Key Points
Question
What is the association between risk of dementia and ischemic stroke, and does it differ by stroke severity and recurrence?
Findings
In this cohort study, among 1378 ischemic strokes and 2860 incident dementia cases occurring in a cohort of 15 379 adults, risk of dementia by adjusted hazard ratio was 1.76 (95% CI, 1.49-2.00) for 1 minor to mild stroke, 3.47 (95% CI, 2.23-5.40) for 1 moderate to severe stroke, 3.48 (95% CI, 2.54-4.76) for 2 or more minor to mild strokes, and 6.68 (95% CI, 3.77-11.83) for 2 or more moderate to severe strokes.
Meaning
Risk of dementia increased after ischemic stroke, independent of vascular risk factors, and results suggest a dose-response association and emphasize the importance of primary and secondary stroke prevention.
This cohort study evaluates the association between risk of dementia and incident ischemic stroke by stroke severity and recurrence.
Abstract
Importance
Ischemic stroke is associated with increased risk of dementia, but the association of stroke severity and recurrence with risk of impaired cognition is not well known.
Objective
To examine the risk of dementia after incident ischemic stroke and assess how it differed by stroke severity and recurrence.
Design, Setting, and Participants
The Atherosclerosis Risk in Communities (ARIC) study is an ongoing prospective cohort of 15 792 community-dwelling individuals from 4 US states (Mississippi, Maryland, Minnesota, and North Carolina). Among them, 15 379 participants free of stroke and dementia at baseline (1987 to 1989) were monitored through 2019. Data were analyzed from April to October 2021. Associations between dementia and time-varying ischemic stroke incidence, frequency, and severity were studied across an average of 4.4 visits over a median follow-up of 25.5 years with Cox proportional hazards models adjusted for sociodemographic characteristics, apolipoprotein E, and vascular risk factors.
Exposures
Incident and recurrent ischemic strokes were classified by expert review of hospital records, with severity defined by the National Institutes of Health Stroke Scale (NIHSS; minor, ≤5; mild, 6-10; moderate, 11-15; and severe, ≥16).
Main Outcomes and Measures
Dementia cases adjudicated through expert review of in-person evaluations, informant interviews, telephone assessments, hospitalization codes, and death certificates. In participants with stroke, dementia events in the first year after stroke were not counted.
Results
At baseline, the mean (SD) age of participants was 54.1 (5.8) years, and 8485 of 15 379 participants (55.2%) were women. A total of 4110 participants (26.7%) were Black and 11 269 (73.3%) were White. A total of 1378 ischemic strokes (1155 incident) and 2860 dementia cases were diagnosed 1 year or more after incident stroke in participants with stroke, or at any point after baseline in participants without stroke, were identified through December 31, 2019. NIHSS scores were available for 1184 of 1378 ischemic strokes (85.9%). Risk of dementia increased with both the number and severity of strokes. Compared with no stroke, risk of dementia by adjusted hazard ratio was 1.76 (95% CI, 1.49-2.00) for 1 minor to mild stroke, 3.47 (95% CI, 2.23-5.40) for 1 moderate to severe stroke, 3.48 (95% CI, 2.54-4.76) for 2 or more minor to mild strokes, and 6.68 (95% CI, 3.77-11.83) for 2 or more moderate to severe strokes.
Conclusions and Relevance
In this study, risk of dementia significantly increased after ischemic stroke, independent of vascular risk factors. Results suggest a dose-response association of stroke severity and recurrence with risk of dementia.
Introduction
Risk of dementia increases after stroke1,2,3 and may be especially high in patients with larger and more severe strokes.3 Reliable estimates for the risk of dementia after stroke (especially ischemic stroke [IS], which is the most common type) are needed to inform clinicians, researchers, and policymakers.
Assessment of cognitive function in stroke survivors must consider several challenges. Timing of dementia onset must be considered, as early cognitive changes after stroke may be transient, and attrition is common in poststroke follow-up.4 Aphasia and neglect, frequent after stroke, could complicate cognitive evaluation; therefore, comprehensive cognitive assessment is recommended.5 Because risk factors for stroke are also risk factors for dementia,6 any prestroke cognitive dysfunction or dementia (present in 10% of hospitalized stroke patients7,8) must be distinguished from new poststroke impairments.
The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS)9 combines longitudinal data on vascular risk factors, cognitive performance, adjudicated stroke, and stroke severity with a thorough dementia surveillance process. Because cognitive status and vascular risk are measured at multiple time points, the effect of stroke on dementia can be evaluated while accounting for shared risk factors. We hypothesize that, compared with individuals without stroke and independent of vascular risk factors, risk of poststroke dementia is higher in patients with IS, more severe strokes, and multiple recurrent strokes.
Methods
Study Population
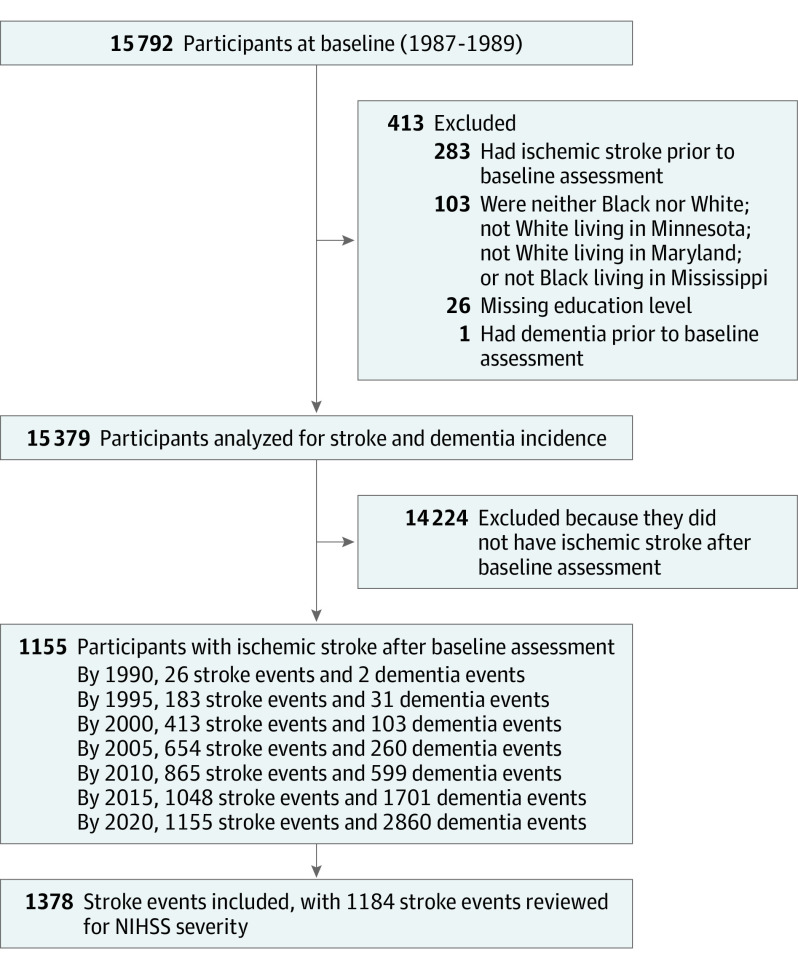
The ARIC study is an ongoing community-based prospective cohort study including 15 792 adults aged 45 to 64 years at baseline (visit 1, 1987-1989) with more than 30 years of follow-up. Participants were sampled from 4 US communities: Forsyth County, North Carolina; suburbs of Minneapolis, Minnesota; Washington County, Maryland; and Jackson, Mississippi10 and reexamined at 6 additional visits: 1990 to 1992 (ARIC visit 2), 1993 to 1995 (ARIC visit 3), and 1996 to 1997 (ARIC visit 4), 2011 to 2013 (ARIC-NCS visit 5), 2016 to 2017 (ARIC-NCS visit 6), and 2018 to 2019 (ARIC-NCS visit 7). Data were analyzed from April to October 2021. Self-reported race data were collected, and participants who were not Black or White, and those who were not White in Minneapolis and Washington County (n = 103), were excluded owing to small subsamples, as were 26 individuals with unknown educational level or with prevalent IS (n = 283) or dementia (n = 1) at ARIC baseline, resulting in the inclusion of 15 379 individuals (Figure 1). Race was classified according to participants’ self-reported race at ARIC baseline. It was evaluated owing to known disparities in some of the conditions that ARIC was designed to evaluate (eg, atherosclerosis-related disease). Ethnicity was not evaluated or reported. Race and center were combined and a race-center variable was generated because of the different distribution of race groups across the ARIC centers. Median (IQR) follow-up was 25.5 (18.0-29.9) years across a median (IQR) of 4 (3-6) visits. Written informed consent was obtained at each visit, with institutional review board approval at each ARIC center. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Figure 1. Flowchart of Participants Selected for Analysis.
Stroke Ascertainment
Stroke hospitalizations from 1987 to 2019 were identified using established methods11,12 from data abstracted from medical records.13 Following preliminary classification by a computer-generated algorithm,12 stroke events were verified and adjudicated by study physicians as ischemic or hemorrhagic, with disagreement with the algorithm triggering a second review. Definitions of stroke subtypes and standardized criteria for IS12 have remained consistent over study follow-up. Definite stroke events require autopsy or imaging confirmation; probable stroke events lack exclusionary findings on imaging and have an appropriate clinical presentation.
Ascertainment of Dementia
Cognitive function14 has been assessed in ARIC from 1990 to 1992 through in-person administration of a 3-test battery and from 2011 to 2013 in ARIC-NCS through an expanded 10-test battery and informant interviews. A computer algorithm generated preliminary diagnoses that were verified by expert review (eMethods in the Supplement).6,15
Among participants who did not return for an in-person follow-up evaluation, dementia was ascertained from telephone cognitive assessments, informant interviews, or hospitalization codes or death certificates (eMethods in the Supplement); for these individuals, dementia onset was defined as 180 days prior to the interview, hospitalization, or death. In participants with IS, incident dementia events in the first year after stroke were censored to exclude cases of short-term changes in cognitive function after stroke.
Stroke Severity
Trained physicians abstracted data on stroke severity from available stroke hospitalization records of definite or probable IS, using an algorithm valid for retrospective National Institutes of Health Stroke Scale (NIHSS) scoring.16,17 Blinded double abstraction of 15% of records was independently conducted by trained neurologists; total NIHSS correlation between reviewers was 0.90. Because of variability in the available data, stroke severity was classified into 4 levels: minor (NIHSS ≤5), mild (NIHSS 6-10), moderate (NIHSS 11-15), and severe (NIHSS ≥16). Additional details are presented in the eMethods in the Supplement.
Definition of Covariates
Statistical models included baseline and time-varying risk factors as covariates. At baseline (1987 to 1989), data were collected on age, sex, combined race and center, education, smoking, diabetes, systolic blood pressure, body mass index (calculated as weight in kilograms divided by height in meters squared), antihypertensive medication, and statin use (eMethods in the Supplement). At visit 2 (1990 to 1992), cognitive function was measured using delayed word recall,18 word fluency,19 and digit symbol substitution20 tests, used to compute a standardized global cognition score.14 Atrial fibrillation, diabetes, use of antihypertensives or statins, and smoking status were considered time-varying covariates and updated at ARIC visits and via self-report during annual (1987 to 2011) and semiannual (2012 to 2019) follow-up calls.
Statistical Analysis
Extended Kaplan-Meier curves of dementia by age were generated to examine time-varying incident IS.21 Kaplan-Meier curves were plotted for individuals without stroke and with stroke by 2 levels of severity (minor to mild [NIHSS ≤10] or moderate to severe [NIHSS ≥11]), as were comparable cumulative incidence curves accounting for competing risk of death. Poisson regression models with robust error variance were used to estimate dementia incidence rates (IRs) with 95% CIs per 100 person-years, adjusted for age, sex, and race and center. Separate models calculated the IRs associated with time-varying stroke incidence, frequency (1 or ≥2 strokes), severity (NIHSS ≤5, 6-10, 11-15, or ≥16), or a combination of frequency (1 or ≥2 strokes) and severity (minor to mild or moderate to severe). We applied the Mantel-Haenszel approach22 to each fully adjusted estimate of IRs to determine the proportion of dementia risk attributable to stroke by severity and number of stroke events.
Cause-specific Cox proportional hazards models23 separately examined the time-varying exposures tested in the Poisson regression models. An additional Cox model tested the association of dementia with each minor to mild or moderate to severe stroke. Follow-up time was measured from baseline to first diagnosis of dementia, censoring owing to death or administrative censoring on December 31, 2019, with ties handled using the Efron method. Supremum tests indicated that the proportional hazards assumption was met, but an inspection of Schoenfeld residuals revealed a nonsignificant trend over time. Consequently, in supplemental analyses, IS was stratified by age at incident stroke (younger than 75 years and 75 years and older1). Interactions between each time-varying exposure and age at stroke, race, sex, education, and apolipoprotein E (APOE) status were evaluated, with comparable subgroup analyses performed. Because the date of incident dementia was estimated for some participants, sensitivity analyses were conducted in which onset was estimated to occur 3 years prior to an informant interview, hospitalization, or death. Additional sensitivity analyses allowed for interval censoring by using discrete-time, cause-specific proportional hazards models with a complimentary log-log link and examined the competing risk of death by fitting Fine-Gray subdistribution hazard models.24
Three covariate-adjusted models were fit to the data. Model 1 adjusted for age, sex, and race and center. Model 2 added education, APOE status, global cognition,14 systolic blood pressure, and antihypertensive medications at baseline as time-invariant covariates. Model 3 treated age, sex, race and center, education, APOE status, global cognition, body mass index, systolic blood pressure, and total cholesterol at baseline as time-invariant, and integrated atrial fibrillation, cigarette use, diabetes, antihypertensive medications, and statin use as time-varying covariates. To address missingness (13.5% for IS severity) while accounting for the repeated measurement of time-varying covariates, multilevel multiple imputation by chained equations25 was performed in Mplus version 8.5 (Muthén & Muthén). Ten imputed data sets were generated (exceeding the 3 imputations suggested by a 2-stage analysis26), and analyzed in SAS version 9.4 (SAS Institute). All tests were 2-sided, and P values <.05 were considered significant.
Results
Participant Characteristics
At baseline, the mean (SD) age of participants was 54.1 (5.8) years, and 8485 of 15 379 participants (55.2%) were women. A total of 4110 participants (26.7%) were Black and 11 269 (73.3%) were White. Significant differences were observed between the stroke and nonstroke groups (Table 1; eTables 1-3 in the Supplement). During the study period, 1378 definite and probable IS events (1155 incident IS events) were identified. Data on NIHSS were missing for 194 events overall (14.1%), among them 156 incident events (13.5%). Severity was primarily mild (NIHSS ≤5, 744 [62.8%]; NIHSS 6-10, 26 [22.1%]; NIHSS 11-15, 94 [7.9%]; NIHSS ≥16, 84 [7.1%]). At the end of follow-up in 2019, 8544 of 15 379 participants (55.6%) were deceased, with 447 of 6835 living participants (6.5%) no longer participating in telephone or in-person assessments.
Table 1. Baseline Characteristics of the Study Population, the Atherosclerosis Risk in Communities (ARIC) Study Visit 1 (1987-1989)a.
| Characteristic | Participants with vs without ischemic stroke (N = 15 379) | Participants with ischemic stroke by severity of incident stroke (N = 999) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (N = 15 379) | No stroke (n = 14 224) | Stroke (n = 1155) | P value | All (N = 999) | NIHSS ≤5 (n = 647) | NIHSS 6-10 (n = 208) | NIHSS 11-15 (n = 73) | NIHSS ≥16 (n = 71) | P trend | |
| Age | ||||||||||
| No. | 15 379 | 14 224 | 1155 | NA | 999 | 647 | 208 | 73 | 71 | NA |
| Mean (SD), y | 54.1 (5.8) | 54 (5.8) | 55.5 (5.7) | <.001 | 55.7 (5.6) | 55.5 (5.7) | 56 (5.7) | 56.2 (5.4) | 57 (4.7) | .09 |
| Age at time of stroke | ||||||||||
| No. | NA | NA | 1155 | NA | 999 | 647 | 208 | 73 | 71 | NA |
| Mean (SD), y | NA | NA | 71.1 (8.7) | NA | 70.2 (8.5) | 70.5 (8.6) | 69.1 (7.7) | 69.5 (9.2) | 70.8 (8.5) | .17 |
| Female, No./total No. (%) | 8485/15 379 (55.2) | 7902/14 224 (55.6) | 583/1155 (50.5) | .001 | 497/999 (49.7) | 319/647 (49.3) | 94/208 (45.2) | 45/73 (61.6) | 39/71 (54.9) | .19 |
| Race and center, No./total No. (%) | ||||||||||
| Black, Jackson, Mississippi | 3649/15 379 (23.7) | 3275/14 224 (23.0) | 374/1155 (32.4) | <.001 | 341/999 (34.1) | 207/647 (32.0) | 82/208 (39.4) | 24/73 (32.9) | 28/71 (39.4) | .09 |
| Black, Forsyth County, North Carolina | 461/15 379 (3.0) | 416/14 224 (2.9) | 45/1155 (3.9) | 41/999 (4.1) | 31/647 (4.8) | 9/208 (4.3) | 1/73 (1.4) | 0 | ||
| White, Forsyth County, North Carolina | 3441/15 379 (22.4) | 3234/14 224 (22.7) | 207/1155 (17.9) | 173/999 (17.3) | 120/647 (18.5) | 35/208 (16.8) | 10/73 (13.7) | 8/71 (11.3) | ||
| White, Minneapolis, Minnesota | 3927/15 379 (25.5) | 3676/14 224 (25.8) | 251/1155 (21.7) | 215/999 (21.5) | 151/647 (23.3) | 36/208 (17.3) | 14/73 (19.2) | 14/71 (19.7) | ||
| White, Washington County, Maryland | 3901/15 379 (25.4) | 3623/14 224 (25.5) | 278/1155 (24.1) | 229/999 (22.9) | 138/647 (21.3) | 46/208 (22.1) | 24/73 (32.9) | 21/71 (29.6) | ||
| Education, No. (%) | ||||||||||
| Did not complete high school | 3651/15 379 (23.7) | 3269/14 224 (23.0) | 382/1155 (33.1) | <.001 | 339/999 (33.9) | 210/647 (32.5) | 80/208 (38.5) | 22/73 (30.1) | 27/71 (38.0) | .59 |
| High school, GED, or vocational school | 6277/15 379 (40.8) | 5841/14 224 (41.1) | 436/1155 (37.7) | 376/999 (37.6) | 254/647 (39.3) | 72/208 (34.6) | 26/73 (35.6) | 24/71 (33.8) | ||
| College, professional, or graduate school | 5451/15 379 (35.4) | 5114/14 224 (36.0) | 337/1155 (29.2) | 284/999 (28.4) | 183/647 (28.3) | 56/208 (26.9) | 25/73 (34.2) | 20/71 (28.2) | ||
| One or more APOE alleles, No./total No. (%) | 4586/14 836 (30.9) | 4223/13 715 (30.8) | 363/1121 (32.4) | .27 | 311/969 (32.1) | 190/633 (30.0) | 73/197 (37.1) | 25/71 (35.2) | 23/68 (33.8) | .18 |
| Smoking status, No./total No. (%) | ||||||||||
| Current | 4006/15 372 (26.1) | 3648/14 218 (25.7) | 358/1154 (31.0) | .001 | 315/998 (31.6) | 194/647 (30.0) | 77/207 (37.2) | 19/73 (26.0) | 25/71 (35.2) | .36 |
| Former | 4951/15 372 (32.2) | 4603/14 218 (32.4) | 348/1154 (30.2) | 300/998 (30.1) | 205/647 (31.7) | 56/207 (27.1) | 21/73 (28.8) | 18/71 (25.4) | ||
| Never | 6415/15 372 (41.7) | 5967/14 218 (42.0) | 448/1154 (38.8) | 383/998 (38.4) | 248/647 (38.3) | 74/207 (35.7) | 33/73 (45.2) | 28/71 (39.4) | ||
| Body mass index, kg/m2 | ||||||||||
| No. | 15 356 | 14 205 | 1151 | NA | 995 | 646 | 208 | 72 | 69 | NA |
| Mean (SD) | 27.7 (5.4) | 27.6 (5.4) | 28.6 (5.5) | <.001 | 28.7 (5.5) | 28.4 (5.0) | 29.3 (6.4) | 29.2 (6.9) | 29.5 (5.5) | .08 |
| Systolic blood pressure, mm HG | ||||||||||
| No. | 15 366 | 14 214 | 1152 | NA | 997 | 646 | 208 | 72 | 71 | NA |
| Mean (SD) | 121.3 (18.8) | 120.7 (18.5) | 128.4 (21.1) | <.001 | 128.9 (21.5) | 127.2 (21.4) | 132.5 (21.2) | 129.2 (21.0) | 133.1 (22.1) | .005 |
| Hypertension medication use, No./total No. (%) | 4667/15 379 (30.4) | 4177/14 224 (29.4) | 490/1155 (42.4) | <.001 | 437/999 (43.7) | 265/647 (41.0) | 97/208 (46.6) | 35/73 (47.9) | 40/71 (56.3) | .006 |
| Diabetes, No./total No. (%) | 1803/15 243 (11.8) | 1536/14 102 (10.9) | 267/1141 (23.4) | <.001 | 247/987 (25.0) | 148/640 (23.1) | 61/205 (29.8) | 17/72 (23.6) | 21/70 (30.0) | .14 |
| Total cholesterol, mg/dL | ||||||||||
| No. | 15 137 | 14 003 | 1134 | NA | 980 | 634 | 205 | 72 | 69 | NA |
| Mean (SD) | 215 (42.1) | 214.6 (41.8) | 220.3 (45.0) | <.001 | 220.2 (45.7) | 219.7 (43.9) | 219.9 (49.1) | 220.9 (49.2) | 225.1 (47.9) | .83 |
| Statin use, No./total No. (%) | 88/15 264 (0.6) | 83/14 120 (0.6) | 5/1144 (0.4) | .52 | 4/990 (0.4) | 3/642 (0.5) | 1/207 (0.5) | 0 | 0 | .48 |
| Global cognition factor score | ||||||||||
| No. | 13 878 | 12 838 | 1040 | NA | 894 | 595 | 180 | 56 | 63 | NA |
| Mean (SD) | 0.7 (0.9) | 0.7 (0.9) | 0.4 (0.9) | <.001 | 0.3 (0.9) | 0.3 (0.9) | 0.2 (0.9) | 0.5 (1.0) | 0.3 (0.9) | .21 |
| Dementia diagnosis by 2019, No./total No. (%) | 2860/15 379 (18.6) | 2591/14 224 (18.2) | 269/1155 (23.3) | <.001 | 234/999 (23.4) | 165/647 (25.5) | 45/208 (21.6) | 17/73 (23.3) | 7/71 (9.9) | .007 |
| Deceased by 2019, No./total No. (%) | 8544/15 379 (55.6) | 7698/14 224 (54.1) | 846/1155 (73.2) | <.001 | 763/999 (76.4) | 466/647 (72.0) | 173/208 (83.2) | 57/73 (78.1) | 67/71 (94.4) | <.001 |
Abbreviations: APOE, apolipoprotein E; NIHSS, National Institutes of Health Stroke Scale.
Study baseline defined as the first assessment (1987-1989) conducted for the ARIC study. Univariate baseline differences in study variables were assessed using χ2 tests, t tests, linear regression, Cochran-Armitage trend tests, and Cochran-Mantel-Haenszel trend tests as appropriate.
Dementia Incidence
In total, 2902 dementia cases were identified through December 31, 2019, 699 through an in-person evaluation (24.1%), 844 through telephone assessment (30.5%), 1058 through hospitalization records (36.5%), and 261 through death certificate (9.0%); of these, 42 were diagnosed in the first year after IS and were excluded. Among the remaining 2860 participants with dementia, 269 had a preceding IS. The median (IQR) time between IS and incident dementia was 7.2 (3.7-12.4) years. Demographic characteristics–adjusted IRs of dementia per 100 person-years were 0.60 (95% CI, 0.57-0.64) in participants without stroke vs 2.49 (95% CI, 2.21-2.81) in those with stroke. The IRs after adjusting for sociodemographic characteristics, APOE, and vascular risk factors were 0.47 (95% CI, 0.44-0.50) in participants without stroke vs 1.21 (95% CI, 1.05-1.40) in those with stroke. Among participants with stroke, the proportion of dementia risk attributable to stroke was 17.4% (95% CI, 4.8 to 28.6), and increased with frequency (9.1% [95% CI, −6.4 to 22.5] for 1 stroke vs 53.0% [95% CI, 35.1 to 65.3] for ≥2 strokes) and severity (7.0% [95% CI, −12.4 to 21.3] for NIHSS ≤5 vs 50.0% [95% CI, 8.3 to 72.9] for NIHSS ≥16). In participants with 2 or more strokes with at least 1 categorized as NIHSS ≥11, the proportion attributable was 63.0% (95% CI, 18.0 to 83.3), 9 times the amount in individuals with 1 stroke and NIHSS ≤10 (7.0% [95% CI, −8.7 to 21.3]).
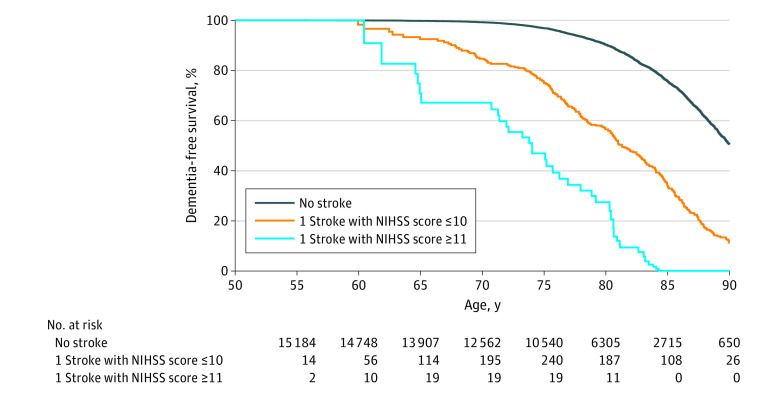
Extended Kaplan-Meier curves of dementia by age are presented in Figure 2. Age without dementia at median survival significantly decreased from 90.0 (95% CI, 88.8-91.2) years in participants without stroke to 81.3 (95% CI, 80.3-82.3) in those with minor to mild stroke and 73.8 (95% CI, 69.8-78.3) in those with moderate to severe stroke. The decrease in the proportion of dementia-free individuals with age is steeper in participants with stroke vs those without stroke (log-rank P < .001; Gehan-Breslow-Wilcoxon P < .001). For individuals older than 80 years, rates of dementia were substantially increased in those with more severe stroke (NIHSS ≥11) vs those without stroke (log-rank trend P < .001; Gehan-Breslow-Wilcoxon trend P < .001). This trend was replicated in cumulative incidence curves accounting for the competing risk of death (eFigure 1 in the Supplement). After adjusting for risk factors, increased hazard rates of dementia were associated with both stroke frequency, increasing from 1.81 (95% CI, 1.57-2.08) for 1 event to 3.91 (95% CI, 2.97-5.16) for ≥2 events, and severity, rising from 1.79 (95% CI, 1.52-2.10) for NIHSS ≤5 to 4.70 (95% CI, 2.23-9.91) for NIHSS ≥16 (Table 2). The same patterns, with modestly attenuated associations, were evident in sensitivity analyses that estimated an earlier date of incident dementia (eTable 4 in the Supplement), used interval censoring (eTable 5 in the Supplement), or examined the competing risk of death (eTable 6 in the Supplement). We assessed the risk of dementia associated with each variable by stroke incidence (eFigure 2 in the Supplement) and severity of the incident stroke (eFigure 3 in the Supplement), as well as estimates for each event categorized as minor to mild or moderate to severe stroke (eFigure 4 in the Supplement). There was a statistically significant interaction between race and moderate to severe stroke with a stronger adjusted relative hazard of dementia among Black participants with a moderate to severe stroke. In subgroup analyses (Figure 3; eFigures 2-4 in the Supplement), relative risk of dementia was greater among participants with IS and older than 75 years.
Figure 2. Extended Kaplan-Meier Curves of Dementia by National Institutes of Health Stroke Scale (NIHSS) Severity of Incident Ischemic Stroke (N = 15 379).
Extended Kaplan-Meier curves generated using the Simon and Makuch method. Severity of incident ischemic stroke is treated as a time-varying exposure. Consequently, participants do not enter the risk set for ≤10 or ≥11 until the age of incident stroke. Differences in the rates of dementia were statistically significant when comparing participants with stroke to those without stroke (log-rank P < .001; Gehan-Breslow-Wilcoxon P < .001). Rates of dementia among participants >80 years were substantially increased among participants with more severe stroke (NIHSS ≥11) vs without stroke (log-rank trend P < .001; Gehan-Breslow-Wilcoxon trend P < .001). Dementia diagnosis was determined by adjudicated review, telephone interviews, informant interviews, hospitalization records, and death certificates. Only dementia diagnoses with a date more than 1 year poststroke were counted.
Table 2. Continuous Time, Cause-Specific Risk Estimates of Dementia by Incidence, Number, and Severity of Ischemic Stroke Events in the Atherosclerosis Risk in Communities (ARIC) Study From Study Visit 1 (1987-1989) to 2019 (N = 15 379).
| Event | No. dementia/No. (IR per 100 person-years) | HR (95% CI) | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| No stroke | 2591/14 224 (0.75) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Dementia by incidence of ischemic stroke | 269/1155 (3.61) | 2.14 (1.88-2.43) | 1.98 (1.74-2.25) | 2.02 (1.78-2.30) |
| Dementia by No. of ischemic stroke events | ||||
| 1 Stroke | 217/973 (3.28) | 1.91 (1.66-2.19) | 1.78 (1.55-2.05) | 1.81 (1.57-2.08) |
| ≥2 Strokes | 52/182 (6.18) | 4.25 (3.23-5.60) | 3.66 (2.78-4.82) | 3.91 (2.97-5.16) |
| Dementia by severity of ischemic stroke, by NIHSS of the first stroke event | ||||
| ≤5 | 165/647 (3.37) | 1.85 (1.58-2.17) | 1.74 (1.48-2.04) | 1.79 (1.52-2.10) |
| 6-10 | 45/208 (3.47) | 3.13 (2.33-4.21) | 2.62 (1.94-3.53) | 2.75 (2.04-3.70) |
| 11-15 | 17/73 (3.45) | 3.50 (2.17-5.63) | 3.35 (2.08-5.40) | 3.28 (2.03-5.30) |
| ≥16 | 7/71 (3.93) | 4.90 (2.33-10.30) | 4.54 (2.16-9.55) | 4.70 (2.23-9.91) |
| Dementia by No. and severity of ischemic stroke events, by NIHSS of most severe stroke event | ||||
| 1 Stroke, NIHSS ≤10 | 167/703 (3.28) | 1.82 (1.57-2.11) | 1.70 (1.46-1.96) | 1.73 (1.49-2.00) |
| 1 Stroke, NIHSS ≥11 | 20/128 (3.30) | 3.59 (2.31-5.57) | 3.41 (2.19-5.31) | 3.47 (2.23-5.40) |
| ≥2 Strokes, all NIHSS ≤10 | 38/137 (5.73) | 3.70 (2.70-5.05) | 3.18 (2.32-4.35) | 3.48 (2.54-4.76) |
| ≥2 Strokes, at least 1 NIHSS ≥11 | 12/40 (8.34) | 8.44 (4.78-14.88) | 7.64 (4.33-13.49) | 6.68 (3.77-11.83) |
| Dementia by No. and severity of ischemic stroke events | ||||
| Each minor to mild stroke (NIHSS ≤10) | NA | 1.86 (1.68-2.06) | 1.73 (1.56-1.92) | 1.76 (1.59-1.94) |
| Each moderate to severe stroke (NIHSS ≥11) | NA | 3.00 (2.12-4.25) | 2.80 (2.01-3.89) | 3.02 (2.23-4.10) |
Abbreviations: HR, hazard ratio; IR, incidence rates; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale.
Figure 3. Adjusted Hazard Ratios (aHRs) of Dementia by National Institutes of Health Stroke Scale (NIHSS) Severity of Each Ischemic Stroke Compared With Not Having a Stroke (N = 15 379).
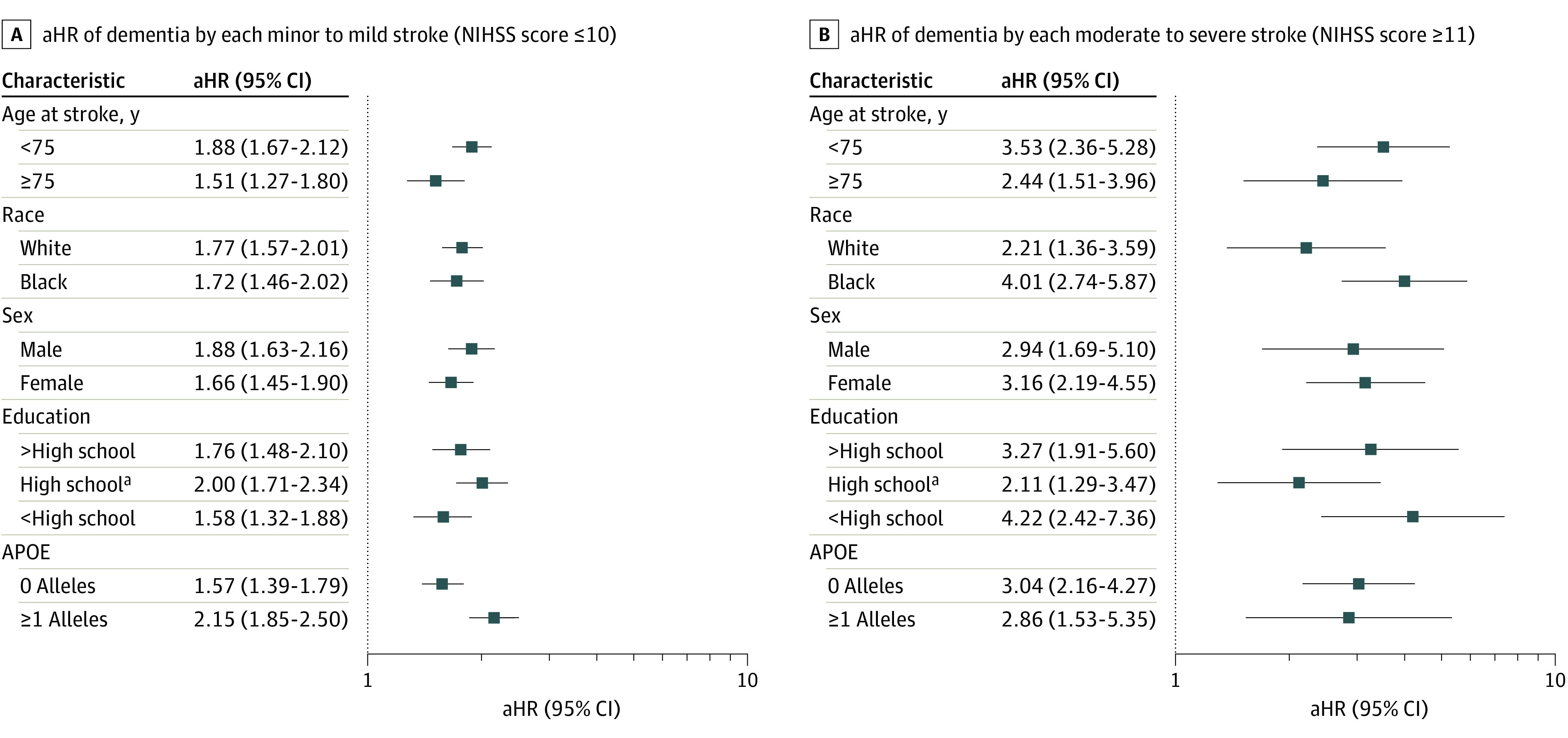
aIndicates a statistically significant interaction was detected between high school education (P = .01) and minor to mild stroke (NIHSS ≤ 10). HRs and 95% CIs were calculated from cause-specific, multivariable Cox proportional hazards regression models. All models were adjusted for age, sex, race and center, education, apolipoprotein E (APOE) alleles, and global cognition as time-invariant covariates. Body mass index (calculated as weight in kilograms divided by height in meters squared), systolic blood pressure, and total cholesterol at baseline were also included as time-invariant covariates. Atrial fibrillation, cigarette use, diabetes, antihypertensive medication use, and statin use were included as time-varying covariates.
Discussion
In this community-based cohort, risk of dementia was elevated in individuals with a history of IS, independent of shared risk factors. This risk was further increased in individuals with more severe stroke and more recurrent strokes. Furthermore, individuals with IS at a younger age (younger than 75 years) had a larger incremental increase in dementia risk than adults who were older at the time of stroke. These data emphasize the value of both primary and secondary stroke prevention for reducing dementia risk.
A faster longitudinal rate of cognitive decline poststroke than that seen prestroke has been reported in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study.27 Although 15% to 25% of patients with stroke meet criteria for dementia 3 months after stroke in some studies,28,29 this early cognitive trajectory may be transient and thus dementia diagnoses should be made cautiously in the early period poststroke. In 1 study, 31% of individuals who were cognitively impaired poststroke had cognitive recovery over the 3 years poststroke,30 whereas other studies demonstrated stable rates of cognitive impairment from 3 months (22%) to 14 years (21%).31 By excluding dementia diagnosed within the first year after stroke, we reduced the likelihood of misdiagnosis or transient early reductions in cognition. Furthermore, dementia diagnoses in ARIC are based on a range of surveillance methods, reducing likelihood of missed or incorrect ascertainment.
Risk of stroke, and thus recurrent stroke and dementia, is increased in individuals with more vascular risk factors. An estimated 39% of recurrent strokes and 10% of poststroke dementia cases are attributable to vascular risk factors present before the stroke.32 By evaluating these risk factors and their changes and treatment over time, we demonstrated that the risk of dementia was elevated independent of these risk factors for both first and recurrent strokes. Even when risk is reduced poststroke by secondary prevention, the effect of these risk factors (likely present for decades) remains important for dementia risk. In fact, even intensive risk-factor management within the first year after stroke does not improve cognitive outcomes,33 indicating the likely need to manage risk factors over years to best impact cognition both poststroke and in the general population.
Prestroke cognition is also critically important in dementia risk. Cognitive status at the time of stroke is associated with poststroke dementia, even among individuals without recurrent stroke.34 Cognitive decline preceding stroke is common35 but infrequently evaluated, complicating diagnosis, but also increasing susceptibility to further decline poststroke. Through ARIC’s careful surveillance methods, dementia events prior to stroke were identified. Therefore any diagnosis of dementia poststroke, especially with our built-in 1-year lag, was more likely to be attributable to new non–short-term effects of the stroke. However, it is still possible that subtle cognitive deficits not meeting criteria for dementia may have preceded the stroke and might impact poststroke dementia risk.
By demonstrating elevated dementia risk even beyond 1 year poststroke and independent of shared risk factors, our data suggest that either (1) risk-factor control is worse in individuals with 1 or several (or more severe) strokes, which is associated with increases in dementia risk; (2) an ongoing, unaccounted for process or confounder may lead to elevated stroke risk as well as risk of dementia; or (3) stroke has a long-term impact on cognition leading to dementia via unmeasured or subclinical ischemic injury. More severe or recurrent stroke might also reduce cognitive reserve, perhaps worsening vulnerability to neurodegenerative pathologies either directly or via modifications in social interactions or lifestyle, which could further impact cognition. Risk-factor management was considered in this analysis, but only whether medications were taken and not their efficacy at controlling risk factors, nor how lifestyle modifications might have been implemented to control risk.
Patients with a greater number of strokes and those with more severe strokes are less likely to survive and be evaluated for long-term outcomes. Therefore, data are limited regarding the importance of initial stroke severity in cognition beyond the first year. Cortical involvement, often resulting in a higher NIHSS score, has been associated with a greater odds of early (3-week) cognitive impairment,36 and an NIHSS score higher than 7 was associated with a 3.4-fold greater odds of cognitive impairment at 3 months.37 Early assessment is particularly problematic for more severe strokes, because early aphasia may interfere with a broader evaluation of cognition. Studies that exclude patients with aphasia undoubtedly miss important cases wherein individuals might develop dementia, and those that consider aphasia as evidence of cognitive impairment are likely misclassifying many of these patients. In the Oxford Vascular Study,3 which evaluated dementia using a range of methodologies and over a longer duration but without a disease-free control group, 1-year dementia rates were highest in individuals with severe strokes (NIHSS >10), lower in those with minor stroke, and lowest in adults with transient ischemic attack.
Stroke severity is not only an indication of the stroke size and location, but may also reflect stroke etiology or patient-level characteristics. Atrial fibrillation may lead to a higher NIHSS scores and has been associated with dementia,38 and large vessel strokes are more likely than lacunar strokes to involve the cortex, thus increasing the likelihood of both higher NIHSS scores and poststroke cognitive impairment. Additionally, NIHSS scores may be impacted by a patient’s age, sex,39 comorbidities,40 lifestyle,41 social determinants of health,42,43 preexisting cognitive impairment,44 or underlying leukoaraiosis.45,46 We have considered many but not all of these factors.
Limitations
Our study is limited by the lack of consistent data in the immediate period poststroke and lack of neuroimaging in the intervening period, which might demonstrate subclinical disease burden and can influence not only the NIHSS score,46 but also the likelihood of recurrent stroke.47 Attrition remains a potential concern, and survivorship may impact dementia rates, especially in individuals with severe or recurrent strokes. We have attempted to account for this potential bias by imputing missing data, by evaluating time-to-event with ongoing cohort surveillance, and by considering competing risk where results remained similar to our primary models. Additionally, although we have measures of comorbidity presence and medication use at repeated time points, these are not comprehensive measures of risk-factor control, and there may be residual confounding in our observed associations. Acute changes in cognitive function early after stroke were excluded in accordance with our conservative approach, but we may have therefore underestimated the rate of early poststroke dementia. Data on stroke severity were not prospectively assessed in ARIC, so severity was based on data in the hospitalization records from admission or as close as possible to admission; however, the exact time of evaluation after stroke onset was not available.
Conclusions
Stroke is common, and despite decreases in stroke incidence rates over time,11,48 dementia remains a major concern for individuals with a prior stroke. Because both stroke severity and recurrent stroke are associated with an elevated risk of dementia, this emphasizes the importance of not only prevention of stroke incidence but also secondary prevention to reduce stroke recurrence. As observational data, these results do not show that treatment or prevention of stroke would reduce the risk of dementia, but they support the value of interventions aimed at reducing strokes and their severity. Future studies should evaluate whether interventions aimed at reducing stroke severity and improving secondary prevention have an impact on dementia rates, and should monitor patients for longer durations poststroke to evaluate impact.
eMethods
eTable 1. Time-varying Characteristics of the Study Population among Participants with Versus without Ischemic Stroke from Visits 1 through 7
eTable 2. Time-varying Characteristics of Participants with Ischemic Stroke by Severity of Incident Ischemic Stroke from Visits 1 through 7
eTable 3. Time-varying Characteristics of Participants with Ischemic Stroke by Number of Ischemic Strokes from Visits 1 through 7
eTable 4. Continuous Time, Cause-Specific Estimates of Risk of Dementia by Incidence, Number, and Severity of Ischemic Stroke Events in ARIC from 1987-1989 to 2019 (n=15,379) With Onset Estimated to Occur 3 Years Prior
eTable 5. Discrete Time, Cause-Specific Estimates of Risk of Dementia by Incidence, Number, and Severity of Ischemic Stroke Events in ARIC from 1987-1989 to 2019 (n=15,379)
eTable 6. Continuous Time, Competing Risk Estimates of Dementia by Incidence, Number, and Severity of Ischemic Stroke Events in ARIC from 1987-1989 to 2019 (n=15,379)
eFigure 1. Competing Risk Cumulative Incidence Curves of Dementia by NIHSS Severity of Incident Ischemic Stroke (n=15,379)
eFigure 2. Adjusted Hazard Ratio (95% Confidence Intervals) of Dementia by Incident Ischemic Stroke Compared to Not Having a Stroke by Age of Stroke, Race, Sex, Education and APOE Subgroups (n=15,379)
eFigure 3. Adjusted Hazard Ratio (95% Confidence Intervals) of Dementia by Severity of Incident Ischemic Stroke Compared to Not Having a Stroke by Age of Stroke, Race, Sex, Education and APOE Subgroups (n=15,379)
eFigure 4. Adjusted Hazard Ratio (95% Confidence Intervals) of Dementia by Number of Ischemic Strokes Compared to Not Having a Stroke by Age of Stroke, Race, Sex, Education and APOE Subgroups (n=15,379)
eReferences
References
- 1.De Ronchi D, Palmer K, Pioggiosi P, et al. The combined effect of age, education, and stroke on dementia and cognitive impairment no dementia in the elderly. Dement Geriatr Cogn Disord. 2007;24(4):266-273. doi: 10.1159/000107102 [DOI] [PubMed] [Google Scholar]
- 2.Mijajlović MD, Pavlović A, Brainin M, et al. Post-stroke dementia—a comprehensive review. BMC Med. 2017;15(1):11. doi: 10.1186/s12916-017-0779-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pendlebury ST, Rothwell PM; Oxford Vascular Study . Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol. 2019;18(3):248-258. doi: 10.1016/S1474-4422(18)30442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pendlebury ST, Chen PJ, Welch SJ, et al. ; Oxford Vascular Study . Methodological factors in determining risk of dementia after transient ischemic attack and stroke: (II) effect of attrition on follow-up. Stroke. 2015;46(6):1494-1500. doi: 10.1161/STROKEAHA.115.009065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol. 2010;9(9):895-905. doi: 10.1016/S1474-4422(10)70164-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74(10):1246-1254. doi: 10.1001/jamaneurol.2017.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callisaya ML, Purvis T, Lawler K, Brodtmann A, Cadilhac DA, Kilkenny MF. Dementia is associated with poorer quality of care and outcomes after stroke: an observational study. J Gerontol A Biol Sci Med Sci. 2021;76(5):851-858. doi: 10.1093/gerona/glaa139 [DOI] [PubMed] [Google Scholar]
- 8.Donnellan C, Werring D. Cognitive impairment before and after intracerebral haemorrhage: a systematic review. Neurol Sci. 2020;41(3):509-527. doi: 10.1007/s10072-019-04150-5 [DOI] [PubMed] [Google Scholar]
- 9.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1-11. doi: 10.1016/j.dadm.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The ARIC investigators . The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 11.Koton S, Schneider AL, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312(3):259-268. doi: 10.1001/jama.2014.7692 [DOI] [PubMed] [Google Scholar]
- 12.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736-743. doi: 10.1161/01.STR.30.4.736 [DOI] [PubMed] [Google Scholar]
- 13.Ruban A, Daya N, Schneider ALC, et al. Liver enzymes and risk of stroke: the Atherosclerosis Risk in Communities (ARIC) study. J Stroke. 2020;22(3):357-368. doi: 10.5853/jos.2020.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross AL, Power MC, Albert MS, et al. Application of latent variable methods to the study of cognitive decline when tests change over time. Epidemiology. 2015;26(6):878-887. doi: 10.1097/EDE.0000000000000379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knopman DS, Gottesman RF, Sharrett AR, et al. Midlife vascular risk factors and midlife cognitive status in relation to prevalence of mild cognitive impairment and dementia in later life: the Atherosclerosis Risk in Communities study. Alzheimers Dement. 2018;14(11):1406-1415. doi: 10.1016/j.jalz.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsell CJ, Alwell K, Moomaw CJ, et al. Validity of a retrospective National Institutes of Health Stroke Scale scoring methodology in patients with severe stroke. J Stroke Cerebrovasc Dis. 2005;14(6):281-283. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 17.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858-862. doi: 10.1161/01.STR.31.4.858 [DOI] [PubMed] [Google Scholar]
- 18.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46(2):141-145. doi: 10.1001/archneur.1989.00520380041011 [DOI] [PubMed] [Google Scholar]
- 19.Benton A., Hamsher K. Multilingual Aphasia Examination. University of Iowa; 1976. [Google Scholar]
- 20.Wechsler, D. Wechsler Memory Scale-Revised. Psychological Corporation; 1987. [Google Scholar]
- 21.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35-44. doi: 10.1002/sim.4780030106 [DOI] [PubMed] [Google Scholar]
- 22.Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10(3):195-216. doi: 10.1177/096228020101000303 [DOI] [PubMed] [Google Scholar]
- 23.Cox DR. Regression models and life-tables. J R Stat Soc Ser A Stat Soc. 1972;34(2):187-202. http://www.jstor.org/stable/2985181 [Google Scholar]
- 24.Fine JP, Gray RJ. A Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 25.Enders CK, Keller BT, Levy R. A fully conditional specification approach to multilevel imputation of categorical and continuous variables. Psychol Methods. 2018;23(2):298-317. doi: 10.1037/met0000148 [DOI] [PubMed] [Google Scholar]
- 26.von Hippel PT. How many imputations do you need? a two-stage calculation using a quadratic rule. Sociol Methods Res. 2020;49(3):699-718. doi: 10.1177/0049124117747303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314(1):41-51. doi: 10.1001/jama.2015.6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Censori B, Manara O, Agostinis C, et al. Dementia after first stroke. Stroke. 1996;27(7):1205-1210. doi: 10.1161/01.STR.27.7.1205 [DOI] [PubMed] [Google Scholar]
- 29.Desmond DW, Moroney JT, Paik MC, et al. Frequency and clinical determinants of dementia after ischemic stroke. Neurology. 2000;54(5):1124-1131. doi: 10.1212/WNL.54.5.1124 [DOI] [PubMed] [Google Scholar]
- 30.Liman TG, Heuschmann PU, Endres M, Flöel A, Schwab S, Kolominsky-Rabas PL. Changes in cognitive function over 3 years after first-ever stroke and predictors of cognitive impairment and long-term cognitive stability: the Erlangen Stroke Project. Dement Geriatr Cogn Disord. 2011;31(4):291-299. doi: 10.1159/000327358 [DOI] [PubMed] [Google Scholar]
- 31.Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995-2010. Stroke. 2013;44(1):138-145. doi: 10.1161/STROKEAHA.112.670844 [DOI] [PubMed] [Google Scholar]
- 32.Portegies ML, Wolters FJ, Hofman A, Ikram MK, Koudstaal PJ, Ikram MA. Prestroke vascular pathology and the risk of recurrent stroke and poststroke dementia. Stroke. 2016;47(8):2119-2122. doi: 10.1161/STROKEAHA.116.014094 [DOI] [PubMed] [Google Scholar]
- 33.Ihle-Hansen H, Thommessen B, Fagerland MW, et al. Multifactorial vascular risk factor intervention to prevent cognitive impairment after stroke and TIA: a 12-month randomized controlled trial. Int J Stroke. 2014;9(7):932-938. doi: 10.1111/j.1747-4949.2012.00928.x [DOI] [PubMed] [Google Scholar]
- 34.Rist PM, Chalmers J, Arima H, et al. Baseline cognitive function, recurrent stroke, and risk of dementia in patients with stroke. Stroke. 2013;44(7):1790-1795. doi: 10.1161/STROKEAHA.111.680728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hénon H, Durieu I, Guerouaou D, Lebert F, Pasquier F, Leys D. Poststroke dementia: incidence and relationship to prestroke cognitive decline. Neurology. 2001;57(7):1216-1222. doi: 10.1212/WNL.57.7.1216 [DOI] [PubMed] [Google Scholar]
- 36.Nys GM, van Zandvoort MJ, de Kort PL, Jansen BP, de Haan EH, Kappelle LJ. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 2007;23(5-6):408-416. doi: 10.1159/000101464 [DOI] [PubMed] [Google Scholar]
- 37.Lin JH, Lin RT, Tai CT, Hsieh CL, Hsiao SF, Liu CK. Prediction of poststroke dementia. Neurology. 2003;61(3):343-348. doi: 10.1212/01.WNL.0000078891.27052.10 [DOI] [PubMed] [Google Scholar]
- 38.Zhou DH, Wang JY, Li J, Deng J, Gao C, Chen M. Study on frequency and predictors of dementia after ischemic stroke: the Chongqing stroke study. J Neurol. 2004;251(4):421-427. doi: 10.1007/s00415-004-0337-z [DOI] [PubMed] [Google Scholar]
- 39.Viticchi G, Falsetti L, Plutino A, Bartolini M, Buratti L, Silvestrini M. Sex influence in ischemic stroke severity and outcome among metabolically unhealthy overweight patients. J Neurol Sci. 2020;416:116955. doi: 10.1016/j.jns.2020.116955 [DOI] [PubMed] [Google Scholar]
- 40.Appelros P, Nydevik I, Seiger A, Terént A. Predictors of severe stroke: influence of preexisting dementia and cardiac disorders. Stroke. 2002;33(10):2357-2362. doi: 10.1161/01.STR.0000030318.99727.FA [DOI] [PubMed] [Google Scholar]
- 41.Hung SH, Ebaid D, Kramer S, et al. Pre-stroke physical activity and admission stroke severity: a systematic review. Int J Stroke. 2021:16(9):1009-1018. doi: 10.1177/1747493021995271 [DOI] [PubMed] [Google Scholar]
- 42.Kleindorfer D, Lindsell C, Alwell KA, et al. Patients living in impoverished areas have more severe ischemic strokes. Stroke. 2012;43(8):2055-2059. doi: 10.1161/STROKEAHA.111.649608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vivanco-Hidalgo RM, Avellaneda-Gomez C, Dadvand P, et al. Association of residential air pollution, noise, and greenspace with initial ischemic stroke severity. Environ Res. 2019;179(Pt A):108725. doi: 10.1016/j.envres.2019.108725 [DOI] [PubMed] [Google Scholar]
- 44.Béjot Y, Duloquin G, Crespy V, et al. Influence of preexisting cognitive impairment on clinical severity of ischemic stroke: the Dijon Stroke Registry. Stroke. 2020;51(6):1667-1673. doi: 10.1161/STROKEAHA.119.028845 [DOI] [PubMed] [Google Scholar]
- 45.Helenius J, Goddeau RP Jr, Moonis M, Henninger N. Impact of leukoaraiosis burden on hemispheric lateralization of the National Institutes of Health Stroke Scale deficit in acute ischemic stroke. Stroke. 2016;47(1):24-30. doi: 10.1161/STROKEAHA.115.011771 [DOI] [PubMed] [Google Scholar]
- 46.Helenius J, Henninger N. Leukoaraiosis burden significantly modulates the association between infarct volume and National Institutes of Health Stroke Scale in ischemic stroke. Stroke. 2015;46(7):1857-1863. doi: 10.1161/STROKEAHA.115.009258 [DOI] [PubMed] [Google Scholar]
- 47.Koton S, Schneider ALC, Windham BG, Mosley TH, Gottesman RF, Coresh J. Microvascular brain disease progression and risk of stroke: the ARIC study. Stroke. 2020;51(11):3264-3270. doi: 10.1161/STROKEAHA.120.030063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koton S, Sang Y, Schneider ALC, Rosamond WD, Gottesman RF, Coresh J. Trends in stroke incidence rates in older US adults: an update from the Atherosclerosis Risk in Communities (ARIC) cohort study. JAMA Neurol. 2020;77(1):109-113. doi: 10.1001/jamaneurol.2019.3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Time-varying Characteristics of the Study Population among Participants with Versus without Ischemic Stroke from Visits 1 through 7
eTable 2. Time-varying Characteristics of Participants with Ischemic Stroke by Severity of Incident Ischemic Stroke from Visits 1 through 7
eTable 3. Time-varying Characteristics of Participants with Ischemic Stroke by Number of Ischemic Strokes from Visits 1 through 7
eTable 4. Continuous Time, Cause-Specific Estimates of Risk of Dementia by Incidence, Number, and Severity of Ischemic Stroke Events in ARIC from 1987-1989 to 2019 (n=15,379) With Onset Estimated to Occur 3 Years Prior
eTable 5. Discrete Time, Cause-Specific Estimates of Risk of Dementia by Incidence, Number, and Severity of Ischemic Stroke Events in ARIC from 1987-1989 to 2019 (n=15,379)
eTable 6. Continuous Time, Competing Risk Estimates of Dementia by Incidence, Number, and Severity of Ischemic Stroke Events in ARIC from 1987-1989 to 2019 (n=15,379)
eFigure 1. Competing Risk Cumulative Incidence Curves of Dementia by NIHSS Severity of Incident Ischemic Stroke (n=15,379)
eFigure 2. Adjusted Hazard Ratio (95% Confidence Intervals) of Dementia by Incident Ischemic Stroke Compared to Not Having a Stroke by Age of Stroke, Race, Sex, Education and APOE Subgroups (n=15,379)
eFigure 3. Adjusted Hazard Ratio (95% Confidence Intervals) of Dementia by Severity of Incident Ischemic Stroke Compared to Not Having a Stroke by Age of Stroke, Race, Sex, Education and APOE Subgroups (n=15,379)
eFigure 4. Adjusted Hazard Ratio (95% Confidence Intervals) of Dementia by Number of Ischemic Strokes Compared to Not Having a Stroke by Age of Stroke, Race, Sex, Education and APOE Subgroups (n=15,379)
eReferences