Abstract
Purpose
To highlight the cellular, matrix, and hydration changes associated with opacity that occurs in the corneal stroma after injury.
Methods
Review of the literature.
Results
The regulated transition of keratocytes to corneal fibroblasts and myofibroblasts, and of bone marrow-derived fibrocytes to myofibroblasts, is in large part modulated by transforming growth factor beta (TGFβ) entry into the stroma after injury to the epithelial basement membrane (EBM) and/or Descemet's membrane. The composition, stoichiometry, and organization of the stromal extracellular matrix components and water is altered by corneal fibroblast and myofibroblast production of large amounts of collagen type I and other extracellular matrix components—resulting in varying levels of stromal opacity, depending on the intensity of the healing response. Regeneration of EBM and/or Descemet's membrane, and stromal cell production of non-EBM collagen type IV, reestablishes control of TGFβ entry and activity, and triggers TGFβ-dependent myofibroblast apoptosis. Eventually, corneal fibroblasts also disappear, and repopulating keratocytes reorganize the disordered extracellular matrix to reestablish transparency.
Conclusions
Injuries to the cornea produce varying amounts of corneal opacity depending on the magnitude of cellular and molecular responses to injury. The EBM and Descemet's membrane are key regulators of stromal cellularity through their modulation of TGFβ. After injury to the cornea, depending on the severity of the insult, and possibly genetic factors, trace opacity to severe scarring fibrosis develops. Stromal cellularity, and the functions of different cell types, are the major determinants of the level of the stromal opacity.
Keywords: cornea; keratocytes, corneal fibroblasts, myofibroblasts, TGF-beta; collagen type IV; corneal opacity; fibrosis
Corneal opacity is a leading cause of vision loss.1 Monocular blindness, attributable to corneal opacity from trauma or corneal ulceration, was estimated to occur with a worldwide incidence of 1.5 to 2.0 million cases per year in 2001.1 Corneal opacity can occur after trauma, chemical injuries, infections, surgeries (Fig. 1), or secondary to other corneal diseases or disorders, including corneal dystrophies. The pathophysiologic processes leading to the corneal opacity after injury are the major determinants of whether the opacity could resolve spontaneously and the probable time course of resolution. This article reviews the pathophysiology of corneal scarring attributable to corneal fibroblast and/or myofibroblast generation, with or without neovascularization, that occurs with trauma, chemical burns, infections, and surgeries. It also distinguishes etiologies where there is at least a possibility of spontaneous resolution and highlights current treatments that can promote the resolution of scarring stromal fibrosis with or without neovascularization.
Figure 1.
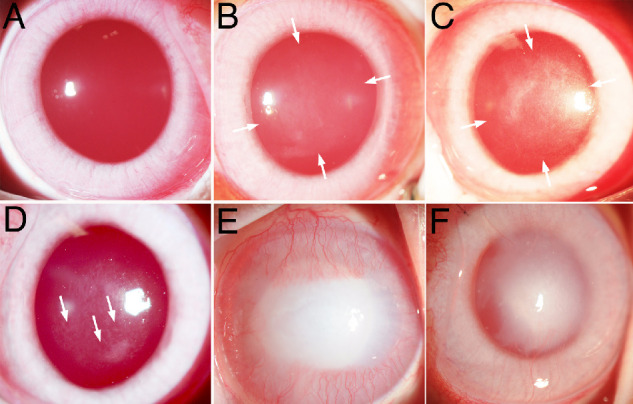
Broad beam slit lamp photos of corneas after injuries that produce opacity. (A) Normal unwounded rabbit cornea with high transparency such that only the red reflex is seen for the most part. (B) Rabbit cornea one month after −3 D PRK with mild opacity (haze) in the area of the excimer laser ablation between the arrows. (C) Rabbit cornea one month after −9 D PRK with moderate opacity (haze) owing to the development of myofibroblasts and fibrosis in the area of the excimer laser ablation between the arrows. (D) Rabbit cornea at six weeks after −9 D PRK with clear areas (arrows) called lacunae developing within the scarring fibrosis. (E) Rabbit cornea at two weeks after 5 mm central 1 M sodium hydroxide exposure for 1 minute with severe fibrosis and CNV. (F) Rabbit cornea at 4 months after 8 mm Descemetorhexis removal of the central endothelium and Descemet's membrane with persistent stromal scarring fibrosis and CNV, but a decrease since the one-month time point (not shown but see Ref. 25). Original magnification ×20.
Corneal Transparency—The Cellular and Extracellular Matrix Contributions
An excellent review of molecular and cellular contributions to corneal transparency, with a major focus on the normal unwounded cornea, has been published previously.2 The present review emphasizes the pathophysiology of corneal opacity after trauma, chemical burns, infections, and surgeries of the cornea. It is necessary to summarize the inputs of the normal contributors to corneal transparency to better appreciate the pathophysiology of stromal opacity.
Contributions of Water to Stromal Transparency and Opacity
Water, and its concentration in the stroma, make major contributions to stromal transparency and opacity despite the clarity of the pure liquid. The pump leak function of the corneal endothelium modulates the flow of water into the stroma and, therefore, the packing of the normal stromal extracellular matrix components.2–4 After corneal endothelial injury or removal, there is an immediate increase in the opacity in the overlying stroma associated with edema. It is likely not the increased water itself, but the effect of excessive water on the packing of the other stromal components that creates the associated opacity. A fascinating aspect of this opacity immediately post endothelial injury is that it is, and remains, localized to the stroma immediately overlying damaged endothelium and does not spread laterally to the adjacent stroma where the endothelium remains intact, unless the endothelial injury is so large that this compartmentalization is overwhelmed (Fig. 2). This localized stromal edema then persists until the endothelium regenerates or is replaced surgically. One might expect that the excess water would diffuse rather freely from the edematous stroma into the adjacent normal stroma and the stromal thickness would increase throughout the cornea, but that is not what is observed. If no endothelial replacement surgery is performed and the endothelium does not regenerate, this demarcation between edematous stroma and normal thickness stroma, where the endothelium remains intact, can persist for years or even decades. Lateral stromal water movement in the corneal stroma is highly restricted by the stromal “ground substance” and follows the laws of irreversible thermodynamics that describe viscous flow.5,6 The ground substance of the corneal stroma is thought to be composed of the proteoglycans and other components that fill the spaces between the collagen fibrils. The lateral flow of water is highly restricted from dimensional considerations so that the endothelium peripheral to a denuded area can maintain local hydration and thickness. That is, the ion and water transport systems of the endothelium can maintain local hydration despite the fluid leak into that region from edematous regions.5,6
Figure 2.
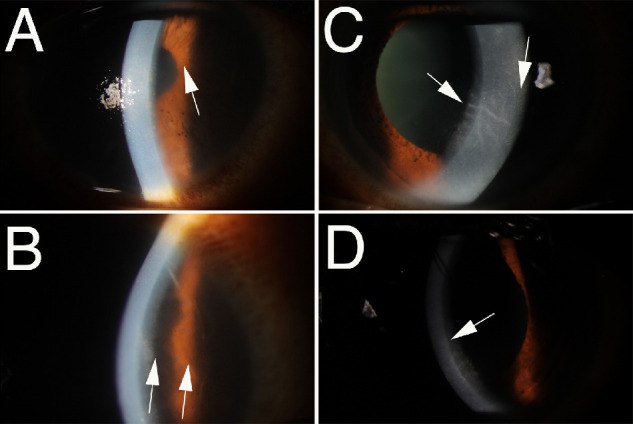
Corneal compartmentalization of stromal edema. Slit lamp photos of corneas with (A, B) corneal endothelial rejection after DMEK or (C, D) herpes simplex virus endotheliitis. Arrows indicate the point to which endothelial damage had extended in each panel with the area of damaged endothelium being inferior to the arrow tip in all panels. Note the sharp demarcation between the edematous stroma overlying the zone of endothelial injury and the normal hydration and thickness in the corneal stroma above with normal endothelium in each of these disorders that damages corneal endothelium. Original magnifications: (A and B) ×20; (C and D) ×30.
The epithelium also has an important role in the maintenance of the normal stromal hydration. Injuries or ischemia of the epithelium commonly cause edema of the underlying stroma owing to the loss of the epithelial barrier function that allows unchecked water passage into the stroma from the tears that overcomes the pumping function of the endothelium until epithelial integrity is restored.7,8
Contributions of Stromal Matrix Components to Transparency
An exceptionally detailed review of the normal corneal stroma components and composition was published recently,9 and only a few highlights relevant to transient haze and fibrosis are provided in this review. The stromal fibrils of the cornea (Fig. 3) are heterotypic (generated from ≥2 or more fibril-forming collagen types) fibrils9 composed of fibril-forming collagen type I (80%–90%) (Fig. 4) and lesser amounts of regulatory fibril-forming collagen type V (10%–20%). Collagen V has been shown to regulate the nucleation of protofibril assembly, and thereby control the number of fibrils and assembly of smaller diameter fibrils in the corneal stroma.10 Smaller amounts of other collagens, such as collagen type XI and collagen type XII, are also found in unwounded corneal stroma.11–13 Surrounding the collagen fibrils in the corneal stroma, in what is sometimes referred to as the ground substance, are proteoglycans. Small leucine-rich proteoglycans found in the corneal stroma are keratocan, lumican, decorin, biglycan, fibromodulin, and osteoglycin.9,13–18 The small leucine-rich proteoglycans serve as critical modulators of cell growth and regulate collagen fibrillogenesis, and thereby are important tissue organizers. They also modulate growth factors, including transforming growth factor (TGF)β-1, TGFβ-2, and possibly TGFβ-3.19–21,16 As mentioned elsewhere in this article, proteoglycans are also important regulators of stromal hydration, and bind water through their glycosaminoglycan chains. There are numerous other components within the stroma of unwounded corneas that include fibrillin-1, fibronectin, and matricellular proteins.9 The precise stoichiometry of collagen type I, collagen type V, the six proteoglycans (keratocan, lumican, decorin, biglycan, fibromodulin, and osteoglycin), and other stromal components, in addition to water, found in normal unwounded adult corneal stroma remains undefined, but is likely important in the maintenance of transparency. Perturbations in this normal stoichiometry, along with the upregulation of components after injury that are normally expressed at lower levels or not at all in the stroma, are likely to lead to disruption of the carefully regulated stromal environment and trigger some level of loss of transparency ranging from nearly imperceptible haze to dense opacity.
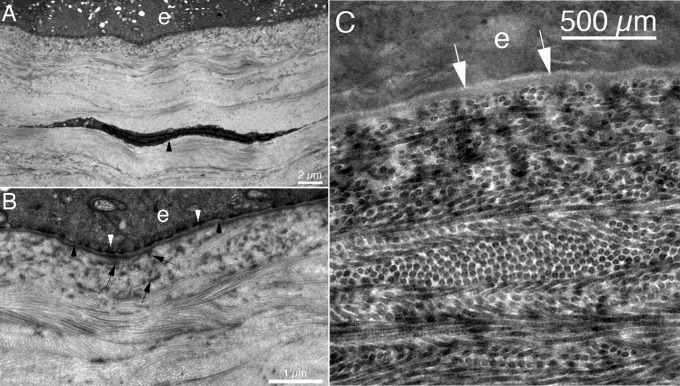
Figure 3.
Ultrastructure of the normal corneal stroma. Transmission electron microscopy of the unwounded rabbit cornea. (A) The arrowhead indicates a keratocyte. Original magnification ×12.8K. (B) The uniform diameter and regular packing of the collagen fibrils associated with transparency. Note some groups of fibrils are seen longitudinally, whereas other groups are seen in cross-section. However, all the fibrils have identical diameter. Lamina densa (black arrowheads) and lamina lucida (immediately anterior to lamina densa) of the epithelial basement membrane (EBM) are well-delineated. In the most anterior stroma, besides fibrils cut in cross-section, many densities are noted (arrows). These likely represent structures that include anchoring fibrils that are part of the adhesion complex of the epithelium, which also includes hemidesmosomes (white arrowheads). Many of these densities in the anterior stroma likely also include exocytic vesicles produced by keratocytes to provide components such as nidogen-1, laminins, and perlecan to maintain the EBM.21,22 Original magnification ×42K. (C) Higher magnification TEM shows the uniformity of the collagen fibrils, with some stromal lamellae cut tangentially, others cut obliquely, and some shown longitudinally. Arrows indicate lamina lucida of the EBM, and lamina densa is just posterior to lamina lucida in the EBM. Original magnification ×88K. Rabbits do not have Bowman's layer.
Figure 4.
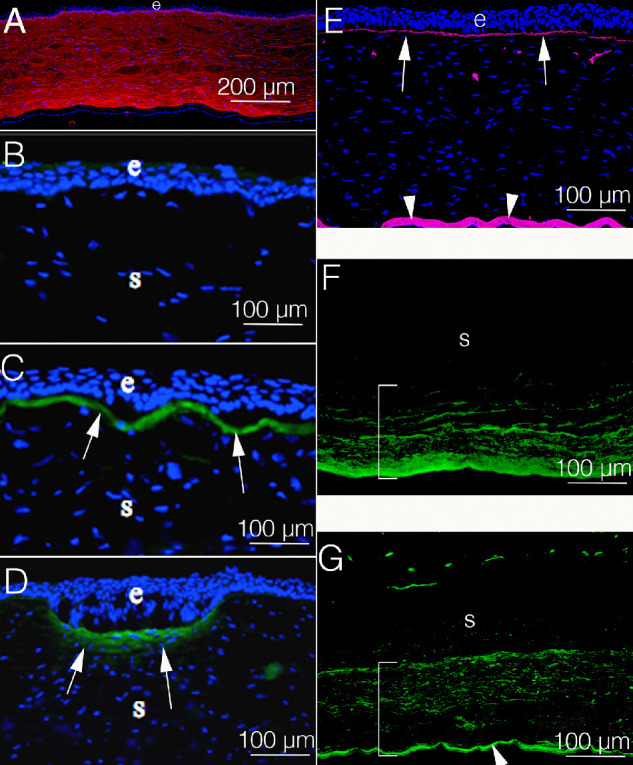
Collagen expression in rabbit corneas. (A) Collagen type I (red) is the most highly expressed collagen in the corneal stroma. (B) Collagen type III (green) is expressed at levels beneath the level of detection by immunohistochemistry in unwounded corneas. (C) At 1 month after PRK, however, a dense layer of collagen type III (green, arrows) is present in the subepithelial stroma. (D) Similarly, at 1 month after a partial thickness incision, collagen type III (green, arrows) is present along the superficial stroma at the gapping and epithelial plugged incision. (E) In the unwounded cornea collagen type IV (pink) is prominent in the epithelial basement membrane (EBM) (arrows) and Descemet's membrane (arrowheads). It is detected in the stroma in a few anterior DAPI-negative membrane bound bodies that are believed to be vesicles produced by keratocytes to maintain the EBM. (F) At 4 months after removal of the central corneal endothelium and Descemet's membrane (Descemetorhexis) there is prominent expression of collagen type IV (green) primarily in corneal fibroblasts in the posterior stroma (bracket). Note that the corneal endothelium and Descemet's membrane have not regenerated at this time point after injury.25 (G) At 6 months after Descemetorhexis, the endothelium and Descemet's membrane have regenerated.25 Collagen type IV (green) is present in the regenerated Descemet's membrane (arrowhead) and persists in the posterior stroma (bracket) after production by corneal fibroblasts. This area of the stroma is not yet repopulated with keratocytes (not shown).25 e is epithelium in all panels. Blue is DAPI in all panels.
Collagen type IV seems to have a special role in the stroma beyond its critical role as a major component of the epithelial basement membrane (EBM) and Descemet's membrane, along with its association with anchoring fibrils in the anterior stroma. Thus, in the normal unwounded cornea, collagen type IV is detected at high levels in the EBM22,23 and Descemet's membrane,24,25 (Fig. 4) and at much lower levels associated with the anchoring fibrils.26,27 Collagen type IV, however, is markedly upregulated deeper in the stroma after either anterior or posterior corneal injury and seems to serve a TGFβ modulatory role associated with its direct binding to TGFβ (Fig. 4).22,23,25 This alternative role for collagen type IV is detailed in the section on corneal fibroblasts.
Collagen type III is expressed at low levels in the unwounded cornea, but is markedly upregulated in the stroma after many types of injuries (Fig. 4).28,29 Collagen type III production is upregulated in corneal fibroblasts by TGFβ,30,31 and it is a major collagen upregulated in the corneal stroma after both fibrotic and non-fibrotic injuries.28,32 Galiacy et al.33 showed that matrix metalloproteinase 14 overexpression in the cornea with a recombinant adeno-associated virus-based vector after injury reduced corneal opacity and expression of collagen type III.
Contributions of Stromal Cells to Transparency
Keratocytes are the workhorses of the normal unwounded corneal stroma (Fig. 5) and serve to maintain the stromal collagen fibrils and ground substance through the production of collagens and proteoglycans throughout life (Table).2,9,34 They are also a major source, likely via secretory vesicles, of at least a portion of the components such as perlecan, nidogen, and collagen type IV that maintains the EBM in coordination with the epithelium22,23 and Descemet's membrane in coordination with the corneal endothelium.25 Keratocytes are also the progenitors to corneal fibroblasts and, therefore, along with bone marrow-derived fibrocytes,35,36 the precursor cells to myofibroblasts that can develop after corneal injuries.37
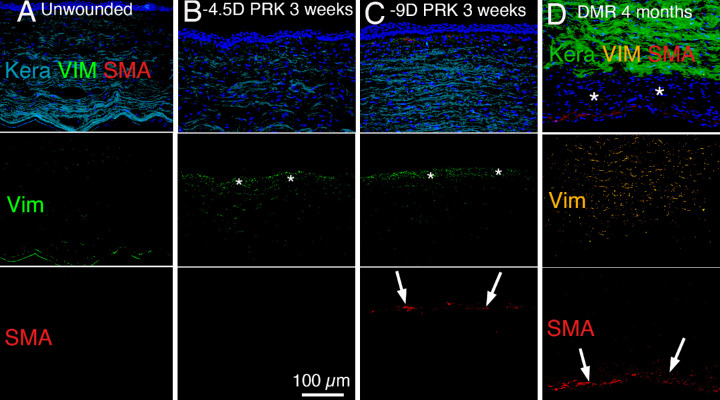
Figure 5.
Triplex immunohistochemistry for keratocan (keratocyte marker), vimentin (mesenchymal cell marker) and alpha-smooth muscle actin (αSMA) (myofibroblast marker). Note that the concentration of vimentin antibody used does not detect the low vimentin expression in most keratocytes.21,52 (A) In the unwounded normal rabbit cornea, the stroma is populated primarily with keratocan-positive keratocytes and only a portion of those cells, especially near the EBM or Descemet's basement membrane expressed sufficient vimentin to be vimentin positive. No αSMA was detected. (B) At 3 weeks after −4.5 D PRK in the rabbit cornea, there was a decrease in keratocan-positive keratocytes and increase in vimentin-positive corneal fibroblasts (*) in the subepithelial stroma. No αSMA-positive myofibroblasts were detected. (C) At 3 weeks after −9 D PRK in the rabbit cornea, there was a decrease in keratocan-positive keratocytes and increase in vimentin-positive corneal fibroblasts (*) in the subepithelial stroma, and some cells differentiated into αSMA-positive myofibroblasts (arrows). (D) At 4 months after 8-mm diameter removal of corneal endothelium and Descemet's membrane (Descemetorhexis) in a rabbit cornea, there are residual αSMA-positive myofibroblasts (arrows) compared to 1 month after Descemetorhexis (not shown, but see Fig. 6 and reference 24). There remains an area (*) filled with keratocan-negative, vimentin-positive, αSMA-negative cells, that are likely primarily corneal fibroblasts, between the anterior stroma populated with keratocan-positive keratocytes and the layer of myofibroblasts. Blue is DAPI-stained cell nuclei in the composite panels. Original magnification ×200 in each panel.
Table.
Stromal Cell Types and Functions
| Stromal Cell Type | Phenotypic Markers | Origin | Function |
|---|---|---|---|
| Keratocytes | Keratocan, vimentin | Neural crest | Maintain collagen fibrils and matrix, EBM, DBM |
| Corneal fibroblasts | Vimentin | Keratocytes | Early matrix production, growth factor production (HGF, KGF), precursor to myofibroblasts |
| Fibrocytes | CD34, CD45, COL I | Bone marrow derived | Precursor to myofibroblasts |
| Immature myofibroblasts | Vimentin | Corneal fibroblasts, fibrocytes | Precursor to myofibroblasts |
| Mature myofibroblasts | Vimentin, SMA, desmin | Immature myofibroblasts | Fibrosis, ECM production |
*HGF, hepatocyte growth factor; *KGF, keratinocyte growth factor; SMA, α-smooth muscle actin; COL I, collagen type I; EBM, epithelial basement membrane; DBM, Descemet's basement membrane.
Keratocytes are transparent relative to corneal fibroblasts and myofibroblasts because of their expression of high amounts of corneal crystallins, such as aldehyde dehydrogenase 1A1, aldehyde dehydrogenase 3A1, and transketolase.38–40 However, the loss of cellular transparency is not the only factor leading to stromal opacity. Altered production of collagens and proteoglycans by corneal fibroblasts and myofibroblasts relative to keratocytes is equally important in the development of opacity, as detailed elsewhere in this review.
Contributions of Corneal Nerves to Transparency
Corneal innervation is critical to the normal function of the epithelium and the blink reflex that continually bathes the ocular surface with tears.41 Damage to the corneal nerve supply in conditions such as herpes simplex keratitis and diabetes mellitus often produces neurotrophic keratopathy, which may be accompanied by persistent epithelial defects and scarring stromal fibrosis.41 Transient damage to corneal nerves produced by refractive surgical procedures often produces neurotrophic epitheliopathy and associated dry eye symptoms.42,43
Most corneal nerves are sensory, but sympathetic and parasympathetic fibers are also present in the stroma.41 The nerve bundles enter the peripheral limbus with an equal distribution around the circumference of the cornea and normally lose their perineurium and myelin sheaths within 1 mm of stromal entry.41 These nerves, surrounded only by Schwann cell sheaths, then subdivide several times to form smaller branches that course through the stromal ground substance before penetrating the subepithelial stroma to terminate in the epithelium.41,42
The cornea is so densely innervated (Fig. 6) that the fibers could decrease the transparency of the normal cornea unless they were themselves transparent. Demyelination facilitates this needed transparency. It is unknown whether corneal axons use other processes, such as the expression of crystallin proteins, similar to the corneal epithelium and keratocytes,38–40 to enhance their transparency. Importantly, normal nerve regeneration is inhibited by myofibroblasts after corneal injuries such as photorefractive keratectomy (PRK).44
Figure 6.
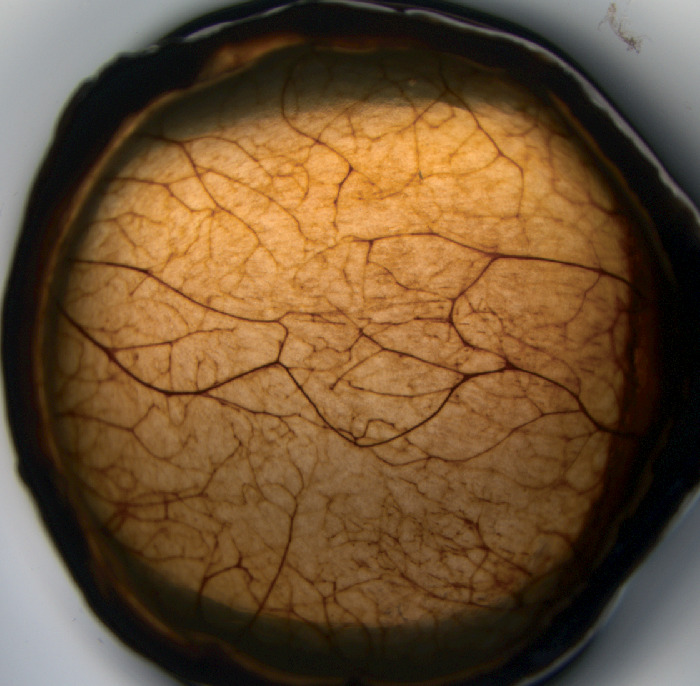
Innervation in the rabbit cornea. Corneal nerves in the normal unwounded rabbit cornea stained using the Karnovsky-Roots acetylcholinesterase (AChE) technique.42 Note the dense network of nerve fibers that are for the most part transparent at the slit lamp. Original magnification ×10.
Mild Transient Corneal Opacity—The Cellular and Extracellular Matrix Contributions
Keratocytes in the adjacent stroma undergo apoptosis after even minor injuries to the corneal epithelium45 or endothelium.46 The keratocyte apoptosis response in the anterior corneal stroma can be triggered by damage as minor as a poorly fit contact lens,47 but severe injuries or infections typically involve large numbers of the adjacent keratocytes.48,49 Injuries such as alkali burns induce greater keratocyte necrosis and thereby a more uncontrolled injury to the affected stroma. The location and extent of the keratocyte apoptosis response, which may also involve small levels of keratocyte necrosis, depends on the characteristics of the specific injury.50 Many surviving keratocytes surrounding the zone of apoptosis are driven by the TGFβ that enters the stroma from the epithelium and tears22 and/or aqueous humor25 after injury to the EBM or Descemet's membrane, respectively, to transform into more metabolically active keratocan-negative, vimentin-positive, alpha-smooth muscle actin (αSMA)-negative corneal fibroblasts. The injury itself, and the activities of corneal fibroblasts, alter the composition, structure, and hydration of the affected stroma and produce stromal opacity (Fig. 1).9,51
Sometimes there is a tendency to think of corneal fibroblasts as merely an intermediate cell between the all-important keratocytes and myofibroblasts. But corneal fibroblasts serve many critical roles in the response to injury. In addition, αSMA-positive myofibroblasts are not generated after many moderate injuries, and when they do develop there are always corneal fibroblasts present nearby (Fig. 5). For example, in both rabbits and humans after PRK for low-to-moderate corrections for myopia less than −6 diopters (D), keratocan-negative, vimentin-positive, αSMA-negative corneal fibroblasts are generated in large numbers, but in most corneas no myofibroblasts are detected (Fig. 4). These corneal fibroblasts, and the changes they produce in the extracellular matrix of the stroma, are the causes of the transient, mild anterior stromal opacity (Fig. 1B), also referred to as haze, observed at varying levels in all corneas after PRK.22,23,50 A small proportion of these lower injury corneas, depending on the extent of the original injury, and likely the genetic makeup of the individual, go on to generate myofibroblasts and more severe scarring fibrosis (referred to clinically as late haze).22,50
The change from keratocytes to corneal fibroblasts phenotype occurs after the entry of TGFβ-1 and TGFβ-2 into the stroma from the epithelium and tears and/or aqueous humor after injury to the EBM22,23 and/or Descemet's membrane,24,25 depending on the injury. Jester et al.52 showed that platelet-derived growth factor and integrin signaling are also involved in this transition from keratocyte to corneal fibroblast. This change in phenotype signals the onset of a cellular developmental pathway that leads to the eventual transition from vimentin-positive, αSMA-negative, desmin-negative corneal fibroblasts to vimentin-positive, αSMA-positive, desmin-negative immature myofibroblasts to vimentin-positive, αSMA-positive, desmin-positive mature myofibroblasts in the cornea,53 if the stromal entry of epithelial/tear TGFβ is not restricted by the regeneration of the epithelium and EBM.22,23 The duration of the developmental transition from corneal fibroblasts to myofibroblasts varies, possibly depending on the localized concentration of TGFβ-1 and TGFβ-2, from 1 week to several months and tends to be species dependent. Thus, in rabbits that undergo high level −9 D PRK, the myofibroblasts peak at approximately 1 month after injury (Fig. 1),22,50 whereas in human corneas when corneal fibrosis develops the myofibroblasts tend to peak 2 to 6 months after the injury,54 but can even develop years after the original PRK surgery if there is a subsequent corneal injury.55 Therefore, even transitioning keratocytes often spend weeks or months as corneal fibroblasts as they develop into mature myofibroblasts. In corneas that do develop myofibroblasts and stromal fibrosis, there is also typically a layer of vimentin-positive, keratocan-negative, αSMA-negative corneal fibroblasts localized between keratocytes and myofibroblasts and other corneal fibroblasts within the myofibroblast-populated tissue itself (Fig. 5). The layer of corneal fibroblasts, and possibly other vimentin-positive, keratocan-negative, αSMA-negative cells, like fibrocytes, persists for weeks to months after injury until keratocytes repopulate the affected stroma.22 A similar process occurs in posterior injuries to the cornea, where myofibroblasts will develop if Descemet's membrane is not regenerated or surgically replaced to impede high level TGFβ movement from the aqueous humor into the corneal stroma.24,25
Corneal fibroblasts are metabolically active cells that produce many structural and regulatory proteins either not produced at all or produced in smaller quantities by keratocytes. The fibroblasts tend to increase in numbers and spread into the adjacent stroma after an injury, depending on the severity.56,57 Corneal fibroblasts produce larger amounts of collagen type I, collagen type V, and proteoglycans, such as lumican, decorin, biglycan, mimecan, syndecan-4, and perlecan relative to keratocytes, which contribute to the low levels of stromal opacity noted after mild injuries.58–62 These components, at least initially, are not in the stoichiometry and/or organization associated with transparency in the normal uninjured corneal stroma and are likely produced to quickly augment the stroma in response to the injury. The corneal fibroblasts themselves produce less corneal crystallins38–40 than keratocytes and, therefore, contribute to the mild to moderate stromal opacity that develops after mild to moderate injuries and to some of the opacity noted in fibrotic corneas with myofibroblasts (Fig. 5).
Corneal fibroblasts produce metalloproteinases, including collagenase, stromelysin, and gelatinases,63,64 as do corneal epithelial cells,65 which are involved in remodeling the normal corneal stroma and in the removal of disordered stromal matrix once it is deposited. The expression of metalloproteinases by corneal fibroblasts is upregulated by IL-1 alpha released from injured corneal epithelial cells.63,64 Little is known about how the degradation of normal corneal stroma or fibrotic matrix in scarred corneas is finely tuned so the degradation process does not produce extensive stromal damage, although in some pathologies, such as severe microbial corneal ulcers, the degradative process is uncontrolled and can progress to corneal perforation.
IL-1 alpha released by epithelial or endothelial injury triggers other important functions in corneal fibroblasts.66 Gene array experiments67 demonstrated that IL-1 alpha (and tumor necrosis factor-alpha) stimulate the transcription of many chemokines, such as monocyte-derived neutrophil chemotactic factor, chemokine (C–C motif) ligand 2, also called monocyte chemoattractant protein-1, granulocyte colony-stimulating factor, and C-X-C motif chemokine 5 (also called neutrophil-activating peptide or ENA-78), that amplify the effect of IL-1 alpha itself in drawing not only fibrocytes, but other bone marrow-derived cells, such as lymphocytes, neutrophils, and macrophages, into the corneal stroma. IL-1 alpha also upregulates the production of hepatocyte growth factor and keratinocyte growth factor (or fibroblast growth factor-7)68,69 that stimulate epithelial healing by modulating the proliferation, motility, differentiation, and apoptosis of corneal epithelial cells.70 The hepatocyte growth factor produced by corneal fibroblasts is also thought to inhibit myofibroblast generation in the corneal stroma,71 as it has been shown to do in other organs.72–74
Corneal fibroblasts, in coordination with corneal epithelial cells22,23 or corneal endothelial cells,24,25 are critical participants in the regeneration of the EBM or Descemet's membrane, respectively, after injury. In rabbit studies, corneal fibroblasts have been found to produce perlecan, collagen type IV, laminin alpha-5, nidogen-1 and nidogen-2—all critical components of both of the corneal basement membranes.22–25,75,76 Myofibroblasts also produce many of these components but are unable to incorporate them in the nascent EBM or Descemet's membrane.22,23,75 Studies performed by Gallego-Muñoz et al.31 confirmed these observations when they noted that after severe alkali burns nidogen-2 was retained in newly secreted, but disordered, matrix produced by myofibroblasts in the injured stroma. Even though nidogen-2 was present in the superficial stroma of these corneas, it could not contribute to the effective regeneration of the EBM. Based on in vitro studies,75,76 and the cellularity of the adjacent stroma during regeneration of these basement membranes, it is apparent that corneal fibroblasts are the major cells involved with corneal epithelium or corneal endothelium in regeneration of EBM or Descemet's membrane, respectively, after injuries, whereas keratocytes, which also produce these components,75,76 are more involved with the maintenance of the EBM or Descemet's membrane.22–25 Both corneal fibroblasts and keratocytes seem to deliver components to the nascent basement membranes via secretory vesicles that contain one or more of the basement membrane components.22,23
Collagen type IV production by corneal fibroblasts after severe injuries, such as Descemetorhexis or high correction PRK, seems to be much greater than what is needed to simply regenerate the basement membranes and is often detected deep within the stroma away from regenerating basement membranes (Fig. 7). TGFβ-1 markedly upregulates collagen type IV mRNAs (Col4a1 and Col4a2) in corneal fibroblasts, but not in myofibroblasts.25 Collagen type IV binds TGFβ in competition with the cognate TGFβ receptors expressed by corneal fibroblasts and myofibroblasts.77,78 We hypothesized that collagen type IV is produced by corneal fibroblasts in the stroma to downregulate the effects of TGFβ-1 and TGFβ-2 that enter from the epithelium, tears and/or aqueous humor after injury, and thereby modulate the fibrosis response.25 Other investigators also noted this collagen type IV production in corneal fibroblasts in the anterior stroma in alkali burns and posterior stroma in lacerations, but concluded it was related to the regeneration of the EBM.79 However, the production by corneal fibroblasts in the stroma far from the nascent regenerating EBM (Fig. 7) speaks to this secondary function for collagen type IV in modulating TGFβ that enters the stroma after injury.
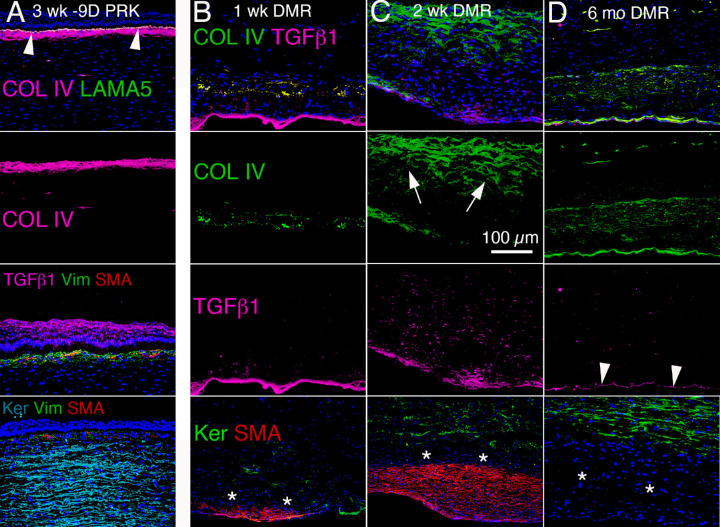
Figure 7.
TGFβ-1 and collagen type IV localization after PRK or Descemetorhexis in rabbit corneas. (A) At 3 weeks after high-correction −9 D PRK, large amounts of collagen type IV (COL IV) are present in the anterior stroma posterior to the nascent EBM labelled with laminin alpha-5 (LAMA-5). COL IV binds TGFβ and likely modulates TGFβ association with cognate TGFβ receptors to downregulate TGFβ activity. At this time point 3 weeks after −9 D PRK, TGFβ-1 is present at high levels in the tears and epithelium and penetrates the nascent EBM into the anterior stroma at levels sufficient to drive the development of corneal fibroblasts and fibrocytes into myofibroblasts. The subepithelial stroma of this cornea is filled with vimentin-positive corneal fibroblasts and fibrocytes that are driven by the high levels of TGFβ-1 to develop into αSMA-positive myofibroblasts. Posterior to this layer of developing myofibroblasts, keratocan-positive keratocytes occupy the balance of the stroma. (B) At 1 week after Descemetorhexis (DMR), high levels of TGFβ-1 accumulate from the aqueous humor at the posterior corneal surface devoid of corneal endothelium and Descemet's membrane. Already, COL IV, that binds TGFβ-1, produced by corneal fibroblasts and myofibroblasts is accumulating in the posterior stroma. * indicates a layer of keratocan-negative, αSMA-negative cells anterior to the accumulating myofibroblasts that are corneal fibroblasts, and possibly fibrocytes. (C) At 2 weeks after Descemetorhexis (DMR), the fibrotic area in the posterior cornea occupied by αSMA-positive myofibroblasts has expanded. TGFβ-1 is present at high levels at the posterior corneal surface, still devoid of corneal endothelium and Descemet's membrane, and throughout the posterior stroma. A large amount of COLIV (arrows) produced by corneal fibroblasts is present in the stroma. Some COLIV is also produced by myofibroblasts. * again indicates a layer of keratocan-negative, αSMA-negative cells anterior to the accumulating myofibroblasts. D. At 6 months after Descemetorhexis (DMR), the corneal endothelium and Descemet's membrane (with concentrated high levels of COLIV) have regenerated.25 Arrowheads indicate TGFβ-1 produced by the regenerated corneal endothelial cells. The αSMA-positive myofibroblasts have disappeared, but the posterior stroma is still occupied by keratocan-negative, αSMA-negative corneal fibroblasts (*) at this time point after the DMR injury. A high level of COLIV produced by these corneal fibroblasts persists in the posterior stroma. Blue is DAPI staining of cell nuclei. Original magnification ×200.
Teleologically speaking, during the response to injury, myofibroblasts strive to maintain their supply of TGFβ-1 and TGFβ-2 by occupying the stroma in proximity to the nascent EBM or Descemet's membrane and preventing its repair, which would cut off the myofibroblasts requisite supplies of TGFβ-1 and TGFβ-2 and trigger their demise. Conversely, corneal fibroblasts endeavor to regenerate the EBM and/or Descemet's, and also deposit large amounts of collagen type IV within the deeper stroma (Fig. 7), to downregulate the functional supply of TGFβ-1 and TGFβ-2 and thereby trigger the apoptosis of the myofibroblasts. At the same time, the corneal fibroblasts control their own development into the fibrotic myofibroblasts through the production of collagen type IV, and possibly other factors yet to be discovered. Once the struggle between these two fibroblastic cells subsides, and, hopefully, at least for functionality, ends in regeneration of the EBM and/or Descemet's membrane and the death of the myofibroblasts, it appears that the keratocytes function to “clean up the mess” by reabsorbing, rearranging, and/or redepositing the stromal components in the appropriate stoichiometry and organization to clear the scarring fibrosis and restore transparency.22,23 These keratocytes either migrate in from the more normal peripheral stroma or possibly arise through retrodifferentiation of the corneal fibroblasts.
Severe Corneal Opacity—The Cellular and Extracellular Matrix Transition to Fibrosis
When an injury involving the EBM and/or Descemet's membrane occurs in the cornea, there is an orchestrated response that, if unchecked, will lead to the development of large numbers of myofibroblasts, and stromal fibrosis (Figs. 1C, E, F). Likely, the systems in place must be prepared to respond to the worst case scenario of a severe bacterial or viral infection that has the potential to lead to stromal destruction, corneal perforation and blindness.49 Therefore, even relatively trivial injuries, such as a corneal abrasion, sets in motion the elements of a full fibrotic response. Thus, TGFβ-1 and TGFβ-2 enter the stroma from either the epithelium/tears and/or the aqueous humor, and keratocytes proximate to the injury differentiate into corneal fibroblasts, while fibrocytes are drawn in from the limbal blood vessels.22–25 Many of these keratocan-negative, vimentin-positive, αSMA-negative corneal fibroblasts, likely dependent on the localized concentration of TGFβ-1 and TGFβ-2, begin their programmed development into myofibroblasts. The time course of this myofibroblast development seems to depend on the severity of the injury and likely is also species related. Thus, a few days is typically the minimum time for the appearance of the earliest myofibroblasts in rabbits after an injury such as PRK or Descemetorhexis.22,23,25,36 In humans, “late haze” stromal fibrosis after PRK usually does not develop until 2 to 6 months after surgery,54 but the corneal fibroblasts began their transition into myofibroblasts in such eyes immediately after the surgical injury.22,23 After high correction PRK, a much lower percentage of human corneas (5%–7%) than rabbit corneas (>99%) develop scarring fibrosis. Similarly, after Descemetorhexis without endothelial replacement, a much smaller percentage of human corneas than rabbit corneas develop scarring stromal fibrosis, although insufficient numbers of these procedures have been performed in either species to obtain reliable percentages. If the EBM and/or Descemet's membrane, depending on the injury, is repaired in a timely manner, then the requisite stromal TGFβ needed to continue this myofibroblast development is cut off and the precursor cells either undergo apoptosis or revert back to their progenitor cells.22 If the affected basement membrane(s) are not repaired, typically within 2 to 3 weeks, then TGFβ entry into the stroma continues and the precursors develop into mature myofibroblasts that excrete greater amounts of disordered extracellular matrix associated with fibrosis (Fig. 5 and Fig. 7).22,23 A schematic diagram depicting the fibrosis response to moderate to severe anterior corneal injury is provided in Figure 8.
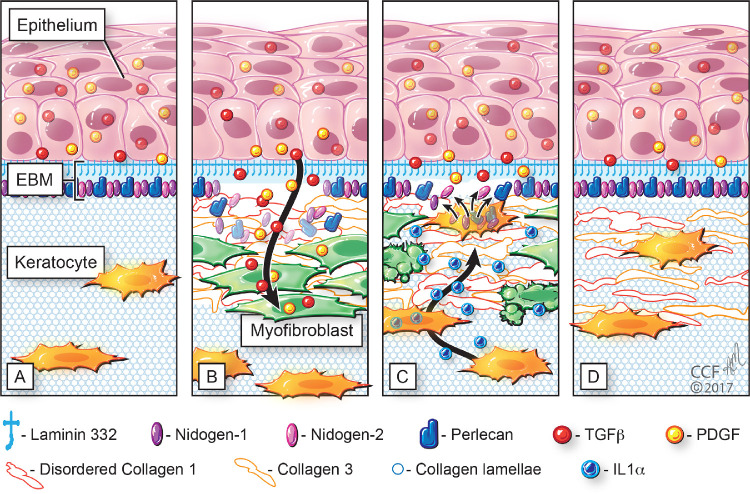
Figure 8.
Fibrosis response to moderate to severe injuries in the anterior cornea. (A) In the unwounded cornea with intact EBM, TGFβ, and platelet-derived growth factor (PDGF) present in the epithelium and tears cannot gain access to the stroma owing to collagen type IV (not shown) and perlecan in the EBM. (B) After injury to the epithelium and EBM, activated TGFβ, and PDGF gain access to the stroma and drive the development of corneal fibroblasts and myofibroblasts that produce disordered extracellular matrix (ECM) that includes collagen types I, III, and IV. The resulting opacity and stromal changes persist from months to years, or in some cases permanently. (C) In some corneas, corneal fibroblasts or keratocytes are eventually able to penetrate the abnormal ECM and myofibroblasts to cooperate with the epithelium in regenerating the EBM. Deprived of requisite TGFβ, myofibroblasts undergo apoptosis driven by autocrine or paracrine IL-1α. (D) Eventually, the keratocytes can reabsorb and reorganize all of the disordered ECM and return complete transparency to the cornea. Milder nonfibrotic injuries begin similarly with injuries to the EBM, entry of TGFβ and PDGF into the anterior stroma and development of corneal fibroblasts and entry of fibrocytes in the stroma. However, the EBM regenerates in a timely manner and shutting off TGFβ and PDGF entry into the stroma. Deprived of requisite TGFβ to continue their development, myofibroblast precursors (corneal fibroblasts and fibrocytes) either revert to keratocytes or undergo apoptosis, and no myofibroblasts are generated. Thus, only a small amount of disordered ECM is produced, and only mild haze is generated. Reprinted with permission from Wilson SE, Marino GK, Torricelli AAM, Medeiros CS. Injury and defective regeneration of the epithelial basement membrane in corneal fibrosis: a paradigm for fibrosis in other organs? Matrix Biol. 2017;64:17–26. © 2017 Elsevier B.V.
After PRK in rabbits, corneal myofibroblasts in situ undergo a transition from vimentin-positive, αSMA-negative, desmin-negative immature, to vimentin-positive, αSMA-positive, desmin-negative intermediate and finally to vimentin-positive, αSMA-positive, desmin-positive mature myofibroblasts.53 These mature myofibroblasts produce high levels of disorganized extracellular matrix materials, including large amounts of collagen type I,80 which disorganize the normal stromal structure to produce fibrotic scarring opacity. In addition, the myofibroblasts themselves express low levels of corneal crystallins and, therefore, contribute directly to the stromal opacity in fibrosis.38
Fibrocytes, also driven by TGFβs, similarly develop into vimentin-positive, αSMA-positive, desmin-positive mature myofibroblasts in rabbit and mouse corneas after injury.35,36,24 However, the myofibroblasts generated from these precursors are not equivalent.80 A proteomic analysis of these two myofibroblasts in vitro found that approximately 29% of the proteins produced by these cells were differentially expressed between the two types of myofibroblasts.80 These studies showed that the canonical pathways related to mitochondrial function, oxidative phosphorylation and sirtuin signaling predominated in keratocyte-derived myofibroblasts, whereas pathways involved in glycolysis, integrin signaling, and the remodeling of epithelial adherens junctions predominated in bone marrow-derived myofibroblasts.80 Bone marrow-derived myofibroblasts produce much more collagen type XI and collagen type III, whereas keratocyte-derived myofibroblasts produce more collagen type VII.80 Thus, after severe corneal injuries, these different populations of myofibroblasts likely cooperate in the generation of a full stromal fibrosis response.
Once established after injury, myofibroblasts, and the disorganized stromal matrix they produce, persist until the injured basement membrane(s) are either regenerated or replaced surgically. The presence of the myofibroblasts in the subepithelial or ante-endothelial areas retards the regeneration of the basement membranes because the restoration of the functional EBM or Descemet's membrane requires the concerted efforts of both the epithelial cells and keratocytes/corneal fibroblasts or the corneal endothelial cells and keratocytes/corneal fibroblasts, respectively, including the incorporation of components such as perlecan, nidogen-1, and laminins into the nascent basement membranes.22–25 Myofibroblasts, in contrast, despite their production of some BM components,22–25 seem to be incapable of incorporating these components into the regenerating BM.22–25 In some corneas that develop scarring fibrosis, stromal transparency can be restored months or years later.22,23,25,26,81 This return of stromal transparency indicates that the injured basement membrane(s) has been regenerated, the myofibroblasts were deprived of TGFβ underwent apoptosis, and keratocytes migrated into the damaged stroma to reabsorb and reorganize the fibrotic extracellular matrix to restore the organization associated with transparency.22,23,25,26,81
Corneal Neovascularization (CNV) and Corneal Opacity
New blood vessels may grow into the stroma after moderate to severe corneal injuries (Figs. 1E, F) affecting either the anterior49 or posterior24,25 cornea, although they can also develop after chronic low-grade injuries such as contact lens wear.82 This CNV, and the associated opacity, often severely compromises vision by decreasing the transparency of the stroma. A recent review details the factors that trigger CNV and the molecular regulation of its development, including the critical role of VEGF.83 CNV can be recognized in immunohistochemistry because the associated pericytes are αSMA positive.24,25,49 Importantly, pericytes have been shown to have the capacity to develop into myofibroblasts in many organs.84–87 Therefore, once CNV develops in a cornea, the associated pericytes likely provide a third precursor cell, in addition to corneal fibroblasts and fibrocytes, for the development of myofibroblasts. After CNV develops, resolution is typically dependent on removing the inciting hypoxia, inflammation, and/or infection that triggered growth of the new blood vessels, although CNV often persists indefinitely despite these therapeutic measures. Pharmacological agents that block VEGF receptors may be useful therapeutic agents in the future.83
Conclusions
The changes associated with the loss of transparency in the cornea that occur in response to injury are complex cellular and molecular changes that are regulated in large part by the entry, and production, of growth factors such as TGFβ and platelet-derived growth factor in the stroma. The regeneration of EBM and/or Descemet's membrane, and their associated components, such as perlecan and collagen type IV, are critical determinants of the development of the corneal fibrosis response mediated through BM regulation of TGFβ entry into the stroma from the tears, epithelium, and aqueous humor. The dynamic modulation of the keratocyte to corneal fibroblast to myofibroblast transition, and the apoptosis or developmental transition of the corneal fibroblast and myofibroblast that occurs with the downregulation of the wound healing response, are key determinates of the development and resolution of corneal opacity after injury. Keratocytes, corneal fibroblasts and myofibroblasts serve differing roles in the response to injury and the downregulation of the corneal wound healing response through their differing qualitative and quantitative production of extracellular matrix components that comprise the extracellular matrix of the stroma, as well as their production of growth factors, chemokines and other regulatory molecules.
Acknowledgments
Supported in part by Department of Defense grant VR180066, US Public Health Service grant P30-EY025585 from the National Eye Institute, National Institutes of Health, Bethesda, MD, and Research to Prevent Blindness, New York, NY.
Disclosure: S.E. Wilson, None; L.P. Sampaio, None; T.M. Shiju, None; G.S.L. Hilgert, None; R.C. de Oliveira, None
References
- 1. Whitcher JP, Srinivasan M, Upadhyay MP.. Corneal blindness: a global perspective. Bull World Health Organ. 2001; 79: 214–221. [PMC free article] [PubMed] [Google Scholar]
- 2. Hassell JR, Birk DE.. The molecular basis of corneal transparency. Exp Eye Res. 2010; 91: 326–335, doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maurice DM. The permeability to sodium ions of the living rabbit's cornea. J Physiol (Lond). 1951; 12: 367–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klyce SD. Endothelial pump and barrier function. Exp Eye Res. 2020; 198: 108068, doi: 10.1016/j.exer.2020.108068. [DOI] [PubMed] [Google Scholar]
- 5. Klyce SD, Russell SR: Numerical solution of coupled transport equations applied to corneal hydration dynamics. J Physiol . 1979; 292: 10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waring GO IV, Klyce SD.. Intrastromal corneal implants for the treatment of presbyopia. In: Copeland RA Jr, Afshari NA, eds. Copeland and Afshari's Principles and Practice of Cornea. New Delhi: Jaypee Brothers Medical Publishers, Ltd, 2013: 1274–1279. [Google Scholar]
- 7. Riley MV. The role of the epithelium in control of corneal hydration. Exp Eye Res. 1971; 12: 128–137. [DOI] [PubMed] [Google Scholar]
- 8. Fischbarg J, Maurice DM.. An update on corneal hydration control. Exp Eye Res. 2004; 78: 537–541, doi: 10.1016/j.exer.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9. Espana EM, Birk DE.. Composition, structure and function of the corneal stroma. Exp Eye Res. 2020; 198: 108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen S, Mienaltowski MJ, Birk DE.. Regulation of corneal stroma extracellular matrix assembly. Exp Eye Res. 2015; 133: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith SM, Birk DE.. Focus on molecules: collagens V and XI. Exp Eye Res. 2012:98: 105–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marchant JK, Zhang G, Birk DE.. Association of type XII collagen with regions of increased stability and keratocyte density in the cornea. Exp Eye Res. 2002; 75: 683–694. [DOI] [PubMed] [Google Scholar]
- 13. Massoudi D, Malecaze F, Galiacy SD.. Collagens and proteoglycans of the cornea: importance in transparency and visual disorders. Cell Tissue Res. 2016; 363: 337–349. [DOI] [PubMed] [Google Scholar]
- 14. Doane KJ, Ting WH, McLaughlin JS, Birk DE.. Spatial and temporal variations in extracellular matrix of periocular and corneal regions during corneal stromal development. Exp Eye Res. 1996; 62: 271–283. [DOI] [PubMed] [Google Scholar]
- 15. Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H.. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998; 141: 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohan RR, Tovey JC, Gupta R, Sharma A, Tandon A.. Decorin biology, expression, function and therapy in the cornea. Curr Mol Med. 2011; 11: 110–128. [DOI] [PubMed] [Google Scholar]
- 17. Chakravarti S, Petroll WM, Hassell JR, et al.. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci. 2000; 41: 3365–3373. [PMC free article] [PubMed] [Google Scholar]
- 18. Chakravarti S, Zhang G, Chervoneva I, Roberts L, Birk DE.. Collagen fibril assembly during postnatal development and dysfunctional regulation in the lumican-deficient murine cornea. Dev Dyn. 2006; 235: 2493–2506. [DOI] [PubMed] [Google Scholar]
- 19. Brown CT, Lin P, Walsh MT, Gantz D, Nugent MA, Trinkaus-Randall V.. Extraction and purification of decorin from corneal stroma retain structure and biological activity. Protein Expr Purif. 2002; 25: 389–399. [DOI] [PubMed] [Google Scholar]
- 20. Hildebrand A, Romarís M, Rasmussen LM, et al.. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994; 302 (Pt 2): 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson SE. TGF beta -1, -2 and -3 in the modulation of fibrosis in the cornea and other organs. Exp Eye Res. 2021; 207: 108594. [DOI] [PubMed] [Google Scholar]
- 22. de Oliveira RC, Tye G, Sampaio LP, et al.. TGFβ1 and TGFβ2 proteins in corneas with and without stromal fibrosis: Delayed regeneration of epithelial barrier function and the epithelial basement membrane in corneas with stromal fibrosis. Exp Eye Res. 2021; 202: 108325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Oliveira RC, Sampaio LP, Shiju TM, Santhiago MR, Wilson SE.. Epithelial basement membrane regeneration after PRK-induced epithelial-stromal injury in rabbits: fibrotic vs. non-fibrotic corneal healing. J Ref Surg. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Medeiros CS, Saikia P, de Oliveira RC, Lassance L, Santhiago MR, Wilson SE.. Descemet's membrane modulation of posterior corneal fibrosis. Invest Ophth Vis Sci. 2019; 60: 1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sampaio LP, Guilherme Hilgert GSL, Shiju TM, et al.. Descemet's membrane injury and regeneration, and posterior corneal fibrosis in rabbits. Exp Eye Res. 2021; 213: 108803. [DOI] [PubMed] [Google Scholar]
- 26. Gipson IK, Spurr-Michaud SJ, Tisdale AS.. Anchoring fibrils form a complex network in human and rabbit cornea. Invest Ophthalmol Vis Sci. 1987; 28: 212–220. [PubMed] [Google Scholar]
- 27. Keene DR, Sakai LY, Lunstrum GP, Morris NP, Burgeson RE.. Type VII collagen forms an extended network of anchoring fibrils. J Cell Biol. 1987; 104: 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marino GK, Santhiago MR, Santhanam A, et al.. Regeneration of defective epithelial basement membrane and restoration of corneal transparency. J Ref Surg. 2017; 33: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lorenzo-Martín E, Gallego-Muñoz P, Mar S, Fernández I, Cidad P, Martínez-García MC. Dynamic changes of the extracellular matrix during corneal wound healing. Exp. Eye Res. 2019; 186: 107704. [DOI] [PubMed] [Google Scholar]
- 30. Guo N, Li X, Mann MM, Funderburgh ML, Du Y, Funderburgh JL.. Hyaluronan synthesis mediates the fibrotic response of keratocytes to transforming growth factor beta. J. Biol. Chem. 2010; 285: 32012–32019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gallego-Muñoz P, Ibares-Frías L, Garrote JA, et al.. Human corneal fibroblast migration and extracellular matrix synthesis during stromal repair: role played by platelet-derived growth factor-BB, basic fibroblast growth factor, and transforming growth factor-β1. J. Tissue Eng. Regen. Med. 2018; 12: e737–e746. [DOI] [PubMed] [Google Scholar]
- 32. Massoudi D, Malecaze F, Galiacy SD.. Collagens and proteoglycans of the cornea: importance in transparency and visual disorders. Cell Tissue Res. 2016; 363: 337–349. [DOI] [PubMed] [Google Scholar]
- 33. Galiacy SD, Fournié P, Massoudi D, et al.. Matrix metalloproteinase 14 overexpression reduces corneal scarring. Gene Ther. 2011; 18: 462–468. [DOI] [PubMed] [Google Scholar]
- 34. Yam GHF, Riau AK, Funderburgh ML, Mehta JS, Jhanji V.. Keratocyte biology. Exp Eye Res. 2020; 196: 108062. [DOI] [PubMed] [Google Scholar]
- 35. Lassance L, Marino GK, Medeiros CS, Thangavadivel S, Wilson SE.. Fibrocyte migration, differentiation and apoptosis during the corneal wound healing response to injury. Exp Eye Res. 2018; 170: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Oliveira RC, Wilson SE.. Fibrocytes, wound healing, and corneal fibrosis. Invest Ophthalmol Vis Sci. 2020; 61: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson SE. Corneal myofibroblasts and fibrosis. Exp Eye Res. 2020; 201: 108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jester JV, Moller-Pedersen T, Huang J, et al.. The cellular basis of corneal transparency: evidence for 'corneal crystallins'. J Cell Sci. 1999; 112(Pt 5): 613–622. [DOI] [PubMed] [Google Scholar]
- 39. Estey T, Piatigorsky J, Lassen N, Vasiliou V.. ALDH3A1: a corneal crystallin with diverse functions. Exp Eye Res. 2007; 84: 3–12. [DOI] [PubMed] [Google Scholar]
- 40. Pei Y, Reins RY, McDermott AM.. Aldehyde dehydrogenase (ALDH) 3A1 expression by the human keratocyte and its repair phenotypes. Exp Eye Res. 2006; 83: 1063–1073. [DOI] [PubMed] [Google Scholar]
- 41. Müller LJ, Marfurt CF, Kruse F, Tervo TM.. Corneal nerves: structure, contents and function. Exp Eye Res. 2003; 76: 521–542. [DOI] [PubMed] [Google Scholar]
- 42. Wilson SE: Laser in situ keratomileusis-induced (presumed) neurotrophic epitheliopathy. Ophthalmology. 2001; 108: 1082–1087. [DOI] [PubMed] [Google Scholar]
- 43. Medeiros CS, Marino GK, Lassance L, Thangavadivel S, Santhiago MR, Wilson SE.. The impact of photorefractive keratectomy and mitomycin C on corneal nerves and their regeneration. J Refract Surg. 2018; 34: 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jeon KI, Hindman HB, Bubel T, et al.. Corneal myofibroblasts inhibit regenerating nerves during wound healing. Sci Rep. 2018; 8: 12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilson SE, He Y-G, Weng J, Li Q, McDowall AW, Vital M, Chwang EL.. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996; 62: 325–328. [DOI] [PubMed] [Google Scholar]
- 46. Medeiros CS, Lassance L, Saikia P, Wilson SE.. Posterior stromal keratocyte apoptosis triggered by mechanical endothelial injury and nidogen-1 production in the cornea. Exp. Eye Res. 2018; 172: 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson SE: Keratocyte apoptosis in refractive surgery: Everett Kinsey Lecture. CLAO Journal. 1998; 24: 181–185. [PubMed] [Google Scholar]
- 48. Wilson SE, Kim W-J: Keratocyte apoptosis: implications on corneal wound healing, tissue organization, and disease. Invest Ophthalmology Vis Sci. 1998; 39: 220–226. [PubMed] [Google Scholar]
- 49. Marino GK, Santhiago MR, Santhanam A, et al.. Epithelial basement membrane injury and regeneration modulates corneal fibrosis after pseudomonas corneal ulcers in rabbits. Exp Eye Res. 2017; 161: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mohan RR, Hutcheon AEK, Choi R, et al.. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res 2003; 76: 71–87. [DOI] [PubMed] [Google Scholar]
- 51. Fukuda K. Corneal fibroblasts: function and markers. Exp Eye Res. 2020; 200: 108229. [DOI] [PubMed] [Google Scholar]
- 52. Jester JV, Huang J, Petroll WM, Cavanagh HD.. TGF beta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGF beta, PDGF and integrin signaling. Exp Eye Res. 2002; 75: 645–657. [DOI] [PubMed] [Google Scholar]
- 53. Chaurasia SS, Kaur H, Medeiros FW, Smith SD, Wilson SE.. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp Eye Res. 2009; 89: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lipshitz I, Loewenstein A, Varssano D, Lazar M.. Late onset corneal haze after photorefractive keratectomy for moderate and high myopia. Ophthalmology. 1997; 104: 369–373. [DOI] [PubMed] [Google Scholar]
- 55. Gomes BA, Smadja D, Espana EM, Ahn ES, Netto MV, Santhiago MR.. Very late-onset corneal scar triggered by trauma after photorefractive keratectomy. J Cataract Refract Surg. 2012; 38: 1694–1697. [DOI] [PubMed] [Google Scholar]
- 56. Miron-Mendoza M, Lin X, Ma L, Ririe P, Petroll WM.. Individual versus collective fibroblast spreading and migration: regulation by matrix composition in 3D culture. Exp Eye Res. 2012; 99: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miron-Mendoza M, Graham E, Kivanany P, Quiring J, Petroll WM.. The role of thrombin and cell contractility in regulating clustering and collective migration of corneal fibroblasts in different ECM environments. Invest Ophthalmol Vis Sci. 2015; 56: 2079–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ren R, Hutcheon AE, Guo XQ, et al.. Human primary corneal fibroblasts synthesize and deposit proteoglycans in long-term 3-D cultures. Dev Dyn. 2008; 237: 2705–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hassell JR, Schrecengost PK, Rada JA, SundarRaj N, Sossi G, Thoft RA.. Biosynthesis of stromal matrix proteoglycans and basement membrane components by human corneal fibroblasts. Invest Ophthalmol Vis Sci. 1992; 33: 547–557. [PubMed] [Google Scholar]
- 60. Brown CT, Nugent MA, Lau FW, Trinkaus-Randall V.. Characterization of proteoglycans synthesized by cultured corneal fibroblasts in response to transforming growth factor beta and fetal calf serum. J Biol Chem. 1999; 274: 7111–7119. [DOI] [PubMed] [Google Scholar]
- 61. Gil ES, Park SH, Marchant J, Omenetto F, Kaplan DL.. Response of human corneal fibroblasts on silk film surface patterns. Macromol Biosci. 2010; 10: 664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guo X, Hutcheon AE, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW.. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2007; 48: 4050–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fini ME, Girard MT.. The pattern of metalloproteinase expression by corneal fibroblasts is altered by passage in cell culture. J Cell Sci. 1990; 97(Pt 2): 373–383. [DOI] [PubMed] [Google Scholar]
- 64. Iwatake A, Murakami A, Ebihara N.. The expression of matrix metalloproteinases and their inhibitors in corneal fibroblasts by alarmins from necrotic corneal epithelial cells. Jpn J Ophthalmol. 2018; 62: 92–100. [DOI] [PubMed] [Google Scholar]
- 65. Couture C, Zaniolo K, Carrier P, et al.. The tissue-engineered human cornea as a model to study expression of matrix metalloproteinases during corneal wound healing. Biomaterials. 2016; 78: 86–101. [DOI] [PubMed] [Google Scholar]
- 66. Wilson SE. Interleukin-1 and transforming growth factor beta: Commonly opposing, but sometimes supporting, master regulators of the corneal wound healing response to injury. Invest Ophthalmol Vis Sci. 2021; 62: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hong JW, Liu JJ, Lee JS, et al.. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Invest Ophthalmol Vis Sci. 2001; 42: 2795–2803. [PubMed] [Google Scholar]
- 68. Wilson SE, Chen L, Mohan RR, Liang Q, Liu J.. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res. 1999; 68: 377–397. [DOI] [PubMed] [Google Scholar]
- 69. Weng J, Mohan RR, Li Q, Wilson SE.. IL-1 upregulates keratinocyte growth factor and hepatocyte growth factor mRNA and protein production by cultured stromal fibroblast cells: interleukin-1 beta expression in the cornea. Cornea. 1997; 16: 465–471. [PubMed] [Google Scholar]
- 70. Wilson SE, He YG, Weng J, Zieske JD, Jester JV, Schultz GS.. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp Eye Res. 1994; 59: 665–678. [DOI] [PubMed] [Google Scholar]
- 71. Miyagi H, Jalilian I, Murphy CJ, Thomasy SM.. Modulation of human corneal stromal cell differentiation by hepatocyte growth factor and substratum compliance. Exp Eye Res. 2018; 176: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cahill EF, Kennelly H, Carty F, Mahon BP, English K.. Hepatocyte growth factor is required for mesenchymal stromal cell protection against bleomycin-induced pulmonary fibrosis. Stem Cells Transl Med. 2016; 5: 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gazdhar A, Fachinger P, van Leer C, et al.. Gene transfer of hepatocyte growth factor by electroporation reduces bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007; 292: L529–L536. [DOI] [PubMed] [Google Scholar]
- 74. Cui Q, Wang Z, Jiang D, Qu L, Guo J, Li Z.. HGF inhibits TGF-beta1-induced myofibroblast differentiation and ECM deposition via MMP-2 in Achilles tendon in rat. Eur J Appl Physiol. 2011; 111: 1457–1463. [DOI] [PubMed] [Google Scholar]
- 75. Santhanam A, Torricelli AA, Wu J, Marino GK, Wilson SE.. Differential expression of epithelial basement membrane components nidogens and perlecan in corneal stromal cells in vitro. Mol Vis. 2015; 21: 1318–1327. [PMC free article] [PubMed] [Google Scholar]
- 76. Santhanam A, Marino GK, Torricelli AA, Wilson SE.. EBM regeneration and changes in EBM component mRNA expression in stromal cells after corneal injury. Mol Vis. 2017; 23: 39–51. [PMC free article] [PubMed] [Google Scholar]
- 77. Paralkar VM, Vukicevic S, Reddi AH.. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev. Biol. 1991; 143: 303–308. [DOI] [PubMed] [Google Scholar]
- 78. Iozzo RV, Zoeller JJ, Nystrom A.. Basement membrane proteoglycans: modulators par excellence of cancer growth and angiogenesis. Mol Cells. 2009; 27: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ishizaki M, Shimoda M, Wakamatsu K, et al.. Stromal fibroblasts are associated with collagen IV in scar tissues of alkali-burned and lacerated corneas. Curr Eye Res. 1997; 16: 339–348. [DOI] [PubMed] [Google Scholar]
- 80. Saikia P, Crabb JS, Dibbin LL, et al.. Quantitative proteomic comparison of myofibroblasts derived from bone marrow or locally from the cornea. Sci Rep. 2020; 10: 16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wilson SE, Marino GK, Torricelli AAM, Medeiros CS.. Corneal fibrosis: injury and defective regeneration of the epithelial basement membrane. A paradigm for fibrosis in other organs? Matrix Biol 2017; 64: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chan WK, Weissman B.. Corneal pannus associated with contact lens wear. Am J Ophthalmol. 1996; 121: 540–546. [DOI] [PubMed] [Google Scholar]
- 83. Nicholas MP, Mysore N.. Corneal neovascularization. Exp Eye Res. 2021; 202: 108363. [DOI] [PubMed] [Google Scholar]
- 84. Hung CF, Wilson CL, Schnapp LM.. Pericytes in the lung. Adv Exp Med Biol. 2019; 1122: 41–58. [DOI] [PubMed] [Google Scholar]
- 85. Kida Y, Duffield JS.. Pivotal role of pericytes in kidney fibrosis. Clin Exp Pharmacol Physiol. 2011; 38: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kostallari E, Shah VH.. Pericytes in the liver. Adv Exp Med Biol. 2019; 1122: 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Alex L, Frangogiannis NG.. Pericytes in the infarcted heart. Vasc Biol. 2019; 1: H23–H31. [DOI] [PMC free article] [PubMed] [Google Scholar]