Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized solid organ and hematologic cancer treatments by improving overall prognoses. However, they can lead to overactivation of the immune system and several immune-related adverse events and sometimes affecting the renal system. Although acute interstitial nephritis is well described, we know little about ICI-associated glomerular injury. Herein, we report an exceptional case of renal ANCA positive-associated vasculitis (AAV) after nivolumab therapy. Three weeks after the last nivolumab injection, the patient presented with proteinuria at 1.73 g/g of creatininuria, hematuria, and acute kidney injury needing dialysis associated with lung hemorrhage; anti-neutrophil cytoplasmic antibody (ANCA titer ≥1,280 with myeloperoxidase specificity of 780 U/mL) was positive, and kidney biopsy confirmed glomerular injury with crescents. The patient underwent treatment with steroid pulses, rituximab, and plasmapheresis, resulting in an improvement of the renal function and lung hemorrhage and produced a negative ANCA titer. Despite the results of the PEXIVAS study and the absence of clear benefit of plasmapheresis demonstrated in idiopathic AAV, we suggest that drug-induced AAV may be effectively treated by plasmapheresis, steroids, and rituximab.
Keywords: Nivolumab, Plasmapheresis, ANCA vasculitis, Kidney failure
Introduction
Immune checkpoint inhibitors (ICIs) have greatly improved the overall prognosis for several malignancies. These monoclonal antibodies act by blocking immune checkpoints that modulate the activation of lymphocytes.
Three checkpoint proteins are responsible for inducing anergy: cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), the programmed death-1 receptor (PD-1), and its ligand PD-L1. These molecules are localized on several immune system cells, such as T cells, B cells, and dendritic cells. PD-1 regulates cytotoxic T-cell proliferation and B-cell overall survival, whereas CTLA4 mostly inhibits memory T-cell activation [1].
Tumor cells, in order to escape the immune system, decrease the expression of tumor-associated antigens and major histocompatibility complex molecules: they secrete immunosuppressive molecules and selectively upregulate checkpoint inhibitor receptor molecules in their microenvironment. Understanding these mechanisms has permitted to specifically target this pathway to treat several malignancies using ICIs.
However, upregulating the immune response may result in immune-related adverse events. ICIs may cause autoimmune diseases due to tissue-specific self-reactive T-cell activation. The most frequent organs affected by ICI-induced autoimmune diseases are the skin, the endocrine system, and the gastrointestinal tract. Yet, adverse events related to the kidneys can also occur [2, 3]. Acute tubulointerstitial nephritis is the most common pathological finding [4, 5]. To date, very few cases of ICI-associated glomerulonephritis have been described, and its specific management and treatment remains unknown [6, 7]. Herein, we report on a case of renal ANCA-associated vasculitis (AAV) associated with nivolumab given to a patient presenting with metastatic non-small-cell lung carcinoma.
Case Presentation
An 81-year-old man was diagnosed with metastatic non-small-cell lung adenocarcinoma in May 2018. The initial treatment was cisplatin and pemetrexed from May 2018 to July 2018 and then pemetrexed alone from July 2018 to September 2019. Remission occurred from September 2019 to February 2020. Then, in February, the tumor mass has increased on chest CT-scan. He was placed on nivolumab therapy (240 mg every 2 weeks); the last dose was given on April 28, 2020. Nivolumab was then discontinued because of asthenia. Kidney function was normal at that time, i.e., serum creatinine (Scr) was 1.09 mg/dL and estimated glomerular filtration rate (CKD-EPI) was 64 mL/min/1.73 m2. There was no microscopic hematuria, and proteinuria level was 0.6 g/L.
At 3 weeks after nivolumab was discontinued, the patient presented with fatigue, fever, and shortness of breath that needed oxygen therapy because of a lung hemorrhage. Scr level had increased to 5.66 mg/dL. Proteinuria was 1.73 g/g of creatininuria with a glomerular profile (46% of albumin) associated with hematuria. Anti-neutrophil cytoplasmic antibodies (ANCA) were positive with a titer ≥1/1,280 (indirect immunofluorescence) with a myeloperoxidase (MPO) specificity of 780 U/mL (enzyme immunoassay, N <5 U/mL).
A kidney biopsy, shown in Figure 1, performed on June 8, revealed crescentic glomerulonephritis. There were 23 glomeruli. Cellular to fibrocellular crescents involved 78% of glomeruli, and 22% of glomeruli were globally sclerosed. Fibrinoid necrosis involved 52% of glomeruli, and slight fibrosis surrounding rare atrophic tubules (5%) was present. Immunofluorescence staining was negative. Kidney failure worsened, and hemodialysis was initiated on June 11. On the day of hemodialysis initiation, nivolumab trough level was 4.1 μg/mL. The patient had no other organ injury apart from renal and lung involvement.
Fig. 1.
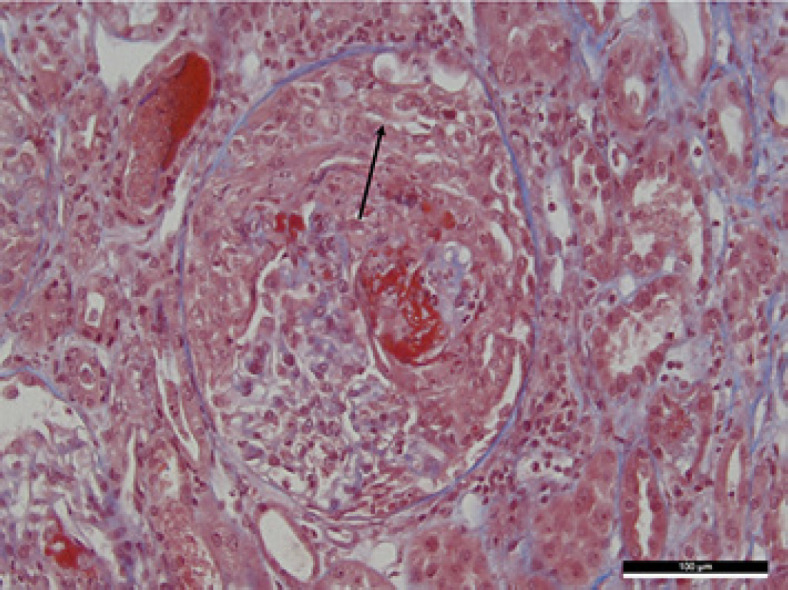
Renal biopsy (trichrome stain) showing the patient's crescentic glomerulonephritis. Bowman's space is occupied by layers of proliferating epithelial cells forming a crescent. Black arrow shows the extracapillary proliferation.
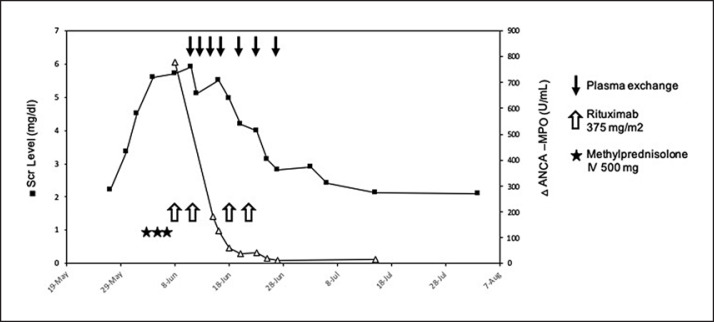
As of June 4, the patient received 3 methylprednisolone (MP) pluses (500 mg), followed by oral prednisolone (1 mg/kg), and 4 perfusions of rituximab at 375 mg/m2 on June 8, 14, 20, and 27. Because there was no renal response after MP pulses, we decided to start plasmapheresis: 7 sessions were performed between June 13 and 27. Scr levels and anti-MPO titers under treatment are shown in Figure 2. At the end of plasmapheresis, nivolumab trough level was <3.0 μg/mL.
Fig. 2.
Outcome of serum creatinine (milligrams per deciliter) and ANCA titers before and after treatment by steroids, rituximab, and plasmapheresis.
Kidney function progressively improved allowing hemodialysis to be discontinued by June 25. A chest CT-scan, performed at the end of treatment, did not show any recurrence of the lung hemorrhage. At nearly 1 year, renal function was stable, i.e., estimated glomerular filtration rate at 20 mL/min/1.73 m2 and proteinuria at 0.6 g/L. To date, there is no sign of adenocarcinoma worsening, and the patient is still alive.
Discussion and Conclusion
The incidence of acute kidney injury associated with ICIs occurs in ∼1.4%–4.9% of cases according to recent reports [4]. The most common pathological disorder reported is acute tubulointerstitial nephritis [8]. Nivolumab has been rarely described to be associated with glomerulopathies: focal and segmental glomerulosclerosis [9], PLA2R-negative membranous nephropathy [10], and mesangial expansion with no crescents or endocapillary hypercellularity [11]. Clinical cases and outcomes of ICI-related glomerulopathies reported in the literature are summarized in Table 1. The temporal association of AAV with the good evolution of the cancer under nivolumab was discordant with the usual time appearance of paraneoplastic AAV described in the literature and made it less plausible than a drug effect [12, 13, 14]. Moreover, ICI therapies are associated in the literature with many autoimmune disorders involving many organs including the kidney. To the best of our knowledge, this is the second case of renal ANCA positive AAV induced by nivolumab.
Table 1.
Summary of published checkpoint inhibitors glomerular toxicities and outcome
| Case report | Renal manifestation | Malignancy | Immunotherapy | Treatment | Renal outcome |
|---|---|---|---|---|---|
| Lin et al. [10] | Membranous nephropathy (PLA2R neg) | Melanoma | Nivolumab | D/C + steroids | Remission (partial) |
| Mamlouk et al. [15] | Membranous nephropathy (PLA2R neg) | RCC | Nivolumab | D/C + steroids | Remission |
| Kitchlu et al. [25] | MCD | Hodgkin lymphoma | Nivolumab | D/C + steroids | Remission (partial) |
| Kitchlu et al. [25] | MCD | Melanoma | Ipilimumab | D/C + steroids | Remission |
| Daanen et al. [9] | FSGS | RCC | Nivolumab | D/C + steroids + MMF | Remission followed by relapse |
| Jung et al. [26] | IgA nephropathy AKI grade 4 Cellular crescents with necrosis Subepithelial deposition |
Clear cell kidney cancer | Nivolumab | DC + steroids + RRT | Recovery (RRT was d/c after 5 months) |
| Kishi et al. [11] | IgA nephropathy AKI grade 2 Mesangial exp. with no crescents or endocapillary hypercellularity |
Lung SCC | Nivolumab | D/C | Remission complete |
| Mamlouk et al. [15] | IgA nephropathy AKI grade 2 endocapillary hypercellularity |
Melanoma | Nivolumab + ipilimumab | D/C + steroids | Remission followed by relapse |
| Mamlouk et al. [15] | IgA nephropathy AKI grade 3 No glomerular proliferative lesions |
Melanoma | Pembrolizumab | D/C + steroids, MMF + infliximab | Partial recovery |
| Mamlouk et al. [15] | Anti-dsDNA AKI with proteinuria ATIN with no immune complex deposition GN | Bladder cancer | Nivolumab | D/C + steroids | Partial renal recovery dsDNA and RNP; not detectable |
| Van den Brom et al. | GPA + PR3-ANCA | Malignant melanoma | Ipilimumab | Cyclosporine and | Remission |
| [27] | Dysmorphic erythrocytes and proteinuria Extrarenal: cutaneous vasculitis, stable lung nodule | followed by pembrolizumab | steroids | ||
| Mamlouk et al. [15] | Focal necrotizing pauci-immune glomerulonephritis ANCA neg with no crescents Extrarenal; n/a | NSCLC (SCC) | Nivolumab | D/C, steroids and rituximab | Complete recovery |
| Mamlouk et al. [15] | Focal segmental pauci-immune necrotizing glomerulonephritis + MPO-ANCA Extrarenal; n/a | mRCC | Tremelimumab | D/C, steroids, plasmapheresis and rituximab | Partial recovery |
| Mamlouk et al. [15] | Granulomatous necrotizing vasculitis ANCA neg Extrarenal; n/a | Uveal melanoma | Nivolumab + ipilimumab | D/C, steroids and rituximab | Complete recovery |
| Gallan et al. [7] | Focally crescentic and sclerosing glomerulonephritis ANCA neg with no immunofluorescence staining for immunoglobulin G (IgG), IgA, IgM, C3, or C1q | Lung adenocarcinoma | Pembrolizumab | D/C + steroids | Complete recovery |
| Current case | Pauci-immune glomerulonephritis + MPO-ANCA of crescentic class type | Lung adenocarcinoma | Nivolumab | D/C + steroids + rituximab and plasmapheresis | Partial recovery |
PLA2R, phospholipase A-2 receptor; D/C, discontinuation of immune checkpoint inhibitor; Neg, negative; mRCC, metastatic renal cell carcinoma; MCD, minimal change disease; FSGS, focal segmental glomerulosclerosis; MMF, mycophenolate mofetil; AKI, acute kidney injury; RRT, renal replacement therapy; SCC, squamous cell carcinoma; dsDNA, double-stranded DNA; RNP, ribonucleoprotein; ATIN, acute tubulointerstitial nephropathy; GN, glomerulonephritis; GPA, granulomatosis with polyangiitis; ANCA, anti-neutrophil cytoplasmic antibodies; MPO, myeloperoxidase; n/a, not available; NSCLC, non-squamous cell lung carcinoma.
The severity of ICI-related glomerulopathies and responses to treatments differ, partly due to variations between cases. Overall, the variety of ICI-induced renal manifestations suggests multiple complex mechanisms, which should be further elucidated.
A rare case of MPO-ANCA-positive glomerulonephritis, supposedly induced by tremelimumab, was effectively treated with plasmapheresis [15]. Direct involvement of ANCA in the mechanisms of pathogenesis is yet to be proven although animal models clearly indicate the pathogenicity of ANCAs [16].
ANCA stimulates the production of reactive oxygen species and the degranulation of proteolytic enzymes by polymorphonuclear, after pre-stimulation by pro-inflammatory cytokines (TNF-alpha) and adhesion to the endothelium of small vessels. Immunization of genetically MPO-deficient mice with murine MPO and the transfer of murine ANCA-MPO IV in mice without functional lymphocytes (Rag2−/−) has demonstrated systemic AAV, which was prevented by depletion of polymorphonuclear [17].
In our patient, it is possible that the favorable outcome when using plasmapheresis in the setting of ANCA vasculitis-associated severe renal disorders may be explained in several ways: i.e., by the clearance of ANCA and/or clearance of other auto-antibodies and/or removal of circulating microparticles and nonspecific inflammatory molecules (complement system and cytokines) and finally by the removal of the responsible drug (i.e., nivolumab) which may act as a trigger of the autoimmune disease. In our case, the nivolumab dosage was still positive at the time of plasmapheresis initiation. The complement system, and in particular the alternative pathway, is implicated in the development of tissue lesions, and plasmapheresis allows efficient and rapid clearance of those complement proteins [18]. Plasma exchange also removes auto-antibodies, and their resynthesis is progressively slowed by medication.
The efficacy of plasmapheresis in AAV was demonstrated in the MEPEX trial [19]. This study reported that in patients with creatinine >500 μmol/L (5.8 mg/dL) at diagnosis of AAV, renal recovery was greater and the need for dialysis reduced after plasma exchange compared to IV MP. All patients of this study received oral prednisolone and oral cyclophosphamide.
Recently, the Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis (PEXIVAS) study assessed the efficacy of plasmapheresis in patients with AAV and kidney involvement and/or pulmonary involvement [20]. The authors found no difference in benefit of plasmapheresis versus standard of care (i.e., without plasma exchange) regarding a composite endpoint including mortality or end-stage kidney failure after a median follow-up of 3 years. They concluded that there was no justification for plasma exchange in patients with AAV and kidney or pulmonary involvement, thus questioning the benefit of plasmapheresis for this indication. Yet, many case series report the potential benefit of plasmapheresis in AAV in a subset of patients presenting with alveolar hemorrhage. In 2021, many authors have not abandoned plasmapheresis, and its utilization is still debated in the literature [21, 22].
In this case of ICI-associated ANCA positive AAV, the efficacy of plasmapheresis on outcome led us to discuss the mechanisms of drug-induced AAV and the difference compared to idiopathic AAV [23, 24]. There may be several hypotheses: (1) nivolumab was directly or indirectly associated with the synthesis of ANCA: therefore, its discontinuation and the natural clearance of antibodies was sufficient to change the course of the disease; (2) nivolumab was associated with the synthesis of ANCA, and plasma exchange helped lowering nivolumab plasma levels and thus ANCA titers; or (3) nivolumab was a trigger for AAV, and the clearance of ANCA and several pro-inflammatory proteins was efficient through plasma exchange. In conclusion, we report the second case of nivolumab-induced ANCA positive AAV. Our finding invites us to investigate the mechanisms involved in the induction of autoimmunity in immunotherapy, including when disease occurs as a rare event. AAVs induced by ICI and associated with renal issues have rarely been described and need to be researched further. Finally, despite the results of the PEXIVAS study, the authors can make the hypothesis that plasmapheresis may be efficient in the subgroup of ICI-induced AAV which will have to be shown in larger studies.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. According to the local ethics committee (French CCP “commission de protection des personnes”), no more document is needed. The manuscript was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Conflict of Interest Statement
The authors of this manuscript have no conflicts of interest to disclose.
Funding Sources
This manuscript did not receive any funding.
Author Contributions
R.L. wrote the manuscript, J.N. and L.R. supervised and corrected the manuscript, C.E. interpreted the pathology findings, H.N.B. performed plasmapheresis, and F.T., A.C.T., and T.P. were the doctors in charge of the patient. All authors read and approved the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.Gremese E, Alivernini S, Ferraccioli ES, Ferraccioli G. Checkpoint inhibitors (CPI) and autoimmune chronic inflammatory diseases (ACIDs): tolerance and loss of tolerance in the occurrence of immuno-rheumatologic manifestations. Clin Immunol. 2020;214:108395. doi: 10.1016/j.clim.2020.108395. [DOI] [PubMed] [Google Scholar]
- 2.Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45((2)):160–9. doi: 10.1159/000455014. [DOI] [PubMed] [Google Scholar]
- 3.Perazella MA, Shirali AC. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol. 2018;29((8)):2039–52. doi: 10.1681/ASN.2018050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90((3)):638–47. doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016 Aug;68((2)):287–91. doi: 10.1053/j.ajkd.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 6.Ashour T, Nakhoul G, Patil P, Funchain P, Herlitz L. Immune check point inhibitor-associated glomerulonephritis. Kidney Int Rep. 2019 Feb;4((2)):355–9. doi: 10.1016/j.ekir.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallan AJ, Alexander E, Reid P, Kutuby F, Chang A, Henriksen KJ. Renal vasculitis and pauci-immune glomerulonephritis associated with immune checkpoint inhibitors. Am J Kidney Dis. 2019;74((6)):853–6. doi: 10.1053/j.ajkd.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Koda R, Watanabe H, Tsuchida M, Iino N, Suzuki K, Hasegawa G, et al. Immune checkpoint inhibitor (nivolumab)-associated kidney injury and the importance of recognizing concomitant medications known to cause acute tubulointerstitial nephritis: a case report. BMC Nephrol. 2018;19((1)):48. doi: 10.1186/s12882-018-0848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daanen RA, Maas RJH, Koornstra RHT, Steenbergen EJ, van Herpen CML, Willemsen AECAB. Nivolumab-associated nephrotic syndrome in a patient with renal cell carcinoma: a case report. J Immunother. 2017 Dec;40((9)):345–8. doi: 10.1097/CJI.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 10.Lin JS, Wang DY, Mamlouk O, Glass WF, Abdelrahim M, Yee C, et al. Immune checkpoint inhibitor associated reactivation of primary membranous nephropathy responsive to rituximab. J Immunother Cancer. 2020 Oct;8((2)):e001287. doi: 10.1136/jitc-2020-001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishi S, Minato M, Saijo A, Murakami N, Tamaki M, Matsuura M, et al. IgA nephropathy after nivolumab therapy for postoperative recurrence of lung squamous cell carcinoma. Intern Med. 2018 May 1;57((9)):1259–63. doi: 10.2169/internalmedicine.9814-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baschinsky DY, Baker PB, Niemann TH, Wilmer WA. Pauci-immune ANCA-positive crescentic glomerulonephritis associated with metastatic adenocarcinoma of the lung. Am J Kidney Dis. 2000 Oct;36((4)):E24. doi: 10.1053/ajkd.2000.17727. [DOI] [PubMed] [Google Scholar]
- 13.Baldeo C, Ali R, Hritani A, Poenariu A. ANCA-negative pauci-immune crescentic glomerulonephritis linked with non-small cell carcinoma of the lung. Case Rep Nephrol Dial. 2015 Jul 4;5((2)):168–72. doi: 10.1159/000435808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morikawa T, Yoshida A, Kobayashi S, Shibata M, Hamada M, Kishida M, et al. AP-VAS 2012 case report: a case of ANCA-negative pauci-immune crescentic glomerulonephritis associated with IL-6-producing adenosquamous cell carcinoma of the lung. CEN Case Rep. 2013 Mar 14;((2)):158–64. doi: 10.1007/s13730-013-0058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mamlouk O, Lin JS, Abdelrahim M, Tchakarov AS, Glass WF, Selamet U, et al. Checkpoint inhibitor-related renal vasculitis and use of rituximab. J Immunother Cancer. 2020 Jul;8((2)):e000750. doi: 10.1136/jitc-2020-000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Little MA, Al-Ani B, Ren S, Al-Nuaimi H, Leite M, Jr, Alpers CE, et al. Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS One. 2012;7((1)):e28626. doi: 10.1371/journal.pone.0028626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002 Oct;110((7)):955–63. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennette JC, Falk RJ, Hu P, Xiao H. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu Rev Pathol. 2013 Jan 24;8:139–60. doi: 10.1146/annurev-pathol-011811-132453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayne DRW, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007 Jul;18((7)):2180–8. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 20.Walsh M, Merkel PA, Peh C-A, Szpirt WM, Puéchal X, Fujimoto S, et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020 13;382((7)):622–31. doi: 10.1056/NEJMoa1803537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronbichler A, Shin JI, Wang CS, Szpirt WM, Segelmark M, Tesar V. Plasma exchange in ANCA-associated vasculitis: the pro position. Nephrol Dial Transplant. 2021 Jan 25;36((2)):227–31. doi: 10.1093/ndt/gfaa311. [DOI] [PubMed] [Google Scholar]
- 22.Specks U, Fussner LA, Cartin-Ceba R, Casal Moura M, Zand L, Fervenza FC. Plasma exchange for the management of ANCA-associated vasculitis: the con position. Nephrol Dial Transplant. 2021 Jan 25;36((2)):231–6. doi: 10.1093/ndt/gfaa312. [DOI] [PubMed] [Google Scholar]
- 23.Grau RG. Drug-induced vasculitis: new insights and a changing lineup of suspects. Curr Rheumatol Rep. 2015 Dec;17((12)):71. doi: 10.1007/s11926-015-0545-9. [DOI] [PubMed] [Google Scholar]
- 24.Nakazawa D, Masuda S, Tomaru U, Ishizu A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat Rev Rheumatol. 2019;15((2)):91–101. doi: 10.1038/s41584-018-0145-y. [DOI] [PubMed] [Google Scholar]
- 25.Kitchlu A, Fingrut W, Avila-Casado C, Chan CT, Crump M, Hogg D, et al. Nephrotic syndrome with cancer immunotherapies: a report of 2 cases. Am J Kidney Dis. 2017;70((4)):581–5. doi: 10.1053/j.ajkd.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Jung K, Zeng X, Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC Nephrol. 2016;17((1)):188. doi: 10.1186/s12882-016-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Brom RRH, Abdulahad WH, Rutgers A, Kroesen B-J, Roozendaal C, de Groot DJA, et al. Rapid granulomatosis with polyangiitis induced by immune checkpoint inhibition. Rheumatology. 2016;55((6)):1143–5. doi: 10.1093/rheumatology/kew063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.