ABSTRACT
Aplasia cutis congenita (ACC) is a rare congenital defect described by the absence of skin and occasionally subcutaneous tissues or bone. The management of ACC varies depending on the lesion size, location and associated abnormalities. Small lesions often heal spontaneously, whereas larger lesions are significant and usually associated with additional anomalies in other organs. This paper reports three cases, which describe large lesions of ACC, presented with other abnormalities (Adams–Oliver syndrome, intestinal obstruction and heart defect). Particular attention should be paid to the patient with large lesions of ACC to investigate more congenital anomalies.
INTRODUCTION
Aplasia cutis congenita (ACC) is a rare cutaneous malformation characterized by a congenital absence of skin and in some cases subcutaneous tissues or bone. Lesions are usually found on the vertex of the scalp [1] and sometimes in the limbs, abdomen or trunk [2]. The appearance of the lesion widely varies from an atrophic, membranous alopecia scar to ulceration [3].
In this case series, we present three cases of ACC associated with limb anomalies, intestinal obstruction and a complex heart defect. Physicians must investigate internal organs involvement once they diagnose large lesions of ACC.
CASE REPORTS
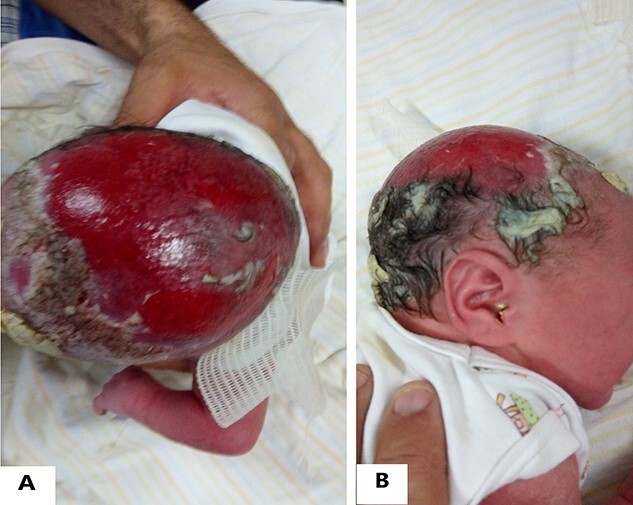
CASE 1: A female newborn was born by vaginal delivery at 40 weeks after a normal pregnancy. Physical examination revealed an irregularly shaped hemorrhagic defect measured (7 × 5) cm on the vertex of the scalp over the parietal occipital region (Fig. 1). She also had many terminal limbs anomalies that include absence of toes, hypoplastic toes nails and one additional dead finger in the right hand (Fig. 2). There was scar tissue on her abdomen (Fig. 3). She was the fifth child of the non-consanguineous marriage, and her brothers did not suffer from any similar anomalies. Her 34-year-old mother did not take any medication during gestation. There were no abnormalities in the family relatives. Computed tomography scan of the head showed an absence of the skin and bone of the scalp (Fig. 4). She died before doing more investigations. The combination of the scalp defect with terminal limbs anomalies suggests a diagnosis of Adams–Oliver syndrome (AOS).
Figure 1 .

(A&B) ACC appears as a hemorrhagic large defect extending over the occipital and parietal regions.
Figure 2 .

(A, B&C) Terminal limb anomalies; absence of toes, hypoplastic toes nails and additional dead finger in the hand.
Figure 3 .
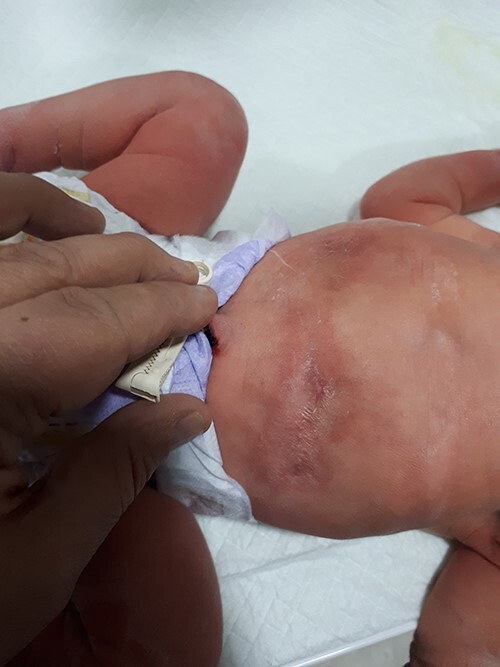
Scar tissue on the abdomen.
Figure 4 .

The CT scan shows an absence of the skin and bone of the scalp.
CASE 2: A female newborn was born at 40 weeks by a caesarian section after an uncomplicated pregnancy. Clinical examination showed an extensive hemorrhagic defect on the vertex of her scalp measuring (8 × 6) cm (Fig. 5). She was admitted to the neonatal intensive care unit. While monitoring her, she suffered from a delay in the passing of meconium. A contrast barium enema showed an intestinal obstruction (Fig. 6), and urgent nasogastric intubation had been done (Fig. 7). She died, so we could not do other investigations. The family history detected that she was the first child of the non-consanguineous marriage, and her mother did not take any medication during her pregnancy. There was no history of congenital anomalies in her brothers or relatives.
Figure 5 .
(A&B) ACC appears as an extensive hemorrhagic lesion on the vertex of the scalp.
Figure 6 .

A nasogastric intubation was performed to treat the intestinal obstruction.
Figure 7 .
(A&B) A contrast barium enema presents intestinal obstruction.
CASE 3: A female newborn was born at 39 weeks by a caesarian section with a fibrotic alopecic scar measuring (4 × 3) cm on the vertex of her scalp (Fig. 8). Chest auscultation showed a high pansystolic murmur at the left lower sternum edge with a fixed split in the second heart sound. She was the third child of the non-consanguineous marriage. The pregnancy progressed without any complications. An Echocardiography was demanded, which revealed (Fig. 9):
Figure 8 .

ACC appears as a fibrotic alopecic scar on the vertex of the scalp.
Figure 9 .

An echocardiogram shows multiple malformations that include PFO, VSD, Tricuspid valve insufficiency and severe pulmonary valve stenosis.
(1) A Patent foramen ovale (PFO) with a left-to-right shunt.
(2) A Ventricular septal defect (VSD) measures 7 mm.
(3) A Low-grade Tricuspid valve insufficiency.
(4) A severe pulmonary valve stenosis and intermediate pulmonary hypertension.
Her mother did not take any medication during her gestation. The family history of congenital anomalies was negative.
DISCUSSION
The specific etiology of Aplasia cutis congenita is not clear yet. Chromosomal abnormalities, especially BMS1, trauma, intrauterine infection and teratogens in pregnancy are possible causes [1, 2, 4]. ACC has been classified into nine types; some are associated with other abnormalities or genetic syndromes, such as AOS [4]. The management of ACC varies depending on the lesion size and location. For small uncomplicated lesions, conservative management includes wound cleaning and using antibiotics. However, larger lesions may need urgent surgery because of the high risk of infection and bleeding.
In addition, large lesions ≥4 cm can also be associated with underlying defects [4].
In our paper, the focus of attention is on three sporadic cases of ACC with large lesions (7 × 5) cm, (8 × 6) cm and (4 × 3) cm, which are accompanied by additional internal abnormalities.
The first case is about a female newborn diagnosed with AOS. AOS is a rare multiple organ disorder with an incidence rate estimated at 1 of 225 000 live births [5]. The typical clinical manifestation of AOS is ACC associated with limb defect. Other extra anomalies include cutis marmorata, telangiectasia congenita and additional malformations in the internal organs [3, 6]. Many genetic mutations predispose to AOS, such as mutations in EOGT, DOCK6 DLL4, ARHGAP31, RBPJ and NOTCH1 [3]. Limb defects are commonly bilateral and asymmetrical, with lower extremities affected more than upper extremities. A Brachydactyly is the most common limb defect [6]. The scar tissue on her abdomen probably represents an ACC lesion, which formed and scarred intrauterine [3].
The second case is about a female newborn diagnosed with ACC associated with intestinal obstruction. Physicians highly suspected intestinal obstruction in a neonate who failed to pass the meconium within the first 24 h. It has many probable causes such as atresia, stenosis, malrotation and others [7]. The association between intestinal obstruction and ACC may be caused by a mutation in ITGP4 [8].
The third case is about a female diagnosed with ACC associated with many congenital heart defects. The appearance of her aplasia cutis lesion was as an alopecic scar, and this is due to its formation early in the gestation so it can heal before the delivery [3]. The incidence rate of congenital heart disease in neonates with heart murmurs ranges from 22 to 86% [9], so echo in neonates with murmurs is very useful for the early diagnosis and treatment. In most cases, congenital heart diseases are isolated, but they may be associated with extracardiac anomalies [9]. The combination of ACC with CHDs may suggest the diagnosis of AOS even though the absence of skeletal malformations [10].
It is evident from this case series that ACC of 4 cm or greater is significantly associated with other underlying congenital defects. Thus, for large lesions of ACC, it is strongly suggested to investigate for other congenital malformations.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
FUNDING
No funding was obtained for this study.
ETHICAL APPROVAL
No approval was required.
PATIENT CONSENT
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
ACKNOWLEDGEMENTS
Not applicable.
Contributor Information
Ihsan Baroudi, Faculty of medicine, University of Hama, Hama, Syria.
Ola Alakhras, Faculty of medicine, University of Hama, Hama, Syria.
Thaer Douri, Department of Dermatology, Al Assad Medical Center, Hama, Syria.
Nedal Alkhani, Department of Pediatrics, Al Assad Medical Center, Hama, Syria.
REFERENCES
- 1. Alexandros B, Dimitrios G, Elias A, Evangelos D, Andreas M, Sotirios P et al. Aplasia cutis congenita: two case reports and discussion of the literature. Surg Neurol Int 2017;8:273 doi: 10.4103/sni.sni_188_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brzezinski P, Pinteala T, Chiriac AE, Foia L, Chiriac A. Aplasia cutis congenita of the scalp- what are the steps to be followed? Case report and review of the literature. An Bras Dermatol 2015;90:100–3 doi: 10.1590/abd1806-4841.20153078. PMCID: PMC4323704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drolet B, Prendiville J, Golden J, Enjolras O, Esterly NB. Membranous aplasia cutis with hair collars. Congenital absence of skin or neuroectodermal defect? Arch Dermatol. 1995;131:1427–31. doi: 10.1001/archderm.1995.01690240091015 [DOI] [PubMed] [Google Scholar]
- 4. Brackenrich J, Brown A. Aplasia Cutis Congenita. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2021, PMID: 30571024 Bookshelf ID: NBK535403. [PubMed] [Google Scholar]
- 5. Saeidi M, Ehsanipoor F. A case of Adams-Oliver syndrome. Adv Biomed Res 2017;6:167 doi: 10.4103/2277-9175.221861. PMID: 29387678 Free PMC article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bakry O, Attia A, El Shafey EN. Adams-Oliver syndrome. A case with isolated aplasia cutis congenita and skeletal defects. J Dermatol Case Rep 2012;6:25–8 doi: 10.3315/jdcr.2012.1092. PMID: 22514587 Free PMC article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hajivassiliou CA. Intestinal obstruction in neonatal/pediatric surgery. Semin Pediatr Surg 2003;12:241–53 doi: 10.1053/j.sempedsurg.2003.08.005. PMID: 14655163 Review. [DOI] [PubMed] [Google Scholar]
- 8. Matyas M, Miclea D. Gabriela Zaharie case report: uncommon association of ITGB4 and KRT10 gene mutation in a case of epidermolysis bullosa with pyloric atresia and. Aplasia Cutis Congenita Front Genet 2021;12:641977. 10.3389/fgene.2021.641977 PMCID: PMC8296908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karatza AA, Panagiotopoulou O, Gkentzi D, Dimitriou G. Congenital heart disease in asymptomatic neonates with extra-cardiac malformations and genetic disorders. Balkan Med J 2019;36:366 doi: 10.4274/balkanmedj.galenos.2019.2019.9.104. PMID: 31597411 Free PMC article. No abstract available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Digilio MC, Marino B, Dallapiccola B. Autosomal dominant inheritance of aplasia cutis congenita and congenital heart defect: a possible link to the Adams-Oliver syndrome. Am J Med Genet A 15 October 2008;146A:2842–4 doi: 10.1002/ajmg.a.32526PMID: 1892417. [DOI] [PubMed] [Google Scholar]



