Abstract
The selective estrogen receptor (ER) modulator, tamoxifen, is the only endocrine agent with approvals for both the prevention and treatment of premenopausal and postmenopausal estrogen-receptor positive breast cancer as well as for the treatment of male breast cancer. Endoxifen, a secondary metabolite resulting from CYP2D6-dependent biotransformation of the primary tamoxifen metabolite, N-desmethyltamoxifen (NDT), is a more potent antiestrogen than either NDT or the parent drug, tamoxifen. However, endoxifen’s antitumor effects may be related to additional molecular mechanisms of action, apart from its effects on ER. In phase 1/2 clinical studies, the efficacy of Z-endoxifen, the active isomer of endoxifen, was evaluated in patients with endocrine-refractory metastatic breast cancer as well as in patients with gynecologic, desmoid, and hormone-receptor positive solid tumors, and demonstrated substantial oral bioavailability and promising antitumor activity. Apart from its potent anticancer effects, Z-endoxifen appears to result in similar or even greater bone agonistic effects while resulting in little or no endometrial proliferative effects compared with tamoxifen. In this review, we summarize the preclinical and clinical studies evaluating endoxifen in the context of breast and other solid tumors, the potential benefits of endoxifen in bone, as well as its emerging role as an antimanic agent in bipolar disorder. In total, the summarized body of literature provides compelling arguments for the ongoing development of Z-endoxifen as a novel drug for multiple indications.
Keywords: endoxifen, tamoxifen, CYP2D6, antiestrogen, hormone therapy, breast cancer
Breast cancer is the most diagnosed cancer worldwide, with nearly 80% of tumors classified as estrogen receptor positive (ER+), based on the expression of ER alpha (ERα). In the United States alone, nearly 284 200 new cases of breast cancer are estimated with a projection of 44 130 deaths in 2021, making it the second most common cause of cancer-related deaths among women (1). Despite these staggering numbers, ER+ breast cancer mortality rates have steadily declined over the years, in part because of the use of highly effective endocrine treatment regimens such as tamoxifen. Tamoxifen, a selective estrogen receptor modulator (SERM), was originally developed as an antifertility drug but failed in this capacity (2). Its efficacy for the treatment of postmenopausal metastatic breast cancer was first reported by Cole et al in the early 1970s, with promising antitumor activity and manageable side effects (3), resulting in the US Food and Drug Administration approval of tamoxifen for the postmenopausal treatment of metastatic ER+ breast cancer in 1977. Results from ensuing randomized clinical trials over the next three decades firmly established the therapeutic utility of tamoxifen for the treatment of early and advanced breast cancer, including both premenopausal and postmenopausal women (4-13), the adjuvant treatment of both pre- and postmenopausal breast cancer (14-17) and breast cancer in men (18, 19). Tamoxifen is also the only US Food and Drug Administration-approved drug for use in the prevention of breast cancer in high-risk women (20, 21). The results of the Early Breast Cancer Trialists’ Collaborative Group, which summarized multiple clinical trials of women with early breast cancer receiving chemotherapy and hormonal therapy, concluded that 5 years of adjuvant tamoxifen therapy significantly improves disease-free survival by reducing the risk of disease recurrence and death by upwards of 50%, as well as decreasing the risk of contralateral breast cancer (22), making tamoxifen a reliable endocrine agent for reducing breast cancer mortality.
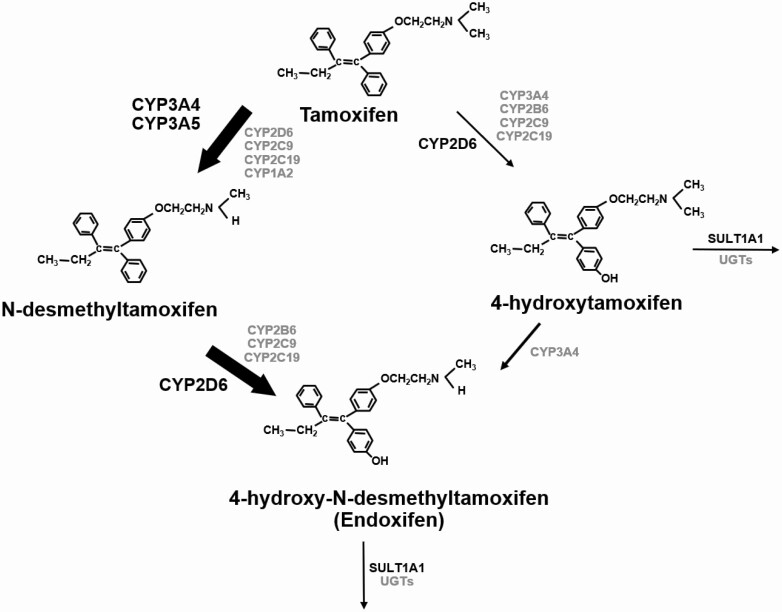
Tamoxifen is extensively metabolized in the liver by the cytochrome P450 enzyme system into the primary metabolites 4-hydroxy-tamoxifen (4HT) and N-desmethyltamoxifen (NDT) (Fig. 1). NDT undergoes secondary catabolism to 4-hydroxy-N-desmethyl-tamoxifen (endoxifen). The cytochrome P450 2D6 (CYP2D6) enzyme mediates the oxidation of tamoxifen to 4HT and NDT to endoxifen (23). Studies have identified 4HT and endoxifen as the most active metabolites of tamoxifen (24-27), with preclinical data demonstrating that both metabolites demonstrate equipotent ER binding affinity as well as suppression of estrogen-dependent growth of ER+ breast cancer cells, with 100-fold greater effects compared with the parent drug, tamoxifen (28, 29). The plasma concentrations of tamoxifen, NDT, 4HT, and endoxifen observed in patients taking the 20 mg/d dose of tamoxifen are 133.8 ± 59.16 ng/mL, 278.1 ± 141.5 ng/mL, 3.0 ± 1.5 ng/mL, and 24.4 ± 16.2 ng/mL (30, 31). Studies directly measuring the plasma concentrations of tamoxifen metabolites in tamoxifen-treated patients have shown that low levels of 4HT or endoxifen associates with poor survival outcomes (32-35). Because the plasma concentrations of 4HT are very low compared with the other metabolites in tamoxifen-treated patients, much of the focus has been on evaluating the association between plasma concentrations of tamoxifen, NDT, and endoxifen levels and clinical outcomes.
Figure 1.
Schematic representation of tamoxifen biotransformation into primary and secondary metabolites by the cytochrome P540 system. The thickness of the arrows represents the relative contribution of each of the pathway to the overall oxidation of tamoxifen. Reprinted from Sideras et al. J Clin Oncol. 2010;28(16):2768-2776, with permission from Wolters Kluwer Health, Inc.
The association between polymorphisms in CYP2D6 genotype and clinical benefit of tamoxifen in patients receiving tamoxifen therapy have been the subject of controversy for years (36, 37). Although many studies have demonstrated an association between decreased CYP2D6 metabolism and poor clinical outcomes including shorter time to recurrence and worse relapse-free survival (38-40), other studies have disputed this notion showing no such association (41-44). Perhaps one of the most important issues confounding the retrospective and even prospective tamoxifen pharmacogenetic studies relates to the fact that differences in the concentrations of the active tamoxifen metabolites are rendered irrelevant when additional anticancer therapies are given either before or after tamoxifen. For example, a secondary analysis of the prospective Austrian Breast and Colorectal Cancer Study Group Trial 8 that randomized postmenopausal women with ER+ breast cancer to tamoxifen for 5 years (arm A) or tamoxifen for 2 years followed by anastrozole for 3 years (arm B), demonstrated that reduced CYP2D6 metabolism was associated with a higher risk of breast cancer recurrence, but only during the period of tamoxifen administration and not after switching to anastrozole (45). In another study, the role of CYP2D6 metabolism in patients treated with either tamoxifen monotherapy or chemotherapy and tamoxifen was evaluated. This study showed that in contrast to patients treated with tamoxifen monotherapy, no significant association between CYP2D6 genotype and recurrence-free survival was observed in patients treated with chemotherapy and tamoxifen (46). A recent prospective study validated this finding, showing that variation in endoxifen levels were not associated with recurrence when women with ER+ breast cancer were treated with adjuvant chemotherapy, a short period of tamoxifen (1-2 years), followed by an aromatase inhibitor (47). Therefore, although CYP2D6 genotype and low concentrations of the active metabolites are a critical determinant of tamoxifen clinical efficacy, they are unlikely to be associated with the risk of recurrence when other treatments are administered, which additionally alter the hazard for recurrence. Other additional differences reported to influence outcomes of the tamoxifen pharmacogenetic studies include differences in cohort size, patient recruitment criteria, use of concomitant CYP2D6 inhibitors, ER status, and CYP2D6 genotyping methods and are often cited as potential confounding factors (48).
Regardless, there is consensus that endoxifen is the most abundant active metabolite of tamoxifen, and the development of endoxifen as a novel drug was pursued to avoid issues related to CYP2D6 polymorphisms. Early phase (1/2) studies have demonstrated substantial antitumor activity of endoxifen in patients with prior progression on tamoxifen, providing definitive evidence for the benefit of endoxifen in tamoxifen-resistant patients (49, 50). In this review, we summarize the published literature surrounding the pharmacology and pharmacodynamics of endoxifen compared with tamoxifen in ER+ breast cancer, its effects on nonbreast cancers and nonbreast organs, as well as its emerging role as an antimanic agent in bipolar disorder.
CYP2D6 Metabolism of Tamoxifen and Endoxifen Plasma Concentrations
Studies have shown that in patients receiving tamoxifen therapy, CYP2D6 genetic variation is significantly associated with endoxifen levels, with endoxifen plasma concentrations ranging from 1.5 to 3.1 ng/mL for poor metabolizers, to above 22.0 ng/mL in ultrarapid metabolizers (51, 52). Furthermore, endoxifen concentrations are lower in tamoxifen-treated patients who are coprescribed CYP2D6-inhibiting drugs (30, 31, 53). In addition to numerous studies evaluating CYP2D6 genotype and tamoxifen clinical outcomes, several studies have reported an association between endoxifen concentrations and disease-free survival in tamoxifen treated early breast cancer patients (38, 39, 54). Taken together, these findings have led to the implementation of clinical guidelines based on CYP2D6 genotype status in deciding tamoxifen therapy recommendations (48). Notably, in patients with predicted decreased CYP2D6 enzyme activity, recommendations include either increasing the tamoxifen dose from 20 mg/d to 40 mg/d, which has been reported to normalize endoxifen concentration (55) or switching from tamoxifen to an aromatase inhibitor (AI) (48). For all patients, recommendation was provided to avoid concomitant usage of moderate and strong CYP2D6-inhibiting drugs (48).
Based on data that lower concentrations of the active metabolites of tamoxifen are associated with inferior breast cancer outcomes, as well as data from murine studies demonstrating that Z-endoxifen administration demonstrated high oral bioavailability (56, 57), the development of endoxifen as a novel therapeutic for the treatment ER+ breast cancer ensued.
Endoxifen Compared With Tamoxifen as a Potent Antiestrogen
Evidence supporting endoxifen’s superior antiestrogenic effects came from studies demonstrating that endoxifen, at concentrations typically observed in CYP2D6 extensive metabolizers, was not only more potent in terms of inhibition of estrogen-dependent tumor growth, but unlike tamoxifen or 4HT, additionally appeared to target ERα for degradation in breast cancer cells (58). This observation suggested that the mechanism of action of endoxifen may be distinct from that of tamoxifen or 4HT. Supporting this notion, global gene expression profiling of MCF7 cells treated with the tamoxifen metabolite 4HT, endoxifen, or the selective estrogen receptor degrader ICI 182 780 (fulvestrant) in the absence or presence of estrogen revealed substantial differences in the transcriptomes of endoxifen-treated cells compared with those of the other treatment groups in both settings (59). Notably, pathway analysis of differentially regulated genes in endoxifen-treated MCF7 cells indicated that cell-cycle arrest and apoptosis pathways were significantly induced at endoxifen concentrations that mirror those observed in CYP2D6 extensive metabolizers but not poor metabolizers. Other independent groups demonstrated that although clinical concentrations of tamoxifen and its metabolites used in the postmenopausal setting partially blocked estrogen-stimulated genes, the addition of endoxifen was necessary to achieve complete blockade of the expression of these estrogen-stimulated genes (60). In another study, clinical levels of tamoxifen and its metabolites in the premenopausal setting only partially inhibited estrogen-dependent growth and gene modulation. However, supplementation of endoxifen at concentrations corresponding to that of extensive metabolizers additionally augmented the inhibitory effects of tamoxifen and its metabolites on estrogen-stimulated actions (61). These data suggest that the endoxifen concentrations, particularly in the premenopausal setting, might be critical owing to its stronger antiestrogenic actions. Collectively, these preclinical studies established that endoxifen is an important component of tamoxifen’s antiestrogenic effects, with its increasing concentrations associating with improved anticancer effects.
Endoxifen’s Superior Antitumor Activity Compared With Tamoxifen
In a collaboration between Mayo Clinic and the National Cancer Institute, the first in-human prospective clinical trial of Z-endoxifen, the active isomer of endoxifen, was tested in patients with ER+ breast cancer who had progressed on multiple endocrine therapies (NCT01327781) (49). In this study, administration of oral Z-endoxifen resulted in plasma concentrations of Z-endoxifen reaching as high as 628.9 to 1886.8 ng/mL, greatly exceeding the Z-endoxifen concentrations achieved with tamoxifen (56, 57, 62, 63) and manageable toxicity. Furthermore, Z-endoxifen demonstrated promising antitumor activity, including in patients with ESR1 mutations, which are reported at a higher frequency in ER+ metastatic breast cancers and associates with worse clinical outcomes (64). A maximum tolerated dose was not identified. Importantly, Z-endoxifen clearance was unaffected by CYP2D6 metabolism, implying that in women with low or nonfunctional CYP2D6 enzyme activity, therapeutic concentrations of Z-endoxifen could be achieved thereby eliminating any role for CYP2D6 genotype in determining treatment outcomes in these women. In a second phase 1 clinical trial evaluating Z-endoxifen in patients with hormone receptor-positive breast cancer, Z-endoxifen dose levels as high as 360 mg/d dose were tested (NCT01273168) (50), more than double the highest dose (160 mg/d) studied in the first phase 1 clinical study (49). Although the maximum tolerated dose was not established, the doses used in this study were well tolerated. Regarding the breast cancer patients enrolled onto this trial, 1 of 9 patients (11%), treated with an aromatase inhibitor for a prolonged period before trial enrollment, experienced a partial response, whereas 3 other patients (33%) who were previously on multiple endocrine therapies including tamoxifen and anastrazole, experienced stable disease for ≥ 6 cycles (50).
These encouraging findings led to a randomized phase 2 clinical trial from the Alliance for Clinical Trials in Oncology (A011203) (NCT02311933) directly comparing the efficacy of tamoxifen and Z-endoxifen in women with ER+ and human epidermal growth factor 2 receptor (HER2) metastatic breast cancer that had progressed during endocrine therapy. Preliminary results from this trial were reported at the San Antonio Breast Cancer Symposium 2019 meeting. Although Z-endoxifen was not superior to tamoxifen (median progression-free survival [PFS], 4.3 months vs 1.8 months; hazard ratio [HR], 0.77; 95% CI, 0.49-1.22), marked differences in Z-endoxifen efficacy were observed according to receipt of prior CDK4/6 inhibitor (CDK4/6i) therapy. In patients with progression on CDK4/6i, Z-endoxifen was not superior to tamoxifen (median PFS 2.4 months vs 1.8 months; HR 1.29; 95% CI, 0.66-2.96). In contrast, in CDK4/6i-naïve patients, Z-endoxifen significantly prolonged PFS compared with tamoxifen (median PFS, 7.2 months vs 2.4 months; HR, 0.42; 95% CI, 0.22-0.80; P = 0.002). For patients that progressed on tamoxifen and crossed over to Z-endoxifen, the clinical benefit rate was 28% (14.0%-46.2%). These findings are consistent with emerging data from other prospective trials wherein endocrine monotherapy exhibited minimal antitumor activity in the post-CDK 4/6 inhibitor space. For example, in the recently reported VERONICA study in which patients with endocrine and CDK4/6i refractory metastatic breast cancer were randomized to fulvestrant alone or fulvestrant plus venetoclax, the median PFS was 2.69 months (95% CI, 1.94-3.71) in the combination arm vs 1.94 months (1.84-3.55) in the fulvestrant arm (65). This study also supports the findings from the A011203 study demonstrating lack of endocrine sensitivity in tumors that progressed on CDK4/6i. Although additional studies are needed to pinpoint the cause for the lack of antitumor activity following CD4/6i treatment, based on the preclinical and phase 1/2 clinical trials, Z-endoxifen is expected to exhibit the greatest clinical benefit in CDK4/6i-naïve ER+ breast cancer.
In preclinical studies that evaluated the antitumor activity of Z-endoxifen with letrozole and tamoxifen in the aromatase-expressing MCF7 (MCF7AC1) breast cancer xenograft model, Z-endoxifen was shown to be superior to tamoxifen and letrozole both in the treatment of naïve xenografts, as well as in xenografts that developed resistance to letrozole (66). The MCF7AC1 model is a clinically relevant tumor model that has faithfully predicted the clinical efficacy of AI therapy over tamoxifen therapy as well as the clinical efficacy of fulvestrant and anastrozole combinatorial therapies over AI monotherapy in vivo (67, 68). Furthermore, in the letrozole-resistant MCF7AC1 xenograft tumor model, Z-endoxifen was superior not only to tamoxifen, but additionally to exemestane monotherapy or exemestane plus everolimus combinatorial therapies (66), all of which have a track record of proven clinical efficacy in the AI-resistant setting. Importantly, in the AI-resistant setting and unlike tamoxifen, Z-endoxifen was not cross-resistant, making Z-endoxifen a promising second-line therapy in the AI-resistant setting. Furthermore, in the MCF7AC1 model resistant to letrozole, a comparison of the transcriptome of tamoxifen and Z-endoxifen-treated tumors revealed that Z-endoxifen more potently inhibits ERα target genes than tamoxifen, further supporting Z-endoxifen’s potent antiestrogenic activity compared with tamoxifen. Ingenuity pathway analysis revealed that Z-endoxifen may uniquely target signaling kinases such as ATM and PI3K/AKT, with tumor tissues confirming reduced expression of phospho and total AKT in Z-endoxifen treated AI-resistant tumors at clinically relevant concentrations (5 µM) but not in tamoxifen-treated AI-resistant tumors (66). Collectively, these data certainly establish that Z-endoxifen is a superior endocrine agent compared with tamoxifen, particularly in suppressing the growth of endocrine-refractory ER+ breast cancer.
Endoxifen’s Antitumor Activity in Other Solid Tumors
The second phase 1 clinical trial study that evaluated the efficacy of Z-endoxifen at doses as high as 360 mg/d also investigated the antitumor activity of Z-endoxifen on other solid cancers including advanced gynecological and desmoid cancers (50). Of the 4 patients diagnosed with desmoid tumors, 1 who had previously progressed on tamoxifen and γ-secretase inhibitor therapy experienced a partial response with Z-endoxifen therapy. Remarkably, this patient remained on the study for as long as 62 cycles (ie, for nearly 5 years of Z-endoxifen treatment) and had improved pain levels. A second patient, who had progressed on multiple therapies as well as another patient who previously progressed on tamoxifen, showed prolonged disease stabilization (50). Given that desmoid tumors lack expression of ERα and progesterone receptors (69), this finding suggests that Z-endoxifen may be a substrate for non-ER cancer targets and may have beneficial effects in the treatment of ER- tumors. Although desmoid tumors lack ERα expression, it is noteworthy that these tumors express ER-beta (ERβ) (69), whose expression is reported to correlate with desmoid tumor growth (70). Preclinical studies have shown that endoxifen can stabilize ERβ protein expression and that ERβ presence enhances the sensitivity of breast cancer cells to antiestrogenic effects of endoxifen (71). Therefore, Z-endoxifen’s antitumor activity in desmoid tumors may involve an ERβ-dependent mechanism. Z-endoxifen therapy also led to partial response or stable disease for ≥ 6 cycles in patients diagnosed with gynecological malignancies (50). These findings call for future investigation of Z-endoxifen antitumor activity in other solid tumors. A comprehensive summary of the study design, clinical outcomes, including adverse effects in the 2 Z-endoxifen phase 1 clinical trials, is provided in Table 1, whereas a summary of Z-endoxifen pharmacokinetics is provided in Table 2.
Table 1.
Summary of the study design, adverse events, and clinical outcomes from the 2 Z-endoxifen phase 1 clinical trials
| Parameters | Goetz et al study, 2017 (49) | Takebe et al study, 2021 (50) |
|---|---|---|
| Patient enrollment details | Patients with metastatic or locally recurrent hormone receptor positive breast cancer. | Patients with hormone receptor positive solid breast tumors, desmoid tumors, or gynecologic tumors. |
| Prior treatment information | Progressed while receiving tamoxifen (if premenopausal) or an AI (if postmenopausal) in either the metastatic or adjuvant setting. | Progressed while receiving at least 1 prior chemotherapy regimen with or without at least 1 hormonal regimen in the metastatic setting. For HER2+ metastatic breast cancer, progressed on at least 1 prior HER2-directed regimen. |
| Limitations on prior therapy | None. Unlimited prior endocrine therapy regimens were allowed. | None. |
| Z-endoxifen dosages evaluated | 20, 40, 60, 80, 100, 120 and 160 mg/d. | 20, 40, 60, 100, 140, 200, 280 and 360 mg/d. |
| MTD | MTD not determined. | MTD not determined. |
| AE | Mostly grade 2 AE. Grade 4 hypertriglyceridemia noted at 60 mg/dose. No major AE reported at the highest dose (160 mg/d). | Most frequent AE were grades 2 and 3 lymphopenia and anemia. Grade 4 colonic perforation reported at the highest 360-mg dose. |
| Clinical benefit | Among the 25 patients with measurable disease, the overall response rate was 12.0% (95% CI, 2.6-31.2). Clinical benefit (stable disease ≥ 6 months) was observed in 19% of patients (3 of 16) who experienced progression during tamoxifen and 32% (7 of 22) who had no prior tamoxifen treatment or did not experience progression with adjuvant tamoxifen. |
Breast cancer: 1/9 patients (11%) showed partial response; 3/9 patients (33%) experienced stable disease for ≥ 6 months. Desmoid tumors: 1/4 patients (25%) showed partial response; 2/4 patients (50%) experienced disease stabilization. Gynecologic tumors: 1/20 patients (5%) showed partial response; 3/20 patients (15%) experience disease stabilization of ≥ 6 cycles. |
Abbreviations: AE, adverse event; AI, aromatase inhibitor; HER2, human epidermal growth factor 2 receptor; MTD, maximum tolerated dose.
Table 2.
Summary of the pharmacokinetics based on the dose level and treatment day from the 2 Z-endoxifen phase 1 clinical trials
| Dose level (mg) | No. of patients | Day | Tmax (h) | Cmax (ng/mL) | C24h (ng/mL) | AUC0-24h (hours × µg/mL) | Accumulation (AUC) | Half-life (h) |
|---|---|---|---|---|---|---|---|---|
| Goetz et al study, 2017 (49) | ||||||||
| 20 | 3 | 1 | 4.3 ± 3.2 | 64.8 ± 13.2 | 38.1 ± 3.7 | 1.09 ± 0.21 | 3.47 ± 1.29 | 49.0 ± 21.7 |
| 2 | 28 | 3.5 ± 3.5 | 215 ± 83 | 167 ± 49 | 4.19 ± 1.46 | |||
| 40 | 8 | 1 | 3.6 ± 2.3 | 169 ± 49 | 86.6 ± 22.6 | 2.49 ± 0.58 | 3.96 ± 0.89 | 57.2 ± 14.8 |
| 8 | 28 | 1.7 ± 1.0 | 499 ± 49 | 414 ± 111a | 9.66 ± 2.32 | |||
| 60 | 8 | 1 | 2.6 ± 0.9 | 348 ± 222 | 132 ± 93 | 4.33 ± 2.69 | 3.94 ± 1.86 | 56.5 ± 31.4 |
| 5 | 28 | 2.8 ± 0.8 | 643 ± 332 | 421 ± 216 | 11.6 ± 5.5 | |||
| 80 | 8 | 1 | 6.8 ± 7.3 | 238 ± 49 | 152 ± 39 | 4.15 ± 0.91 | 4.14 ± 1.28 | 60.2 ± 21.4 |
| 7 | 28 | 2.6 ± 1.7 | 913 ± 142 | 577 ± 122b | 15.8 ± 2.3 | |||
| 100 | 8 | 1 | 4.0 ± 2.8 | 344 ± 104 | 194 ± 43 | 5.62 ± 1.18 | 4.62 ± 1.10 | 68.1 ± 18.4 |
| 7 | 28 | 4.2 ± 3.1 | 1284 ± 364 | 952 ± 246 | 25.7 ± 6.8 | |||
| 120 | 3 | 1 | 3.0 ± 1.0 | 378 ± 155 | 243 ± 89 | 6.03 ± 2.73 | 3.64 ± 1.18 | 51.7 ± 19.7 |
| 3 | 28 | 2.7 ± 1.1 | 1261 ± 453 | 813 ± 235 | 20.7 ± 6.3 | |||
| 160 | 3 | 1 | 3.7 ± 2.1 | 635 ± 39 | 333 ± 62 | 9.81 ± 6.3 | 3.81 ± 0.76 | 54.6 ± 12.8 |
| 3 | 28 | 5.1 ± 4.0 | 1874 ± 633 | 1362 ± 379 | 37.9 ± 12.3 | |||
| Takebe et al study, 2021 (50) | ||||||||
| 20 | 3 | 1 | 3.7 ± 0.6 | 68.6 ± 7.9 | 30.2 ± 4.3 | 1.08 ± 0.07 | 3.9 ± 0.9 | 55.9 ± 15.0 |
| 3 | 28 | 0.7 ± 0.6 | 286.1 ± 86.8 | 131.7 ± 79.2 | 4.25 ± 1.19 | |||
| 40 | 5 | 1 | 4.0 ± 0.0 | 112.2 ± 30.2 | 52.5 ± 15.4 | 1.65 ± 0.36 | 3.0 ± 0.3 | 40.4 ± 4.4 |
| 3 | 28 | 3.3 ± 2.5 | 257.9 ± 55.0 | 147.3 ± 42.6 | 4.76 ± 0.85 | |||
| 60 | 3 | 1 | 12.0 ± 10.4 | 121.6 ± 39.7 | 82.7 ± 9.3 | 2.14 ± 0.63 | 3.7 ± 1.5 | 52.4 ± 25.2 |
| 3 | 28 | 10.8 ± 12.0 | 417.3 ± 188.6 | 345.4 ± 175.9 | 8.08 ± 4.05 | |||
| 100 | 3 | 1 | 4.3 ± 2.9 | 296.0 ± 30.0 | 155 ± 21 | 5.00 ± 0.46 | 2.7 ± N/A | 36.8 ± N/A |
| 3 | 28 | 3.0 ± 1.4 | 1010.0 ± 411.5 | 428.0 ± N/A | 13.29 ± N/A | |||
| 140 | 3 | 1 | 4.3 ± 1.5 | 610.0 ± 306.0 | 311.0 ± 171.0 | 9.76 ± 4.24 | 2.4 ± 0.2 | 30.6 ± 3.5 |
| 3 | 28 | 2.0 ± 1.0 | 1113.0 ± 325.0 | 563.0 ± 49.0 | 17.40 ± 1.15 | |||
| 200 | 3 | 1 | 4.0 ± 3.5 | 517.0 ± 232.0 | 336.0 ± 105.0 | 9.22 ± 3.11 | 3.1 ± 0.9 | 43.4 ± 15.8 |
| 3 | 28 | 2.0 ± 0.0 | 1641.0 ± 830.0 | 1069.0 ± 652.0 | 30.75 ± 19.43 | |||
| 280 | 7 | 1 | 3.0 ± 0.8 | 930.0 ± 224.0 | 468.0 ± 128.0 | 14.96 ± 4.64 | N/Ac | N/Ac |
| 360 | 7 | 1 | 3.9 ± 1.6 | 1309.0 ± 349.0 | 676.0 ± 297.0 | 20.55 ± 7.33 | N/Ac | N/Ac |
Data presented as mean ± SD.
Abbreviations: AUC, area under the curve; AUC0-24h, area under the curve over 24 h; C24h, serum concentration after 24 h; Cmax, peak serum concentration; N/A, not available; Tmax, time to maximum serum concentration.
a n = 7 as reported in Goetz et al study.
b n = 5 as reported in Goetz et al study.
c Pharmacokinetics on day 28 not reported for these doses in the Takebe et al study.
Additional in vitro studies have documented a role for endoxifen in effectively inhibiting growth of melanoma cancer cells and in vivo studies demonstrated superior antitumor activity over tamoxifen in reducing metastatic melanoma nodules (72). Endoxifen has also been shown to be superior over tamoxifen both as a single agent as well as in combination with all-trans-retinoic acid in inhibiting proliferation and migration of melanoma cancer cells, without inducing drug toxicity (73). These data suggest that endoxifen might be a promising treatment for melanoma. Like desmoid tumors, ERβ is expressed in melanoma, and thus is a potential target for therapeutic intervention (74). Further studies are needed to determine whether endoxifen’s therapeutic effects in melanoma are mediated via ERβ.
Endoxifen Effects on the Bone, Lipids, and Uterus
Long-term endocrine therapy is often associated with concerning side effects (75). Prolonged AI therapy is known to cause debilitating effects on bone health by inducing accelerated bone loss and increased rates of vertebral and hip fractures (76). In contrast, tamoxifen and the newer SERMs, including raloxifene, lasofoxifene, and arzoxifene (77), generally elicit beneficial effects on the bone by reducing bone loss and the risk of bone fractures and may exhibit some mild lipid-lowering effects (78-83). Given these data, it was of interest to evaluate the effects of endoxifen on these phenotypes. In ovariectomized mice, Z-endoxifen oral administration was shown to increase cancellous and cortical bone mass (84), eliciting similar beneficial effects on bone as noted with the other SERMs. Also, oral administration of Z-endoxifen not only reduced bone turnover in both ovary-intact and ovariectomized rats but also protected ovariectomized rats against bone loss (85), further corroborating endoxifen’s agonistic effects on the bone. Regarding the effect of endoxifen on lipids, there are few if any preclinical data pertaining to the effects of endoxifen on lipids. However, early data from the first phase 1 clinical study suggests that endoxifen may reduce total cholesterol levels (49).
An additional concerning side effect of prolonged tamoxifen therapy is the increased risk of uterine cancer (86, 87). This is a serious clinical problem and often an important consideration in the development of novel SERMs (88). Evaluation of Z-endoxifen’s uterotropic effects in both ovary-intact and ovariectomized rats have shown that endoxifen, unlike tamoxifen, elicits minimal proliferative activity in the uterus and notably its partial agnostic effects are less potent compared with estradiol (85, 89). Collectively, these data suggest that endoxifen’s beneficial effects may extend beyond its potent anticancer activity. Furthermore, these findings also suggest that in patients receiving AI or tamoxifen therapy, replacing with or switching over to endoxifen therapy for long-term treatment might be a safer option as it would alleviate some of the health concerns associated with the former treatments.
Endoxifen’s Antimanic Activity in Bipolar Disorder
Bipolar I disorder is a chronic mental illness characterized by the occurrence of acute mania or mixed episodes of shifts in mood, energy, and activity levels and the ability to carry out day-to-day tasks, thereby disabling normal life. The role of protein kinase C (PKC) signaling in the pathophysiology of bipolar disorder is well documented (90). Although clinical trials have previously demonstrated antimanic activity for tamoxifen, a known PKC inhibitor, in patients with bipolar I disorder (91, 92), reports have indicated that endoxifen is a more potent inhibitor of PKC compared with tamoxifen (93). Considering these data, endoxifen’s efficacy in the treatment of bipolar I disorder has been evaluated. A double-blind, active-controlled trial has demonstrated that endoxifen is well tolerated and exhibits promising antimanic activity in bipolar disorder patients, with its efficacy similar to that of divalproex, the commonly prescribed drug for the treatment of this disease (94). Furthermore, endoxifen treatment resulted in numerically fewer side effects compared with divalproex. The phase 3 multicenter, double-blind, active-controlled clinical trial that ensued further validated endoxifen safety and efficacy for the treatment of bipolar I disorder (Clinical Trials Registry-India/2017/07/009163) (95). This trial additionally showed that remission was achieved earlier with endoxifen, compared with divalproex, as early as day 4. Collectively, these findings highlight a novel antimanic activity for endoxifen in bipolar disorder and calls for further exploration of endoxifen actions in this disease.
Concluding Remarks
The current body of literature has clearly demonstrated that endoxifen is a potent antiestrogen with a unique mechanism of action that differs from tamoxifen. Phase 1/2 clinical trials evaluating Z-endoxifen have demonstrated encouraging antitumor activity in endocrine-refractory breast cancer as well as in nonbreast cancers. Early findings from the phase 2 clinical study directly comparing the efficacy of endoxifen vs tamoxifen monotherapy in ER+ metastatic breast cancer patients suggest that endoxifen may be an efficacious drug compared with tamoxifen.
In premenopausal women with early-stage, ER+ breast cancer, an updated analysis of the International Breast Cancer Study Group 24-02 study demonstrated that therapies that more deeply suppress estrogen such as ovarian function suppression (OFS) drugs (eg, goserelin [Zoladex]) in addition to tamoxifen or AI therapy are superior to tamoxifen monotherapy (96, 97). However, deep estrogen deprivation can cause significant morbidity, often resulting in detrimental effects on women’s overall health. Follow-up studies of patients enrolled on the Suppression of Ovarian Function Trial and Tamoxifen and Exemestane Trial extending 8 years after surgery have reported that side effects associated with accelerated aging were doubled in premenopausal women treated with AI plus OFS compared with tamoxifen plus OFS (98). The superior estrogen-suppressive effects of endoxifen compared with tamoxifen (66) calls for future clinical investigation comparing the efficacy of endoxifen without OFS with that of tamoxifen or AI in combination with OFS therapy in premenopausal breast cancer patients. The results from such a study would determine whether endoxifen could be a replacement for the current standard of care that includes OFS, potentially eliminating the debilitating side effects associated with OFS therapy and considerably improving the quality of life in these women.
Additionally, the utility of combining Z-endoxifen with CDK4/6i is another interesting area of clinical investigation. Currently, CDK4/6i in combination with AI or fulvestrant is the standard of care for the treatment of metastatic hormone receptor positive breast cancer in patients, owing to their significant improvement in PFS (99-103). Although the PFS benefit in MONALEESA7 appeared similar comparing the tamoxifen/ribociclib and AI/ribociclib arms, given issues with prolonged QT, the standard approach is to use either fulvestrant or an AI in combination with CDK 4/6 inhibitors (104). Given that Z-endoxifen antitumor activity is superior to that of letrozole (66), further studies are needed to determine whether endoxifen and CDK 4/6i combinations provide better antitumor activity compared with either tamoxifen, fulvestrant, or AI combinations.
Finally, comprehensive transcriptome studies have indicated that endoxifen is a more potent inhibitor of estrogen-regulated genes compared with tamoxifen in ER+ breast cancer. However, endoxifen’s effect on the proteome remains poorly understood and calls for further investigation. Although emerging studies indicate that endoxifen may impact oncogenic signaling kinases (66), more in-depth phospho and total proteome-based mass spectroscopy analyses and kinase screening assays are necessary to comprehensively identify the signaling kinases and cellular proteins that are targeted by endoxifen. These findings are critical not only to our understanding of the molecular basis of endoxifen’s potent antitumor activity in ER+ breast cancer, but also will provide insights into its potential mode of action in nonbreast malignancies. Meanwhile, based on the encouraging findings from phase 1/2 clinical trials, the ongoing efforts to develop endoxifen for the treatment of ER+ breast cancer should continue (105).
Acknowledgments
Funding: This work has been supported by grants from the Mayo Clinic Breast Cancer Specialized Program of Research Excellence Grant (P50CA 116201 to M.P.G., J.M.R., and J.R.H.), the Mayo Clinic Cancer Center Support Grant (P30 CA15083 to M.P.T. and J.M.R.), the Prospect Creek Foundation (to M.P.G. and J.R.H.), the George M. Eisenberg Foundation for Charities (to M.P.G. and J.R.H.), the Regis Foundation (to M.P.G.) and the Eagles 5th District Cancer Telethon Funds for Cancer Research (to S.J.).
Author Contributions: S.J. reviewed the literature and wrote the manuscript. J.M.R., J.R.H., and M.P.G. reviewed and edited the manuscript. M.P.G. approved the final version for publication.
Glossary
Abbreviations
- 4HT
4-hydroxy tamoxifen
- AI
aromatase inhibitor
- CDK4/6i
CDK4/6 inhibitor
- CYP2D6
cytochrome P450 2D6
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor-beta
- ER+
estrogen receptor positive
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
- MCF7AC1
aromatase-expressing MCF7
- NDT
N-desmethyltamoxifen
- OFS
ovarian function suppression;
- PFS
progression-free survival
- PKC
protein kinase C
- SERD
selective estrogen receptor degrader
- SERM
selective estrogen receptor modulator
Contributor Information
Swaathi Jayaraman, Department of Oncology, Mayo Clinic, Rochester, MN 55905, USA.
Joel M Reid, Department of Oncology, Mayo Clinic, Rochester, MN 55905, USA; Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, Rochester, MN 55905, USA.
John R Hawse, Department of Biochemistry and Molecular Biology, Mayo Clinic, Rochester, MN 55905, USA.
Matthew P Goetz, Department of Oncology, Mayo Clinic, Rochester, MN 55905, USA; Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, Rochester, MN 55905, USA.
Additional Information
Disclosures: M.P.G. is the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, MD; and reports personal fees continuing medical education activities from Research to Practice and Clinical Education Alliance, consulting fees to Mayo Clinic from Eagle Pharmaceuticals, Lilly, Biovica, Novartis, Pfizer, Sermonix, AstraZeneca, Blueprint Medicines, and Biotheranostics; and grant funding to Mayo Clinic from Pfizer, Lilly, and Sermonix. The other authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed for this review.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. [DOI] [PubMed] [Google Scholar]
- 2. Quirke VM. Tamoxifen from failed contraceptive pill to best-selling breast cancer medicine: a case-study in pharmaceutical innovation. Front Pharmacol. 2017;8:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25(2):270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiang DT, Kennedy BJ. Tamoxifen (antiestrogen) therapy in advanced breast cancer. Ann Intern Med. 1977;87(6):687-690. [DOI] [PubMed] [Google Scholar]
- 5. Ingle JN, Ahmann DL, Green SJ, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981;304(1):16-21. [DOI] [PubMed] [Google Scholar]
- 6. Rose C, Mouridsen HT. Treatment of advanced breast cancer with tamoxifen. Recent Results Cancer Res. 1984;91:230-242. [DOI] [PubMed] [Google Scholar]
- 7. van Veelen H, Willemse PH, Tjabbes T, Schweitzer MJ, Sleijfer DT. Oral high-dose medroxyprogesterone acetate versus tamoxifen. A randomized crossover trial in postmenopausal patients with advanced breast cancer. Cancer. 1986;58(1):7-13. [DOI] [PubMed] [Google Scholar]
- 8. Ingle JN, Krook JE, Green SJ, et al. Randomized trial of bilateral oophorectomy versus tamoxifen in premenopausal women with metastatic breast cancer. J Clin Oncol. 1986;4(2):178-185. [DOI] [PubMed] [Google Scholar]
- 9. Buchanan RB, Blamey RW, Durrant KR, et al. A randomized comparison of tamoxifen with surgical oophorectomy in premenopausal patients with advanced breast cancer. J Clin Oncol. 1986;4(9):1326-1330. [DOI] [PubMed] [Google Scholar]
- 10. Manni A. Tamoxifen therapy of metastatic breast cancer. J Lab Clin Med. 1987;109(3):290-299. [PubMed] [Google Scholar]
- 11. Ingle JN, Mailliard JA, Schaid DJ, et al. A double-blind trial of tamoxifen plus prednisolone versus tamoxifen plus placebo in postmenopausal women with metastatic breast cancer. A collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic. Cancer. 1991;68(1):34-39. [DOI] [PubMed] [Google Scholar]
- 12. Castiglione-Gertsch M, Pampallona S, Varini M, et al. Primary endocrine therapy for advanced breast cancer: to start with tamoxifen or with medroxyprogesterone acetate? Ann Oncol. 1993;4(9):735-740. [DOI] [PubMed] [Google Scholar]
- 13. Kimmick G, Muss HB. Current status of endocrine therapy for metastatic breast cancer. Oncology (Williston Park). 1995;9(9):877-86, 889. [PubMed] [Google Scholar]
- 14. Adjuvant tamoxifen in the management of operable breast cancer: the Scottish Trial. Report from the Breast Cancer Trials Committee, Scottish Cancer Trials Office (MRC), Edinburgh. Lancet. 1987;2(8552):171-175. [PubMed] [Google Scholar]
- 15. Controlled trial of tamoxifen as a single adjuvant agent in the management of early breast cancer. ‘Nolvadex’ Adjuvant Trial Organisation. Br J Cancer. 1988;57(6):608-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ribeiro G, Swindell R. The Christie hospital adjuvant tamoxifen trial–status at 10 years. Br J Cancer. 1988;57(6):601-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320(8):479-484. [DOI] [PubMed] [Google Scholar]
- 18. Cutuli B, Lacroze M, Dilhuydy JM, et al. Male breast cancer: results of the treatments and prognostic factors in 397 cases. Eur J Cancer. 1995;31A(12):1960-1964. [DOI] [PubMed] [Google Scholar]
- 19. Borgen PI, Wong GY, Vlamis V, et al. Current management of male breast cancer. A review of 104 cases. Ann Surg. 1992;215(5):451-457; discussion 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371-1388. [DOI] [PubMed] [Google Scholar]
- 21. Cuzick J, Sestak I, Cawthorn S, et al. ; IBIS-I Investigators . Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16(1):67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davies C, Godwin J, Gray R, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310(3):1062-1075. [DOI] [PubMed] [Google Scholar]
- 24. Jordan VC, Collins MM, Rowsby L, Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977;75(2):305-316. [DOI] [PubMed] [Google Scholar]
- 25. Lien EA, Solheim E, Lea OA, Lundgren S, Kvinnsland S, Ueland PM. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989;49(8):2175-2183. [PubMed] [Google Scholar]
- 26. Lien EA, Anker G, Lønning PE, Solheim E, Ueland PM. Decreased serum concentrations of tamoxifen and its metabolites induced by aminoglutethimide. Cancer Res. 1990;50(18):5851-5857. [PubMed] [Google Scholar]
- 27. Lien EA, Solheim E, Ueland PM. Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res. 1991;51(18):4837-4844. [PubMed] [Google Scholar]
- 28. Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85(2):151-159. [DOI] [PubMed] [Google Scholar]
- 29. Lim YC, Desta Z, Flockhart DA, Skaar TC. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005;55(5):471-478. [DOI] [PubMed] [Google Scholar]
- 30. Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97(1):30-39. [DOI] [PubMed] [Google Scholar]
- 31. Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80(1):61-74. [DOI] [PubMed] [Google Scholar]
- 32. Helland T, Henne N, Bifulco E, et al. Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res. 2017;19(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Helland T, Naume B, Hustad S, et al. Low Z-4OHtam concentrations are associated with adverse clinical outcome among early stage premenopausal breast cancer patients treated with adjuvant tamoxifen. Mol Oncol. 2021;15(4):957-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madlensky L, Natarajan L, Tchu S, et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89(5):718-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saladores P, Mürdter T, Eccles D, et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J. 2015;15(1):84-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beverage JN, Sissung TM, Sion AM, Danesi R, Figg WD. CYP2D6 polymorphisms and the impact on tamoxifen therapy. J Pharm Sci. 2007;96(9):2224-2231. [DOI] [PubMed] [Google Scholar]
- 37. Kiyotani K, Mushiroda T, Zembutsu H, Nakamura Y. Important and critical scientific aspects in pharmacogenomics analysis: lessons from controversial results of tamoxifen and CYP2D6 studies. J Hum Genet. 2013;58(6):327-333. [DOI] [PubMed] [Google Scholar]
- 38. Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302(13):1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goetz MP, Knox SK, Suman VJ, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101(1):113-121. [DOI] [PubMed] [Google Scholar]
- 40. Zembutsu H, Nakamura S, Akashi-Tanaka S, et al. Significant effect of polymorphisms in CYP2D6 on response to tamoxifen therapy for breast cancer: a prospective multicenter study. Clin Cancer Res. 2017;23(8):2019-2026. [DOI] [PubMed] [Google Scholar]
- 41. Nowell SA, Ahn J, Rae JM, et al. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat. 2005;91(3):249-258. [DOI] [PubMed] [Google Scholar]
- 42. Wegman P, Elingarami S, Carstensen J, Stål O, Nordenskjöld B, Wingren S. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. 2007;9(1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rae JM, Drury S, Hayes DF, et al. ; ATAC trialists . CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104(6):452-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Regan MM, Leyland-Jones B, Bouzyk M, et al. ; Breast International Group (BIG) 1-98 Collaborative Group . CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1-98 trial. J Natl Cancer Inst. 2012;104(6):441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goetz MP, Suman VJ, Hoskin TL, et al. CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8. Clin Cancer Res. 2013;19(2):500-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kiyotani K, Mushiroda T, Hosono N, et al. Lessons for pharmacogenomics studies: association study between CYP2D6 genotype and tamoxifen response. Pharmacogenet Genomics. 2010;20(9):565-568. [DOI] [PubMed] [Google Scholar]
- 47. Sanchez-Spitman A, Dezentjé V, Swen J, et al. Tamoxifen pharmacogenetics and metabolism: results from the prospective CYPTAM study. J Clin Oncol. 2019;37(8):636-646. [DOI] [PubMed] [Google Scholar]
- 48. Goetz MP, Sangkuhl K, Guchelaar HJ, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin Pharmacol Ther. 2018;103(5):770-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goetz MP, Suman VJ, Reid JM, et al. First-in-human phase I study of the tamoxifen metabolite Z-endoxifen in women with endocrine-refractory metastatic breast cancer. J Clin Oncol. 2017;35(30):3391-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takebe N, Coyne GO, Kummar S, et al. Phase 1 study of Z-endoxifen in patients with advanced gynecologic, desmoid, and hormone receptor-positive solid tumors. Oncotarget. 2021;12(4):268-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mürdter TE, Schroth W, Bacchus-Gerybadze L, et al. ; German Tamoxifen and AI Clinicians Group . Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89(5):708-717. [DOI] [PubMed] [Google Scholar]
- 52. Safgren SL, Suman VJ, Kosel ML, et al. Evaluation of CYP2D6 enzyme activity using a 13C-dextromethorphan breath test in women receiving adjuvant tamoxifen. Pharmacogenet Genomics. 2015;25(4):157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95(23):1758-1764. [DOI] [PubMed] [Google Scholar]
- 54. Hwang GS, Bhat R, Crutchley RD, Trivedi MV. Impact of CYP2D6 polymorphisms on endoxifen concentrations and breast cancer outcomes. Pharmacogenomics J. 2018;18(2):201-208. [DOI] [PubMed] [Google Scholar]
- 55. Hertz DL, Deal A, Ibrahim JG, et al. Tamoxifen dose escalation in patients with diminished CYP2D6 activity normalizes endoxifen concentrations without increasing toxicity. Oncologist. 2016;21(7):795-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reid JM, Goetz MP, Buhrow SA, et al. Pharmacokinetics of endoxifen and tamoxifen in female mice: implications for comparative in vivo activity studies. Cancer Chemother Pharmacol. 2014;74(6):1271-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gong IY, Teft WA, Ly J, et al. Determination of clinically therapeutic endoxifen concentrations based on efficacy from human MCF7 breast cancer xenografts. Breast Cancer Res Treat. 2013;139(1):61-69. [DOI] [PubMed] [Google Scholar]
- 58. Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res. 2009;69(5):1722-1727. [DOI] [PubMed] [Google Scholar]
- 59. Hawse JR, Subramaniam M, Cicek M, et al. Endoxifen’s molecular mechanisms of action are concentration dependent and different than that of other anti-estrogens. PLoS One. 2013;8(1):e54613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maximov PY, McDaniel RE, Fernandes DJ, et al. Pharmacological relevance of endoxifen in a laboratory simulation of breast cancer in postmenopausal patients. J Natl Cancer Inst. 2014;106(10):dju283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maximov PY, McDaniel RE, Fernandes DJ, et al. Simulation with cells in vitro of tamoxifen treatment in premenopausal breast cancer patients with different CYP2D6 genotypes. Br J Pharmacol. 2014;171(24):5624-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dahmane E, Mercier T, Zanolari B, et al. An ultra performance liquid chromatography-tandem MS assay for tamoxifen metabolites profiling in plasma: first evidence of 4’-hydroxylated metabolites in breast cancer patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(32):3402-3414. [DOI] [PubMed] [Google Scholar]
- 63. Sanchez-Spitman AB, Swen JJ, Dezentje VO, Moes DJAR, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics and pharmacogenetics of tamoxifen and endoxifen. Expert Rev Clin Pharmacol. 2019;12(6):523-536. [DOI] [PubMed] [Google Scholar]
- 64. Zundelevich A, Dadiani M, Kahana-Edwin S, et al. ESR1 mutations are frequent in newly diagnosed metastatic and loco-regional recurrence of endocrine-treated breast cancer and carry worse prognosis. Breast Cancer Res. 2020;22(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lindeman GL, Bowen R, Jerzak KJ, et al. J Clin Oncol. 2021;39(15_suppl):1004. [Google Scholar]
- 66. Jayaraman S, Hou X, Kuffel MJ, et al. Antitumor activity of Z-endoxifen in aromatase inhibitor-sensitive and aromatase inhibitor-resistant estrogen receptor-positive breast cancer. Breast Cancer Res. 2020;22(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Long BJ, Jelovac D, Handratta V, et al. Therapeutic strategies using the aromatase inhibitor letrozole and tamoxifen in a breast cancer model. J Natl Cancer Inst. 2004;96(6):456-465. [DOI] [PubMed] [Google Scholar]
- 68. Macedo LF, Sabnis GJ, Goloubeva OG, Brodie A. Combination of anastrozole with fulvestrant in the intratumoral aromatase xenograft model. Cancer Res. 2008;68(9):3516-3522. [DOI] [PubMed] [Google Scholar]
- 69. Santos GA, Cunha IW, Rocha RM, et al. Evaluation of estrogen receptor alpha, estrogen receptor beta, progesterone receptor, and cKIT expression in desmoids tumors and their role in determining treatment options. Biosci Trends. 2010;4(1):25-30. [PubMed] [Google Scholar]
- 70. Santti K, Ihalainen H, Rönty M, et al. Estrogen receptor beta expression correlates with proliferation in desmoid tumors. J Surg Oncol. 2019;119(7):873-879. [DOI] [PubMed] [Google Scholar]
- 71. Wu X, Subramaniam M, Grygo SB, et al. Estrogen receptor-beta sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen. Breast Cancer Res. 2011;13(2):R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen P, Sheikh S, Ahmad A, Ali SM, Ahmad MU, Ahmad I. Orally administered endoxifen inhibits tumor growth in melanoma-bearing mice. Cell Mol Biol Lett. 2018;23:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ribeiro MP, Silva FS, Paixão J, Santos AE, Custódio JB. The combination of the antiestrogen endoxifen with all-trans-retinoic acid has anti-proliferative and anti-migration effects on melanoma cells without inducing significant toxicity in non-neoplasic cells. Eur J Pharmacol. 2013;715(1-3):354-362. [DOI] [PubMed] [Google Scholar]
- 74. Marzagalli M, Montagnani Marelli M, Casati L, Fontana F, Moretti RM, Limonta P. Estrogen receptor β in melanoma: from molecular insights to potential clinical utility. Front Endocrinol (Lausanne). 2016;7:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299-1309. [DOI] [PubMed] [Google Scholar]
- 76. Ligibel JA, O’Malley AJ, Fisher M, Daniel GW, Winer EP, Keating NL. Patterns of bone density evaluation in a community population treated with aromatase inhibitors. Breast Cancer Res Treat. 2012;134(3):1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Silverman SL. New selective estrogen receptor modulators (SERMs) in development. Curr Osteoporos Rep. 2010;8(3):151-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Aihara T, Suemasu K, Takei H, et al. Effects of exemestane, anastrozole and tamoxifen on bone mineral density and bone turnover markers in postmenopausal early breast cancer patients: results of N-SAS BC 04, the TEAM Japan substudy. Oncology. 2010;79(5-6):376-381. [DOI] [PubMed] [Google Scholar]
- 79. Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337(23):1641-1647. [DOI] [PubMed] [Google Scholar]
- 80. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Jama. 1999;282(7):637-645. [DOI] [PubMed] [Google Scholar]
- 81. McClung MR, Siris E, Cummings S, et al. Prevention of bone loss in postmenopausal women treated with lasofoxifene compared with raloxifene. Menopause. 2006;13(3):377-386. [DOI] [PubMed] [Google Scholar]
- 82. Deshmane V, Krishnamurthy S, Melemed AS, Peterson P, Buzdar AU. Phase III double-blind trial of arzoxifene compared with tamoxifen for locally advanced or metastatic breast cancer. J Clin Oncol. 2007;25(31):4967-4973. [DOI] [PubMed] [Google Scholar]
- 83. Cummings SR, Ensrud K, Delmas PD, et al. ; PEARL Study Investigators . Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362(8):686-696. [DOI] [PubMed] [Google Scholar]
- 84. Gingery A, Subramaniam M, Pitel KS, et al. The effects of a novel hormonal breast cancer therapy, endoxifen, on the mouse skeleton. Plos One. 2014;9(5):e98219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gingery A, Iwaniec UT, Subramaniam M, et al. Skeletal and uterotrophic effects of endoxifen in female rats. Endocrinology. 2017;158(10):3354-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bernstein L, Deapen D, Cerhan JR, et al. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91(19):1654-1662. [DOI] [PubMed] [Google Scholar]
- 87. Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet. 2000;356(9233):881-887. [DOI] [PubMed] [Google Scholar]
- 88. Pinkerton JV, Goldstein SR. Endometrial safety: a key hurdle for selective estrogen receptor modulators in development. Menopause. 2010;17(3):642-653. [DOI] [PubMed] [Google Scholar]
- 89. Schweikart KM, Eldridge SR, Safgren SL, et al. Comparative uterotrophic effects of endoxifen and tamoxifen in ovariectomized Sprague-Dawley rats. Toxicol Pathol. 2014;42(8):1188-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Saxena A, Scaini G, Bavaresco DV, et al. Role of protein kinase C in bipolar disorder: a review of the current literature. Mol Neuropsychiatry. 2017;3(2):108-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zarate CA Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561-570. [DOI] [PubMed] [Google Scholar]
- 92. Yildiz A, Guleryuz S, Ankerst DP, Ongür D, Renshaw PF. Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch Gen Psychiatry. 2008;65(3):255-263. [DOI] [PubMed] [Google Scholar]
- 93. Ali SM, Ahmad A, Shahabuddin S, Ahmad MU, Sheikh S, Ahmad I. Endoxifen is a new potent inhibitor of PKC: a potential therapeutic agent for bipolar disorder. Bioorg Med Chem Lett. 2010;20(8):2665-2667. [DOI] [PubMed] [Google Scholar]
- 94. Ahmad A, Sheikh S, Shah T, et al. Endoxifen, a new treatment option for mania: a double-blind, active-controlled trial demonstrates the antimanic efficacy of endoxifen. Clin Transl Sci. 2016;9(5):252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ahmad A, Sheikh S, Khan MA, et al. Endoxifen: a new, protein kinase C inhibitor to treat acute and mixed mania associated with bipolar I disorder. Bipolar Disord. 2020. doi: 10.1111/bdi.13041. [DOI] [PubMed] [Google Scholar]
- 96. Pagani O, Regan MM, Walley BA, et al. ; TEXT and SOFT Investigators; International Breast Cancer Study Group . Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Francis PA, Regan MM, Fleming GF, et al. ; SOFT Investigators; International Breast Cancer Study Group . Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Francis PA, Pagani O, Fleming GF, et al. ; SOFT and TEXT Investigators and the International Breast Cancer Study Group . Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35. [DOI] [PubMed] [Google Scholar]
- 100. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425-439. [DOI] [PubMed] [Google Scholar]
- 101. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925-1936. [DOI] [PubMed] [Google Scholar]
- 102. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738-1748. [DOI] [PubMed] [Google Scholar]
- 103. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646. [DOI] [PubMed] [Google Scholar]
- 104. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904-915. [DOI] [PubMed] [Google Scholar]
- 105. Goetz MP. The development of endoxifen for breast cancer. Clin Adv Hematol Oncol. 2018;16(2):102-105. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed for this review.