Abstract
Macrophage phagocytosis plays an important role hematoma clearance in intracerebral hemorrhage (ICH). This study examined the characteristics of multinucleated giant cells (MGCs), a group of macrophages with multiple nuclei, in a mouse ICH model. Whether MGCs could be increased by treatment with a CD47 blocking antibody and decreased by treatment with clodronate liposomes were also examined.
ICH was induced via autologous blood injection. Male adult C57BL/6 mice in different groups had: 1) ICH alone; 2) ICH with anti-CD47 blocking antibody or control IgG; and 3) ICH with anti-CD47 antibody combined with clodronate liposomes or control liposomes. The effect of anti-CD47 antibody on MGC formation was also tested in females. Brains were harvested at days 3 or 7 for brain histology. Many MGCs were found at day 3 post-ICH, but were reduced at day 7. MGCs phagocytosed many red blood cells and were heme oxygenase-1, ferritin, YM-1 and iNOS positive. CD47 blocking antibody injection increased MGC numbers in the perihematomal zone and in the hematoma in both sexes. Co-injection of clodronate liposomes depleted MGCs in both the hematoma core and the peri-hematomal area.
In conclusion, MGCs represent a macrophage/microglia subtype with strong phagocytosis capacity. MGCs exhibited not only an M2 but also an M1 phenotype and appeared involved in hemoglobin degradation. Anti-CD47 antibody boosted the number of MGCs, which may contribute to enhanced hematoma clearance. Understanding the exact roles of MGCs in ICH may reveal novel targets for ICH treatment.
Keywords: intracerebral hemorrhage, multinucleated giant cells, CD 47, clodronate liposomes
Introduction
Intracerebral hemorrhage (ICH) is a devastating form of stroke with great morbidity and mortality and no current effective therapy1. After an ICH, the hematoma itself causes mechanical disruption and increased intracranial pressure (mass effect), but blood components also cause secondary injury2, 3. Prior research has indicated that release of hemoglobin degradation products, such as heme and iron, after hemolysis of the hematoma contributes greatly to secondary injury4. While hematoma evacuation might be a method of reducing mass effects and hematoma-related neurotoxicity5, 6, surgical hematoma has so far failed to provide definitive improvements in patients7. There has, therefore, been interest in alternative methods of enhancing hematoma clearance. Erythrocyte phagocytosis is another way of hematoma clearance and it may protect neurons from toxic hemoglobin and other blood cell components. Phagocytosis by macrophages and activation of resident microglia in the brain helps in hematoma clearance and brain recovery8. One method to enhance hematoma clearance is to block CD47, a ‘do-not-eat-me’ signal normally expressed on erythrocytes. A CD47 blocking antibody enhanced hematoma clearance and improved functional outcome in a mouse ICH model9.
Circulating macrophages and resident microglia exhibit various morphologies and functions in central nervous system pathologies. One special macrophage morphology is the multinucleated giant cell (MGC). MGCs were first described in 1868 and were seen in many pathological processes including sarcoidosis, arthritis and neoplasia10, 11. Very recently MGCs have been found in the brain of patients after ICH12. Since macrophage recruitment and microglia activation can be either hazardous or neuroprotective13–16, understanding the microglia/macrophage morphology traits after ICH may unveil concepts on the pathophysiological role of these phagocytes and potential therapeutic interventions.
The current study had three aims. 1) To examine if MGCs are present in the mouse brain after ICH and elucidate their characteristics. 2) To determine if blocking CD47 enhances the formation of MGCs. 3) To determine if the number of MGCs can be depleted by clodronate liposome treatment.
Materials and Methods
Animals and ICH
The protocols used in this study were approved by the University of Michigan Committee on Use and Care of Animals. The study complies with the ARRIVE guidelines for reporting in vivo experiments. Young C57BL/6 mice (The Jackson Laboratory), aged 2–4 months, were given water and food ab libitum. A total of 36 male and 12 female mice were used in this study. The ICH modeling was as previously described 9. In brief, mice were anesthetized using ketamine (80 mg/kg, i.p.) and xylazine (5mg/kg, i.p.) and body temperature maintained at 37°C with a heating pad. After autologous blood was acquired from the right femoral artery, the mice were placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA). A burr hole was made and autologous blood (30µl) injected at a rate of 3µl/min using a 26-gauge needle at the coordinates: 0.2 mm anterior, 2.5 mm lateral and 3.5 mm ventral to the bregma. The needle was removed 10 minutes after injection to prevent reflux. The burr hole was then sealed with bone wax and the skin was sutured closed. Randomization was carried out using odd/even numbers.
Experimental groups
The experiment included 3 parts. In the first part, male mice had an ICH and they were euthanized at day 3 (n=6) and 7 (n=6). In the second part, mice were injected in the right basal ganglia with 30µl blood mixed with either anti-CD47 antibody (Invitrogen, 16–0479-85, 10µg/ml in blood, n=6 in male, n=6 in female) or IgG (Invitrogen, 16–4714-852, 10µg/ml in blood, n=6 in male, n=6 in female). Mice were euthanized at day 7 after ICH ictus. In the third part, male mice had an injection of anti-CD47 antibody (10µg/ml) in 30µl blood with either 5µl clodronate liposomes (to deplete macrophages/microglia; n=6) or 5µl control liposomes (n=6) and were euthanized at day 7. In all parts, brains were used for histological analysis.
Brain Histology and Hematoxylin and Eosin (H&E) Staining
Mice were anesthetized with pentobarbital (100 mg/kg) before undergoing transcardiac perfusion with 4% paraformaldehyde. After decapitation, brains were further fixed by immersion in paraformaldehyde, dehydrated with 30% glucose, and sectioned (18μm) coronally on a cryostat. Hematoxylin and eosin staining was used to confirm the morphology of interested cells, as described previously17.
Immunohistochemistry
Immunohistochemistry was conducted using the avidin-biotin technique as described previously17, 18. The primary antibodies were polyclonal rat anti-macrophages/ monocytes (MOMA-2, Abcam, ab33451, 1:200), polyclonal rabbit anti-inducible nitric oxide synthase (iNOS, Abcam, ab15323, 1:200), polyclonal rabbit anti-YM-1 (Abcam, ab93034, 1:200), monoclonal rabbit anti-ferritin (Abcam, ab75973, 1:200), and polyclonal rabbit anti-heme oxygenase-1 (HO-1, Enzo, SPA-895-F, 1:500). Negative controls omitted the primary antibody. In this study, we also use hematoxylin counterstaining for the nucleus detection. Briefly, the sections used for immunohistochemistry was immersed into hematoxylin for 10 seconds right after the 3,3’-diaminobenzidine (DAB) incubation. An oil immersion lens was adopted for morphological observations.
Immunofluorescence
HO-1 (Enzo, SPA-895-F, 1:500) and donkey anti-rabbit IgG (H + L) Alexa Fluor 488 (1:500; Invitrogen) were the primary and secondary antibodies for immunofluorescent labeling. Sections were blocked with 15% donkey serum for 30 minutes and then incubated at 4°C overnight with the primary antibody. They were then incubated with secondary antibody for 2 hours. Nucleus staining was performed with 4’, 6-diamidino-2-phenylindole (DAPI) before mounting.
Cell counting
Cell counting was performed in a blinded manner. Three images at different areas either in the hematoma or perihematoma were obtained at 40x magnification in each brain section at the bregma level. All the counting was repeated three times and the mean values calculated.
Statistical Analysis
All data are presented as means ± standard deviation (SD). Statistical differences among groups were analyzed using Student’s t test. Statistical differences were considered significant if P<0.05.
Results:
1. MGC morphology after intracerebral hemorrhage
In the mouse ICH model (male mice), H&E staining showed erythrocytes were phagocytosed by macrophage/microglia-like cells at the peripheral regions of the hematoma. The diameter of those phagocytic cells could reach more than 20µm. High magnification images showed engulfed red blood cells in MGCs (Figure 1A). Immunohistochemistry staining using the macrophage/microglia marker Iba-1 and hematoxylin showed Iba-1 positive cells with multiple nuclei (Figure 1B).
Figure 1.

Multinucleated giant cell (MGC) morphology after intracerebral hemorrhage. (A) H&E staining of male mice in the ipsilateral and contralateral basal ganglia at day 3 and day 7 after ICH. (B) Immunohistochemistry staining using Iba-1 and hematoxylin staining in the ipsilateral and contralateral basal ganglia 3 days after ICH. The high magnification images showed morphology of an Iba-1 positive cell with multiple nuclei. Black scale bar = 20 µm, white scale bar = 10 µm.
2. Changes MOMA-2 labeled MGCs after ICH
Iba-1 was used to stain macrophages/microglia in ICH brain. Iba-1 positive cells exhibited either a round shape with a larger cell body or a ramified shape with a smaller cell body, indicative of active and inactive phagocytes, respectively. To better observe activated macrophages/ microglia, MOMA-2 immunohistochemistry was used with hematoxylin counterstaining for MGCs. MOMA-2 positive cells at day 3 and day 7 post-ICH were round shape with large cell body with some MOMA-2 positive cells having multi-nuclei. MGCs were seen at both day 3 and day 7 but decreased at day 7 (25 ± 5% at day 3 vs. 11 ± 3% of total MOMA-2 positive cells at day 7, P<0.001; Figure 2). Soma sizes of MGCs were larger at day 3 than those at day 7 (262 ± 26 µm2 at day 3 vs. 175 ± 26 µm2 at day 7, P<0.001; Figure 2).
Figure 2.
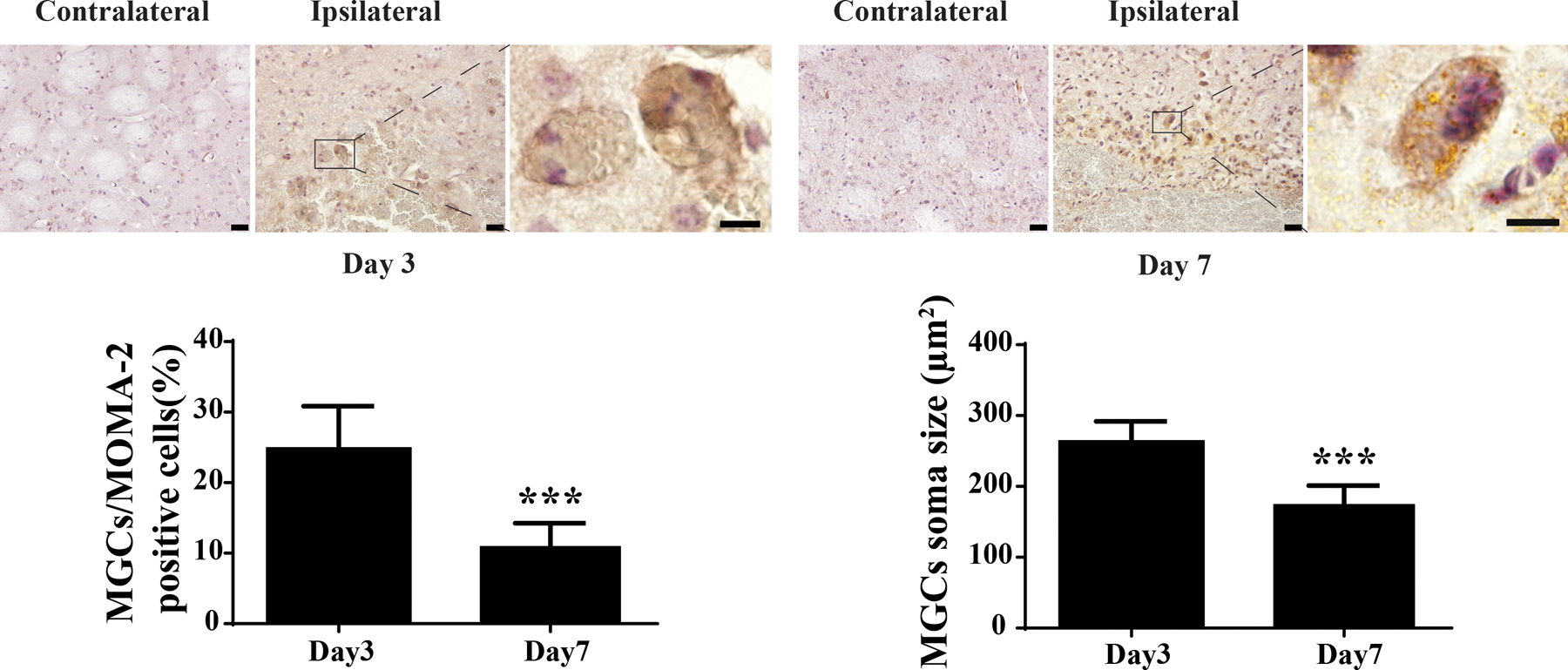
MOMA-2 positive MGCs in the ipsilateral but not in the contralateral basal ganglia after ICH. Examples of cells positive for MOMA-2 in male mice at day 3 and day 7 after ICH. The high magnification images showed morphology of MOMA-2 positive cells with multiple nuclei. Asterisk indicates the hematoma. Scale bar: low magnification images = 20µm, high magnification images = 10µm. MGCs as a percentage of all MOMA-2 cells was calculated at both time points, as was the average soma size of the MGCs. Values are means ± SD, n=6 for each group; ***P<0.001 vs. the other group.
3. MGC phenotypes after ICH
Various markers were used to test the phenotype of the MGCs in the basal ganglia at days 3 and 7 after ICH. MGCs were labeled by hemoglobin metabolism related markers, HO-1 and ferritin. Whether MGCs were positive for markers of macrophage polarization was also examined. MGCs at days 3 and 7 following ICH were labeled by the M1 marker iNOS and the M2 marker YM-1 (Figure 3).
Figure 3.
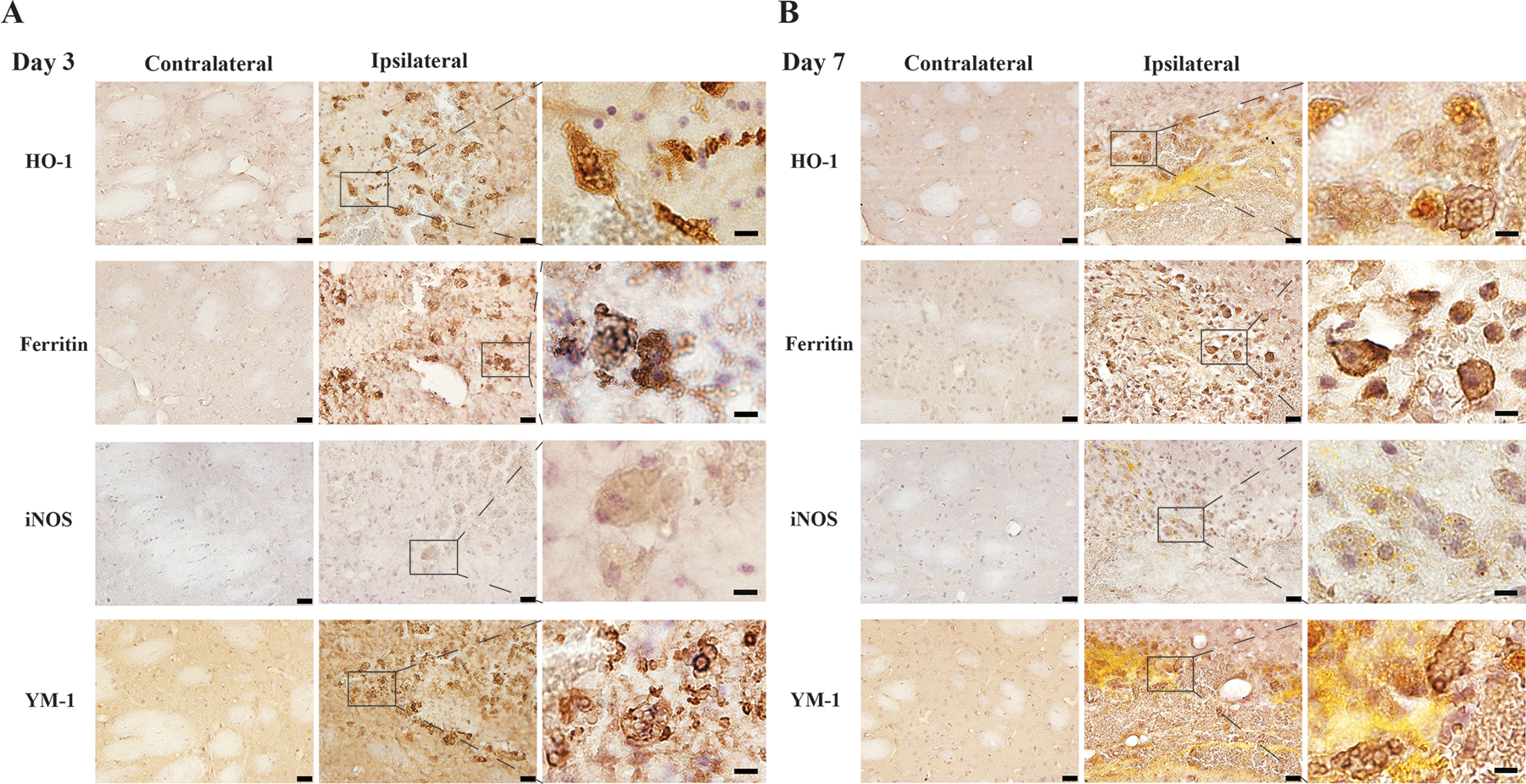
Immunohistochemistry staining using HO-1, ferritin iNOS and YM-1 counterstained with hematoxylin in the ipsilateral and contralateral basal ganglia in male mice 3 days (A) and 7 days (B) after ICH. The high magnification images showed morphology of positive cells with multiple nuclei. Scale bars: low magnification images =20 µm, high magnification images =10 µm.
4. HO-1 labeled MGCs following ICH co-injected with CD47 blocking antibody in male and female mice
CD47 is a suppressor of erythrocyte phagocytosis (a ‘don’t eat me’ signal) 9. An anti-CD47 antibody/blood co-injection model was used to examine whether blocking CD47 would affect MGC formation in male and female mice. HO-1 immunofluorescence staining was performed 7 days after ICH. In male mice, some HO-1 positive cells were MGCs (with two or more nuclei labeled by DAPI) and there were more HO-1 positive MGCs in the anti-CD47 antibody-treated group in both the hematoma (13 ± 9 vs. 2 ± 5 /mm2 in IgG treated group, P<0.05) and in the peri-hematomal area (75 ± 19 vs. 21 ± 15/mm2 in IgG treated group, P<0.001, Figure 4). Similarly, in female mice, there were more HO-1 positive MGCs in the anti-CD47 antibody treated group in both the hematoma (20 ± 14/mm2 vs. none in IgG treated group, P<0.05) and the peri-hematomal area (63 ± 5 vs. 21 ± 17/mm2 in IgG treated group, P<0.001, Figure 4).
Figure 4.
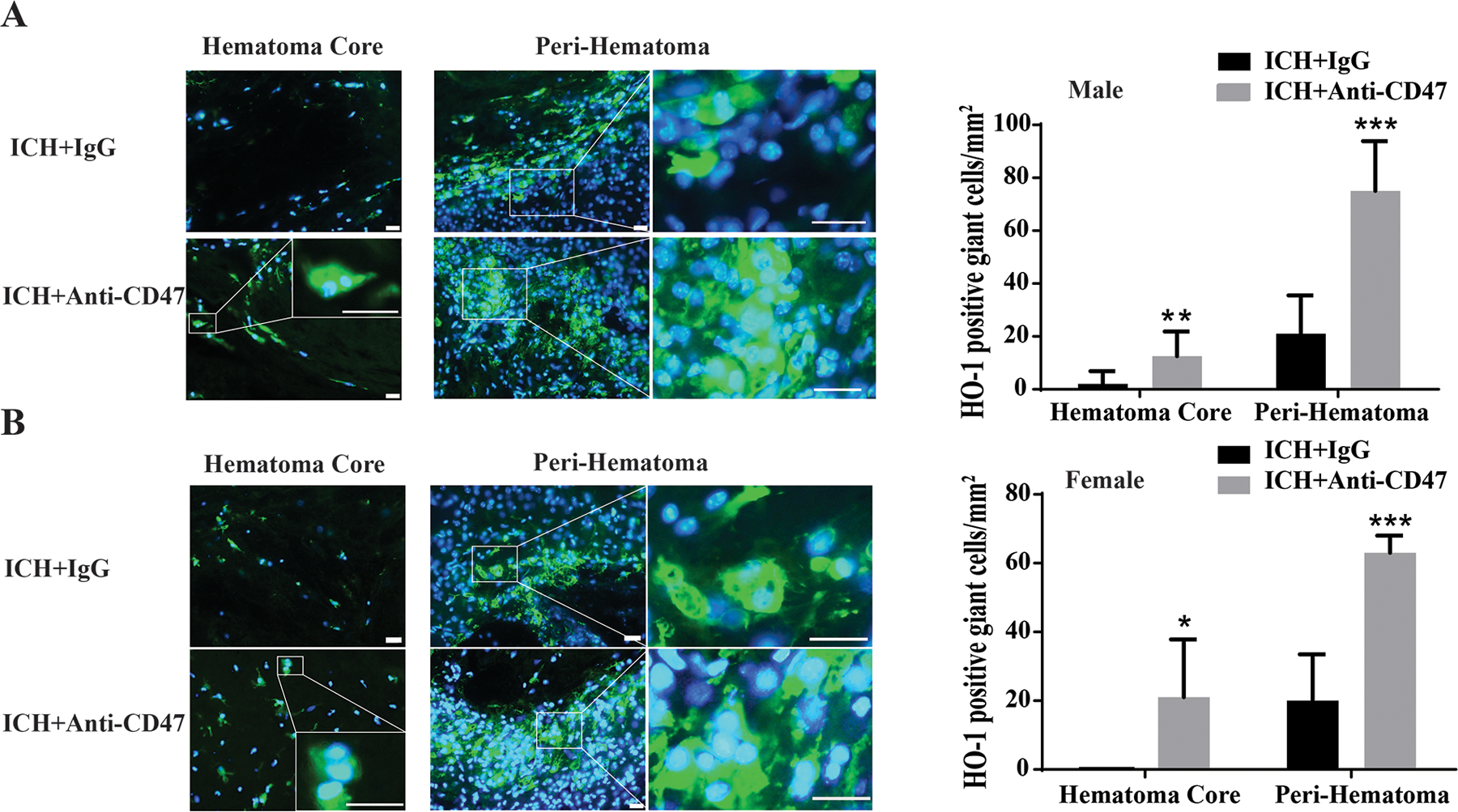
Examples of immunofluorescence staining using HO-1 and DAPI in (A) male and (B) mice 7 days after an ICH with a co-injection of either anti-CD47 antibody or control IgG. The high magnification images showed morphology of HO-1 positive cells with multiple nuclei in the hematoma and the peri-hematomal area. Scale bar = 20µm. The number of HO-1 giant cells was quantified in male and female mice with anti-CD47 antibody treatment or control IgG. Values are means ± SD, n=6 for each group; *P<0.05, **P<0.01, ***P<0.001 vs. ICH + IgG group.
5. Clodronate liposomes reduced the number of HO-1 positive MGCs
Clodronate liposomes have been used to deplete microglia/macrophages in the brain 9. A co-injection model in male mice was used to test whether they would also deplete MGCs. HO-1 immunofluorescence staining was performed 7 days after ICH where animals where blood was co-injected with CD47 blocking antibody (to enhance MGC formation) and either clodronate or control liposomes. Clodronate liposomes co-administration eliminated HO-1 positive MGCs from the hematoma core (Figure 5) and drastically decreased their number in the peri-hematomal area (3 ± 5 vs. 41 ± 15/mm2 in control liposome group, P<0.001; Figure 5).
Figure 5.
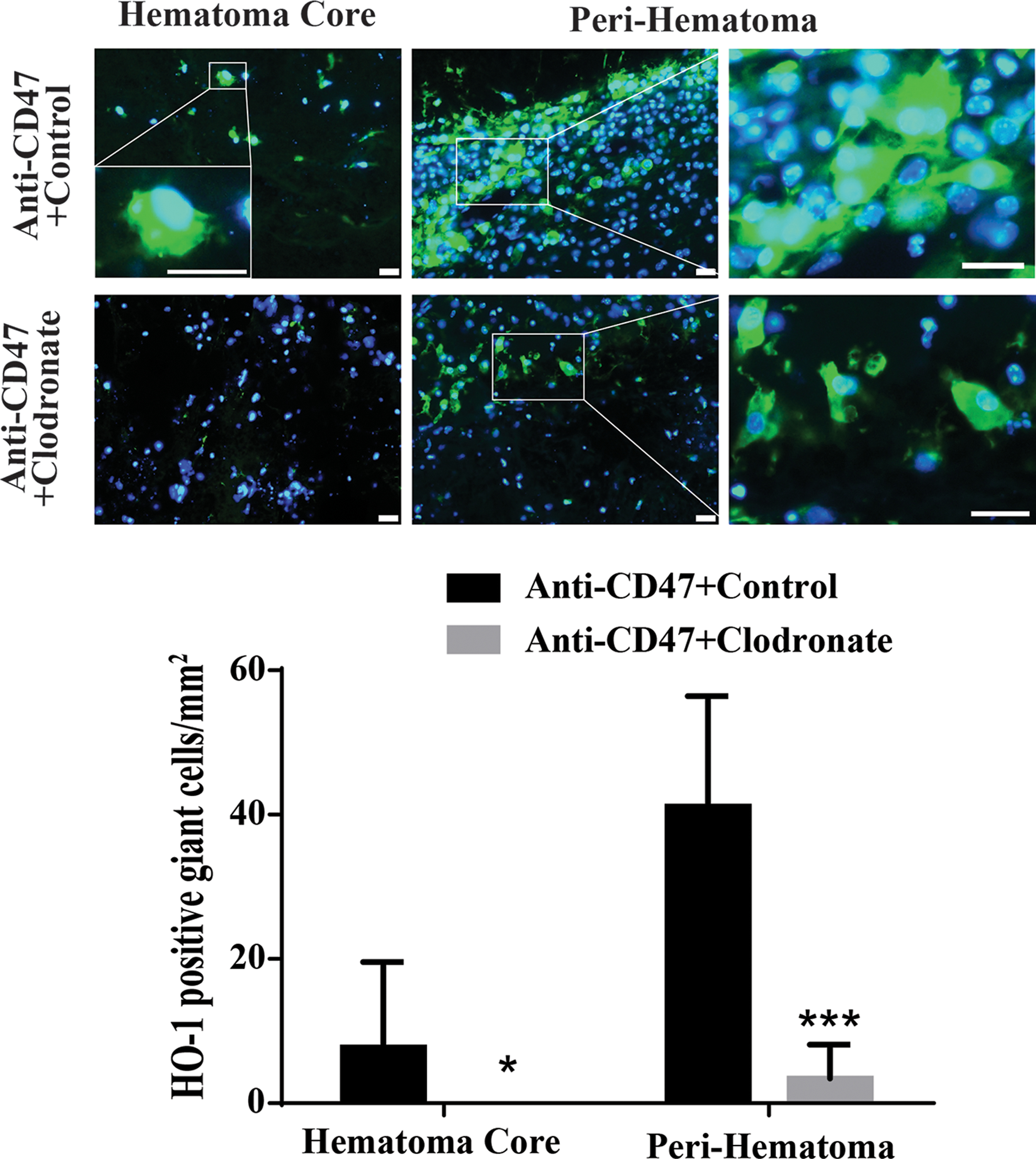
Examples of immunofluorescence staining using HO-1 and DAPI in male mice 7 days after an ICH with a co-injection CD47 antibody (to enhance MGC formation) and either clodronate or control liposomes. The high magnification images showed morphology of positive cells with multiple nuclei in hematoma core and peri-hematoma area. Scale bar = 20µm. The number of HO-1 positive MGCs was determined in both the hematoma core and the peri-hematomal zone. Values are means ± SD, n=6 for each group; *P<0.05, ***P<0.001 vs. Anti-CD47+control group.
Discussion
This study found that: 1) in a mouse ICH model, many MGCs were found at day 3 and that this number decreased at day 7; 2) MGCs phagocytosed red blood cells, were immune-positive for iron handling proteins and exhibited a mixed M1 and M2 phenotype; 3) a CD47 blocking antibody increased the number of MGCs in both male and female mice; and 4) Clodronate liposomes significantly depleted MGCs in both the hematoma and the peri-hematomal area.
The formation of MGCs may enhance hematoma clearance. MGCs can be found in many nervous system diseases such as viral encephalitis, HIV associated dementia and amyotrophic lateral sclerosis 19, 20, and are thought to represent macrophages differentiation termination21. The exact roles of MGCs are still unknown. Some research has shown that MGCs may help remodel the extracellular matrix and clear pathogens and apoptotic debris22. However, others think that MGCs may be dysfunctional thus contribute to pathological processes10, 23. The mechanisms of MGCs formation are still unclear, they may be due to macrophage fusion21, phagocytosis24 or cyto-dyskinesis 25. A recent study found MGCs around the hematoma 5 days after ICH in humans, indicating a potential role of these giant cells in ICH12. In the current study, MGCs were found in the mouse brain after ICH particularly within the perihematoma zone but also within the hematoma. In order to better estimate what portion of activated macrophage/microglia were MGCs, MOMA-2 was used to identify macrophage/microglia around hematoma at different time point post-ICH. Results showed that roughly one fifth of the MOMA-2 positive cells were MGCs at day 3, but this decreased to one tenth at day 7. Day 7 was chosen for the follow-up experiment because our previous study found that the hematoma was not totally removed in this mouse ICH model. 9 Like studies in different models26, 27, the MGCs in ICH mice exhibited an amoeboid-like morphology. They phagocytosed more red blood cells than their mononucleate peers suggesting MGCs may be more capable scavengers than regular macrophages/microglia.
To examine MGC function, immunostaining was performed. HO-1 is an enzyme involving heme degradation and ferritin is a protein involving iron storage. MGCs were HO-1 and ferritin positive, suggesting that the MGCs found in mice after ICH brain were involved in hemoglobin degradation. The expression of macrophage/microglia polarization markers was also examined. Unlike CD16 and CD206 that only stained smaller cells (data not shown), MGCs were immunopositive for iNOS, a M1 marker, and YM-1, a M2 marker. Studies suggested that activated microglia and macrophage can be polarized into two types: M1 (classic) and M2 (alternative). M1 type macrophage/microglia is thought to be proinflammatory and are contributes to cell loss and other pathologies. M2 type is considered as anti-inflammatory phenotype that participates in tissue repair, function recovery and phagocytosis28. Different from these studies, our data suggests that the phagocytic MGCs exhibit not only an M2 but also a M1 phenotype,
CD47 is a widely expressed “don’t eat me” signal found on erythrocytes. Previously, the CD47 blocking antibody employed here was shown to enhance hematoma resolution and reduce brain injury and neurological deficits in mice9. Interaction between CD47 on the target cell and SIRPα on the phagocyte is a key of inhibitor of phagocytosis (e.g. for preventing erythrocyte phagocytosis)29. The CD47 blocking antibody can prevent this inhibition hence facilitating macrophage phagocytosis. Research using cultured macrophages showed that CD47 combined with integrin-associated protein could interact with macrophage fusion receptor preventing macrophage multinucleation30. In line with those results, the present study using HO-1 and DAPI double staining showed increased MGCs in the hematoma and perihematomal area with the CD47 blocking antibody. The enhanced phagocytosis via CD47-blockade we previously described may be partially due to these MGCs changes9. The effects of CD47 on MGCs were similar in male and female mice, an important parameter in therapy development.
In addition, clodronate is found an efficient scavenger of macrophage in several disease models31, 32, and our previous finding also showed the similar effects in mice ICH model9. In the present study, clodronate drastically reduced the number of MGCs both within the hematoma and in the peri-hematoma zone. Our previous study found that clodronate liposomes inhibit hematoma clearance and worsen brain damage in this ICH model9. Taken all together, formation of MGCs may be beneficial for brain recovery after ICH although clodronate liposomes may also affect other non-MGC macrophage/microglial phenotypes.
There are limitations of our study. 1) Though MOMA-2 is a better marker than Iba-1 for activated phagocytosis cells, it still cannot distinguish active monocyte/macrophages from activated microglia. 2) This is a proof-of-concept study. The CD47 blocking antibody was co-injected with blood. In future studies, it is important to develop a systemic method that can enhance MGC formation and potentially speed hematoma clearance. 3) The ultimate fate of the MGCs after ICH is still unknown. 4) Using multiple nuclei as the sole method to identify MGCs might include overlapped cells since, currently, there are no specific MGC markers.
In conclusion, our study revealed the formation of many MGCs after ICH in mice. Further studies are needed for better understanding of the exact roles of MGCs in ICH and whether it may be a therapeutic target.
Funding:
YH, RFK and GX are supported by grants NS-091545, NS-090925, NS-096917, NS-106746 and NS-112394 from the National Institutes of Health (NIH).
Footnotes
Compliance with Ethical Standards
Conflict of interest: Jialiang Wei, Ming Wang, Chaohui Jing, Richard F. Keep, Ya Hua and Guohua Xi declare that they have no conflict of interest.
Ethical approval: All institutional and national guidelines for the care and use of laboratory animals were followed.
References:
- 1.Hankey GJ. Stroke. Lancet 2017;389:641–654 [DOI] [PubMed] [Google Scholar]
- 2.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral hemorrhage. Lancet Neurol 2006;5:53–63 [DOI] [PubMed] [Google Scholar]
- 3.Keep RF, Andjelkovic AV, Xiang J, Stamatovic SM, Antonetti DA, Hua Y, et al. Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J Cereb Blood Flow Metab 2018;38:1255–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurology 2012;11:720–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson DA, Keep RF, Hua Y, Xi G. Hematoma clearance as a therapeutic target in intracerebral hemorrhage: From macro to micro. J Cereb Blood Flow Metab 2018;38:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu R, Li H, Hua Y, Keep RF, Xiao J, Xi G, et al. Early hemolysis within human intracerebral hematomas: An mri study. Transl Stroke Res 2019;10:52–56 [DOI] [PubMed] [Google Scholar]
- 7.Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (mistie iii): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet 2019;393:1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CF, Goods BA, Askenase MH, Hammond MD, Renfroe SC, Steinschneider AF, et al. Erythrocyte efferocytosis modulates macrophages towards recovery after intracerebral hemorrhage. J Clin Invest 2018;128:607–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jing C, Bian L, Wang M, Keep RF, Xi G, Hua Y. Enhancement of hematoma clearance with cd47 blocking antibody in experimental intracerebral hemorrhage. Stroke 2019;50:1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn MT, Schepetkin IA. Role of nadph oxidase in formation and function of multinucleated giant cells. J Innate Immun 2009;1:509–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers TJ, Spector WG. Inflammatory giant cells. Immunobiology 1982;161:283–289 [DOI] [PubMed] [Google Scholar]
- 12.Shtaya A, Bridges LR, Esiri MM, Lam-Wong J, Nicoll JAR, Boche D, et al. Rapid neuroinflammatory changes in human acute intracerebral hemorrhage. Ann Clin Transl Neurol 2019;6:1465–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: Therapeutic targets. Neurotherapeutics 2010;7:378–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat Rev Neurosci 2007;8:57–69 [DOI] [PubMed] [Google Scholar]
- 15.Nakajima K, Yamamoto S, Kohsaka S, Kurihara T. Neuronal stimulation leading to upregulation of glutamate transporter-1 (glt-1) in rat microglia in vitro. Neuroscience Letters 2008;436:331–334 [DOI] [PubMed] [Google Scholar]
- 16.Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia 2009;57:835–849 [DOI] [PubMed] [Google Scholar]
- 17.Ni W, Mao S, Xi G, Keep RF, Hua Y. Role of erythrocyte cd47 in intracerebral hematoma clearance. Stroke 2016;47:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Hua Y, Keep RF, Xi G. Brain ceruloplasmin expression after experimental intracerebral hemorrhage and protection against iron-induced brain injury. Transl Stroke Res 2019;10:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghorpade A, Persidsky Y, Swindells S, Borgmann K, Persidsky R, Holter S, et al. Neuroinflammatory responses from microglia recovered from hiv-1-infected and seronegative subjects. J Neuroimmunol 2005;163:145–156 [DOI] [PubMed] [Google Scholar]
- 20.Fendrick SE, Xue QS, Streit WJ. Formation of multinucleated giant cells and microglial degeneration in rats expressing a mutant cu/zn superoxide dismutase gene. Journal of neuroinflammation 2007;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vignery A Macrophage fusion: Molecular mechanisms. Methods Mol Biol 2008;475:149–161 [DOI] [PubMed] [Google Scholar]
- 22.Ruibal-Ares B, Riera NE, de Bracco MM. Macrophages, multinucleated giant cells, and apoptosis in hiv+ patients and normal blood donors. Clin Immunol Immunopathol 1997;82:102–116 [DOI] [PubMed] [Google Scholar]
- 23.McNally AK, Anderson JM. Macrophage fusion and multinucleated giant cells of inflammation. Adv Exp Med Biol 2011;713:97–111 [DOI] [PubMed] [Google Scholar]
- 24.McNally AK, Anderson JM. Multinucleated giant cell formation exhibits features of phagocytosis with participation of the endoplasmic reticulum. Exp Mol Pathol 2005;79:126–135 [DOI] [PubMed] [Google Scholar]
- 25.Normand G, King RW. Understanding cytokinesis failure. Adv Exp Med Biol 2010;676:27–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachstetter AD, Van Eldik LJ, Schmitt FA, Neltner JH, Ighodaro ET, Webster SJ, et al. Disease-related microglia heterogeneity in the hippocampus of alzheimer’s disease, dementia with lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Commun 2015;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada J, Jinno S. Novel objective classification of reactive microglia following hypoglossal axotomy using hierarchical cluster analysis. J Comp Neurol 2013;521:1184–1201 [DOI] [PubMed] [Google Scholar]
- 28.Zhao H, Garton T, Keep RF, Hua Y, Xi G. Microglia/macrophage polarization after experimental intracerebral hemorrhage. Transl Stroke Res 2015;6:407–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R. Cd47 functions as a molecular switch for erythrocyte phagocytosis. Blood 2012;119:5512–5521 [DOI] [PubMed] [Google Scholar]
- 30.Han X, Sterling H, Chen Y, Saginario C, Brown EJ, Frazier WA, et al. Cd47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. The Journal of biological chemistry 2000;275:37984–37992 [DOI] [PubMed] [Google Scholar]
- 31.Griesmann H, Drexel C, Milosevic N, Sipos B, Rosendahl J, Gress TM, et al. Pharmacological macrophage inhibition decreases metastasis formation in a genetic model of pancreatic cancer. Gut 2017;66:1278–1285 [DOI] [PubMed] [Google Scholar]
- 32.Danenberg HD, Fishbein I, Gao J, Monkkonen J, Reich R, Gati I, et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation 2002;106:599–605 [DOI] [PubMed] [Google Scholar]


