FIG 1.
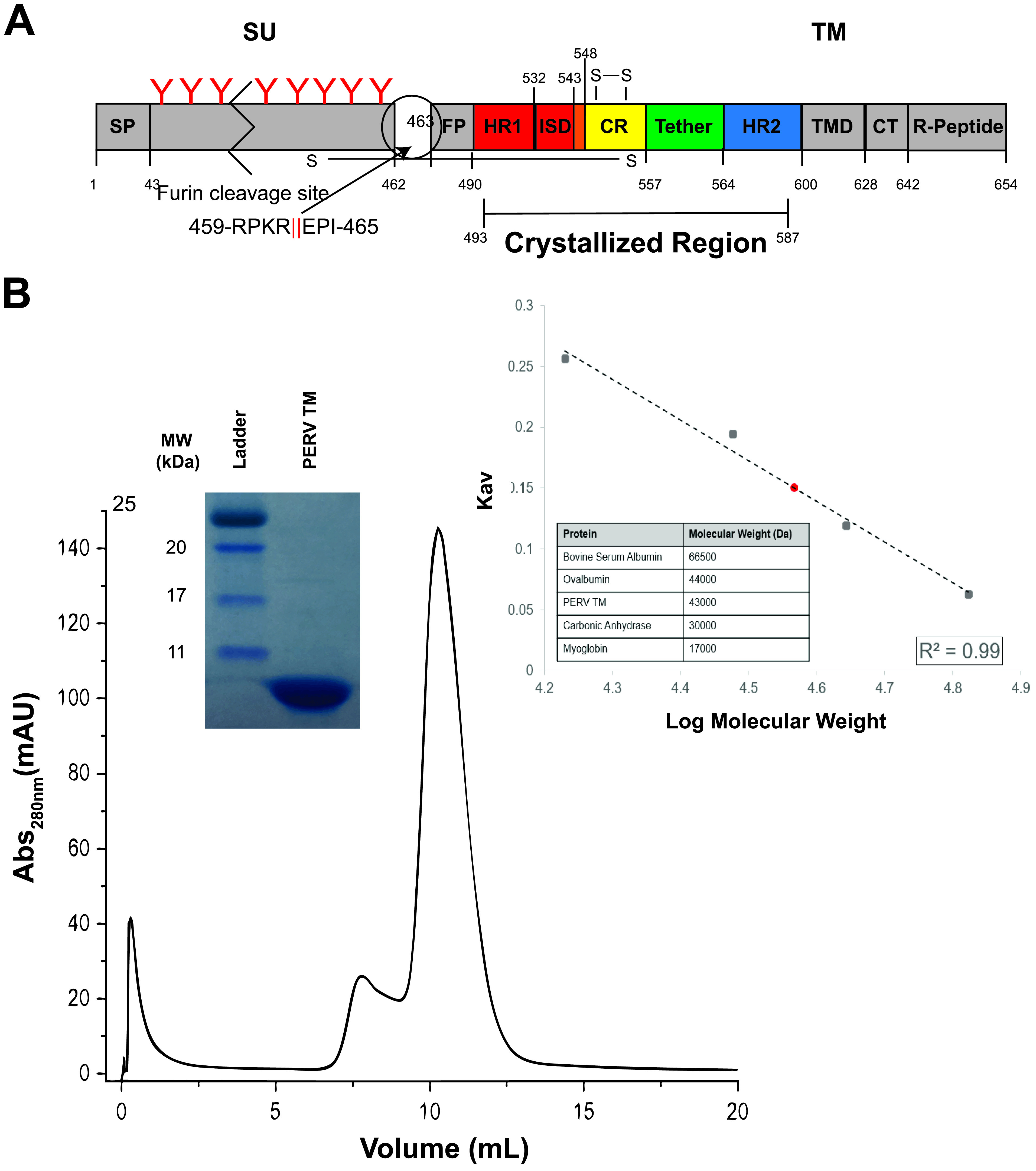
Postfusion PERV TM is a trimer in solution. (A) Overview of key regions of the PERV TM. The PERV envelope GP is translated as a single polypeptide that contains a signal peptide (SP) that allows the secretion and display of the GP on the membrane surface. The attachment subunit (SU) is N-linked glycosylated and is thought to be shed from the fusion subunit (TM) following receptor binding. The red “Y” symbols denote predicted N-linked glycans, and the zigzag indicates the SU is not drawn to scale. The TM fusogen contains the fusion peptide (FP) and the α-helical heptad repeats (HR1 and HR2) separated by a CX6CC-containing CR region and short six-residue tether. The PERV immunosuppressive domain (ISD) is present in the transition region from HR1 to the CR. A cysteine pair is formed between the first and second cysteine residues of the CX6CC motif, and the third cysteine makes an intermolecular disulfide bond with the SU. At the C-terminal end of the TM subunit are the transmembrane domain (TMD) and the cytoplasmic tail (CT), which contains the inhibitory R peptide. The crystallized region of the protein is indicated. (B) Size exclusion chromatogram of PERV TM493–587 on a Superdex 75 10/300 GL column. Purity of the peak fraction was assessed with a 16% Coomassie-stained gel (inset). A calibration Kav standard curve (inset) with bovine serum albumin (66,500 Da), ovalbumin (45,000 Da), carbonic anhydrase (30,000 Da), and myoglobin (17,000 Da) standards displayed as black squares was used to assess molecular weight. γ-Globulin (158,000 Da) was used to measure the void volume (Vo). The apparent tag-intact PERV TM molecular weight was estimated to be ∼43 kDa (shown as a red circle), consistent with a trimeric biological assembly (theoretical trimeric MW, 39.9 kDa).
