Abstract
Paraneoplastic neurological syndromes (PNS) are rare presentations of an underlying oncological disease and more unusual during an oncological disease. They most likely present in small-cell lung carcinomas and thymomas, but present in <1% of the gynecological neoplasms. Acknowledging the pathophysiology is essential for management, explaining its clinical presentation, and future research. We present a patient with an underlying gynecological cancer that during her disease developed a PNS with an unusual autoantibody (anti-CV2/CRMP5) mediating the disease. We report a case of a 62-year-old female diagnosed with ovarian cancer who in the course of her disease developed neurological symptoms associated with cerebellar degeneration. After ruling out differential diagnoses such as metastases, a PNS was suspected and studied, in which anti-CV2/CRMP5 antibodies were positive. With her clinical presentation, radiological features, autoantibody positivity on cerebrospinal fluid, and an underlying oncological disease, cerebellar degeneration was diagnosed. The pathophysiology of PNS is not fully understood; therefore, its diagnosis and management are complex. Diagnosis is based on clinical presentation and specific antibodies associated. Unfortunately, patients have a bad prognosis and diminished quality of life, and therefore a multidisciplinary approach is needed. It is important to mention that the presentation of PNS does not mandatorily appear before the diagnosis of cancer, and multiple cases have been reported in which patients with an underlying oncological disease develop these syndromes. As medical oncologists and neurologists, we must consider and study these syndromes as a possible etiology in cases with an underlying cancer who develop neurological symptoms in the course of their disease.
Keywords: Paraneoplastic neurological syndrome, Paraneoplastic cerebellar degeneration, Ovarian cancer, Onconeural antibodies, Anti-CV2/CRMP5
Introduction
Paraneoplastic neurological syndromes (PNS) are a presentation of signs and symptoms mediated by an immune response associated with an underlying neoplasm and antineuronal antibodies. These syndromes commonly occur as the first sign of an underlying neoplasm leading to its detection and rarely occur during an oncologic disease. Its epidemiology is not well known, but recent studies report an incidence of 3 cases per million person-years, and a population-based study reported an incidence of 1/100,000 person-years, a prevalence of 4/100,000, and an average of 68 years of age [1]. Tumors associated commonly express neuroendocrine proteins such as small-cell lung carcinoma (SCLC) (3–5%), being also the one commonly associated with other paraneoplastic syndromes. Other cancers include thymomas (15–20%), B-cell neoplasms (3–10%), and <1% of gynecological neoplasms [2]. The most common PNS is limbic encephalitis in 31% of cases, followed by paraneoplastic cerebellar degeneration (PCD) with 28% and encephalomyelitis with 20%. PNS presentation in gynecological tumors is extremely rare, and understanding the pathophysiology and presentation is essential to guide management.
Case Presentation
A 62-year-old female presented in June 2018 with abdominal pain, increased abdominal circumference, 5-kg weight loss in the last month, and asthenia. The diagnostic workup included a pelvic ultrasound, CT scan, and CA-125 levels, which confirmed the presence of ascites, a pelvic mass with malignant characteristics in the left ovary, and CA-125 levels of 2,400 U/mL (shown in Fig. 1). A diagnostic laparoscopy with biopsy was performed, which confirmed a stage IIIC ovarian cancer and no germline BRCA mutation.
Fig. 1.
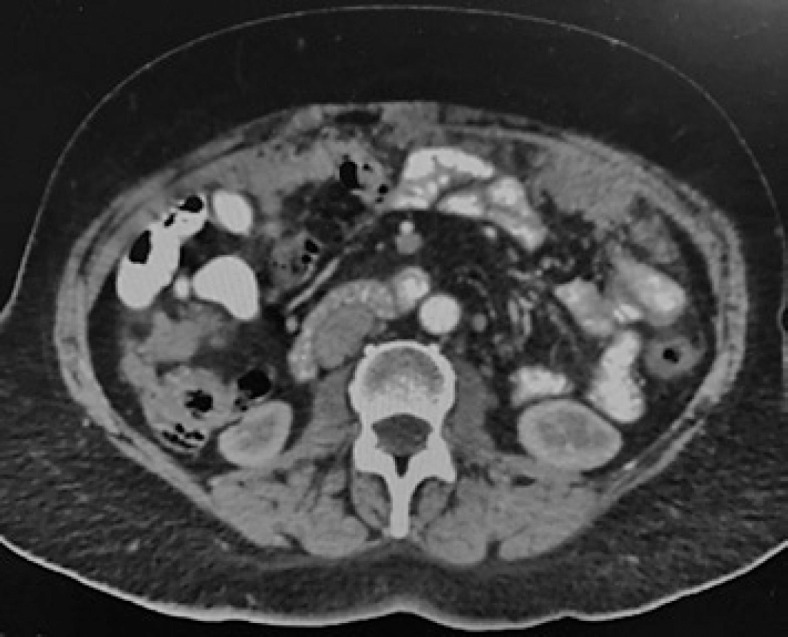
Abdomen and pelvis CT scan at the time of diagnosis, showing peritoneal disease “omental cake.”
A total of 6 cycles of neoadjuvant chemotherapy with paclitaxel plus carboplatin and bevacizumab were given. The patient showed partial response with CA-125 levels lowering to 120 U/mL and underwent debulking surgery in January 2019, achieving suboptimal cytoreduction, with 3-month postsurgical CA-125 levels of 372 U/mL. She continued treatment with bevacizumab as maintenance therapy. Follow-up test with PET-CT showed persistent disease in the right paracolic recesses, positive bilateral parainternal iliac lymph nodes, CA-125 levels of 180 U/mL, and partial response by Response Evaluation Criteria on Solid Tumors (RECIST) criteria.
Two months after her last checkup, the patient developed dysarthria, dizziness, right lateropulsion, tinnitus, and gait instability, causing multiple falls from standing height. A brain MRI was performed, showing cortico-subcortical atrophy as shown in Figure 2, and PET-CT continued showing a partial response to treatment by RECIST. Having a stable oncological disease on PET-CT and brain MRI findings unable to explain the sudden neurological symptoms, a PNS was suspected.
Fig. 2.
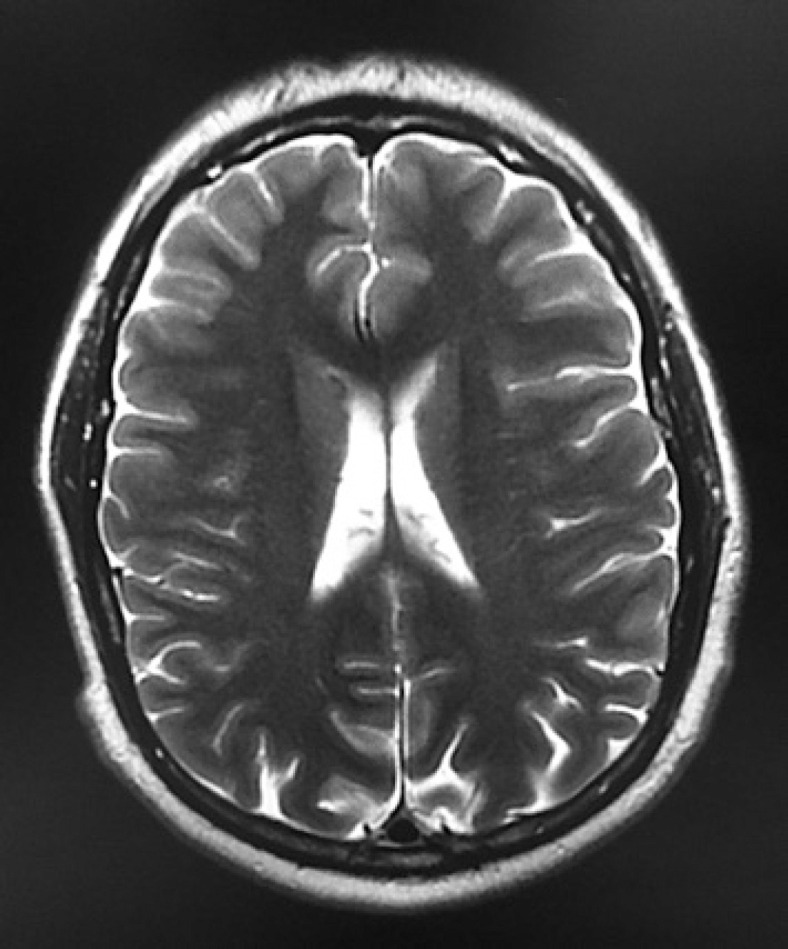
Brain MRI showing cortico-subcortical atrophy and no evidence of brain metastasis or leptomeningeal disease.
Workup for diagnosis included corticospinal fluid (CSF) analysis for multiple onconeural and neuronal cell surface antibodies including anti-Zic 4, anti-NMDAR-D, anti-mGlur1, anti-PCA-1, anti-PCA-2, anti-TR, delta-notch-like, ANNA-1, ANNA-2, ANNA-3, and anti-CV2. The only positive antibody found was anti-CV2. Based on these antibodies, the characteristic cerebellar symptoms, and her known oncological disease, we diagnosed the patient with PCD secondary to an ovarian carcinoma.
Oncology and Neurology medical services proposed continuing oncological treatment, with the goal of maintaining a stable disease and reducing neurological symptoms. Also, treatment was focused on PNS management with high-dose methylprednisolone, plasmapheresis, and rehabilitation.
Discussion and Review of the Literature
Pathophysiology
PNS pathophysiology is immune mediated, and these syndromes damage the nervous system via antineuronal antibodies via onconeural antibodies or neuronal cell surface antibodies. Antibody positivity is not always present; in fact, 60% of PNS affecting the central nervous system (CNS) and 20% affecting peripheral nervous system are antibody positive [3]. However, the presence of antibodies helps make a more specific diagnosis and identify the course and prognosis.
Onconeural antibodies indicate that the disorder is paraneoplastic and mediate damage via cytotoxic T cells, making neuronal damage almost always irreversible [4]. PNS that present these types of antibodies have the worse prognosis. Some examples include anti-Hu, anti-CV2/CRMP5, anti-Yo, anti-Ri, anti-Tr, and anti-Ma. Contrastingly, neuronal surface antibodies cannot differentiate between cases that are paraneoplastic or not, and the mediated damage is via antibodies targeting specific antigens in the CNS. Examples include anti-NMDAR, anti-AMPAR, anti-GABA, anti-mGlur1, anti-mGlur5, and anti-Zic4.
Paraneoplastic Cerebellar Degeneration
Generally, it presents before the diagnosis of a neoplasm and rarely during an oncological disease. The tumors commonly involved are ovarian, breast, SCLC, and Hodgkin's lymphoma [5]. PCD is predominantly associated with anti-Yo antibodies being the main antibody mediating the disease, especially in gynecological neoplasms. Other antibodies associated include anti-Ri antibodies in gynecological neoplasms and SCLC, anti-Tr antibodies in Hodgkin's lymphoma, and anti-CV2/CRMP5 in thymoma and SCLC [6]. All previously mentioned antibodies are onconeuronal and therefore have high syndrome specificity. Comparatively, anti-mGlur1 and anti-Zic4 antibodies have been associated with PCD in Hodgkin's lymphoma and SCLC, respectively, and these 2 are partly characterized paraneoplastic antibodies.
Clinical Presentation and Diagnosis
PCD begins with dizziness, positional vertigo, and nausea. Shortly after, patients develop subacute onset of symmetric truncal and limb ataxia, dysarthria, and nystagmus; CSF cytology shows signs of an inflammatory process. Imaging studies in the acute phase show a normal MRI or increased T2 signal within the cerebellar hemispheres, and fluorodeoxyglucose-PET (FDG-PET) may show signs of cerebellar hypermetabolism. MRI in the chronic phase will show T2 hyperintensity improvement and cerebellar atrophy, and FDG-PET shows cerebellar hypometabolism [7].
Based on the Diagnostic Criteria for PNS by the Paraneoplastic Neurological Syndrome Euronetwork, our case is considered a definite diagnosis which includes a classical presentation, a tumor present, and onconeural antibody positivity [3]. The absence of onconeural antibodies does not rule out a PNS, but their presence helps the diagnosis. Differential diagnosis to keep in mind includes cerebellar metastases, chronic degenerative diseases, Creutzfeldt-Jakob disease, infections, leptomeningeal disease, vitamin deficiencies, and alcohol damage.
Prognosis and Management
PCD progresses fast, usually causing irreversible damage and poor prognosis since it is associated with onconeural antibodies, which cause neuronal damage and loss of Purkinje cells via cytotoxic T cells leading to pancerebellar dysfunction. Comparing disability between different antibodies, those with anti-Tr antibodies have less disability compared with anti-Yo antibodies. Due to its poor prognosis, treatment is based on removing the primary tumor if possible and managing symptoms with rehabilitation, with the goal of offering a better life quality.
The use of corticosteroids, intravenous immunoglobulin, cyclophosphamide, or tacrolimus has not shown significant improvement in most cases [8]. These agents must be a personalized treatment with a multidisciplinary group including neuro-oncologists and associated physicians treating the primary neoplasm. Symptomatic treatment is focused on rehabilitation, and some reports have shown improvement with clonazepam [9]. Immunotherapy treatments include the use of steroids, intravenous immunoglobulin, and cyclophosphamide. A commonly used method is IV methylprednisolone and prednisone daily. Contrastingly, immunoglobulin G and cyclophosphamide help by reducing T-cell proliferation suppressing proinflammatory cytokines [10]. Plasma exchange patients might not respond as expected as it does not remove immunoglobulins from the CSF. Unfortunately, most patients become wheelchair bound. Thus, new therapies focused on mediating immune response at the CNS level are needed.
Patients with anti-Yo, anti-Hu, and anti-CV2/CRMP5 are the most refractory to treatment and have the worst prognosis. PCD with anti-Hu has an average survival of 7 months, followed by anti-Yo antibodies with 13 months, anti-Ri with 69 months, and anti-Tr with 113 months. Survival and prognosis also depend on location and stage of the cancer.
Anti-CV2/CRMP5 Antibody and PCD
Antibodies against collapsin-response-mediator-protein-5 (CRMP5 or CV2), a 66-kDa cytoplasmic protein member of the CRMP family, participate in dendrite and axon formation and regeneration [11] CV2/CRMP5 is involved in brain development via the semaphorin-3A signaling and is mainly located in dendrites of pyramidal neurons, hippocampal pyramidal cells, Purkinje cells, and oligodendrocytes [12]. Anti-CV2/CRMP5 is mainly associated with PCD. Other syndromes include encephalomyelitis, optic neuritis, uveo-retinal symptoms, and myasthenic syndromes. The most common tumors associated with these antibodies are SCLC and thymoma. However, cases in the prostate, head, and neck and uterine cancer have been reported [13, 15, 16]. A summary of PCD with anti-CV2/CRMP5 cases reported in the literature and an underlying cancer, including the case presented, is shown in Table 1. In this table, cases with thymoma or SCLC are excluded since the association between anti-CV2/CRMP5 and PCD is well documented.
Table 1.
PCD and anti-CV2 reported cases with an underlying cancer diagnosis (SCLC and thymoma not included)
| Age | Gender | Type of tumor | Reference |
|---|---|---|---|
| 62 | Female | High-grade ovarian serous carcinoma | Juárez |
| 70 | Female | Uterine sarcoma | Rogemond and Honnorat [11] |
| 72 | Female | Uterine sarcoma | Rogemond and Honnorat [11] |
| 60 | Female | Squamous cell carcinoma of the tongue | Saloustros et al. [14] |
| 80 | Male | Prostate adenocarcinoma | Aliprandi et al. [15] |
| 50 | Female | Ovarian adenocarcinoma | Hezer et al. [16] |
Conclusion
PNS are a presentation of signs and symptoms mediated by an immune response associated with an underlying neoplasm and antineuronal antibodies. It is presented in <1% of the gynecological neoplasms.
The pathophysiology of PNS is not fully understood; therefore, its diagnosis and management are complex, and a multidisciplinary approach is needed. It is important to mention that the presentation of PNS does not mandatorily appear before the diagnosis of cancer, and multiple cases have been reported in which patients with an underlying oncological disease develop these syndromes.
In addition to having little evidence of PNS associated with gynecological tumors, it is relevant to present this case because it developed during a previously diagnosed cancer, while responding to oncological treatment, and the uncommon relationship with anti-CV2 antibodies. A multidisciplinary team for management is essential, as well as ruling out differential diagnoses, mainly central nervous system metastases, but not losing sight of possible chronic degenerative diseases. As medical oncologists and neurologists, we must consider and study these syndromes as a possible etiology in cases with an underlying cancer who develop neurological symptoms in the course of their disease. Acknowledging the pathophysiology is essential for management, understanding the clinical presentation, future research, and, last but not least, to improve the life quality of patients and their families.
Statement of Ethics
The Mexico Centre for Clinical Research, S.A. de C.V. Review Board, approved this study under reference number: CONBIOÉTICA-09-CEI-016-20180921. Written informed consent for publication of this case report and any accompanying images has been obtained from the patient. All identifying information has been removed to preserve confidentiality.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors did not have any funding sources.
Author Contributions
J.J.J.V.W. helped in conception, design, writing, and proofreading of the manuscript. K.G.H.G. helped in translation and writing of the manuscript. J.R.R.C. helped in writing and analysis of the manuscript. M.E.O.C. helped in a neurological approach of the case and review of the medical file. V.G.M. and A.C.M. helped in proofreading, review of the medical file, obtaining informed consent, and provision of imaging.
Data Availability Statement
No dataset was required. A preprint version of this manuscript is available [17].
Acknowledgments
We would like to thank our patient and her family for allowing us to use her medical information for their contribution to this article.
References
- 1.Vogrig A, Gigli GL, Segatti S, Corazza E, Marini A, Bernardini A, et al. Epidemiology of paraneoplastic neurological syndromes: a population-based study. J Neurol. 2020;267((1)):26–35. doi: 10.1007/s00415-019-09544-1. [DOI] [PubMed] [Google Scholar]
- 2.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7((4)):327–40. doi: 10.1016/S1474-4422(08)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75((8)):1135–40. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yshii L, Bost C, Liblau R. Immunological bases of paraneoplastic cerebellar degeneration and therapeutic implications. Front Immunol. 2020;11:991. doi: 10.3389/fimmu.2020.00991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shams'Ili S, Grefkens J, De Leeuw B, Van den Bent M, Hooijkaas H, Van der Holt B, et al. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain. 2003;126((6)):1409–18. doi: 10.1093/brain/awg133. [DOI] [PubMed] [Google Scholar]
- 6.Greene M, Lai Y, Baella N, Dalmau J, Lancaster E. Antibodies to delta/notch-like epidermal growth factor-related receptor in patients with anti-Tr, paraneoplastic cerebellar degeneration, and Hodgkin lymphoma. JAMA Neurol. 2014;71((8)):1003–8. doi: 10.1001/jamaneurol.2014.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madhavan AA, Carr CM, Morris PP, Flanagan EP, Kotsenas AL, Hunt CH, et al. Imaging review of paraneoplastic neurologic syndromes. AJNR Am J Neuroradiol. 2020;41((12)):2176–87. doi: 10.3174/ajnr.A6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keime-Guibert F, Graus F, Fleury A, René R, Honnorat J, Broet P, et al. Treatment of paraneoplastic neurological syndromes with antineuronal antibodies (Anti-Hu, Anti-Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. J Neurol Neurosurg Psychiatry. 2000;68((4)):479–82. doi: 10.1136/jnnp.68.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grativvol RS, Cavalcante WCP, Castro LHM, Nitrini R, Simabukuro MM. Updates in the diagnosis and treatment of paraneoplastic neurologic syndromes. Curr Oncol Rep. 2018;20((11)):92. doi: 10.1007/s11912-018-0721-y. [DOI] [PubMed] [Google Scholar]
- 10.Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. 2012;367((21)):2015–25. doi: 10.1056/NEJMra1009433. [DOI] [PubMed] [Google Scholar]
- 11.Rogemond V, Honnorat J. Anti-CV2 autoantibodies and paraneoplastic neurological syndromes. Clin Rev Allergy Immunol. 2000;19((1)):51–9. doi: 10.1385/CRIAI:19:1:51. [DOI] [PubMed] [Google Scholar]
- 12.Honnorat J, Cartalat-Carel S, Ricard D, Camdessanche JP, Carpentier AF, Rogemond V, et al. Onco-neural antibodies and tumour type determine survival and neurological symptoms in paraneoplastic neurological syndromes with Hu or CV2/CRMP5 antibodies. J Neurol Neurosurg Psychiatry. 2009;80((4)):412–6. doi: 10.1136/jnnp.2007.138016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huemer F, Melchardt T, Tränkenschuh W, Neureiter D, Moser G, Magnes T, et al. Anti-Hu antibody associated paraneoplastic cerebellar degeneration in head and neck cancer. BMC Cancer. 2015;15((1)):996–6. doi: 10.1186/s12885-015-2020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saloustros E, Zaganas I, Mavridis M, Vamvakas L, Plaitakis A, Georgoulias V, et al. Anti-CV2 associated cerebellar degeneration after complete response to chemoradiation of head and neck carcinoma. J Neurooncol. 2009 doi: 10.1007/s11060-009-0022-2. [DOI] [PubMed] [Google Scholar]
- 15.Aliprandi A, Terruzzi A, Rigamonti A, Bazzigaluppi E, Tremolizzo L, Ferrarese C, et al. Paraneoplastic cerebellar degeneration with anti-CV2/CRMP5 antibodies and prostate adenocarcinoma. Neurol Sci. 2015;36((8)):1501–3. doi: 10.1007/s10072-015-2113-5. [DOI] [PubMed] [Google Scholar]
- 16.Hezer S, Descargues G, Vignals C, Imbert Y. [Paraneoplastic cerebellar degeneration and primary fallopian cancer] Gynecol Obstet Fertil Senol. 2019;47((7–8)):610–2. doi: 10.1016/j.gofs.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 17. Available from: https://doi.org/10.21203/rs.3.rs-789310/v1 https://doi.org/10.21203/rs.3.rs-789310/v1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No dataset was required. A preprint version of this manuscript is available [17].


