Abstract
Alopecia areata (AA), an autoimmune disease with a relapsing-remitting course, represents the second cause of nonscarring alopecia worldwide and is associated with several comorbidities, notably atopic dermatitis (AD). In particular, AD is related to its more severe forms alopecia totalis (AT) and alopecia universalis (AU) [Nat Rev Dis Primers. 2017;3:17011]. Considering that AA has been classified as T helper 1-driven disease, whereas AD is the prototypical T helper 2 (Th2)-driven skin disorder, recent studies suggest that these forms may underlie a different chemokine expression resulting in a Th2 skewing as a key pathomechanism that could explain this association [JAMA Dermatol. 2015 May;151(5):522–8]. Several reports showed that dupilumab, a fully human monoclonal antibody targeting the interleukin 4α receptor and thus downregulating Th2 response, led to an improvement of AA associated with AD; most of these patients were females with AT or AU, early-onset AD, and atopic comorbidities [Exp Dermatol. 2020 Aug;29(8):726–32]. We report here a case to further support this hypothesis.
Keywords: Alopecia areata, Atopic dermatitis, Dupilumab, Hair, Therapy treatment
Established Facts
Alopecia areata is an autoimmune disease with a relapsing-remitting course that primarily affects hair follicles for which several treatments are currently available with a variable clinical response rate, often insufficient in its most severe forms.
There is a strong association between alopecia areata (especially in its more severe forms) and atopic diseases, noteworthy atopic dermatitis.
Dupilumab, a full human monoclonal antibody against the interleukin-4α receptor, represents nowadays the gold standard in the treatment of severe atopic dermatitis.
Novel Insights
Recent studies regarding the pathophysiology of alopecia areata suggest that a cytokine skewing from a T helper 1 to T helper 2 response occurs in its more severe forms alopecia totalis and universalis, especially when associated with early-onset atopic comorbidities.
We present here a case of remission of alopecia areata universalis in a patient treated with dupilumab for severe atopic dermatitis.
This case demonstrating a response after 1 year of treatment could be helpful in the management of AA and suggest a new therapeutic option for the disease.
Case Report
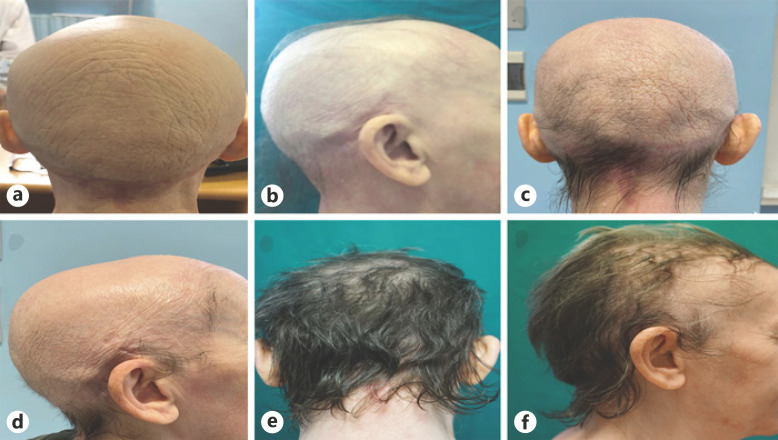
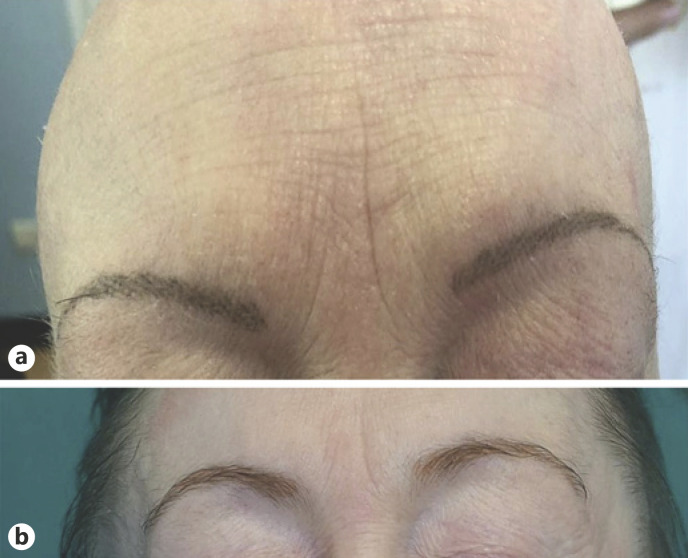
We report here a case of a 49-year-old woman followed in our center with alopecia universalis (AU) since 2011 treated with oral betamethasone 0.5 mg per day without clinical benefit. Moreover, she suffered from early-onset severe atopic dermatitis (AD), allergic rhinitis, asthma, and polysensitization for which she has been previously treated with oral and topical corticosteroids and ciclosporin with poor clinical response and a chronic-relapsing course. In January 2019, we decided to start dupilumab therapy with 600 mg induction dose followed by 300 mg injection every 2 weeks and recorded some validated scores to assess the disease severity: Eczema Area and Severity Index (EASI) was 24, Numerical Rating Scale of itch (NRSi) was 10/10, and Severity Alopecia Tool (SALT) score was 100 (Fig. 1a, b, 2a). In May 2020, we observed a partial regrowth of terminal hairs and body hairs with an EASI score of 0, NRSi 1/10, and SALT score 70/100 (Fig. 1c, d). In her last follow-up, in December 2020, the clinical response markedly improved achieving a SALT score of 10/100 (Fig. 1e, f, 2b).
Fig. 1.
a, b Back and lateral view of the scalp of the patient in January 2019 (first administration of dupilumab). c, d Back and lateral view of the scalp in May 2019. e, f Back and lateral view of the scalp in her last follow-up (December 2020).
Fig. 2.
a Eyebrows in January 2019; the patient had a microblading tattoo to cover her alopecia. b Complete eyebrow regrowth in December 2020.
Discussion
Alopecia areata (AA), an autoimmune disease with a relapsing-remitting course, represents the second cause of nonscarring alopecia worldwide and is associated with several comorbidities, notably AD. In particular, AD is related to its more severe forms alopecia totalis (AT) and AU [1, 2]. Considering that AA has been classified as T helper 1-driven disease, whereas AD is the prototypical T helper 2 (Th2)-driven skin disorder, recent studies suggest that these forms may underlie a different chemokine expression resulting in a Th2 skewing as a key pathomechanism that could explain this association [3]. Several reports showed that dupilumab, a fully human monoclonal antibody targeting the interleukin (IL)-4α and IL-13 receptors and thus downregulating Th2 response, led to an improvement of AA associated with AD: the first report by Penzi et al. [4] observed a full hair regrowth in a 13-year-old girl affected by AT and severe AD after 11 months of treatment with dupilumab; subsequently, several authors described an improvement of AA in their patients treated with dupilumab [5, 6, 7, 8, 9, 10]. Although cases of AA induced by dupilumab have been described [11, 12, 13], it is possible that sex difference, AD duration, and immunological profile of the patients play a crucial role in the treatment's response; in fact as outlined by Marks et al. [14], most of the patients in which dupilumab improved AA were females with AT or AU, early-onset AD, and atopic comorbidities, suggesting a predominant role of Th2 skewing in this subset of patients. The case we present support this hypothesis as our patient is a female, suffering from a long-standing AD associated with atopic comorbidities and AU refractory to treatments. Moreover, it demonstrates that hair regrowth could be observed even in the long term, in our case after 1 year of treatment.
In a recent review of Sachdeva et al. [15], a total of 11 patients showed an improvement of AA during dupilumab therapy, and 4 of them had AU; our report adds another case to this series. Further clinical research studies are needed to assess the efficacy of dupilumab to treat recalcitrant AA, but our case suggests that dupilumab could represent a therapeutic option in patients with severe forms of AA associated with AD. In the near future, a better understanding of AA pathophysiology combined with the stratification of patients based on clinical history, laboratory examinations, cytokine expression, and immunophenotype profile could identify different subsets of patients and thus helping the therapeutic approach, sorting the patients that could benefit for specific therapies, and moving toward a more targeted and personalized medicine.
Statement of Ethics
The authors have no ethical conflicts to disclose. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict of Interest Statement
Silvia Mariel Ferrucci has been the principal investigator in clinical trials for AbbVie, Novartis, and Eli Lilly. The other authors declare no conflicts of interest.
Funding Sources
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contributions
M.R. wrote the article, M.B. and S.M.F. designed the article, and S.T. and L.A. revised it.
Availability of Data and Material
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.Pratt CH, King LE, Jr, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. doi: 10.1038/nrdp.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohan GC, Silverberg JI. Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2015 May;151((5)):522–8. doi: 10.1001/jamadermatol.2014.3324. [DOI] [PubMed] [Google Scholar]
- 3.Ito T, Kageyama R, Nakazawa S, Honda T. Understanding the significance of cytokines and chemokines in the pathogenesis of alopecia areata. Exp Dermatol. 2020 Aug;29((8)):726–32. doi: 10.1111/exd.14129. [DOI] [PubMed] [Google Scholar]
- 4.Penzi LR, Yasuda M, Manatis-Lornell A, Hagigeorges D, Senna MM. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018 Nov 1;154((11)):1358–60. doi: 10.1001/jamadermatol.2018.2976. [DOI] [PubMed] [Google Scholar]
- 5.Gruenstein D, Malik K, Levitt J. Full scalp hair regrowth in a 4-year-old girl with alopecia areata and atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020 Oct 15;6((12)):1286–7. doi: 10.1016/j.jdcr.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida H, Kamata M, Watanabe A, Agematsu A, Nagata M, Fukaya S, et al. Dupilumab improved alopecia areata in a patient with atopic dermatitis: a case report. Acta Derm Venereol. 2019 Jun 1;99((7)):675–6. doi: 10.2340/00015555-3183. [DOI] [PubMed] [Google Scholar]
- 7.Patruno C, Napolitano M, Ferrillo M, Fabbrocini G. Dupilumab and alopecia: a janus effect. Dermatol Ther. 2019 Sep;32((5)):e13023. doi: 10.1111/dth.13023. [DOI] [PubMed] [Google Scholar]
- 8.Magdaleno-Tapial J, Valenzuela-Oñate C, García-Legaz-Martínez M, Martínez-Domenech Á, Pérez-Ferriols A. Improvement of alopecia areata with Dupilumab in a patient with severe atopic dermatitis and review the literature. Australas J Dermatol. 2020 May;61((2)):e223–5. doi: 10.1111/ajd.13208. [DOI] [PubMed] [Google Scholar]
- 9.Ludriksone L, Elsner P, Schliemann S. Simultaneous effectiveness of dupilumab in atopic dermatitis and alopecia areata in two patients. J Dtsch Dermatol Ges. 2019 Dec;17((12)):1278–80. doi: 10.1111/ddg.13990. [DOI] [PubMed] [Google Scholar]
- 10.Harada K, Irisawa R, Ito T, Uchiyama M, Tsuboi R. The effectiveness of dupilumab in patients with alopecia areata who have atopic dermatitis: a case series of seven patients. Br J Dermatol. 2020 Aug;183((2)):396–7. doi: 10.1111/bjd.18976. [DOI] [PubMed] [Google Scholar]
- 11.Maloney NJ, Worswick S, Cheng K. Development of alopecia in patients treated with dupilumab. Dermatol Ther. 2019 May;32((3)):e12869. doi: 10.1111/dth.12869. [DOI] [PubMed] [Google Scholar]
- 12.Yazdanyar S, Jemec GBE. Alopecia areata after treatment with dupilumab. Dermatitis. 2019 Mar;30((2)):175–6. doi: 10.1097/DER.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 13.Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019 Dec 6;5((1)):54–6. doi: 10.1016/j.jdcr.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks DH, Mesinkovska N, Senna MM. Cause or cure? Review of dupilumab and alopecia areata. J Am Acad Dermatol. 2019 Jun 13;S0190-9622((19)):30973–9. doi: 10.1016/j.jaad.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Sachdeva M, Witol A, Mufti A, Maliyar K, Yeung J. Alopecia areata related paradoxical reactions in patients on dupilumab therapy: a systematic review. J Cutan Med Surg. 2021 Feb 13;:1203475421995186. doi: 10.1177/1203475421995186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.