibition of shuttle effect. Furthermore, the carbon shell contains abundant pores which provide sufficient spaces for the absorption of polysulfides.[ 30 ] The X‐ray absorption near edge structure (XANES) spectra of C K‐edge and O K‐edge (Figure S7, Supporting Information) further reveal remarkable changes of the chemical structure after cycling.[ 31 ] In addition, the comparison of impedance between In2O3@S@C and In2O3@S also verifies that the carbon enhances the electron transport (Figure S8, Supporting Information). Electrochemical studies of bare In2O3 and In2O3@C without sulfur indicate that the capacity contributions from In2O3 and carbon are extremely neglectable (Figure S9, Supporting Information). Cycling at different charge versus discharge rates was also performed (Figure S10, Supporting Information). After 100 cycles, the capacities for the two cases of charge/discharge rates of 0.3 C/0.6 C and 0.6 C/0.3 C remain 922.9 and 747.7 mAh g−1, respectively.
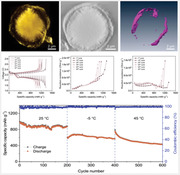
The electrochemical performance of the In2O3@S@C at different temperatures was further evaluated. The first‐cycle discharge–charge profiles of the In2O3@S@C at 0.2 C under different temperatures are presented in Figure 2d. The In2O3@S@C shows an excellent cyclability at room temperature, keeping a reversible capacity of 703.3 mAh g−1 after 400 cycles. At the initial stage at high temperature, high‐temperature environment stimulates the internal electrochemical activity, making the In2O3@S@C exhibits high capacities (Figure 2e). As the cycling proceeds, the intensified dissolution of lithium polysulfides and the increased polarization reduce the capacity. Under a relatively low temperature, the capacity maintains a stable level, which is related to the low polarization and the effective conversion reaction of high‐order polysulfides. The low initial discharge capacity is ascribed to downward shift of the low discharging potential plateau caused by the slow solid phase deposition kinetics.[ 32 ] The capacity‐contribution distribution under high and low potential plateaus reflects the changing process obviously (Figure S11, Supporting Information).
In addition, a series of cycles at different discharge rates with the same charge rate under −10 °C were measured (Figure 2f). When the cycling rates were turned back to 0.2 C, the capacity recovered to a high level, indicating the wide temperature adaptability. Figure 2g shows the specific capacities of In2O3@S@C with S contents of 80, 84, and 90 wt% at 0.5 C after 200 cycles. Moreover, the cathode with a relatively high sulfur loading of ≈4.5 mg cm−2 with a low electrolyte/sulfur ratio of 8 µL mg–1 was further investigated, which displayed a good reversible capacity, as shown in Figure S12 (Supporting Information). We also studied the prolonged cyclic performance with an 84 wt% sulfur content at different temperatures. Continuous testing was carried out at room temperature, high temperature of 50 °C and low temperature of −10 °C (Figure 2h and Figure S13, Supporting Information). In2O3@S@C remains 431.2 mAh g−1 after 600 cycles, which shows a good wide temperature adaptability, indicating a potential for applications. Of course, considering the price of indium, the content of In2O3 in the composite should be decreased for practical use, which could be engineered by reducing the thickness of In2O3 lamellas. Impressively, In2O3@S@C achieves a high capacity of 440.8 mAh g−1 even after 1000 cycles at 1.0 C with a low capacity‐fading rate of 0.038% per cycle, and the Coulombic efficiency keeps exceeding 99.5% (Figure 2i).
In Figure 3a, we conducted ex situ XRD studies on the In2O3@S@C cathode. Excepting for the S8 and Li2S reflections, no other diffraction peaks are observed, which is consistent with previous report.[ 33 ] The crystal form of In2O3 with obvious sharp peak remains fixed in the process of potential change, which shows high crystallinity and stability. To further investigate a precise 3D spatial structure information, we used an X‐ray nano‐CT to reconstruct the In2O3@C. The 2D projection and 3D reconstruction images in Figure 3b,c show the obvious hierarchy of yolk–shell structure through the tomography of the hemispherical cross section of In2O3@C. Furthermore, we isolated the carbon layer by detecting the transition difference before and after the carbon absorption edge to obtain further depth distribution information (Figure 3d,e). The void between the In2O3 core and carbon layer would provide stable sites for sulfur loading. A 3D reconstruction of a sphere and a 3D rendering of the carbon layer are displayed in Movies S1 and S2 (Supporting Information), respectively. Moreover, such Li2S restricted at a controllable area is beneficial for continuous kinetic conversion to achieve a reversible capacity. [ 34 ]
Figure 3.
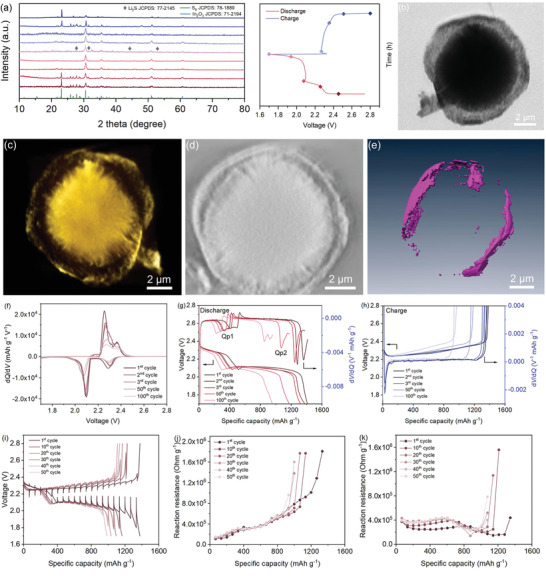
a) Ex situ XRD patterns of the In2O3@S@C when charging and discharging at various potentials. b) Synchrotron X‐ray tomography image of In2O3@C. c) 3D computed tomography image. d) Sliced image of the In2O3@C. e) 3D rendering image of carbon layer. f) dQ/dV plots (Q, capacity; V, voltage). g,h) dV/dQ versus capacity curves during discharge and charge. dV/dQ curves were obtained from the capacity test operated at a C/20 rate at 25 °C. i) Potential response curves of the In2O3@S@C electrodes during GITT measurement at room temperature with 20 min charge/discharge at 0.2 A g−1 followed by 1 h at rest. j,k) In situ reaction resistances of In2O3@S@C during discharge/charge processes.
A differential potential (dV/dQ) curve obtained by differentiating the charge/discharge potential with corresponding capacity and the change of impedance during cycling, have been indicated as two indicators of the degradation of electrode.[ 35 ] In our study, the capacities were measured at C/20 to minimize the contribution of the increase polarization, which were verified by dQ/dV plots (Figure 3f). The stable peak position indicates the consistency of the potential of charging and discharging plateaus. The peak shift of dV/dQ and the change in the peak‐to‐peak capacity were used to understand the capacity fade. Figure 3g,h shows the shifts in the charge and discharge curves and the dV/dQ curves of initial three cycles after 50 and 100 cycles. The slightly shift of Qp1 corresponds to effective conversion of high‐order polysulfides, the peak‐to‐peak capacity decreases with the dramatic shift of Qp2, which is consistent with the result of capacity contribution under high and low potential plateaus (Figure S11, Supporting Information). The passivation of Qp2 peak reflects the inhomogeneity caused by sluggish solid deposition reaction.
The GITT was used to in situ monitor the interfacial reaction resistances at different charge/discharge stages under various temperatures. From the first cycle, a constant current density of 0.2 A g−1 with 20 min charge/discharge and followed by 1 h at rest was applied to collect the potential response in every ten cycles during the GITT measurements, as presented in Figure S14a,b (Supporting Information). The interfacial reaction resistances during discharge/charge processes at room temperature always maintains a low value and a small overpotential change (Figure 3i–k). The low reaction resistance of the battery at the first cycle under high temperature corresponds to the high capacity in the initial stage; and the gradually increasing overpotential increases the interface reaction barrier and intensifies the capacity decay (Figure S14c–e, Supporting Information). At a low temperature, the initially high interface resistance reduces the reaction activity, subsequently the stable conversion of high‐efficiency polysulfides in the high potential plateau becomes the main factor for the stability of the capacity (Figure S14f–h, Supporting Information).
The electrochemical kinetics were investigated by using the CV curves between 0.1 and 1.0 mV s−1 (Figure 4a). The peak currents (i) and rates (v) are as follows: I = avb , and log(i) = blog(v) + log(a).[ 36 ] Figure 4b shows that the b values for the cathodic and anodic peaks are 0.50 and 0.64, respectively, indicating a common kinetics process by diffusion‐controlled and capacitive characteristics. Based on equation i(v) = k 1 v + k 2 v 1/2, the total contribution is obtained, where k 1 v and k 2 v 1/2 represent the contributions caused by the capacitive effect and diffusion‐controlled intercalation, respectively. The ratio of the capacitive contribution increases depending on the increase of scan rates, as displayed in Figure 4c. At a scan rate of 1.0 mV s−1, the capacitive contribution is about 51.6%, which indicates the capacitance‐control effect under a high rate is gradually becoming significant.
Figure 4.
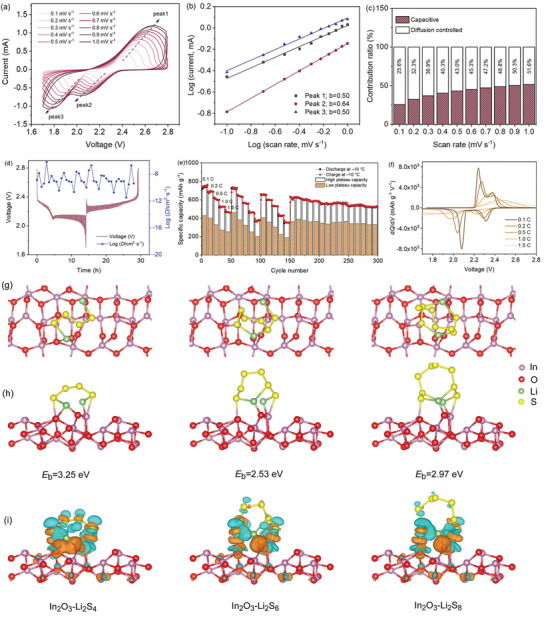
a) CV curves of the In2O3@S@C composite at a series of scan rates. b) Plots of log (i) versus log (v) and the fitting results at different redox states. c) Ratios of capacitive contributions at different rates. d) Temporal voltage evolution in GITT studies with the corresponding Li‐ion diffusion coefficients. e) Rate performance and different plateau capacity of the In2O3@S@C with S feeding ratio of 84.3 wt% cycling under −10 °C. f) dQ/dV plots of different rates. Optimized configurations of polysulfides (Li2S4, Li2S6, and Li2S8) adsorbed on In2O3 (110) surfaces: g) top view, h) side view, and i) electron density differences of corresponding structures (the gold and cyan regions represent negative and positive change of the isovalue of ±0.002).
The diffusion‐controlled reaction kinetics through the electrochemical process were further analyzed by using GITT. As shown in Figure 4d and Figure S15 (Supporting Information), the Li+ ion diffusion coefficients of the In2O3@S@C are calculated to be 3.28 × 10−12 to 6.26 × 10−7 cm2 s−1, suggesting fast Li+ ion diffusion kinetics.[ 37 ] Moreover, several rounds of rate tests were performed with a higher sulfur content at low temperatures (Figure 4e and Figure S16, Supporting Information). By comparing the capacity contribution between high‐ and low‐plateau capacities at different current densities, it is noted that the contribution of high discharge plateau to the total capacity gradually increases in the process of increasing the current density, which is consistent with the high b value at the high discharge plateau. In contrast, the low discharge plateau is mainly affected by diffusion‐controlled process, which provides limited capacity due to the slow solid‐phase reaction kinetics. In addition, the cathode shows a small polarization at high rate under a low temperature (Figure 4f).
In addition, the adsorption between In2O3 and polysulfides was studied through DFT calculations. The adsorption models are displayed in Figure 4g,h. In our study, a common In2O3 facet of (110) was selected for simulations. The binding energies of polysulfides on the In2O3 are −3.2528, −2.5272, and −2.9741 eV for Li2S4, Li2S6, and Li2S8, respectively. The large adsorption energy implies that the shift of polysulfides is reduced efficiently. In Figure 4i, the bader charges analysis was further conducted to demonstrate the electron transfer. Taking the In atom as an example, the bader charges are 11.477, 11.485, and 11.461 after adsorbing Li2S4, Li2S6, and Li2S8, respectively. The brown and blue colors show the accumulation and consumption of charges. The shifts of charge verify the strong adsorption between In2O3 and polysulfides, which is able to efficiently suppress the shuttle effect of polysulfides and reduce the capacity decay caused by the loss of soluble polysulfides. Moreover, the Li2S6 solution adsorbed by In2O3@C exhibits a transparent color. It indicates the strong adsorption capability toward the polysulfides, which is confirmed by the UV–vis spectra (Figure S17, Supporting Information). The robust structure is further confirmed by the post‐cycled composite (Figure S18, Supporting Information). After cycling 1000 times, the composite keeps stable morphology and phase compared to the initial one.
3. Conclusions
In summary, we develop a yolk–shell In2O3@void@carbon composite as the efficient host for Li‐S battery. Owing to the lamellar inner configuration and enhanced adsorption capacity of In2O3 for polysulfides confirmed by DFT calculations, the In2O3@S@C cathode displays a good rate‐performance, long‐term cycling life with a low capacity decay rate after 1000 cycles at 1.0 C, and stable electrochemical performances in a wide temperature range. Furthermore, the rapid kinetic characteristics of continuous transformation during reversible reactions is proved by nano‐CT. The capacity decay behavior is explained by dV/dQ analysis and periodic GITT, which would provide some new insights for engineering high‐performance energy‐storage materials and their battery systems.
4. Experimental Section
Synthesis of In2O3 Microspheres
The flower‐shaped microsphere precursors were fabricated by a hydrothermal strategy. Typically, 1 mmol InCl3·4H2O, 2 mmol CS(NH2)2, and 1 mmol CTAB were dissolved in 40 mL ethanol to form a uniform solution. The solution was transferred into a Teflon‐lined stainless steel autoclave and maintained at 150 °C for 12 h. And afterward, the reactor was dropped to room temperature naturally. The precipitate was collected and washed several times with absolute ethanol and deionized water. The samples were annealed in air atmosphere at 650 °C for 2 h with a ramping rate of 5 °C min−1.
Preparation of In2O3@C
First of all, cover two layers of SiO2 on the surface of the In2O3 core. Typically, the prepared 0.2 g In2O3 was dispersed in a solution consisting of 40 mL absolute ethanol and 8 mL of deionized water by ultrasound. Then, 2 mL of ammonia aqueous solution was added under stirring. Subsequently, 1.6 mL of tetraethyl orthosilicate solution was slowly poured, and being stirred for 2 h. After standing for a few minutes, the precipitate was collected and dried. The steps shown above were repeated twice. 0.2 g of In2O3@SiO2 prepared above was dispersed in 50 mL of deionized water. Then, 1.211 g of tris(hydroxymethyl)aminomethane was added under stirring while hydrochloric acid was added dropwise to adjust the pH to 8.5. Subsequently, 75 mg of dopamine hydrochloride was added to the solution and stirred continuously for 24 h. 10 mL of ammonium hydroxide solution (28%–30% NH3) was added to 25 mL deionized water to form a dilute solution, then the In2O3@SiO2@C was put into the solution and transferred into a Teflon‐lined stainless steel autoclave which was then maintained at 150 °C for 8 h. Finally, the samples were calcined in nitrogen gas at 650 °C for 4 h.
Preparation of In2O3@S@C
In2O3@C and sulfur powders were mixed in a mass ratio of 1:3 in an argon atmosphere and sealed in a heat‐resistant Teflon bottle, maintained at 155 °C for 15 h. After cooling to room temperature naturally, the In2O3@S@C was collected.
Characterization
The morphologies, structure, and compositions of the samples were investigated on a scanning electron microscopy (FESEM, Hitachi Regulus8100, operated at 5kV) and TEM (HT‐7700). HRTEM images and the selected area electron diffraction (SAED) patterns were obtained on a JEOL JEM‐2010F microscope at an acceleration voltage of 200 kV to get the information of microstructure and crystallinity of products. The crystal structure and phase were determined by XRD (Bruker D8 Advance) with Cu Kɑ radiation at a wavelength of 1.5418 Å and XPS (ESCALAB 250Xi). Elemental analysis was measured by EDS. The surface area was measured by using BET (ASAP 2460). The specific gravity of each component was determined by TGA (LabRAM HR800) with a heating rate of 10 °C min−1. Raman spectra were measured by using a spectroscopy (Renishaw in Via). The ultraviolet–visible spectroscopy (U‐2910) was used to check the existence of soluble polysulfide products. XANES were carried out at the Catalysis and Surface Science Endstation at the BL11U beamline in the National Synchrotron Radiation Laboratory (NSRL) in Hefei, China. The soft X‐ray computed nanotomography was conducted on the beamline BL07W of National Synchrotron Radiation Laboratory (NSRL), Hefei, China. For the sample preparation of synchrotron‐based X‐ray nano‐CT, 10 mg of the sample powder was uniformly dispersed in 1 mL of ethanol solution by ultrasonic treatment. Then, 0.1 mL of the mixed solution was dropped on 100‐mesh, carbon‐coated copper‐finder grids (Beijing Zhongjingkeyi Technology Co., Ltd.) followed by natural drying. Finally, the grids loaded with samples were transferred into vacuum chamber for X‐ray nano‐CT tests through a transmission mode. An elliptical capillary condenser was used for the focus of the X‐ray beam. A total of 121 projections in each sample were performed at tilt angles ranging from −60° to 60° at 1° increments with the exposure to X‐ray (445 eV) within 2.0 s. For the tomographic reconstruction, the projections were reconstructed by using the total variation based simultaneous algebraic reconstruction technique (SART‐TV). All projections were corrected based on a reference image with a flat field intensity and aligned to the rotation axis. Then, the reconstructed images were imported into Amira (FEI Visualization Sciences Group, MA) for segmentation and 3D visualization.
Electrochemical Tests
The Li‐S battery cathodes were prepared by coating the slurry of the composite (70 wt%), super P (20 wt%), and polyvinylidene fluoride binding agent (10 wt%) onto an Al foil, dried at 70 °C in a vacuum oven for 12 h. The thickness of electrode was about 250 µm. The sulfur loading and the diameter of the sulfur cathode were 2.1 mg cm−2 and 1.2 cm, respectively. The prepared electrodes were mounted into a coin‐cell (CR2032 type) with pure lithium foil which was used as the anode and Celgard 2400 as the separator as well as the electrolyte (1.0 m LiTFSI in a 1:1 (v/v) 1,3‐dioxolane/dimethyl ether mixture with 1.0 wt% LiNO3). The thickness of Li metal anode was about 500 µm, the dosage of the electrolyte used in cells were about 15 and 8 µL mg–1 of electrolyte/sulfur (E/S) ratio for the electrodes with sulfur loadings of 2.1 and 4.5 mg cm−2, respectively. All batteries were assembled in an argon‐filled glovebox (Mikrouna, Super 1220/750/900) and cycled in the potential range of 1.7–2.8 V through a galvanostatic charge/discharge method on a Neware battery test system (Shenzhen Neware Technology Co., Ltd, CT‐4008). The capacities of the coin‐type cells are calculated by the mass of the corresponding sulfur. CV curves were performed on an electrochemical workstation (CHI660E). GITT measurements were performed by discharging/charging the cell with a 20 min current pulse at 0.2 A g−1 followed by a 1 h rest to collect the potential response. This protocol was designed so that an equilibrium voltage (or close to equilibrium) was obtained in a reasonable experimental time. The difference between quasi‐open‐circuit potential and closed‐circuit potential is used to calculate the overpotentials, and then the in situ reaction resistance can be calculated as R = ΔU overpotential/(M 2 mass loading × I current density). The GITT tests of the diffusion coefficients of Li ions at 0.1 C with 10 min discharge–charge followed by 10 min at rest. A total of 50 current pulses was used for the discharge.
Binding Energy Calculations
The DFT calculations were performed by using CASTEP program. The generalized gradient approximation Perdew–Burke–Ernzerh of functional was used to describe the exchange‐correlation energy. For all the calculations, the plane‐wave energy cut‐off was set to be 500 eV. For surface adsorption calculations, the Brillouin zone was sampled with 3 × 3 × 1 k‐point mesh. At least 12 Å vacuum layer was applied in z‐direction of the slab models to prevent the vertical interactions between slabs. The convergence thresholds for structural optimization were set at 0.01 eV Å–1 in force and 10–5 in energy. The binding energy between polysulfides and In2O3 surface was defined in the following equation
| (1) |
where was the total energy of substrate with adsorbed polysulfides, E surface was the total energy of substrate, and was the energy of polysulfides.
Statistical Analysis
The images were processed on a Fireworks software (Adobe, San Jose, CA). The XRD, XPS, HRTEM, Raman, and electrochemical impedance spectroscopy (EIS) spectra were analyzed on Jade software (Materials Data, Livermore, CA), Peak Fit software (Systat software, Inc., San Jose, CA), Digital Micrography software (Gatan Inc, Pleasanton, CA), Labspec software (Horiba, Kyoto, JP), OMNIC (Thermo Fisher Scientific Inc., Waltham, MA), and ZView (Scribner Associates, Inc., Charlottesville, VA), respectively. The 3D synchrotron X‐ray tomography images were reconstructed on Amira software (FEI Visualization Sciences Group, MA). Results were analyzed on an OriginPro software (Origin Lab, Northampton, MA).
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supporting Information
Supplemental Movie 1
Supplemental Movie 2
Acknowledgements
J.L. and Y.D. contributed equally to this work. This work was supported by the National Key Research and Development Program of China (2017YFA0402904, 2020YFA0406104), Science and Technology Major Project of Anhui Province (18030901093), Key Research and Development Program of Wuhu (2019YF07), National Natural Science Foundation of China (22075131, 11775224, and U2032148), University Synergy Innovation Program of Anhui Province (GXXT‐2020‐073). The authors also thank the National Synchrotron Radiation Lab for the synchrotron beam time at the University of Science and Technology of China.
Liu J., Ding Y., Shen Z., Zhang H., Han T., Guan Y., Tian Y., Braun P. V., A Lamellar Yolk–Shell Lithium‐Sulfur Battery Cathode Displaying Ultralong Cycling Life, High Rate Performance, and Temperature Tolerance. Adv. Sci. 2022, 9, 2103517. 10.1002/advs.202103517
Contributor Information
Jinyun Liu, Email: jyliu@iim.ac.cn.
Yangchao Tian, Email: ychtian@ustc.edu.cn.
Data Availability Statement
Research data are not shared.
References
- 1. Zhang L. L., Zhao D. B., Muhammad Z., Wan F., Xie W., Wang Y. J., Song L., Niu Z. Q., Chen J., Adv. Mater. 2019, 31, 1903955. [DOI] [PubMed] [Google Scholar]
- 2. Bhargav A., He J., Gupta A., Manthiram A., Joule 2020, 4, 285. [Google Scholar]
- 3. Pang Q., Liang X., Kwok C. Y., Nazar L. F., Nat. Energy 2016, 1, 16132. [Google Scholar]
- 4. Xiao F., Yang X., Wang H., Xu J., Liu Y., Yu D. Y. W., Rogach A. L., Adv. Energy Mater. 2020, 10, 2000931. [Google Scholar]
- 5. Yu J., Xiao J., Li A., Yang Z., Zeng L., Zhang Q., Zhu Y., Guo L., Angew. Chem., Int. Ed. 2020, 59, 13071. [DOI] [PubMed] [Google Scholar]
- 6. Li Y. J., Guo S. J., Matter 2021, 4, 1142. [Google Scholar]
- 7. Seh Z. W., Li W. Y., Cha J. J., Zheng G. Y., Yang Y., McDowell M. T., Hsu P.‐C., Cui Y., Nat. Commun. 2013, 4, 1331. [DOI] [PubMed] [Google Scholar]
- 8. Wu Q., Yao Z., Zhou X., Xu J., Cao F., Li C., ACS Nano 2020, 14, 3365. [DOI] [PubMed] [Google Scholar]
- 9. He Y., Li M., Zhang Y., Shan Z., Zhao Y., Li J., Liu G., Liang C., Bakenov Z., Li Q., Adv. Funct. Mater. 2020, 30, 2000613. [Google Scholar]
- 10. Lu C., Chen Y., Yang Y., Chen X., Nano Lett. 2020, 20, 5522. [DOI] [PubMed] [Google Scholar]
- 11. Yao Y., Wang H., Yang H., Zeng S., Xu R., Liu F., Shi P., Feng Y., Wang K., Yang W., Wu X., Luo W., Yu Y., Adv. Mater. 2020, 32, 1905658. [DOI] [PubMed] [Google Scholar]
- 12. Lin L. L., Pei F. P., Peng J., Fu A., Cui J. Q., Fang X. L., Zheng N. F., Nano Energy 2018, 54, 50. [Google Scholar]
- 13. Liu H. D., Chen Z. L., Zhou L., Pei K., Xu P. D., Xin L. S., Zeng Q. W., Zhang J., Wu R. B., Fang F., Che R. C., Sun D. L., Adv. Energy Mater. 2019, 9, 1901667. [Google Scholar]
- 14. Park S. K., Lee J. K., Kang Y. C., Adv. Funct. Mater. 2018, 28, 1705264. [Google Scholar]
- 15. Liu J. Y., Luo T., Meng F. L., Qian K., Wan Y. T., Liu J. H., J. Phys. Chem. C 2010, 9, 4887. [Google Scholar]
- 16. Shi M. M., Bao D., Li S. J., Wulan B. R., Yan J. M., Jiang Q., Adv. Energy Mater. 2018, 8, 1800124. [Google Scholar]
- 17. Xu D. H., Luo G. E., Yu J. F., Chen W. Y., Zhang C. C., Yang D. O., Fang Y. P., Yu X. Y., J. Alloys Compd. 2017, 702, 499. [Google Scholar]
- 18. Li F., Luo G. E., Chen W. Y., Chen Y. C., Fang Y. P., Zheng M. T., Yu X. Y., ACS Appl. Mater. Interfaces 2019, 11, 36949. [DOI] [PubMed] [Google Scholar]
- 19. Kim A. Y., Ardhi R. E. A., Liu G. C., Kim J. Y., Shin H. J., Byun D. J., Lee J. K., Carbon 2019, 153, 62. [Google Scholar]
- 20. Sun L., Liu X. H., Ma T. T., Zheng L. L., Xu Y. S., Guo X. X., Zhang J., Solid State Ionics 2019, 329, 8. [Google Scholar]
- 21. Selvamani V., Suryanarayanan V., Velayutham D., Gopukumar S., New J. Chem. 2017, 41, 3297. [Google Scholar]
- 22. Ye F., Wang C., Du G., Chen X., Zhong Y., Jiang J. Z., J. Mater. Chem. 2011, 21, 17063. [Google Scholar]
- 23. Zhou J., Tian G. H., Chen Y. J., Shi Y. H., Tian C. G., Pan K., Fu H. G., Sci. Rep. 2015, 4, 4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osiak M., Khunsin W., Armstrong E., Kennedy T., Sotomayor Torres C. M., Ryan K. M., O'Dwyer C., Nanotechnology 2013, 24, 065401. [DOI] [PubMed] [Google Scholar]
- 25. Chen Y., Ma W., Cai K., Yang X., Huang C., Electrochim. Acta 2017, 246, 615. [Google Scholar]
- 26. Jin Q., Wang K., Feng P., Zhang Z., Cheng S., Jiang K., Energy Storage Mater. 2020, 27, 43. [Google Scholar]
- 27. Yu P., Feng L. X., Ma D. C., Sun X. R., Pei J. K., Zha X. J., Bao R. Y., Wang Y., Yang M. B., Guo Z. P., Yang W., Adv. Funct. Mater. 2021, 31, 2008652. [Google Scholar]
- 28. Li Z., Zhou C., Hua J., Hong X., Sun C., Li H. W., Xu X., Mai L., Adv. Mater. 2020, 32, 1907444. [DOI] [PubMed] [Google Scholar]
- 29. Zhou G. M., Zhao S. Y., Wang T. S., Yang S. Z., Johannessen B., Chen H., Liu C. W., Ye Y. S., Wu Y. C., Peng Y. C., Liu C., Jiang S. P., Zhang Q. F., Cui Y., Nano Lett. 2020, 20, 1252. [DOI] [PubMed] [Google Scholar]
- 30. Xie J., Li B. Q., Peng H. J., Song Y. W., Zhao M., Chen X., Zhang Q., Huang J. Q., Adv. Mater. 2019, 31, 1903813. [DOI] [PubMed] [Google Scholar]
- 31. Feng X. F., Song M. K., Stolte W. C., Gardenghi D., Zhang D., Sun X. H., Zhu J. F., Cairns E. J., Guo J. H., Phys. Chem. Chem. Phys. 2014, 16, 16931. [DOI] [PubMed] [Google Scholar]
- 32. Deng D. R., Xue F., Bai C. −D., Lei J., Yuan R. M., Zheng M. S., Dong Q. F., ACS Nano 2018, 12, 11120. [DOI] [PubMed] [Google Scholar]
- 33. Pang Q., Shyamsunder A., Narayanan B., Kwok C. Y., Curtiss L. A., Nazar L. F., Nat. Energy 2018, 3, 783. [Google Scholar]
- 34. Zhou J. B., Liu X. J., Zhu L. Q., Zhou J., Guan Y., Chen L., Niu S. W., Cai J. Y., Sun D., Zhu Y. C., Du J., Wang G. M., Qian Y. T., Joule 2018, 2, 2681. [Google Scholar]
- 35. Kato H., Kobayashi Y., Miyashiro H., J. Power Sources 2018, 398, 49. [Google Scholar]
- 36. Tian W., Hu H., Wang Y. X., Li P., Liu J. Y., Liu J. L., Wang X. B., Xu X. D., Li Z. T., Zhao Q. S., Ning H., Wu W., Wu M., ACS Nano 2018, 12, 1990. [DOI] [PubMed] [Google Scholar]
- 37. Wang J., Polleux J., Lim J., Dunn B., J. Phys. Chem. C 2007, 111, 14925. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supplemental Movie 1
Supplemental Movie 2
Data Availability Statement
Research data are not shared.


