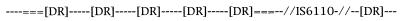Abstract
Spoligotyping is a major tool for molecular typing of Mycobacterium bovis. This technique is based on the polymorphism of spacers that separate direct repeats (DRs) in the M. tuberculosis complex DR region. Numerous M. bovis strains show a lack of several spacers which appears as a gap in the spoligotyping pattern. To determine whether these gaps contain alternative spacers not included in the spoligotyping membrane, PCRs using primers that hybridize to the spacers adjacent to the gaps were performed. Comparing the sizes of products obtained by PCR with those deduced from spoligotyping patterns, fragments were selected and sequenced to look for alternative spacers. Upon analysis of the sequences, five alternative spacers were detected, although deletions of spacers are mainly responsible for the observed gaps. The alternative spacers, which are more frequent in M. bovis than in M. tuberculosis, may contribute to increased M. bovis differentiation.
Bovine tuberculosis (TB) is a chronic zoonotic disease whose etiological agent is Mycobacterium bovis. It constitutes a serious animal health problem, causing economic losses due to decreased meat and milk production and to low exportation of cattle products.
While the main host of M. bovis is cattle, other animals, including humans, may be affected. Argentina, with approximately 5% of its cattle affected, has one of the highest prevalence rates in South America (9). Tuberculous lesions were also found in 3% of pigs and recently in goats and deer (S. Underwood, S. Pinto, M. Rey Moreno, and J. C. Carfagnini, Abstr. 1st Workshop on Human and Animal Infections Provoked by Chlamydia, Mycobacteria, Brucella, and Borrelia, 1997). In addition, M. bovis can affect domestic animals such as dogs and cats, where the infection route may be food of bovine origin, such as uncooked lung tissue (F. Fernández and E. Morici, Abstr. 1st Workshop on Human and Animal Infections Provoked by Chlamydia, Mycobacteria, Brucella, and Borrelia, 1997). A study performed in the main milk production region of Argentina showed that in the period 1984 to 1989, M. bovis was responsible for 2.4 to 6.2% of the TB cases in human beings, of which 64% were rural and meat workers (11). The AIDS epidemic has also increased the risk of transmission of M. bovis to humans. Nosocomial transmission of TB produced by multiresistant M. bovis strains among human immunodeficiency virus-positive individuals was recently described in Spain (16).
Phenotypic typing methods (serotyping, biotyping, etc.) cannot efficiently discriminate among strains of the M. tuberculosis complex. On the other hand, molecular biology tools, such as restriction fragment length polymorphism (RFLP) or the more recent spoligotyping technique (8, 13), are highly efficient for typing of mycobacteria. In human TB, molecular typing is advanced by the use of the insertion sequence IS6110 (6, 7, 18), which is repeated many times in the M. tuberculosis genome, producing a genotypic heterogeneity of isolates. In M. bovis, IS6110 is less useful because the genome of most strains contains only one or very few IS6110 copies (6, 14, 20). The insertion element IS6110 is frequently found in a unique locus of the M. tuberculosis complex genome called the direct repeat (DR) region (7). The DR region was completely sequenced in M. tuberculosis H37RV (7), in an M. bovis isolate from the United State (3), and in different BCG substrains (19). In addition, the DR region sequence from the M. bovis isolate currently undergoing genome sequencing in England is available (http://www.sanger.ac.uk/projects/M_bovis). The DR region consists of 36-bp repetitive sequences separated by nonrepetitive spacers whose lengths vary from 27 to 41 bp. Deletions in this region alter the spacer composition in each strain. Spoligotyping has been developed based on these DR region properties (8).
Several studies have demonstrated that the degree of differentiation achieved by spoligotyping is higher than that of IS6110 RFLP for strains with a low IS6110 copy number, such as M. bovis (1, 2, 5, 20, 22). In contrast, for strains with high IS6110 copy numbers, like most M. tuberculosis strains, IS6110 RFLP is the more discriminative test (4, 10).
Considering that spoligotyping is a rapid and easy-to-apply technique, it is important to improve its capacity for differentiation. The present study analyzed the DR region of M. bovis isolates for the presence of alternative spacers not included in the spoligotyping membrane. The addition of these alternative spacers to the membrane would probably improve its discriminatory power.
MATERIALS AND METHODS
Strains.
M. bovis DNA from bovine isolates from different parts of South America were used. The strains selected for the study were M. bovis 539 and 540 (pattern B), 554 (pattern E), 541 (pattern A), and 563 (pattern C). M. bovis BCG Pasteur was used as the reference strain. Mycobacterial DNA from wild seals found on the coast of Argentina was also included (15). Alternative-spacer frequency analysis was performed on 20 M. tuberculosis strains from humans and 20 M. bovis strains from cattle.
DNA extraction.
Genomic DNA was extracted using proteinase K, lysozyme, and N-cetyl-N,N,N,-trimethylammonium bromide as described by Kamerbeek et al. (8) and Bunschoten et al. (4).
PCR.
Primers used for amplification corresponded to the 25 bp of the spacer sequence adjacent to the gaps observed in spoligotyping patterns. Each of these was named according to the number of the spacer it hybridized with. The sequences are as follows: sp1, 5′ ATAGAGGGTCGCCGGTTCTGGATCA3′; sp2, 5′CCTCATAATTGGGCGACAGCTTTTG3′; sp5, 5′TTTTCTGACCACTTGTGCGGGATTA3′; sp7, 5′GAGGAGAGCGAGTACTCGGGGCTGC3′; sp13, 5′GGGAGAGGGAATGGCAATGATGGTC3′; sp17, 5′CGGAGTCATCCGCGCGGGCCGGCGC3′; IS-left, TGACCCACCTGACATGACCCCAT (corresponds to the 5′ end of IS6110). Primers corresponding to alternative spacers were as follows: sp790, 5′CATGGCACGGCAGGCGTGGCTA3′; sp863, 5′GGGCCGTGGGGCACTTACGG3′; sp1080, 5′GGAGCCGTGCACATGCCGTGGCTCAGG3′; sp1377, 5′GCATGCAGCATGCCGTCCCCGTT3′; sp1453, 5′CGCCATCATCCGGCGCCGCAGCTCCGC3′.
PCR were performed as described by van Soolingen et al. (21). Briefly, amplification reactions were carried out in a Trio-Thermoblock thermocycler (Biometra) using the following program: 1 cycle of 94°C for 3 min; 30 cycles of 94°C for 1 min, 55 to 60°C for 1 min, and 72°C for 30s; and finally, 1 cycle of 70°C for 10 min. The amplification products were resolved by electrophoresis in 1.2% agarose gels. The vector used to clone the PCR products was pGEM-T (Promega). The resulting ligations were used to transform Escherichia coli DH5α. For plasmid minipreps, the Wizard Plus Minipreps kit (Promega) was used.
Sequencing.
The inserts in the pGEM-T vector were sequenced by the dideoxynucleotide chain termination method (17) using the fmol DNA Sequencing System kit (Promega).
Sequence analysis.
Sequences were analyzed using the DNA Strider program for Macintosh (12) and compared with those of M. tuberculosis H37Rv and an M. bovis isolate from the United States (3). This analysis was aimed at finding alternative spacers not included in the spoligotyping membrane.
Analysis of spacer frequency.
PCR analysis of spacer frequencies was performed on 20 strains of M. tuberculosis and 20 strains of M. bovis. Pairs of primers corresponding to alternative spacers 790, 863, 1080, 1377, and 1453 and to the 5′ end of IS6110 (primer IS-left) were used.
RESULTS
Analysis of spacer frequency in M. bovis spoligotyping patterns.
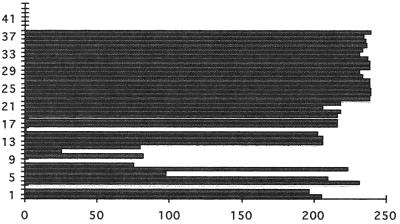
Analysis of the presence of individual spacers in the spoligotyping patterns of 246 M. bovis isolates, mostly from Argentina, (Fig. 1) indicated that the most frequent spacers are those downstream of IS6110. These are present in almost all of the strains, making them relatively useless for differentiation. Conversely, the spacers upstream of IS6110 are less frequent and therefore more polymorphic. Finally, spacers 39 to 43 are absent in all of the M. bovis strains studied in our laboratory. A summary of the structure of the DR region is depicted in Fig. 2.
FIG. 1.
Frequency of spacers among 246 M. bovis isolates submitted to spoligotyping. Spacer numbers are shown on the y axis, and the number of isolates containing a given spacer (frequency) is depicted on the x axis.
FIG. 2.
Gross structure of the DR region. Spacers with bound primers (===), intervening spacers (–––), direct repeats [DR], and IS6110 elements are shown.
Amplification of gap fragments.
To identify the presence of alternative spacers in selected strains, an approach consisting of amplification of the gap region between two known spacers was employed. Analysis of PCR was performed comparing the size of the band obtained by PCR to that of the minimum expected size (MES) estimated from the spoligotyping pattern and according to the combination of primers used. The MES of an amplicon between any pair of primers was calculated as the number of spacers that hybridized (observed spots in the spoligotype pattern) between and inclusive of the primers × 70 (the average length of the DR and spacer). Therefore, when the amplicon size exceeded the MES, the presence of an alternative spacer(s) was suspected and the amplicon was sequenced.
Spoligotyping patterns.
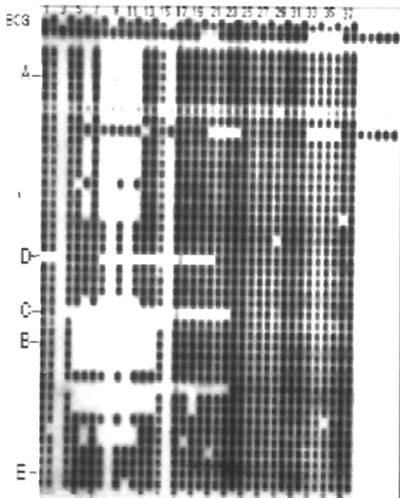
Based on the M. tuberculosis and M. bovis spoligotype patterns observed in our laboratory, strains presenting an absence of several spacers were selected and their spoligotype patterns were arbitrarily named A with 5 missing spacers (from 8 to 12), B with 10 missing spacers (from 5 to 14), C with 20 missing spacers (from 4 to 23), and E with no major gaps. Mycobacteria isolated from wild seals found on the Argentine coast were also included. The wild-seal spoligotype pattern (D) shows a trait of 15 spacers missing (spacers 8 to 22). Strains representative of each spoligotype pattern were chosen (Fig. 3). Pattern A, called pattern 34 in a previous publication (22), is the major spoligotype pattern of Argentina.
FIG. 3.
Representative sample of spoligotype patterns observed with M. bovis isolates. The names of selected spoligotype patterns are marked at the left.
Each strain was amplified by PCR, and the size of the PCR product was compared to the MES (Table 1). M. bovis strain 541 pattern A produced a band of 300 bp (band 5, Table 1) with primers sp5 and sp13, longer than the MES (approximately 210 bp) and a band 50 bp longer than the MES using primers sp7 and sp17 (band 8). A difference of about 200 bp was also observed for sp5 and IS-left. Bands 5 and 8 were selected for further analysis. DNA from M. bovis strain 540 (spoligotype pattern B) was amplified using primers sp2 and sp17 and gave a 250-bp band (band 1) similar in size to the MES. When amplification was performed using primers sp5 and IS-left, a band of 1,110 bp (band 11) was obtained, which was selected to be sequenced since it is larger than the MES. In addition, M. bovis 563 (pattern C) was amplified by using primers sp1 and IS-left and primers sp2 and IS-left (Fig. 3). Both amplifications showed bands (band 15 and band 16, Table 1) with important differences in length with respect to the MES, and the amplification products were therefore sequenced. Wild-seal strain 2009 (pattern D) was amplified using primers sp5 and IS-left and gave a band (band 13) 200 bp longer than the MES that was consequently sequenced. M. bovis strains 554 (pattern E) and BCG were used as controls since they do not lack more than three consecutive spacers. Amplifications using primers sp5 and sp13 (band 6) primers and 7 and 17 (band 9) in the pattern E strain showed no significant difference between the PCR product size and the MES. Differences of about 100 to 150 bp between the PCR product (band 7) and the MES size were observed for BCG using the sp5-sp13 primer combination. This band was also sequenced. No amplification products were obtained using primers sp2 and sp17.
TABLE 1.
PCR and MSE values obtained with different primer pairsa
| Strain | Band no. (PCR product size, MES [bp])
|
|||||
|---|---|---|---|---|---|---|
| sp2-sp17 (55°C) | sp5-sp13 (55°C) | sp7-sp17 (60°C) | sp5–IS-left (60°C) | sp1–IS-left (62°C) | sp2–IS-left (60°C) | |
| B | bd1 (250, 280) | ND | bd11 (1,110, 630) | ND | ND | |
| A | NA | bd5 (300, 210) | bd8 (400, 350) | bd12 (1,200, 980) | ND | ND |
| E | NA | bd6 (640, 560) | bd9 (680, 630) | ND | ND | ND |
| D | ND | ND | ND | bd13 (550, 350) | ND | ND |
| BCG | NA | bd7 (640, 560) | bd10 (700, 630) | bd14 (1,350, 1,260) | ND | ND |
| C | ND | ND | ND | ND | bd15 (400, 210) | bd16 (300, 140) |
PCR annealing temperatures are in parentheses. Values are given in base pairs. bd, band (in boldface are those selected for cloning and sequencing). NA, no amplification. ND, not done.
Sequence analysis.
Sequences were analyzed to identify alternative spacers. For this purpose, the sequences of cloned amplified fragments were compared to the DR region sequences of M. tuberculosis H37 Rv and an M. bovis isolate from the United States. The sequence from the pattern A strain showed alternative spacer 1377 and repeated spacer 7 (Table 2) in the relative order sp7-sp7-sp1377-sp13, with spacer 1377 situated after the second spacer 7. Upon analysis of the sequence from the M. bovis strain with pattern B, alternative spacer 1080 (Table 2) was found. This spacer is located after spacer 2. Two alternative spacers, 790 and 863, were present in the M. bovis strain with pattern C (Table 2) in the order sp1-sp2-sp790-sp863. The wild-seal strain also showed alternative spacer 1453 (Table 2) located after spacer 7. The BCG fragment showed two alternative spacers, 1377 and 1453, located after spacer 7. The naming of alternative spacers corresponds to their position in the sequence of the M. bovis U.S. strain (3).
TABLE 2.
Sequences of alternative spacers detected in this study
| Spacer | Sequence | Source | Spoligotype pattern | Name according to reference 19 |
|---|---|---|---|---|
| 790 | 5′TCGCGGCGCGGCATGGCACGGCAGGCGTGGCTAGGG3′ | M. bovis 563 | C | 5 |
| 863 | 5′TGTGCGCCGTCGCCGTAAGTGCCCCACGGCCCGT3′ | M. bovis 563 | C | 6 |
| 1080 | 5′TTGAACACGGAGCCGTGCACATGCCGTGGCTCAGGGGT3′ | M. bovis 540 | B | 11 |
| 1377 | 5′ACGACGTTAGGGCATGCAGCATGCCGTCCCCGTTTT3′ | M. bovis 541 | A | 16 |
| 1453 | 5′GCTCTTGAGCAACGCCATCATCCGGCGCCGCAGCTCCGC3′ | Wild-seal strain | D | 17 |
Analysis of alternative-spacer frequency of occurrence.
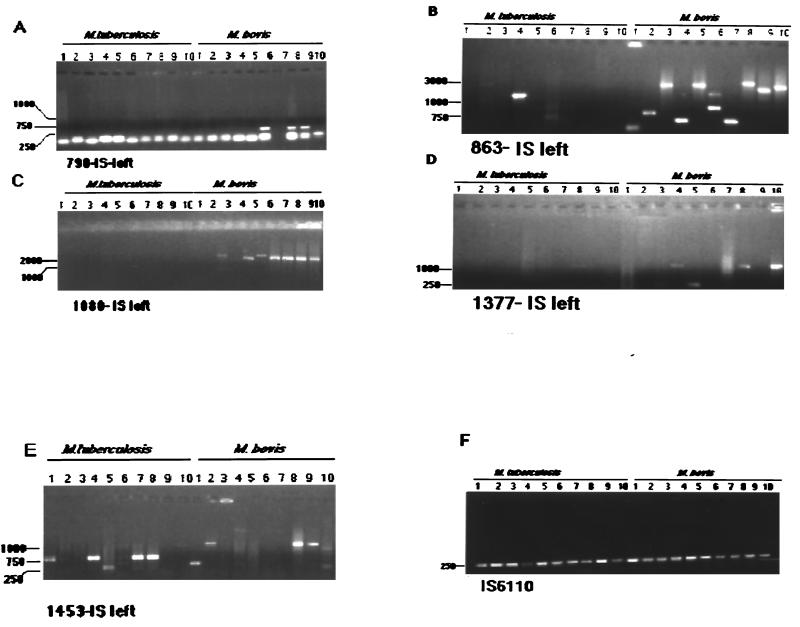
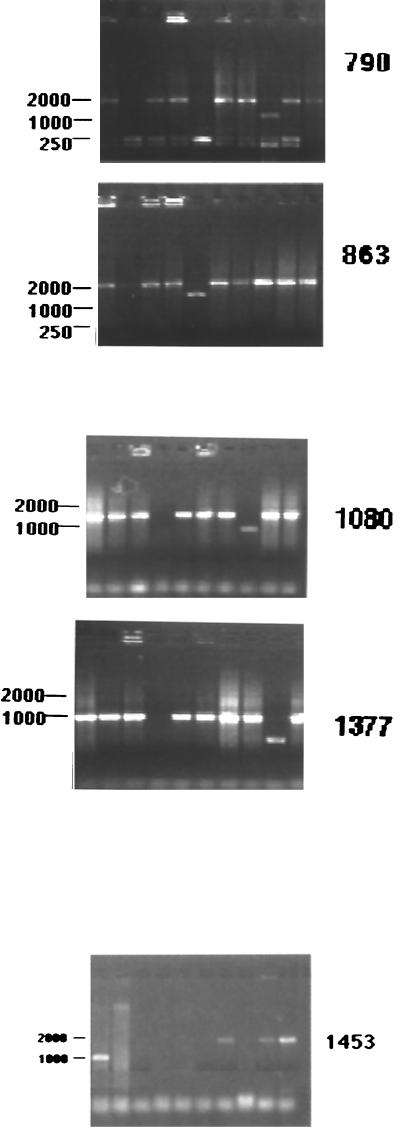
To study the frequency of occurrence of alternative spacers in M. tuberculosis and M. bovis strains, amplifications with primers derived from the five alternative spacers, in combination with primer IS-left, were performed (Fig. 4). Twenty different strains each of M. tuberculosis and M. bovis were used. Amplification with sp790 and IS-left was positive in 6 (30%) of the 20 M. bovis strains, while M. tuberculosis gave no amplification; with primers sp863 and IS-left, 18 (90%) of the 20 M. bovis strains and 8 (40%) of the 20 M. tuberculosis strains were amplified. The pair sp1080 and IS-left yielded positive amplification of 16 (80%) of 20 M. bovis strains and only 1 M. tuberculosis strain. Using primers sp1377 and IS-left 9 (45%) of 20 M. bovis strains were positive while only 1 M. tuberculosis strain gave an amplification product. Finally, the combination sp1453–IS-left resulted in 60% of M. bovis and 70% of M. tuberculosis strains amplified.
FIG. 4.
Agarose gel showing PCR amplification of DNA from randomly selected M. bovis and M. tuberculosis isolates with primer IS-left in combination with primers derived from alternative spacers. Amplifications of 10 strains of M. bovis and M. tuberculosis are shown. Annealing temperatures for amplifications (in parentheses): A, sp790 (63°C); B, sp863 (63°C); C, sp1080 (65°C); D, sp1377 (63°C); E, sp1453 (55°C); F, IS6110 (65°C) (positive control).
Discriminatory power of alternative spacers.
To assess the discriminatory power of alternative spacers, PCRs with combinations of alternative spacers and IS-left primers were performed on 10 pattern A strains (Fig. 5). Using the pair sp790–IS-left, 8 of 10 strains were positive; with sp863–IS-left, all 10 strains were positive; with sp1080–IS-left, 9 of 10 strains were positive; with sp1377–IS-left, 9 of 10 strains were positive; and finally, using the sp1453–IS-left primer pair, 4 of 10 strains were positive (Fig. 5). These results indicated that spacer 1453 may be useful for M. bovis typing, while the other alternative spacers allow moderate discrimination.
FIG. 5.
Agarose gel showing PCR amplification of DNA of M. bovis with pattern A using primer IS-left in combination with primers derived from alternative spacers.
DISCUSSION
M. bovis strains commonly lack several spacers. These missing spacers appear as gaps in their spoligotyping patterns. To determine whether these gaps are mere deletions of DR-spacer units or whether they contain alternative spacers, these regions were amplified using primers hybridizing to spacers adjacent to the gaps; the resulting fragments were then cloned and sequenced. Five alternative spacers were found in this way. However, all of the amplified fragments contain only one or two alternative spacers, indicating that the gaps can be explained mostly by deletions. Mutated spacers were not observed, and in only one case was a repeated spacer observed. The five alternative spacers were previously described by Beggs et al. (3) in an M. bovis isolate. These spacers were present in an average of 55% of the M. bovis isolates and in only 8% of the M. tuberculosis strains, with the exception of spacer 1453, which was present in 70% of the M. tuberculosis strains tested. It is interesting that this alternative spacer is the only one found in the wild-seal strains, which are genetically intermediate between M. tuberculosis and M. bovis. The fact that certain spacers are found more frequently, or that they are exclusive to a given M. tuberculosis complex species, is emphasized by the fact that spacers 3, 9, 16, and 39 to 43 are not observed in M. bovis (Fig. 1). In this work, the absence of an alternative spacer was detected by negative amplification using an alternative-spacer–IS-left primer pair. Another explanation for a no-amplification event could be that the IS6110 element may be inverted in a given strain. However, we believe that this is not likely because in most cases the same strain that gave negative amplification rendered positive amplification with another primer pair, meaning that the IS6110 orientation is not inverted.
As noted previously by other authors (19), the current DR region seems to have evolved from an ancestor possessing all of the present spacers and probably others. The deletions seem to involve the entire DR in addition to the spacer units. The fact that the sequence of the 36-bp DR element is highly conserved between strains and species suggests that deletions have probably arisen by recombination between DR sequences. Another probable mechanism is the duplication and insertion of primordial DR-plus-spacer units, followed later by sequence divergence. However, this mechanism seems unlikely since, with the exception of a few spacers, their sequences are significantly different from each other and in consequence they do not seem to have evolved from a common ancestor. The spacers of the DR region have a conserved order and sequence. In addition, the repeated spacers found in this study are in tandem with the original spacer. The alternative spacers also conserve the order they present in the previously studied M. bovis isolated in the United States (3).
In accordance with the aim of the present study, how much discrimination would be gained by including new spacers in the spoligotyping membrane? Isolates with pattern A, the major spoligotype pattern in Argentina, were selected to test the discriminative power of the five alternative spacers in this cluster of strains. Spacer 1453 showed the highest discriminative power, since only 40% of the strains were positive. These results suggest than some alternative spacers may be more discriminative for M. bovis typing than those already included in the membrane.
ACKNOWLEDGMENTS
The valuable suggestions of Carlos Martin are gratefully acknowledged. We thank Haydee Gil for technical help.
M.I.R., F.B., and A.A.C. are fellows of the National Research Council of Argentina (CONICET). The group is a member of the Latin American and Caribbean Network of Tuberculosis (RELACTB). This work was supported by the Centro Argentino Brasileño de Biotecnología (CABBIO).
REFERENCES
- 1.Aranaz A, Liébana E, Mateos A, Dominguez L, Vidal D, Domingo M, Gonzolez O, Rodriguez-Ferri E F, Bunschoten A F, van Embden J D A, Cousins D. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. J Clin Microbiol. 1996;34:2734–2740. doi: 10.1128/jcm.34.11.2734-2740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer J, Andersen Å, Kremer K, Miörner H J. Usefulness of spoligotyping to discriminate IS6110 low-copy-number Mycobacterium tuberculosis complex strains cultured in Denmark. J Clin Microbiol. 1999;37:2602–2606. doi: 10.1128/jcm.37.8.2602-2606.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beggs M L, Cave M D, Marlowe C, Cloney L, Duck P, Eisenach K D. Characterization of Mycobacterium tuberculosis complex direct repeat sequence for use in cycling probe reaction. J Clin Microbiol. 1996;34:2985–2989. doi: 10.1128/jcm.34.12.2985-2989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De La Salmoniere Y O G, Li H M, Torrea G, Bunschoten A, van Embden J D A, Giequel B. Evaluation of spoligotyping in a study of the transmission of Mycobacterium tuberculosis. J Clin Microbiol. 1997;35:2210–2214. doi: 10.1128/jcm.35.9.2210-2214.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisanotti J C, Alito A, Bigi F, Latini O, Roxo E, Cicuta E, Zumarraga M, Cataldi A, Romano M I. Molecular epidemiology of Mycobacterium bovis isolates from South America. Vet Microbiol. 1988;60:251–257. doi: 10.1016/s0378-1135(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 6.Hermans P W M, van Soolingen D, Bik E M, de Haas P E W, Dale J W, van Embden J D A. Insertion element IS987 from Mycobacterium bovis BCG is located in a hot-spot integration region for insertion elements in Mycobacterium tuberculosis complex strains. Infect Immun. 1991;59:2695–2705. doi: 10.1128/iai.59.8.2695-2705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermans P W M, van Soolingen D, Dale J W, Schuitema A R J, McAdam R A, Catty D, van Embden J D A. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol. 1990;28:2051–2085. doi: 10.1128/jcm.28.9.2051-2058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J D A. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantor I N, Ritacco V. Bovine tuberculosis in Latin America and the Caribbean: current status, control and eradication programs. Vet Microbiol. 1994;40:5–14. doi: 10.1016/0378-1135(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 10.Kremer K, van Soolingen D, Frothingham R, Haas W H, Hermans P W, Martin C, Palittapongarnpim P, Plikaytis B B, Riley L W, Yakrus M A, Musser J M, van Embden J D. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol. 1999;37:2607–2618. doi: 10.1128/jcm.37.8.2607-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latini M S, Latini O, Lopez M L, Cecconi J O. Tuberculosis bovina in seres humanos. Rev Argent Torax. 1990;51:13–16. [Google Scholar]
- 12.Marck C. ‘DNA Strider’: a ‘C’ program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988;16:1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molhuizen H O, Bunschoten A E, Schouls L M, van Embden J D A. Rapid detection and simultaneous strain differentiation of Mycobacterium tuberculosis complex bacteria by spoligotyping. Methods Mol Biol. 1998;101:381–394. doi: 10.1385/0-89603-471-2:381. [DOI] [PubMed] [Google Scholar]
- 14.Romano M I, Alito A, Fisanotti J C, Bigi F, Kantor I, Cicuta M E, Cataldi A. Comparison of different genetic markers for molecular epidemiology of bovine tuberculosis. Vet Microbiol. 1996;50:59–71. doi: 10.1016/0378-1135(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 15.Romano M I, Alito A, Bigi F, Fisanotti J C, Cataldi A. Genetic characterization of mycobacteria from South American wild seals. Vet Microbiol. 1995;47:89–98. doi: 10.1016/0378-1135(95)00103-h. [DOI] [PubMed] [Google Scholar]
- 16.Samper S, Martín C, Pinedo A, Rivero A, Blázquez J, Baquero F, van Soolingen D, van Embden J D A. Transmission between HIV-infected patients of multidrug-resistant tuberculosis caused by Mycobacterium bovis. AIDS. 1997;11:1237–1242. doi: 10.1097/00002030-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thierry D, Brisson-Noël A, Vincent-Lévy-Frébault V, Nguyen S, Guesdon J-L, Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol. 1990;28:2668–2673. doi: 10.1128/jcm.28.12.2668-2673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Embden J D A, van Gorkom T, Kremer K, Jansen R, van der Zeijst B A M, Schouls L M. Genetic variation and evolutionary origin of the direct repeat locus of Mycobacterium tuberculosis complex bacteria. J Bacteriol. 2000;182:2393–2401. doi: 10.1128/jb.182.9.2393-2401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Soolingen D, de Haas P E W, Haagsma J, Eger T, Hermans P W M, Ritacco V, Alito A, van Embden J D A. Use of various genetic markers in differentiation of Mycobacterium bovis strains from animals and humans and for studying epidemiology of bovine tuberculosis. J Clin Microbiol. 1994;32:2425–2433. doi: 10.1128/jcm.32.10.2425-2433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Soolingen D, de Haas P E W, Hermans P W M, van Embden J D A. Manual for fingerprinting of M. tuberculosis strains. Bilthoven, The Netherlands: National Institute of Public Health and Environmental Protection; 1995. [Google Scholar]
- 22.Zumarraga M, Martin C, Samper S, Cataldi A, Alito A, Latini O, Bigi F, Roxo E, Castro Ramos M, Errico F, van Soolingen D, Romano M I. Usefulness of spoligotyping in molecular epidemiology of Mycobacterium bovis-related infection in South America. J Clin Microbiol. 1999;37:296–303. doi: 10.1128/jcm.37.2.296-303.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]