Key Points
Question
What is the effect of initial noninvasive respiratory strategies using continuous positive airway pressure (CPAP) or high-flow nasal oxygen (HFNO), compared with an initial strategy of conventional oxygen therapy, on the risk of tracheal intubation or mortality among hospitalized adults with acute hypoxemic respiratory failure due to COVID-19?
Findings
In this randomized clinical trial of 1273 patients, the composite primary outcome of tracheal intubation or mortality within 30 days occurred in 36% of the patients in the CPAP group compared with 44% in the conventional oxygen therapy group, a difference that was statistically significant, and occurred in 44% in the HFNO group compared with 45% in the conventional oxygen therapy group, a difference that was not significantly different.
Meaning
Among patients with acute hypoxemic respiratory failure and COVID-19, an initial strategy of CPAP significantly reduced the risk of tracheal intubation or mortality compared with conventional oxygen therapy, but there was no significant difference between an initial strategy of HFNO compared with conventional oxygen therapy.
Abstract
Importance
Continuous positive airway pressure (CPAP) and high-flow nasal oxygen (HFNO) have been recommended for acute hypoxemic respiratory failure in patients with COVID-19. Uncertainty exists regarding the effectiveness and safety of these noninvasive respiratory strategies.
Objective
To determine whether either CPAP or HFNO, compared with conventional oxygen therapy, improves clinical outcomes in hospitalized patients with COVID-19–related acute hypoxemic respiratory failure.
Design, Setting, and Participants
A parallel group, adaptive, randomized clinical trial of 1273 hospitalized adults with COVID-19–related acute hypoxemic respiratory failure. The trial was conducted between April 6, 2020, and May 3, 2021, across 48 acute care hospitals in the UK and Jersey. Final follow-up occurred on June 20, 2021.
Interventions
Adult patients were randomized to receive CPAP (n = 380), HFNO (n = 418), or conventional oxygen therapy (n = 475).
Main Outcomes and Measures
The primary outcome was a composite of tracheal intubation or mortality within 30 days.
Results
The trial was stopped prematurely due to declining COVID-19 case numbers in the UK and the end of the funded recruitment period. Of the 1273 randomized patients (mean age, 57.4 [95% CI, 56.7 to 58.1] years; 66% male; 65% White race), primary outcome data were available for 1260. Crossover between interventions occurred in 17.1% of participants (15.3% in the CPAP group, 11.5% in the HFNO group, and 23.6% in the conventional oxygen therapy group). The requirement for tracheal intubation or mortality within 30 days was significantly lower with CPAP (36.3%; 137 of 377 participants) vs conventional oxygen therapy (44.4%; 158 of 356 participants) (absolute difference, −8% [95% CI, −15% to −1%], P = .03), but was not significantly different with HFNO (44.3%; 184 of 415 participants) vs conventional oxygen therapy (45.1%; 166 of 368 participants) (absolute difference, −1% [95% CI, −8% to 6%], P = .83). Adverse events occurred in 34.2% (130/380) of participants in the CPAP group, 20.6% (86/418) in the HFNO group, and 13.9% (66/475) in the conventional oxygen therapy group.
Conclusions and Relevance
Among patients with acute hypoxemic respiratory failure due to COVID-19, an initial strategy of CPAP significantly reduced the risk of tracheal intubation or mortality compared with conventional oxygen therapy, but there was no significant difference between an initial strategy of HFNO compared with conventional oxygen therapy. The study may have been underpowered for the comparison of HFNO vs conventional oxygen therapy, and early study termination and crossover among the groups should be considered when interpreting the findings.
Trial Registration
isrctn.org Identifier: ISRCTN16912075
This randomized clinical trial compares the effect of continuous positive airway pressure or high-flow nasal oxygen vs conventional oxygen therapy on clinical outcomes in hospitalized patients with COVID-19 and acute hypoxemic respiratory failure (AHRF).
Introduction
Acute hypoxemic respiratory failure is a key clinical characteristic of COVID-19 pneumonitis. In a study of 63 792 patients with COVID-19 hospitalized in the UK between March and August 2020, 76% required supplemental oxygen and 9% required tracheal intubation and invasive mechanical ventilation.1 Early in the pandemic, international experiences highlighted the potential risk that intensive care units (ICUs) might become overwhelmed, and high mortality was observed in patients requiring invasive mechanical ventilation.2,3,4 This drove an urgent public health need to identify strategies to reduce the demand for invasive mechanical ventilation.
In patients with COVID-19 and increasing oxygen requirements, noninvasive respiratory strategies such as continuous positive airway pressure (CPAP) and high-flow nasal oxygen (HFNO) provide potentially attractive strategies for avoiding invasive mechanical ventilation. In other respiratory diseases, particularly community-acquired pneumonia, both CPAP and HFNO may improve clinical outcomes; however, patients treated with CPAP experience more adverse events.5,6 In the context of COVID-19, however, there was concern that these strategies might serve only to delay tracheal intubation due to high failure rates, while exacerbating lung injury through generation of large tidal volumes.7,8,9,10
The absence of evidence to support use of CPAP and HFNO in patients with COVID-19 led to significant variability both in international guidelines and clinical practice.9,11 On this basis, there was a need for a randomized clinical trial to determine whether either CPAP or HFNO, compared with conventional oxygen therapy, reduces the need for tracheal intubation or mortality within 30 days in hospitalized patients with acute hypoxemic respiratory failure due to COVID-19.
Methods
Study Design
The Randomized Evaluation of COVID-19 Therapy–Respiratory Support (RECOVERY-RS) clinical trial was conducted across 48 acute care hospitals in the UK and Jersey. The trial protocol was approved by the London-Brighton and Sussex research ethics committee and the Health Research Authority, sponsored by the University of Warwick, coordinated by the Warwick Clinical Trials Unit, and funded by the National Institute for Health Research. An independent trial steering committee and data and safety monitoring committee provided trial oversight. The RECOVERY-RS trial was conducted in accordance with Good Clinical Practice guidelines, local regulations, and the ethical principles described in the Declaration of Helsinki.12 In keeping with regional regulations, consent from patients or agreement from their family or another surrogate was obtained orally, with a written record maintained by the researcher. The trial protocol has been published.13 The trial protocol and statistical analysis plan appear in Supplement 1.
The trial was a parallel group, open-label, adaptive, 3-group, randomized clinical trial designed to evaluate the clinical effectiveness of CPAP or HFNO, compared with conventional oxygen therapy, in hospitalized patients with acute hypoxemic respiratory failure due to COVID-19 (Figure 1). The multigroup design was essentially conducted as 2 separate trials comparing CPAP and HFNO with a common shared control group of conventional oxygen therapy. A group sequential design allowed early study termination of 1 or both interventions if they were found to be more effective than conventional oxygen therapy, with the final analysis for each comparison adjusted to control the pairwise α value of .05.
Figure 1. Patient Screening, Eligibility, and Enrollment in the RECOVERY-RS Trial.
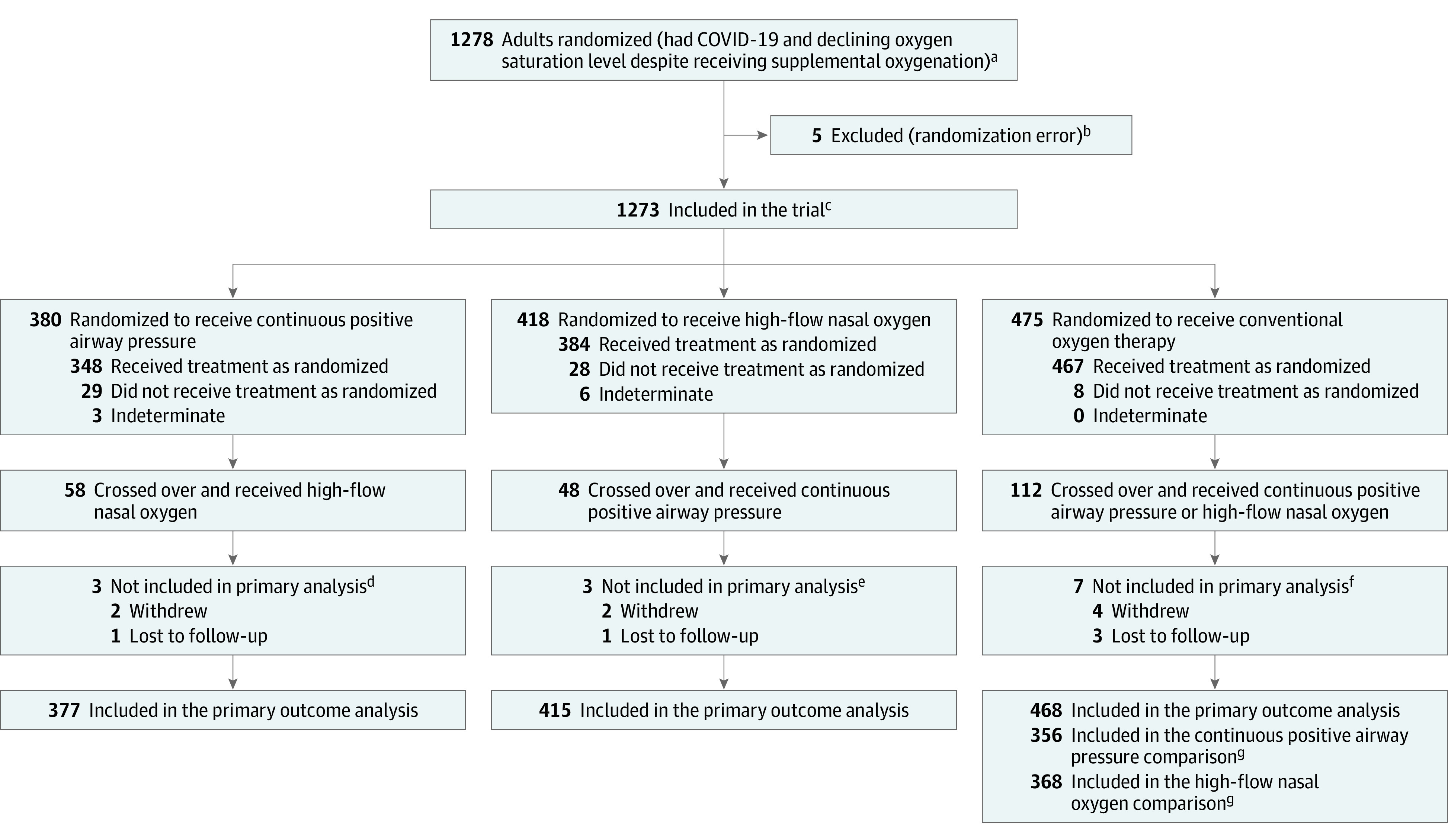
RECOVERY-RS indicates Randomized Evaluation of COVID-19 Therapy–Respiratory Support.
aGiven the COVID-19 pandemic circumstances, investigators did not have hospitals track everyone who was approached or considered but not randomized.
bFive patients were mistakenly randomized twice (3 to the conventional oxygen therapy group and 2 to the high-flow nasal oxygen group), but did not receive treatment. These patients were excluded from the analyses.
cThere were 114 patients randomized to continuous positive airway pressure (CPAP) and 103 patients randomized to conventional oxygen therapy when high-flow nasal oxygen (HFNO) was not available; 109 patients randomized to HFNO and 113 to conventional oxygen therapy when CPAP was not available; and 266 patients randomized to CPAP, 309 to HFNO, and 259 to conventional oxygen therapy when all therapies were available.
dTwo patients did not receive adequate treatment information or a crossover treatment (1 withdrew and 1 lost to follow-up). One patient withdrew and did not receive CPAP or a crossover treatment.
eThe 2 patients who withdrew did not receive HFNO or a crossover treatment. One patient had insufficient data regarding receipt of HFNO or a crossover treatment (lost to follow-up).
fAll 7 patients received treatment. Six patients (including the 4 patients who withdrew) did not receive a crossover treatment. One patient had insufficient data regarding receipt of a crossover treatment.
gComparisons excluded patients who did not have an opportunity to be randomized to the alternative intervention based on hospital site availability.
Participants
Adult hospitalized patients (aged ≥18 years) with known or suspected COVID-19 were eligible if they had acute hypoxemic respiratory failure, defined as an oxygen saturation as measured by pulse oximetry (Spo2) of 94% or less despite receiving a fraction of inspired oxygen (Fio2) of at least 0.40, and were deemed suitable for tracheal intubation if treatment escalation was required. Patients with an immediate (<1 hour) need for invasive mechanical ventilation, known pregnancy, or planned withdrawal of treatment were excluded. Based on the judgment of the treating clinician, a contraindication to the intervention precluded randomization to that specific trial group.
Randomization and Blinding
Eligible patients were randomized using an internet-based system with allocation concealment. We anticipated that either CPAP or HFNO might be unavailable at the hospital sites on a temporary or permanent basis. As such, the randomization system allowed the hospital site to randomize patients to (1) CPAP, HFNO, or conventional oxygen therapy (on a 1:1:1 basis) or (2) a single intervention (CPAP or HFNO) or conventional oxygen therapy (on a 1:1 basis). These 2 systems were integrated and constantly updated to ensure that the allocation ratio was maintained within the permitted thresholds.
The planned sample size was inflated to account for minor imbalances in the allocation ratio and, if it had been needed, the system allowed randomization weightings to be adjusted. Hospital sites could not randomize only between CPAP and HFNO. Randomization was stratified by hospital site, sex, and age and the allocation was generated by a minimization algorithm, which did not include any random component. Due to the nature of the trial interventions and context, it was not possible to blind patients, treating clinicians, or outcome assessors.
Procedures
Patients randomized to CPAP or HFNO started treatment as soon as possible. Breaks from treatment were permitted for comfort. Patients randomized to conventional oxygen therapy received oxygen via a standard face mask or low-flow nasal cannula. The patients in the HFNO group received heated humidified HFNO. The patients in the CPAP group received CPAP that did not permit the incorporation of any inspiratory positive airway pressure. Across all groups, local policies and clinical discretion informed decisions regarding device choice and setup, titration (eg, Fio2, flow, positive end-expiratory pressure), treatment targets (eg, Spo2), and treatment discontinuation. Tracheal intubation was performed when clinically indicated based on the judgment of the treating clinician. Treatment crossover was defined as a patient who received a nonallocated intervention (CPAP or HFNO) for a period of more than 6 hours unless the intervention was used as a bridge to tracheal intubation or for palliative care.
At enrollment, we collected information on demographics (including investigator-classified sex and race and ethnicity), comorbid state, and physiological levels (including blood pressure, respiratory rate, peripheral oxygen saturation, and blood gas measurements). Collection and reporting of race and ethnicity was based on fixed categories and mandated by the funder due to the disproportionate effect of COVID-19 infection on non-White populations.14 Participants were followed up throughout their hospital stay to record intervention use, crossover, adverse events, and outcomes. We undertook data linkage with national data sets to support collection of demographic information and outcomes, including death after hospital discharge.
Outcomes
The primary outcome was a composite of tracheal intubation or mortality within 30 days of randomization. Tracheal intubation, as an outcome, reflects the need for invasive mechanical ventilation, which is typically delivered in high-resource ICUs. Secondary outcomes included the individual incidence of tracheal intubation or mortality within 30 days, time to tracheal intubation, duration of invasive mechanical ventilation, time to death, mortality (during ICU or hospital stay), admission to the ICU, length of stay in the ICU, and length of stay in the hospital (included time from emergency department arrival to hospital discharge).
Sample Size Calculation
Early data on COVID-19 informed the anticipated event rate in the conventional oxygen therapy group.15 Assuming a conservative incidence of 15% for the composite outcome of tracheal intubation or mortality (with a 2-sided significance level of .05 and 90% power), a total of 3000 participants (1000 per group across 3 groups) were required. This equated to detecting a reduction of 5% for tracheal intubation or mortality or an odds ratio of 0.625. This minimally important clinical difference for the primary outcome aligns with what was used in the RECOVERY trial.16,17 The sample size was inflated to 4002 because of the uncertainties in relation to COVID-19 and event rates.
Efficacy monitoring of each pairwise comparison with conventional oxygen therapy was based on an α spending function approach with a 1-sided pairwise type I error rate of 0.025 and a type I error spent at the interim analyses that was proportional to the observed Fisher information. This allowed the trial to be stopped early if 1 or both interventions were more effective than conventional oxygen therapy. Any decision to stop the trial or drop a group due to futility or safety was determined by the data and safety monitoring committee. The sample size calculation assumed the conduct of 11 interim analyses and 1 final analysis.
Statistical Analysis
The primary and secondary analyses were performed for all participants based on their randomized intervention. Outcome data were compared between each intervention group and the conventional oxygen therapy group. Participants in the conventional oxygen therapy group were only included in a comparison with HFNO or CPAP if they had the opportunity to be randomized to that intervention. For the primary outcome, we undertook a post hoc analysis that compared the CPAP and HFNO groups.
Continuous data were summarized using the number of participants (percentage), mean (SD or 95% CI), and median (IQR). Categorical data were summarized with frequency count, percentage, and missing. Odds ratios (95% CIs) were reported for categorical outcomes using logistic regression models. Mean or median differences (95% CIs) were reported for continuous outcomes. Proportional (absolute) differences (95% CIs) were reported for categorical outcomes. For the time to event analysis, hazard ratios (95% CIs) were reported and the proportional hazards assumption was assessed using the score test. In accordance with the statistical analysis plan (Supplement 1), we planned multiple imputation only if there was substantial missingness (≥20%) in relation to the primary outcome.
The primary analysis was unadjusted. For the adjusted secondary analyses, the covariates of age, sex, morbid obesity (defined as a body mass index >35), race and ethnicity, Fio2, respiratory rate, and treatment phases were used with hospital site included as a random effect.18,19 Treatment phases were defined as before July 2020, July 2020 to January 2021, and after January 2021 based on the introduction of dexamethasone as standard care in June 2020 and tocilizumab in January 2021.17,20,21 Due to the unavailability of data from NHS Digital, we could not include social deprivation in the adjusted analyses.
We used inverse probability weighting as a secondary exploratory analysis. This method corrects for bias that may be introduced into the treatment effect as a result of the crossover. Weights were estimated using propensity scores with the response variable as those participants who did crossover or who did not crossover. These weights were then introduced into the main logistic regression models to diminish the bias introduced by treatment change. Subgroup analyses were performed using logistic regression models, with the primary outcome as the response variable and the subgroup × treatment interaction term included in the model. No adjustment was made for the multiple comparisons. Thus, the type I error control was the same as if CPAP and HFNO had each been compared with conventional oxygen therapy in separate trials.
Cutoff values for the final P value for the primary analysis were calculated to correct for the type I error spent at the interim analyses.22 The final cutoff values depended on the information available at the interim analyses and are reported in the Results section below. No correction for the interim analyses was made to the cutoff values for the secondary outcomes or analyses, with a significance threshold of .05 used. All significance testing was 2-sided. Because of the potential for type I error due to multiple comparisons, the findings for the analyses of the secondary outcomes should be interpreted as exploratory. Analyses were conducted using SAS version 9.4 (SAS Institute Inc) and R version 4.0.3 (R Foundation for Statistical Computing).
Results
Trial recruitment was stopped early. Toward the end of the funded 12-month recruitment period, there was a rapid decline in hospitalized patients with COVID-19 in the UK. During the trial period, recruitment was closely tracked with the overall cases of hospitalized patients with COVID-19 in the UK (eFigure 1 in Supplement 2). The trial management group decided not to seek additional funding and to prioritize the sharing of accumulated data to inform international clinical care.
Prior to stopping trial recruitment, 3 formal interim analyses had been conducted of 36, 160, and 387 participants and the trial continued after each analysis. The results of the interim analyses (other than the decision to continue the trial) were not known to the trial management group, trial steering committee, trial sponsor, or trial funder. The trial management group’s recommendation to stop trial recruitment was agreed on by the trial’s steering committee and funder. The trial sponsor made the decision to stop recruitment and the trial closed recruitment on May 3, 2021.
Participant Recruitment
Between April 6, 2020, and May 3, 2021, there were 1278 patients who were randomized across 48 acute care hospitals in the UK and Jersey. Five patients mistakenly underwent double randomization, leaving 1273 patients included in the trial (380 in the CPAP group, 418 in the HFNO group, and 475 in the conventional oxygen therapy group) (Figure 1). Final follow-up occurred on June 20, 2021. Eight patients withdrew and 5 were lost to follow-up. Primary outcome data were available for 99.0% (1260/1273) of participants.
A total of 733 participants (377 in the CPAP group and 356 in the conventional oxygen therapy group) were included in the comparison of CPAP with conventional oxygen therapy. A total of 783 participants (415 in the HFNO group and 368 in the conventional oxygen therapy group) were included in the comparison of HFNO with conventional oxygen therapy (Figure 1 and eTable 1 in Supplement 2).
Participant characteristics were similar at baseline (Table 1 and eTable 2 in Supplement 2). The mean age was 57.4 years (95% CI, 56.7-58.1 years), 66.3% were male, and 65.3% of White race. The median time from first COVID-19 symptoms to randomization was 9 days (IQR, 7.0-12.0 days). The baseline median Spo2 was 93% (IQR, 91%-95%) and Fio2 was 0.60 (IQR, 0.40-0.80).
Table 1. Baseline Characteristics of the Participants.
| Continuous positive airway pressure (n = 380) |
High-flow nasal oxygen (n = 418) |
Conventional oxygen therapy (n = 475) |
|
|---|---|---|---|
| Treatment period, No. (%) | |||
| Before July 2020 | 47 (12.4) | 44 (10.5) | 47 (9.9) |
| July 2020-January 2021 | 262 (69.0) | 289 (69.1) | 331 (69.7) |
| After January 2021 | 71 (18.7) | 85 (20.3) | 97 (20.4) |
| Age, mean (SD), y | 56.7 (12.5) | 57.6 (13.0) | 57.6 (12.7) |
| Sex, No. (%) | |||
| Male | 260 (68.4) | 272 (65.1) | 312 (65.7) |
| Female | 120 (31.6) | 146 (34.9) | 163 (34.3) |
| Race and ethnicity, No. (%)a | |||
| Asianb | 73 (19.2) | 77 (18.4) | 90 (18.9) |
| Blackc | 16 (4.2) | 14 (3.3) | 19 (4.0) |
| Multipled | 3 (0.8) | 4 (1.0) | 6 (1.3) |
| Whitee | 243 (63.9) | 276 (66.0) | 312 (65.7) |
| Otherf | 11 (2.9) | 12 (2.9) | 9 (1.9) |
| Not given | 33 (8.7) | 34 (8.1) | 35 (7.4) |
| Time from symptom onset, median (IQR), d | |||
| To hospital admission | (n = 376); 7.0 (5.5-10.0) | (n = 407); 8.0 (5.0-10.0) | (n = 466); 7.0 (5.0-10.0) |
| To randomization | (n = 378); 9.0 (7.0-12.0) | (n = 414); 9.0 (7.0-12.0) | (n = 470); 9.0 (6.0-12.0) |
| COVID-19 status, No. (%) | (n = 379) | (n = 417) | (n = 473) |
| Confirmed | 326 (85.8) | 355 (84.9) | 409 (86.1) |
| Suspected | 53 (13.9) | 62 (14.8) | 64 (13.5) |
| Comorbidities, No. (%) | |||
| Other (none of the below) | 148 (38.9) | 141 (33.7) | 188 (39.6) |
| Hypertension | 131 (34.5) | 164 (39.2) | 153 (32.2) |
| Diabetes requiring medication | 86 (22.6) | 98 (23.4) | 91 (19.2) |
| Morbid obesity (body mass index >35)g | 62 (16.3) | 81 (19.4) | 75 (15.8) |
| Chronic lung disease | 65 (17.1) | 52 (12.4) | 66 (13.9) |
| Coronary heart disease | 34 (8.9) | 26 (6.2) | 44 (9.3) |
| Uncontrolled or active malignancy | 7 (1.8) | 10 (2.4) | 7 (1.5) |
| Dementia | 4 (1.1) | 1 (0.2) | 3 (0.6) |
| End-stage kidney disease requiring kidney replacement therapy | 2 (0.5) | 6 (1.4) | 5 (1.1) |
| Congestive heart failure | 2 (0.5) | 4 (1.0) | 5 (1.1) |
| Clinical Frailty Scale, No. (%)h | |||
| Very fit to managing welli | 351 (92.4) | 376 (90.0) | 430 (90.5) |
| Very mild frailty to terminally illj | 19 (5.0) | 35 (8.4) | 39 (8.2) |
| Respiratory rate, median (IQR), /min | (n = 377); 24 (21-30) | (n = 414); 24 (20-29) | (n = 472); 23 (20-28) |
| Fio2, median (IQR) | (n = 363); 0.60 (0.40-0.80) | (n = 404); 0.60 (0.40-0.80) | (n = 459); 0.60 (0.40-0.80) |
| Spo2, median (IQR), % | (n = 378); 94.0 (92.0-95.0) | (n = 409); 93.0 (91.0-95.0) | (n = 470); 94.0 (92.0-95.0) |
| Ratio of Spo2 to Fio2, median (IQR), % | (n = 363); 155.0 (110.6-232.5) | (n = 399); 156.7 (113.8-232.5) | (n = 457); 156.7 (115.0-230.0) |
| Pao2, median (IQR), mm Hg | (n = 238); 67.5 (60.0-77.3) | (n = 287); 66.0 (59.3-74.3) | (n = 317); 66.8 (58.5-80.3) |
| Ratio of Pao2 to Fio2, median (IQR), mm Hg | (n = 229); 112.5 (80.0-161.3) | (n = 284); 115.0 (80.9-168.4) | (n = 308); 113.8 (84.8-150.9) |
| Paco2, median (IQR), mm Hg | (n = 252); 33.0 (30.0-36.8) | (n = 306); 33.0 (30.0-36.0) | (n = 331); 33.8 (30.8-36.8) |
Abbreviations: Fio2, fraction of inspired oxygen; Spo2, oxygen saturation as measured by pulse oximetry.
Categories were based on the NHS Data Model and Dictionary.
Asian-Indian, Asian-Pakistani, Asian-Bangladeshi, multiple-White and Asian, or any other Asian background.
Black/African/Caribbean/Black British-African, Black/African/Caribbean/Black British-Caribbean, Black/African/Caribbean/Black British, or any other Black/African/Caribbean/Black British background.
Any other multiple racial and ethnic combination.
White British, White Irish, multiple-White and Black Caribbean, multiple-White and Black African, or any other White background.
Chinese or any other racial and ethnic combination not listed in footnotes b through e.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Based on functional status prior to hospital admission and determined through chart review or patient assessment. It is measured on a 9-point scale (very fit to terminally ill) with lower scores indicating better functional status and a lower level of frailty.
Categories correspond to scores of 1 to 3.
Categories correspond to scores of 4 to 9.
The allocated intervention was received by 91.6% (348/380) of participants in the CPAP group, 91.9% (384/418) in the HFNO group, and 98.3% (467/475) in the conventional oxygen therapy group (Figure 1). In the CPAP group, initial positive end-expiratory pressure was set at a mean of 8.3 cm H2O (95% CI, 8.1-8.5 cm H2O; Table 2). In the HFNO group, initial flow was set at a mean of 52.4 L/min (95% CI, 51.4-53.5 L/min). Of those who required tracheal intubation, their preprocedure clinical conditions appear in Table 2.
Table 2. Initial Intervention Details, Prone Positioning, and Clinical Conditions Prior to Tracheal Intubation.
| Continuous positive airway pressure (n = 380) |
High-flow nasal oxygen (n = 418) |
Conventional oxygen therapy (n = 475) |
|
|---|---|---|---|
| Initial intervention details and prone positioning | |||
| Continuous positive airway pressure | |||
| Positive end-expiratory pressure, mean (95% CI), cm H20 | (n = 304) 8.3 (8.1-8.5) |
NA | NA |
| Delivery device, No. (%) | NA | NA | |
| Noninvasive ventilationa | 147 (38.7) | NA | NA |
| Continuous positive airway pressure | 173 (45.5) | NA | NA |
| Otherb | 24 (6.3) | NA | NA |
| Initial flow for high-flow nasal oxygen, mean (95% CI), L/min | NA | (n = 323) 52.4 (51.4-53.5) |
NA |
| Treatment delivery duration, mean (SD), d | (n = 340) 3.5 (4.6) |
(n = 378) 3.7 (4.1) |
NA |
| Awake and in prone position, No./total (%)c | 207/327 (63.3) | 243/341 (71.3) | 252/374 (67.4) |
| Clinical condition prior to tracheal intubationd | |||
| Alert (conscious), No./total (%) | 72/80 (90.0) | 91/103 (88.3) | 112/122 (91.8) |
| Respiratory rate, median (IQR), /min | (n = 73) 34 (26-39) |
(n = 86) 28 (24-37) |
(n = 103) 30 (25-38) |
| Fio2, median (IQR) | (n = 88) 0.80 (0.65-0.98) |
(n = 100) 0.90 (0.70-0.99) |
(n = 117) 0.90 (0.80-0.98) |
| Spo2, median (IQR), % | (n = 86) 92.0 (89.0-95.0) |
(n = 100) 92.0 (88.0-94.0) |
(n = 122) 92.0 (88.0-93.0) |
| Ratio of Spo2 to Fio2, median (IQR), % | (n = 81) 118.8 (95.9-146.7) |
(n = 89) 103.4 (92.6-135.7) |
(n = 109) 98.0 (92.0-116.3) |
| Pao2, median (IQR), mm Hg | (n = 69) 66.0 (57.0-81.8) |
(n = 71) 63.0 (56.3-72.8) |
(n = 94) 64.5 (54.0-77.3) |
| Ratio of Pao2 to Fio2, median (IQR), mm Hg | (n = 65) 89.0 (69.5-111.0) |
(n = 65) 75.0 (60.0-98.1) |
(n = 84) 76.0 (60.0-98.0) |
| Paco2, median (IQR), mm Hg | (n = 70) 43.1 (34.5-49.5) |
(n = 76) 36.8 (30.8-46.1) |
(n = 97) 40.5 (34.5-47.3) |
Abbreviations: Fio2, fraction of inspired oxygen; NA, not applicable; Spo2, oxygen saturation as measured by pulse oximetry.
In continuous positive airway pressure mode.
Classified by hospital site as “other” and included noninvasive ventilation device in continuous positive airway pressure mode (n = 17) or the specific type of continuous positive airway pressure device was missing (n = 7).
Use of prone positioning was recorded during follow-up and was defined as any use during the hospital stay (both prior to and after randomization). Does not include use of prone positioning after tracheal intubation.
Data reflect the worst physiological levels within the 60 minutes prior to undergoing tracheal intubation.
Treatment crossover occurred in 17.1% of participants (15.3% [58/380] in the CPAP group, 11.5% [48/418] in the HFNO group, and 23.6% [112/475] in the conventional oxygen therapy group; Figure 1 and eTable 3 in Supplement 2).
Primary Outcome
For the comparison of CPAP vs conventional oxygen therapy, the primary composite outcome of tracheal intubation or mortality within 30 days occurred in 36.3% (137/377) of the participants in the CPAP group vs 44.4% (158/356) of the participants in the conventional oxygen therapy group (absolute difference, −8% [95% CI, −15% to −1%], P = .03; Table 3).
Table 3. Primary and Secondary Outcomes in the Continuous Positive Airway Pressure Group vs the Conventional Oxygen Therapy Group .
| Continuous positive airway pressure | Conventional oxygen therapy | Difference (95% CI)a | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|---|
| Effect estimate (95% CI) | P valueb | Effect estimate (95% CI)c | P valueb | ||||
| Primary composite outcome | |||||||
| Tracheal intubation or mortality within 30 d, No./total (%) | 137/377 (36.3) | 158/356 (44.4) | AD, −8 (−15 to −1) | OR, 0.72 (0.53 to 0.96) | .03 | OR, 0.68 (0.48 to 0.94) | .02 |
| Secondary outcomes | |||||||
| Individual components of the primary composite outcome, No./total (%) | |||||||
| Tracheal intubation within 30 d | 126/377 (33.4) | 147/356 (41.3) | AD, −8 (−15 to −1) | OR, 0.71 (0.53 to 0.96) | .03 | OR, 0.67 (0.48 to 0.93) | .02 |
| Mortality within 30 d | 63/378 (16.7) | 69/359 (19.2) | AD, −3 (−8 to 3) | OR, 0.84 (0.58 to 1.23) | .37 | OR, 0.91 (0.59 to 1.39) | .65 |
| Tracheal intubation rate, No./total (%)d | 126/377 (33.4) | 147/356 (41.3) | AD, −8 (−15 to −1) | OR, 0.71 (0.53 to 0.96) | .03 | OR, 0.67 (0.48 to 0.93) | .02 |
| Admission to intensive care unit, No./total (%) | 204/368 (55.4) | 219/348 (62.9) | AD, −7 (−15 to −3) | OR, 0.73 (0.54 to 0.99) | .04 | OR, 0.69 (0.49 to 0.96) | .03 |
| Duration of invasive mechanical ventilation after tracheal intubation, median (IQR), de | (n = 126) 15.0 (8.0 to 25.0) |
(n = 147) 11.0 (6.0 to 23.0) |
MDND, 4.0 (0.04 to 8.0) | HR, 0.82 (0.61 to 1.09) | .17 | HR, 0.83 (0.61 to 1.12) | .22 |
| Time to event, median (IQR), d | |||||||
| Tracheal intubationf | (n = 126) 2.0 (1.0 to 4.0) |
(n = 147) 1.0 (0 to 4.0) |
MDND, 1.0 (0.2 to 1.8) | HR, 0.77 (0.61 to 0.98) | .03 | HR, 0.71 (0.56 to 0.91) | .01 |
| Deathg | (n = 74) 17.0 (11.0 to 26.0) |
(n = 79) 17.0 (11.0 to 24.0) |
MDND, 0 (−3.8 to 3.8) | HR, 0.86 (0.61 to 1.21) | .38 | HR, 0.93 (0.65 to 1.33) | .69 |
| Mortality, No./total (%) | |||||||
| During intensive care unit stay | 62/204 (30.4) | 66/219 (30.1) | AD, 3 (−9 to 9) | OR, 1.01 (0.67 to 1.53) | .95 | OR, 1.10 (0.69 to 1.75) | .68 |
| During hospital stay | 72/364 (19.8) | 78/346 (22.5) | AD, −3 (−9 to 3) | OR, 0.85 (0.59 to 1.22) | .37 | OR, 0.92 (0.62 to 1.38) | .69 |
| Length of stay, mean (SD), d | |||||||
| Intensive care unith | (n = 368) 9.5 (15.6) |
(n = 348) 9.6 (13.6) |
MD,−0.08 (−2.23 to 2.07) | .94 | MD, −0.16 (−2.30 to 1.99) | .88 | |
| Hospitali | (n = 364) 16.4 (17.5) |
(n = 346) 17.3 (18.1) |
MD,−0.96 (−3.59 to 1.67) | .47 | MD, −1.14 (−3.84 to 1.55) | .41 | |
Abbreviations: AD, absolute difference; HR, hazard ratio; MD, mean difference; MDND, median difference; OR, odds ratio.
Expressed as a percentage unless otherwise indicated.
The cutoff values for the P values to define statistical significance for the primary comparison of continuous positive airway pressure vs conventional oxygen therapy corrected for the interim analyses were equivalent to .044 for 2-sided P values (calculated using the method described by Jennison and Turnbull22). For the secondary outcomes (not corrected for the interim analyses), the significance threshold of .05 was used.
Adjusted for age, sex, morbid obesity (defined as a body mass index >35), race and ethnicity, Fio2, respiratory rate, and treatment phases. Hospital site was included as a random effect.
Outcome included tracheal intubation during the index hospital admission.
The proportional hazards assumption was tested in the unadjusted model; the P value was .54.
The proportional hazards assumption was tested in the unadjusted model; the P value was .01.
The proportional hazards assumption was tested in the unadjusted model; the P value was .89.
Patients who were not admitted to the intensive care unit were allocated a stay of 0 days.
Included time from emergency department arrival to hospital discharge.
For the comparison of HFNO vs conventional oxygen therapy, the primary composite outcome occurred in 44.3% (184/415) of the participants in the HFNO group vs 45.1% (166/368) of the participants in the conventional oxygen therapy group (absolute difference, −1% [95% CI, −8% to 6%], P = .83; Table 4).
Table 4. Primary and Secondary Outcomes in the High-Flow Nasal Oxygen Group vs the Conventional Oxygen Therapy Group.
| High-flow nasal oxygen | Conventional oxygen therapy | Difference (95% CI)a | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|---|
| Effect estimate (95% CI) | P valueb | Effect estimate (95% CI)c | P valueb | ||||
| Primary composite outcome | |||||||
| Tracheal intubation or mortality within 30 d, No./total (%) | 184/415 (44.3) | 166/368 (45.1) | AD, −1 (−8 to 6) | OR, 0.97 (0.73 to 1.29) | .83 | OR, 0.94 (0.68 to 1.29) | .69 |
| Secondary outcomes | |||||||
| Individual components of the primary composite outcome, No./total (%) | |||||||
| Tracheal intubation within 30 d | 170/415 (41.0) | 153/368 (41.6) | AD, −1 (−8 to 6) | OR, 0.98 (0.73 to 1.30) | .86 | OR, 0.94 (0.69 to 1.30) | .72 |
| Mortality within 30 d | 78/416 (18.8) | 74/370 (20.0) | AD, −1 (−7 to 4) | OR, 0.92 (0.65 to 1.32) | .66 | OR, 0.97 (0.65 to 1.46) | .90 |
| Tracheal intubation rate, No./total (%)d | 169/415 (40.7) | 154/368 (41.8) | AD, −1 (−8 to 6) | OR, 0.95 (0.72 to 1.27) | .75 | OR, 0.92 (0.67 to 1.27) | .62 |
| Admission to intensive care unit, No./total (%) | 252/408 (61.8) | 214/361 (59.3) | AD, 2 (−4 to 9) | OR, 1.11 (0.83 to 1.48) | .48 | OR, 1.04 (0.75 to 1.45) | .81 |
| Duration of invasive mechanical ventilation after tracheal intubation, median (IQR), de | (n = 169) 15.0 (8.0 to 26.0) |
(n = 154) 12.0 (6.0 to 23.0) |
MDND, 3.0 (−1.0 to 7.0) | HR, 0.92 (0.71 to 1.20) | .56 | HR, 1.01 (0.76 to 1.34) | .96 |
| Time to event, median (IQR), d | |||||||
| Tracheal intubationf | (n = 169) 1.0 (0 to 3.0) |
(n = 154) 1.0 (0 to 3.0) |
MDND, 0 (−0.4 to 0.4) | HR, 0.98 (0.78 to 1.21) | .82 | HR, 0.92 (0.74 to 1.16) | .49 |
| Deathg | (n = 88) 16.5 (9.0 to 22.5) |
(n = 85) 17.0 (11.0 to 24.0) |
MDND, 0 (−3.4 to 3.4) | HR, 0.94 (0.68 to 1.29) | .69 | HR, 0.94 (0.67 to 1.32) | .74 |
| Mortality, No./total (%) | |||||||
| During intensive care unit stay | 72/251 (28.7) | 65/214 (30.4) | AD, −2 (−10 to 7) | OR, 0.92 (0.62 to 1.38) | .69 | OR, 0.98 (0.63 to 1.54) | .94 |
| During hospital stay | 86/405 (21.2) | 80/359 (22.3) | AD, −1 (−7 to 5) | OR, 0.94 (0.67 to 1.33) | .73 | OR, 0.99 (0.67 to 1.47) | .97 |
| Length of stay, mean (SD), d | |||||||
| Intensive care unith | (n = 407) 10.5 (15.6) |
(n = 361) 9.6 (14.1) |
MD, 0.95 (−1.16 to 3.07) | .38 | MD, 0.47 (−1.57 to 2.50) | .65 | |
| Hospitali | (n = 405) 18.3 (20.0) |
(n = 359) 17.1 (18.0) |
MD, 1.21 (−1.50 to 3.93) | .38 | MD, 0.33 (−2.28 to 2.94) | .80 | |
Abbreviations: AD, absolute difference; HR, hazard ratio; MD, mean difference; MDND, median difference; OR, odds ratio.
Expressed as a percentage unless otherwise indicated.
The cutoff values for the P values to define statistical significance for the primary comparison of high-flow nasal oxygen vs conventional oxygen therapy corrected for the interim analyses were equivalent to .044 for 2-sided P values (calculated using the method described by Jennison and Turnbull22). For the secondary outcomes (not corrected for the interim analyses), the significance threshold of .05 was used.
Adjusted for age, sex, morbid obesity (defined as a body mass index >35), ethnicity, Fio2, respiratory rate, and treatment phases. Hospital site was included as a random effect.
Outcome included tracheal intubation during the index hospital admission.
The proportional hazards assumption was tested in the unadjusted model; the P value was .32.
The proportional hazards assumption was tested in the unadjusted model; the P value was .81.
The proportional hazards assumption was tested in the unadjusted model; the P value was .64.
Patients who were not admitted to intensive care unit were allocated a stay of 0 days.
Included time from emergency department arrival to hospital discharge.
The cutoff values for the P values to define statistical significance for the primary comparisons of CPAP and HFNO vs conventional oxygen therapy (corrected for the interim analyses) were equivalent to .044 for 2-sided P values.
Secondary Outcomes
The secondary outcomes appear in Tables 3-4 and in eFigures 2-4 in Supplement 2. There was a significant difference in the individual incidence of tracheal intubation within 30 days in the CPAP group (33.4% vs 41.3% in the conventional therapy group; absolute difference, −8% [95% CI, −15% to −1%]). However, a significant difference was not observed for the individual incidence of mortality within 30 days in the CPAP group (16.7% vs 19.2% in the conventional therapy group; absolute difference, −3% [95% CI, −8% to 3%]). Compared with conventional oxygen therapy, neither CPAP nor HFNO significantly reduced mortality in the ICU or in the hospital.
Significantly fewer participants in the CPAP group required admission to the ICU compared with participants in the conventional oxygen therapy group (55.4% vs 62.9%, respectively; absolute difference, −7% [95% CI, −15% to −3%]). Among the participants who required tracheal intubation, there was a statistically significant increase in the median time to tracheal intubation in the CPAP group (2.0 days [IQR, 1.0 to 4.0 days]) compared with the conventional oxygen therapy group (1.0 day [IQR, 0 to 4.0 days]) (median difference, 1.0 day [95% CI, 0.2 to 1.8 days]; Table 3). For all other outcomes and comparisons, there was no statistically significant difference between study groups.
Exploratory Outcomes
The findings from both the adjusted analyses and the inverse probability weighting analysis were consistent with the primary analysis (eTable 4 in Supplement 2). The majority of tests for interaction in the subgroup analyses were not statistically significant (Figure 2 and Figure 3). However, the comparison of the HFNO group vs the conventional oxygen therapy group for Fio2 was significant (P = .02; Figure 3). The findings were broadly consistent between the unadjusted and adjusted subgroup analyses (Figures 2-3 and eFigure 5 in Supplement 2).
Figure 2. Unadjusted Subgroup Analyses for Tracheal Intubation or Mortality Within 30 Days in the Continuous Positive Airway Pressure (CPAP) Group vs the Conventional Oxygen Therapy Group.
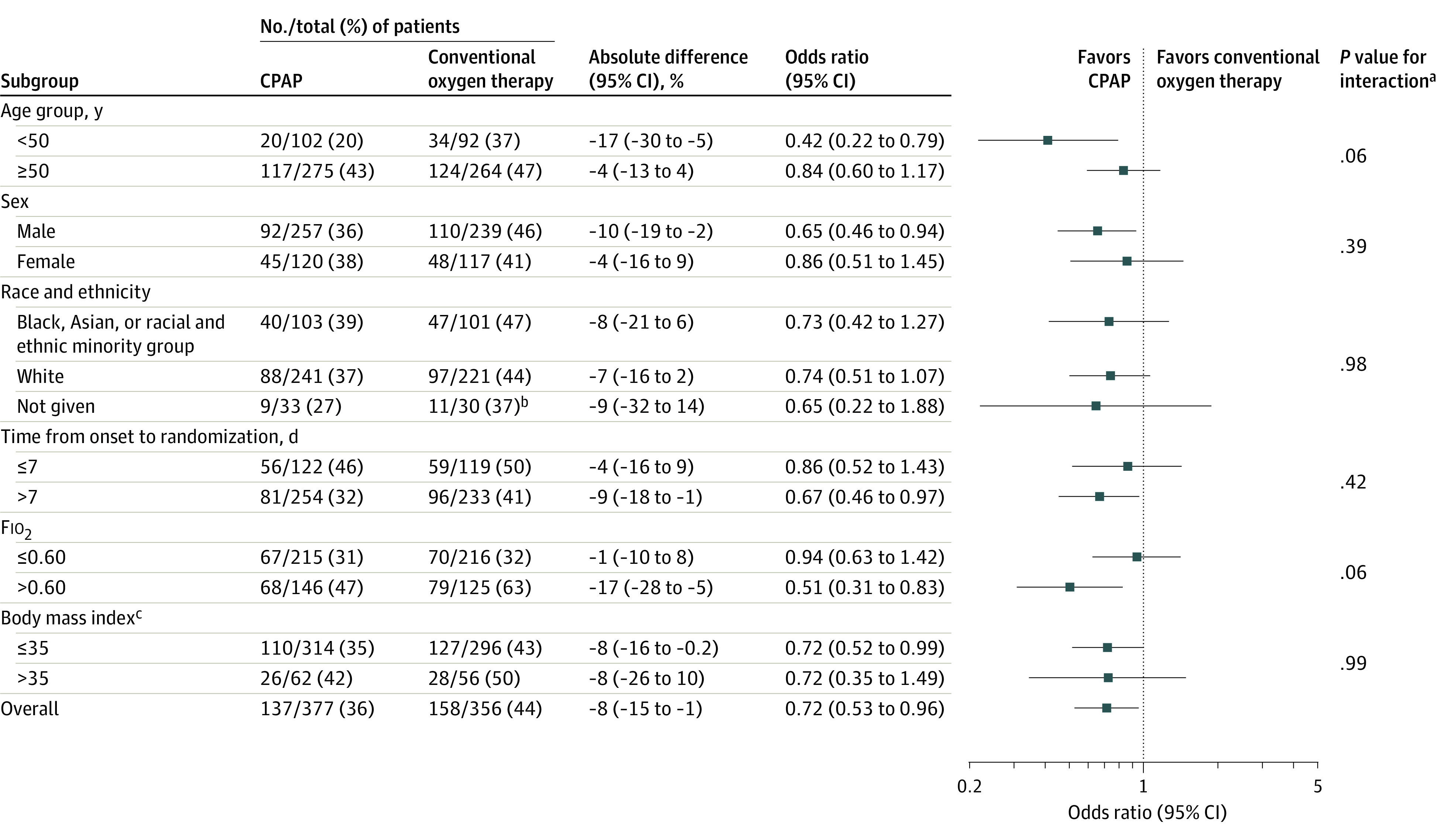
Fio2 indicates fraction of inspired oxygen.
aThe P values were calculated using the test for the subgroup × treatment interaction.
bComparison is limited to those who might have been randomized based on equipment availability.
cCalculated as weight in kilograms divided by height in meters squared. Morbid obesity was defined as a body mass index greater than 35.
Figure 3. Unadjusted Subgroup Analyses for Tracheal Intubation or Mortality Within 30 Days in the High-Flow Nasal Oxygen (HFNO) Group vs the Conventional Oxygen Therapy Group.
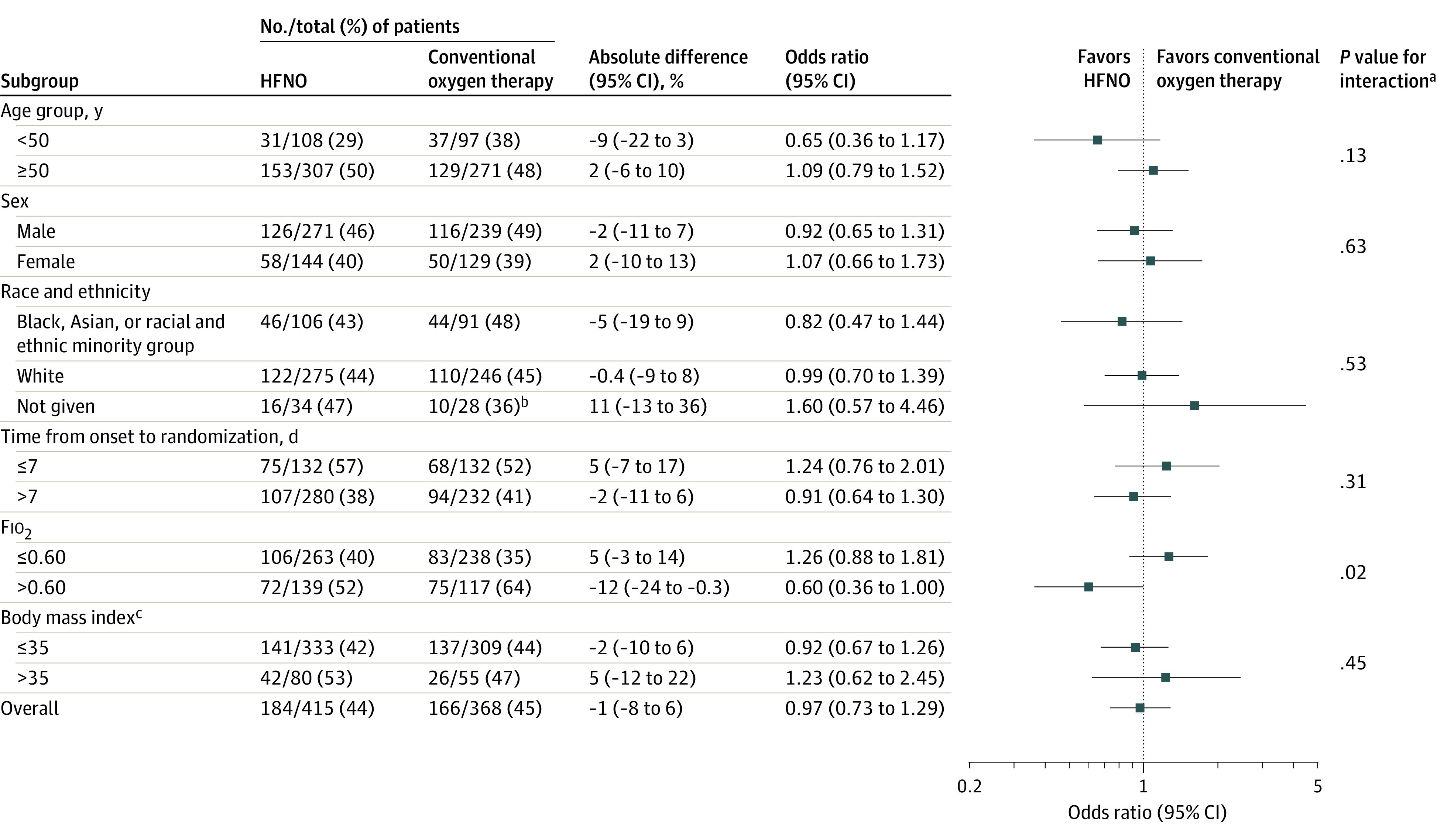
Fio2 indicates fraction of inspired oxygen.
aThe P values were calculated using the test for the subgroup × treatment interaction.
bComparison is limited to those who might have been randomized based on equipment availability.
cCalculated as weight in kilograms divided by height in meters squared. Morbid obesity was defined as a body mass index greater than 35.
Post Hoc Outcomes
A post hoc analysis, which compared CPAP and HFNO, included 570 participants who were randomized across all 3 groups. The primary outcome occurred in 34.6% (91/263) of participants in the CPAP group and in 44.3% (136/307) of participants in the HFNO group (absolute difference, −10% [95% CI, −18% to −2%], P = .02; eTable 5 in Supplement 2).
Adverse Events
Adverse events occurred most frequently in the CPAP group; 34.2% (130/380) of participants in the CPAP group experienced adverse events, followed by 20.6% (86/418) of participants in the HFNO group, and 13.9% (66/475) of participants in the conventional oxygen therapy group (eTable 6 in Supplement 2). Eight participants experienced serious adverse events (7 in the CPAP group and 1 in the conventional oxygen therapy group). Four of the serious adverse events were classified as probably or possibly linked to the trial intervention and all of these events occurred in the CPAP group (1 patient with surgical emphysema and pneumomediastinum, 2 patients with pneumothorax and pneumomediastinum, and 1 patient with vomiting requiring emergency tracheal intubation).
Discussion
In this randomized clinical trial of patients with acute hypoxemic respiratory failure due to COVID-19, an initial strategy of CPAP, compared with conventional oxygen therapy, significantly reduced the composite outcome of tracheal intubation or mortality within 30 days. In contrast, there was no significant difference between an initial strategy of HFNO and conventional oxygen therapy, although given the width of the 95% CI, the trial may have been underpowered to detect small but clinically important treatment effects.
This decrease in the incidence of the primary outcome with CPAP was attributable to a significant decrease in the need for tracheal intubation. Neither HFNO nor CPAP reduced mortality compared with conventional oxygen therapy. More adverse events were reported in the CPAP group.
This pragmatic trial was designed to be deliverable in the context of a pandemic and tested interventions that precluded blinding of either the participant or treating clinician. The decision to perform tracheal intubation, and thereby commence invasive mechanical ventilation, was not standardized.11 It is possible that the lower tracheal intubation rate in the CPAP group may have been driven by a greater willingness among clinicians and patients to delay tracheal intubation, and this may be supported by the finding that the time to tracheal intubation was longer in the CPAP group. However, physiological levels at the time of tracheal intubation were similar across groups, suggesting that, irrespective of treatment strategy, clinicians used a similar threshold to determine the need for tracheal intubation. Furthermore, this effect was not observed with HFNO, which should have been susceptible to the same risk of performance bias.
The decision to not standardize escalation to tracheal intubation was driven by clinical uncertainty regarding the optimal timing and threshold of tracheal intubation in patients with COVID-19.11,23 Although rapidly building clinical consensus may be achievable in trials recruiting patients from a small number of hospitals, such as in the Helmet Noninvasive Ventilation Versus High-Flow Oxygen Therapy in Acute Hypoxemic Respiratory Failure (HENIVOT) trial,24 the RECOVERY-RS trial management group determined that any attempt to stipulate specific criteria might influence clinical equipoise and patient acceptability, affect trial recruitment, and, more importantly, reduce trial generalizability. Previous large trials of noninvasive respiratory strategies have differed in their approach to protocolization of tracheal intubation, which likely reflects these specific challenges, even in respiratory conditions for which the pathophysiology has been well described.25,26,27
A recent systematic review and meta-analysis5 of 25 randomized clinical trials (3804 patients) summarized evidence on the clinical effectiveness of noninvasive ventilation (with or without pressure support) and HFNO, compared with conventional oxygen therapy, in patients with acute respiratory failure. Across 14 trials (1275 patients), noninvasive ventilation via a face mask was significantly associated with a lower risk of both mortality and tracheal intubation. In contrast, HFNO was significantly associated with a lower risk of tracheal intubation (5 trials; 1479 patients), but not mortality (3 trials; 1279 patients). The RECOVERY-RS trial found that CPAP significantly reduced tracheal intubation, but not mortality, although the wide 95% CI precludes the drawing of a specific conclusion about the effect on mortality. The current trial further found that HFNO did not significantly reduce the need for tracheal intubation. One explanation for these discordant findings is differences in pathophysiology between COVID pneumonitis and other causes of acute respiratory failure.5,28 Furthermore, in the RECOVERY-RS trial, some hospitals modified care pathways to deliver CPAP and HFNO outside an ICU, which may have influenced the clinical effectiveness of the interventions.
The current trial builds on the findings of 2 other recently published randomized clinical trials that examined the use of noninvasive respiratory strategies in patients with COVID-19.24,29 The High-Flow Nasal Cannula in Severe COVID-19 With Acute Hypoxemic Respiratory Failure (HiFLo-Covid) trial29 compared HFNO with conventional oxygen therapy in 220 adults with severe COVID-19 across 3 Colombian hospitals. The trial reported that HFNO both reduced the need for tracheal intubation (hazard ratio, 0.62; 95% CI, 0.39-0.96) and time to clinical recovery. In contrast to the current trial (RECOVERY-RS) and the HiFLo-Covid trial,29 the HENIVOT trial24 directly compared 2 noninvasive respiratory strategies, namely noninvasive ventilation via a helmet (with pressure support) vs HFNO. In 110 patients with COVID-19 recruited across 4 ICUs, there was no significant difference for the primary outcome of days free of respiratory support, although significantly fewer patients in the helmet noninvasive ventilation group required tracheal intubation (odds ratio, 0.41; 95% CI, 0.18-0.89). The protocolized approach to the setup and weaning of trial interventions and the decision to perform tracheal intubation in both the HENIVOT and HiFLo-Covid trials potentially limits their generalizability.
Limitations
This trial has several limitations. First, the trial did not achieve its planned sample size with the decision to stop recruitment driven by the end of the funded recruitment period, together with declining numbers of patients with COVID-19 in the UK, and an ethical obligation to share accumulated data with the international clinical community. The decision to stop trial recruitment early did not involve the members of the data and safety monitoring committee, which was the only group that had seen the interim analyses, such that the risk of bias arising from stopping the trial early is likely to be minimal. However, the trial may have been underpowered to detect small but clinically important treatment effects for the comparison of HFNO vs conventional oxygen therapy.
Second, there was crossover between the allocated treatment groups, principally from the conventional oxygen therapy group to 1 or both interventions. This is a common challenge in trials of noninvasive respiratory strategies26,27 and reduces the observed effect size of a clinically effective treatment. Nevertheless, the findings from the inverse probability weighting analysis were consistent with the primary analysis. Third, it was determined that it would be impractical to collect screening data, therefore, it was not possible to describe the reasons and number of patients who were not randomized.
Fourth, the trial’s definition of acute hypoxemic respiratory failure was based on objective criteria of oxygenation and oxygen use. In clinical practice, the decision to commence noninvasive respiratory strategies may be based both on objective criteria, such as these, and subjective criteria, such as respiratory distress.
Fifth, the study population, particularly in terms of racial and ethnic groups, may not be generalizable across all populations. Sixth, there were some minor differences across groups at baseline in relation to comorbid state.
Seventh, the trial was rapidly setup early in the pandemic, prior to the development of a core outcome set for COVID-19 trials.30 Even though the outcome list aligns closely to most of the core outcomes subsequently identified, the trial did not capture information on patient recovery after hospital discharge.
Conclusions
Among patients with acute hypoxemic respiratory failure due to COVID-19, an initial strategy of CPAP significantly reduced the risk of tracheal intubation or mortality compared with conventional oxygen therapy, but there was no significant difference between an initial strategy of HFNO compared with conventional oxygen therapy. The study may have been underpowered for the comparison of HFNO vs conventional oxygen therapy, and early study termination and crossover among the groups should be considered when interpreting the findings.
Trial protocol and statistical analysis plan
eFigure 1. RECOVERY-RS trial recruitment and UK hospitalized COVID-19 patients
eFigure 2. Kaplan Meier curve by treatment arm: time to tracheal intubation (all participants)
eFigure 3. Kaplan Meier curve by treatment arm: duration of invasive ventilation (intubated participants only)
eFigure 4. Kaplan Meier curve by treatment arm: time to death (all participants)
eFigure 5. Adjusted sub-group analyses: tracheal Intubation or mortality within 30 days
eTable 1. Summary of randomizations and data for pairwise comparisons
eTable 2. Additional participant baseline characteristics
eTable 3. Summary of trial crossover by treatment group
eTable 4. Inverse probability weighting analysis
eTable 5. Primary outcome comparison between continuous positive airway pressure and high-flow nasal oxygen
eTable 6. Adverse events and serious adverse events by treatment arm
Nonauthor collaborators
Data sharing statement
References
- 1.Docherty AB, Mulholland RH, Lone NI, et al. ; ISARIC4C Investigators . Changes in in-hospital mortality in the first wave of COVID-19: a multicentre prospective observational cohort study using the WHO clinical characterisation protocol UK. Lancet Respir Med. 2021;9(7):773-785. doi: 10.1016/S2213-2600(21)00175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Docherty AB, Harrison EM, Green CA, et al. ; ISARIC4C Investigators . Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545-1546. doi: 10.1001/jama.2020.4031 [DOI] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreyro BL, Angriman F, Munshi L, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324(1):57-67. doi: 10.1001/jama.2020.9524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000;284(18):2352-2360. doi: 10.1001/jama.284.18.2352 [DOI] [PubMed] [Google Scholar]
- 7.Carteaux G, Millán-Guilarte T, De Prost N, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med. 2016;44(2):282-290. doi: 10.1097/CCM.0000000000001379 [DOI] [PubMed] [Google Scholar]
- 8.Esquinas AM, Egbert Pravinkumar S, Scala R, et al. ; International NIV Network . Noninvasive mechanical ventilation in high-risk pulmonary infections: a clinical review. Eur Respir Rev. 2014;23(134):427-438. doi: 10.1183/09059180.00009413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorman E, Connolly B, Couper K, Perkins GD, McAuley DF. Non-invasive respiratory support strategies in COVID-19. Lancet Respir Med. 2021;9(6):553-556. doi: 10.1016/S2213-2600(21)00168-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marciniak SJ, Farrell J, Rostron A, et al. COVID-19 pneumothorax in the UK: a prospective observational study using the ISARIC WHO clinical characterisation protocol. Eur Respir J. Published online September 16, 2021. doi: 10.1183/13993003.00929-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azoulay E, de Waele J, Ferrer R, et al. International variation in the management of severe COVID-19 patients. Crit Care. 2020;24(1):486. doi: 10.1186/s13054-020-03194-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 13.Perkins GD, Couper K, Connolly B, et al. RECOVERY–Respiratory Support: respiratory strategies for patients with suspected or proven COVID-19 respiratory failure; continuous positive airway pressure, high-flow nasal oxygen, and standard care: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):687. doi: 10.1186/s13063-020-04617-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathur R, Rentsch CT, Morton CE, et al. ; OpenSAFELY Collaborative . Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021;397(10286):1711-1724. doi: 10.1016/S0140-6736(21)00634-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. doi: 10.1016/S1473-3099(20)30195-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kow CS, Hasan SS. Pitfalls in reporting sample size calculation across randomized controlled trials involving ivermectin for the treatment of COVID-19. Am J Ther. 2021;28(5):e616-e619. doi: 10.1097/MJT.0000000000001441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev. 2017;85(2):185-203. doi: 10.1111/insr.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13-22. doi: 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 20.RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi: 10.1016/S0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators . Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491-1502. doi: 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jennison C, Turnbull BW. Group Sequential Methods With Applications to Clinical Trials. Chapman & Hall/CRC; 2000. [Google Scholar]
- 23.Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care. 2021;25(1):121. doi: 10.1186/s13054-021-03540-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grieco DL, Menga LS, Cesarano M, et al. ; COVID-ICU Gemelli Study Group . Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325(17):1731-1743. doi: 10.1001/jama.2021.4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemiale V, Mokart D, Resche-Rigon M, et al. ; Groupe de Recherche en Réanimation Respiratoire du patient d’Onco-Hématologie (GRRR-OH) . Effect of noninvasive ventilation vs oxygen therapy on mortality among immunocompromised patients with acute respiratory failure: a randomized clinical trial. JAMA. 2015;314(16):1711-1719. doi: 10.1001/jama.2015.12402 [DOI] [PubMed] [Google Scholar]
- 26.Frat J-P, Thille AW, Mercat A, et al. ; FLORALI Study Group; REVA Network . High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185-2196. doi: 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 27.Azoulay E, Lemiale V, Mokart D, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018;320(20):2099-2107. doi: 10.1001/jama.2018.14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osuchowski MF, Winkler MS, Skirecki T, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9(6):622-642. doi: 10.1016/S2213-2600(21)00218-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ospina-Tascón GA, Calderón-Tapia LE, García AF, et al. ; HiFLo-Covid Investigators . Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: a randomized clinical trial. JAMA. 2021;326(21):2161-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong A, Elliott JH, Azevedo LC, et al. ; COVID-19-Core Outcomes Set (COS) Workshop Investigators . Core outcomes set for trials in people with coronavirus disease 2019. Crit Care Med. 2020;48(11):1622-1635. doi: 10.1097/CCM.0000000000004585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eFigure 1. RECOVERY-RS trial recruitment and UK hospitalized COVID-19 patients
eFigure 2. Kaplan Meier curve by treatment arm: time to tracheal intubation (all participants)
eFigure 3. Kaplan Meier curve by treatment arm: duration of invasive ventilation (intubated participants only)
eFigure 4. Kaplan Meier curve by treatment arm: time to death (all participants)
eFigure 5. Adjusted sub-group analyses: tracheal Intubation or mortality within 30 days
eTable 1. Summary of randomizations and data for pairwise comparisons
eTable 2. Additional participant baseline characteristics
eTable 3. Summary of trial crossover by treatment group
eTable 4. Inverse probability weighting analysis
eTable 5. Primary outcome comparison between continuous positive airway pressure and high-flow nasal oxygen
eTable 6. Adverse events and serious adverse events by treatment arm
Nonauthor collaborators
Data sharing statement


