Abstract
Rotavirus strains that caused severe diarrhea in 4,634 (2,533 male) children aged less than 5 years and admitted to major hospitals in eight centers throughout Australia from 1993 to 1996 were subject to antigenic and genetic analyses. The G serotypes of rotaviruses were identified in 81.9% (3,793 of 4,634) children. They included 67.8% (from 3,143 children) serotype G1 isolates (containing 46 electropherotypes), 11.5% (from 531 children) serotype G2 isolates (27 electropherotypes), 0.8% (from 39 children) serotype G3 isolates (8 electropherotypes), and 1.6% (from 76 children) serotype G4 isolates (9 electropherotypes). G6 (two strains) and G8 (two strains) isolates were identified during the same period. G1 serotypes were predominant in all centers, with intermittent epidemics of G2 serotypes and sporadic detection of G3 and G4 strains. With the exception of two strains (typed as G1P2A[6] and G2P2A[6]) all serotype G1, G3, and G4 strains were P1A[8] and all serotype G2 strains were P1B[4]. Two contrasting epidemiological patterns were identified. In all temperate climates rotavirus incidence peaked during the colder months. The genetic complexity of strains (as judged by electropherotype) was greatest in centers with large populations. Identical electropherotypes appeared each winter in more than one center, apparently indicating the spread of some strains both from west to east and from east to west. Centers caring for children in small aboriginal communities showed unpredictable rotavirus peaks unrelated to climate, with widespread dissemination of a few rotavirus strains over distances of more than 1,000 km. Data from continued comprehensive etiological studies of genetic and antigenic variations in rotaviruses that cause severe disease in young children will serve as baseline data for the study of the effect of vaccination on the incidence of severe rotavirus disease and on the emergence of new strains.
Group A rotaviruses are the single most important cause of severe acute diarrhea in young children throughout the world. Hospital-based studies reveal that they are the cause of acute diarrhea in 20 to 70% of children less than 5 years old in developed and developing countries and the cause of death in approximately 800,000 children annually in developing countries (28).
Rotaviruses are members of the family Reoviridae. Most human infections are caused by group A rotaviruses that are classified into serotypes by a dual classification system based on neutralizing antigens on two outer capsid proteins, VP7 (G serotype) and VP4 (P serotype) (7, 15). To date, 10 G types and more than 5 P types have been identified in infected humans. There is great genetic diversity within each G and P type on the basis of the gel electrophoretic analysis of gene patterns (electropherotypes). Epidemiological and molecular studies in many countries show complex patterns of change from year to year in the serotypes and electropherotypes that cause diarrhea in hospitalized children from the same geographical areas (2, 9). To date, the majority of severe disease worldwide has been caused by serotypes G1, G2, G3, G4, and P1A (genotype P[8]) and serotype P1B (genotype P[4]) (19). Recent epidemiological studies in Bangladesh (33), Brazil (17, 30), India (1), Kenya (19), and the United States (29) show that other G and P types (G5, G6, G8, G9, G10, P2A[6], P8[11]) can be common and may be of emerging importance in some communities (12).
Rotavirus vaccines are being developed to reduce the huge impact of this disease. The current live oral vaccines focus on the prevention of severe disease caused by the four major human rotavirus serotypes, serotypes G1, G2, G3, and G4 (15). These vaccines could show reduced effectiveness in countries where “novel” rotavirus strains are common. Detailed worldwide epidemiological studies are required to identify the rotavirus serotypes that cause severe disease and to map annual changes in strains in different communities. Results can be used to select areas for vaccine trials and to serve as baselines for identification of new strains should they emerge.
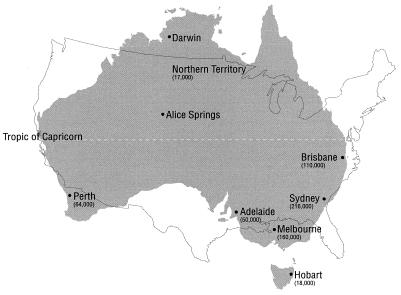
Australia is a large island continent whose area is approximately equal to that of the continental United States, with a total population of 19 million residing predominantly in urban centers separated by distances ranging from 800 to 2,000 km (Fig. 1). The populations served by these centers include 17,000 to 216,000 children <5 years of age. Approximately 10,000 Australian children are admitted to a hospital annually for treatment of severe acute rotavirus diarrhea (4). Australia provides an ideal setting in which to conduct annual surveillance of rotavirus strains in widely dispersed urban centers, with different population densities experiencing tropical (Darwin), hot and arid (Alice Springs), and temperate climates and having different lifestyles; these populations include children living in isolated aboriginal communities (Darwin, Alice Springs). This study aimed to collect all rotavirus-positive fecal specimens from children less than 5 years of age who were admitted to a hospital in eight urban centers during 4 successive years from January 1993 to December 1996. The results show different epidemiological patterns in relation to population size, climate, and/or lifestyle and establish the existence of widespread dissemination of some rotavirus strains. The results also establish baseline data that could be used to choose appropriate centers for testing of the efficacies of rotavirus vaccines and monitoring of the changes in the prevalent rotavirus strains after introduction of rotavirus vaccines.
FIG. 1.
Locations of urban centers in Australia from which rotavirus-positive stool specimens were obtained. The map is superimposed on a map of the continental United States drawn to the same scale. The values in parentheses represent numbers of children aged <5 years residing in each center or in the Northern Territory (inclusive of Alice Springs and Darwin). The values are approximate and are calculated on the basis of 1996 census results.
MATERIALS AND METHODS
Patients and sample collection.
Feces were obtained from all children (aged <5 years) admitted for treatment of acute diarrhea in hospitals in eight Australian cities. Fecal specimens were collected between January 1993 and December 1996. Specimens were obtained within 48 h of admission to a hospital from children with a primary diagnosis of acute diarrhea and were initially processed in the routine diagnostic laboratories of each participating hospital. Children with nonsocomial infections were excluded. Diagnosis of rotavirus infection was made by a variety of assays, including electron microscopy, enzyme immunoassay (EIA), and latex agglutination. All rotavirus-positive fecal specimens were stored at −20 or −70°C in each laboratory for 2 to 3 months, transported frozen to Royal Children's Hospital Melbourne, stored at −70°C, and thawed immediately prior to further testing.
During the 4 years, rotavirus-positive fecal specimens were received from 4,634 patients of whom 2,533 (55.7%) were male. The numbers of rotavirus-positive specimens examined each year from each center are listed in Table 1.
TABLE 1.
Numbers of rotavirus-positive fecal specimens from hospitalized children in eight urban centers in Australia, 1993 to 1996
| Center | No. of rotavirus-positive specimens
|
|||
|---|---|---|---|---|
| 1993 | 1994 | 1995 | 1996 | |
| Perth | 255 | 293 | 307 | 290 |
| Darwin | 41 | 97 | 61 | 21 |
| Alice Springs | 101 | 109 | 77 | 91 |
| Adelaide | 196 | 180 | 342 | 206 |
| Brisbane | 113 | 73 | 135 | 61 |
| Sydney | 134 | 89 | 160 | 74 |
| Melbourne | 165 | 331 | 242 | 246 |
| Hobart | 24 | 32 | 30 | 58 |
| Total | 1,029 | 1,204 | 1,354 | 1,047 |
Assays.
The presence of detectable rotavirus antigen was confirmed in all specimens after transport to the Royal Children's Hospital laboratories by an in-house EIA that incorporates monoclonal antibodies specific for group A subgroup I and subgroup II antigens (5). Rotaviruses were serotyped by an EIA that incorporates neutralizing monoclonal antibodies specific for G1, G2, G3, and G4 antigens and for P1A, P1B, and P2 antigens (5, 18); the EIA was supplemented by reverse transcription-PCR assays (10, 11) for nonserotypeable strains. The genetic compositions of rotavirus-positive specimens were analyzed by polyacrylamide gel electrophoresis of extracted genomic double-stranded RNA (6). Rotaviruses were assigned an electropherotype on the basis of the pattern formed by migration of the 11 genes. Assignment of electropherotype was done visually. Coelectrophoresis of extracted genomic double-stranded RNA was used to compare apparently identical electropherotypes that appeared in more than one geographical location.
All rotavirus-positive fecal specimens were assayed to determine the rotavirus G type. Polyacrylamide gel electrophoresis was performed with specimens confirmed to be EIA positive in our laboratories and included all EIA-positive specimens identified in 1993, all nontypeable strains identified from 1994 to 1996, and representative G-typeable strains identified from 1994 to 1996 (i.e., each fifth rotavirus strain sequentially identified), provided that sufficient fecal material was available. A total of 232 representative rotavirus-positive specimens (selected on the basis of G type and electropherotype) were assayed to determine the rotavirus P type. These included specimens positive for at least one representative of all of the most common rotavirus electropherotypes identified and comprised 155 strains of G1 (representing 27 electropherotypes), 54 strains of G2 (15 electropherotypes), 7 strains of G3 (5 electropherotypes), and 16 strains of G4 (5 electropherotypes).
RESULTS
Peak rates of incidence of rotavirus infection occurred in the colder months (April to October) in all six centers located in temperate regions of the country (Perth, Adelaide, Hobart, Melbourne, Sydney, Brisbane). Patterns of occurrence have been published elsewhere for each of these centers (4). Peaks of rotavirus disease were consistently observed 1 to 2 months earlier in the western city of Perth compared with the times of peak incidence in the eastern states.
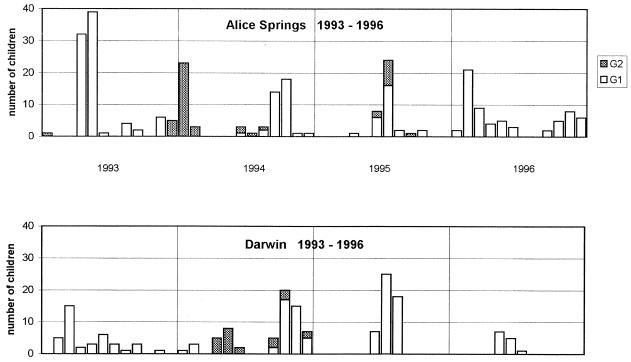
There was no consistent seasonal pattern in the tropical center of Darwin or the hot, arid center of Alice Springs (Fig. 2). They had similar temporal occurrences of peaks that varied from January to November in different years, including two separate peaks of rotavirus activity during 1994 in both centers and in 1996 in Alice Springs alone. Epidemic peaks occurred simultaneously in late 1994 and in 1995. Sequential epidemic peaks implied the spread of rotavirus from Darwin to Alice Springs in 1993 and from Alice Springs to Darwin in 1993 and 1994, despite the 1,200-km distance between the two centers. Characterization of strains (see below) confirmed the spread of strains between these centers during the latter epidemics.
FIG. 2.
Frequencies of rotavirus G1 and G2 serotypes in diarrheal stools from children hospitalized each month in Alice Springs and Darwin.
Characterization of strains.
The G serotypes of rotavirus were identified overall in 81.9% (3,793 of 4,634) of the children, including serotype G1 rotaviruses in 3,143 (67.8%), serotype G2 in 531 (11.5%), serotype G3 in 39 (0.8%), and serotype G4 in 76 (1.6%). In addition, a single G6 strain was identified in Melbourne in 1993 and in Adelaide in 1996 (24). A single G8 strain was identified in Darwin in 1996 and in Brisbane in 1996 (26). All except one of the strains of serotypes G1, G3, and G4 were identified as type P1A[8]; the exception was a Melbourne G1 strain (1995), identified as P2A[6]. All except one of the strains of serotype G2 were type P1B[4]; the exception was an Alice Springs strain (1995), identified as P2A[6]. The rotaviruses in 841 (18.1%) fecal specimens were not typeable.
Electropherotypes were assigned to the rotaviruses in 1,845 of 2,206 (83.6%) of the specimens examined. Overall, 90 different electropherotypes were identified, including 46 within serotype G1, 27 within serotype G2, 8 within serotype G3, and 9 within serotype G4. In general, the electropherotypes of untypeable strains were identical to those of the typeable strains simultaneously present in the same locations.
Composite results indicating an electropherotype that was inconsistent with the subgroup, together with a failure to react with G1 to G4 neutralizing monoclonal antibodies, led to the identification of unusual rotaviruses that were investigated further by RT (reverse transcription)-PCR and by determination of the nucleic acid sequences of the relevant genes. Electropherotypes present in Alice Springs and Darwin in 1994 were shown to be antigenic variants of G2 strains that resulted from human-human G1 and G2 reassortment and have been described elsewhere (25).
Patterns of occurrence of rotavirus strains.
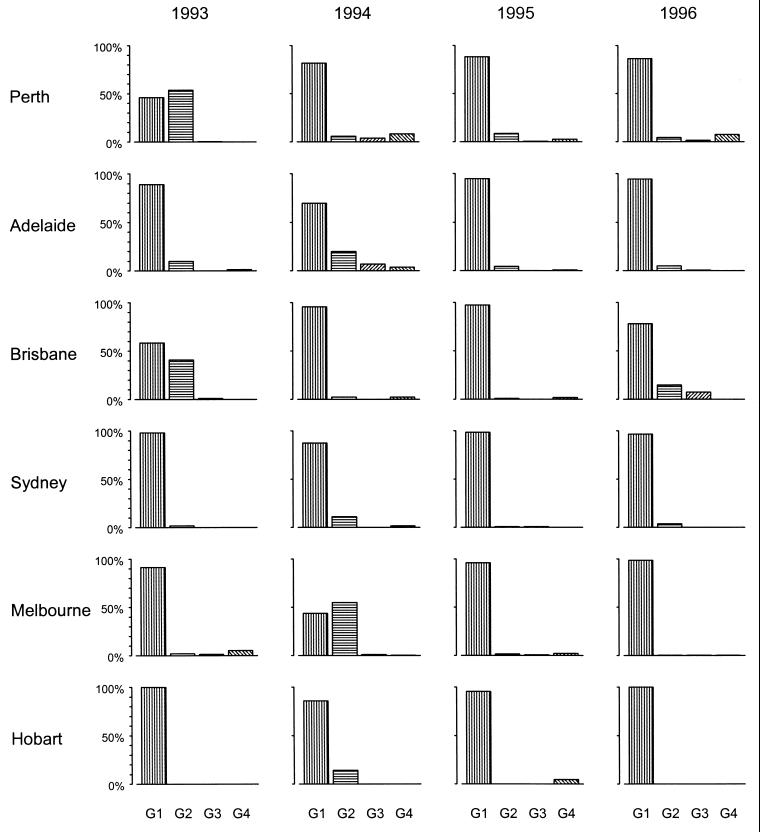
The relative frequencies of individual serotypes varied from year to year in the same center and from center to center during the same year (Fig. 2 and 3).
FIG. 3.
Relative frequencies of occurrence of rotavirus G1, G2, G3, and G4 serotypes in hospitalized children in temperate urban centers throughout Australia, 1993 to 1996.
(i) G1 serotypes.
Strains of the G1 serotype were predominant (prevalence, 30 to 98%) in all centers in all years with the exception of Perth in 1993 and Melbourne in 1994. Most centers showed the coexistence of 3 to 11 G1-associated electropherotypes, with >50% of the strains identified during an epidemic peak being of one to two dominant electropherotypes. In general, fewer electropherotypes (one to three) were identified simultaneously in centers with smaller populations (Alice Springs, Darwin, Hobart), whereas five or more electropherotypes were identified during annual epidemics in the larger centers. Within each center dominant electropherotypes coexisted with less-common electropherotypes that appeared to be unique to that locality.
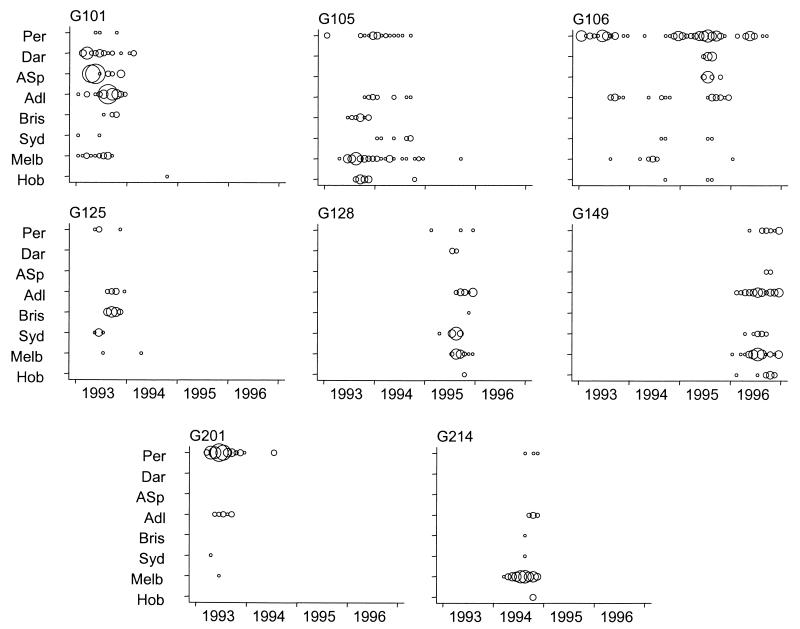
There was no evidence that dominant electropherotypes had appeared in small numbers in the same city during the preceding winter epidemic. The gene patterns of the dominant electropherotypes that occurred sequentially in the same center showed variations in mobility involving 2 to 11 genes, with the most common visible changes occurring in genes 7 and 8. Twenty-one of the total 46 G1-associated electropherotypes identified Australia-wide appeared concurrently in more than one state during a single year. The patterns of occurrence of the six most common G1-associated electropherotypes identified from 1993 to 1996 are shown in Fig. 4. There was no evidence of a consistent direction of spread of individual electropherotypes either from west to east or from south to north. Three electropherotypes (types G105, G106, and G128) were first identified in Perth prior to their identification in the eastern states. Three electropherotypes (types G101, G125, and G149) were identified in eastern states before they appeared in Perth. Only two of the electropherotypes (types G105 and G106) persisted in the same center for more than one epidemic season. One electropherotype (type G106) persisted in Perth during all 4 years of surveillance and in Adelaide, Sydney, Melbourne, and Hobart during 2 or 3 of the 4 years. Dual peaks in the incidence of rotavirus disease in Perth noted in 1994, in April and in December, were associated with two different electropherotypes (types G105 and G106).
FIG. 4.
Monthly occurrence of dominant rotavirus electropherotypes of serotypes G1 (six strains) and G2 (two strains) identified in Australian urban centers from 1993 to 1996. The areas of the circles are proportional to the number of isolates. Per, Perth; Dar, Darwin; ASp, Alice Springs; Adl, Adelaide; Bris, Brisbane; Syd, Sydney; Mel, Melbourne; Hob, Hobart.
(ii) G2 serotype.
Strains of the G2 serotype were identified in most urban centers in most years but showed marked fluctuations in frequencies (1 to 52%) and were more common overall during 1993 and 1994 than in the following 2 years. G2 strains were consistently present throughout the 4 years in Perth and Adelaide, with intermittent epidemics in each of the other centers. G2 strains (of different electropherotypes) were the dominant strains in Perth in 1993 and in Melbourne in 1994 with the number of G2 strains identified exceeding the number of G1 strains identified. Seven of the 27 electropherotypes of G2 identified during the period of surveillance appeared concurrently in more than one state. The G2 electropherotypes that arose in Alice Springs in 1993–1994 and 1995 were later identified in Darwin and Adelaide, respectively. In general, strains with G2-associated electropherotypes exhibited more limited geographic spread than G1-associated electropherotypes and did not persist in the same center for more than one epidemic season (Fig. 4).
G3 and G4 serotypes.
Strains of the G3 and G4 serotypes were uncommon in all centers throughout the 4 years studied. Electropherotypes associated with G3 (8 types) and G4 (9 types) appeared sporadically in small numbers. There was no apparent spread of any electropherotypes between centers.
DISCUSSION
The study described here was a month-by-month, 4-year-long, comprehensive view of rotavirus infection in hospitalized children living in widely separate areas on an island land mass equal in size to the continental United States. The 4,632 children resided in tropical, arid, or temperate climates in centers with widely different populations of susceptible children less than 5 years old, ranging from less than 100 in aboriginal communities in central and northern Australia to more than 100,000 in centers on the eastern coast. Rotaviruses of the G1 to G4, P1A[8], and P1B[4] types caused disease in >80% of patients in all centers during the 4 years studied. Overall the epidemiological patterns identified in temperate climates (associated with urban populations) differed from the patterns in tropical centers caring for children from small dispersed aboriginal communities.
All centers in temperate climates showed seasonal peaks of rotavirus infections that coincided with the cooler months of the year. These alternated with periods of 2 or more consecutive months (including most summer months) when rotaviruses were seldom detected or not detected at all (4). The western city of Perth had peaks of prevalence in the colder months that always preceded those in the eastern states by 1 or 2 months. A similar (unexplained) phenomenon has been recorded in multicenter studies in North America, where the peak prevalence of rotavirus disease occurs 3 to 4 months earlier in Mexico and the southwestern United States than in the northeastern United States (32). Seasonal differences in ambient temperature alone cannot account for the occurrence of epidemic peaks. For example, the timing of annual winter peaks in Brisbane and Melbourne is similar, despite mean midwinter (July) temperature ranges of 9.4 to 20.6 and 5.2 to 12.9°C, respectively. Perhaps diurnal fluctuations in minimum and maximum temperatures at a particular center exert an important influence on seasonal patterns by influencing host susceptibility. Such an effect has been observed in pigs infected with transmissible gastroenteritis virus, in which diarrhea is most severe in animals reared at temperatures that fluctuate between 4 and 20°C every 24 h (31).
Centers located within the tropics and caring predominantly for aboriginal children (Alice Springs, Darwin) showed epidemiologic patterns different from those of all other Australian centers and from the year-round patterns of rotavirus disease usually seen in tropical areas (2, 15, 28). Rotavirus disease in Alice Springs and Darwin exhibited unpredictable peaks (sometimes twice per year) in months ranging from January to November interspersed with periods of 1 to 8 months when no severe rotavirus disease was detected. Factors other than ambient temperature, humidity, and rainfall must have influenced the rotavirus prevalence since the two centers have disparate hot, arid (Alice Springs) and tropical, humid (Darwin) climates. The explosive nature of rotavirus disease outbreaks with relatively long intervening absences of rotavirus disease could be due to temporary eradication of rotaviruses from these small communities once all susceptible children have been infected, followed by the rapid spread of newly introduced rotavirus strains once the numbers of susceptible infants have increased. Further, more detailed study of sequential epidemic strains in Alice Springs and Darwin could throw light on the contribution of genetic change to the epidemiology of rotavirus disease.
The majority (82%) of rotavirus strains could be assigned a G serotype and a P serotype, with more than 95% of typeable strains identified as G1P1A[8] or G2P1B[4]. Rotaviruses of type G1P1A[8] were ubiquitous, usually dominant in all centers, and genetically heterogeneous with some strains that spread Australia-wide. There was no consistent direction of spread of individual strains from city to city. Only two strains persisted Australia-wide for more than 12 months. G2P1B[4] strains were also ubiquitous, but they were less persistent and exhibited limited intercity spread. G3 and G4 strains were relatively uncommon. Sporadic epidemics of G2, G3, and G4 rotaviruses appear to be common in many locations worldwide including the United Kingdom (20), the United States (35), France (8), Japan (34), Ireland (21), Brazil (9, 30), and Chile (22). Previous statistical analysis of the monthly incidence of rotavirus infections in Melbourne from 1977 to 1993 presents evidence for the existence in Australia of a biennial peak in the incidence of rotavirus, with evidence of an interepidemic cycle of 4.6 to 5.2 years' duration (13). Identification of G1, G2, G3, and G4 rotaviruses as a cause of severe pediatric diarrhea over almost 30 years (3, 9) emphasizes that rotavirus vaccines must (at least) be effective in protecting against disease due to these serotypes. The occasional identification of G6 and G8 rotaviruses in Australia together with the occurrence of G5, G8, G9, and G10 strains in many countries (9) emphasizes the potential for some of the currently minor strains to become dominant in many communities, as illustrated by the recent emergence and spread of G9 rotaviruses in Bangladesh (33), North America (9, 29), and Australia (27) after completion of this study.
It was not possible in this study to predict the dominant serotypes (or electropherotypes) likely to emerge from strains present during the preceding winter. The mobilities of 6 or more of the 11 genes of dominant strains that appeared sequentially each year were different from those of the genes of strains from the preceding year. Alterations in the migration of genes 7 and 8 (which code for nonstructural proteins implicated in RNA binding) occurred most commonly. The importance of changes in these genes should be investigated further since gene substitution involving genes that code for nonstructural proteins have also been detected in G2 strains that appear sequentially in Japan (14). The extent of sequence changes within individual genes could not be assessed by the comparatively crude technique of gel electrophoresis. Previous analysis of genes that code for outer capsid structural proteins VP7 and VP4 has shown limited nucleotide (and deduced amino acid) changes over 4 to 5 years in Australian rotaviruses of the same serotype (23). Factors that influence the continual generation of genetically different strains from year to year in the same locality are unexplained. Dominant strains may emerge as escape mutants selected during replication in adults who possess preexisting neutralizing antibody, as described previously for influenza virus (16). New dominant strains can also arise as the result of reassortment of genes between different strains of the same or different G types. The unusual G2 strains that caused outbreaks in Alice Springs and Darwin in 1993 and 1994 were shown to be derived by reassortment between subgroup I and subgroup II human strains (25). The extent of reassortment between human strains and between human and animal strains in the generation of epidemiologically dominant rotaviruses requires further study.
This study supports the need for multicenter surveillance of rotavirus strains, particularly those that cause severe disease in young children. Such studies may give an incomplete picture of the rotavirus strains in a community, since they will not identify strains that cause mild and/or asymptomatic infections in children and/or adults. Nevertheless, these studies are justified since they are relevant to the selection of strains for inclusion in vaccines aimed at prevention of severe rotavirus disease. Surveillance studies should be ongoing to provide baseline data against which vaccine effectiveness can be continually evaluated in order to monitor the emergence of new rotavirus strains.
ACKNOWLEDGMENTS
The study was funded by the Public Health Research and Development Committee of NHMRC and the Royal Children's Hospital Research Institute.
The study would not have been possible without the participation and skilled, careful assistance of the following microbiologists and pediatricians: G. Davidson, P. Goldwater, and A. Lawrence (Women's and Children's Hospital, Adelaide); T. Kok, L. Mickan, and S. Weir (Institute for Medical and Veterinary Science, Adelaide); G. Clift, J. Erlich, J. Hagger, F. Morey, and R. Matters (Alice Springs Hospital, Alice Springs); J. Faogali, J. Farrah, R. Shepherd, and M. Witt (Royal Children's Hospital, Brisbane); G. Lum, A. Lowe, A. Ruben, B. Dwyer, and K. Withnall (Royal Darwin Hospital, Darwin); A. Carmichael, A. Claridge, K. Dahlenburg, R. Fang, R. Tucker, and E. Fair (Royal Hobart Hospital, Hobart); B. Crawford, G. Hogg, B. Ross, P. Ward, and S. Politis (Royal Children's Hospital, Melbourne); R. Hill, A. May, G. O'Connor, and B. Wild (Princess Margaret Hospital for Children, Perth); P. Amin, T. Borg, A. Cunningham, J. MacRae, P. Mclntyre, and G. Sandico (New Children's Hospital, Sydney); and C. Mclver and K. McPhie (Prince of Wales Hospital, Sydney). We thank P. Chondros and S. Vidmar for assistance with statistical analysis and preparation of the figures and Roger Schnagl for generous provision of data from Alice Springs.
REFERENCES
- 1.Aijas S, Gowda K, Jagannath H V, Reddy R R, Maiya P P, Wood R L, Greenberg H B, Raju M, Babu A, Rao C D. Epidemiology of symptomatic human rotaviruses in Bangladore and Mysore, India from 1988 to 1994 as determined by electropherotype, subgroup and serotype analysis. Arch Virol. 1996;141:715–726. doi: 10.1007/BF01718329. [DOI] [PubMed] [Google Scholar]
- 2.Bishop R F. Natural history of rotavirus infections. In: Kapikian A Z, editor. Viral infections of the gastrointestinal tract. 2nd ed. New York, N.Y: Marcel Dekker, Inc; 1994. pp. 131–167. [Google Scholar]
- 3.Bishop R F, Unicomb L E, Barnes G L. Epidemiology of rotavirus serotypes in Melbourne, Australia 1973–1989. J Clin Microbiol. 1991;29:862–868. doi: 10.1128/jcm.29.5.862-868.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlin J B, Chondros P, Masendycz P, Bugg H, Bishop R F, Barnes G L. Rotavirus infection and rates of hospitalisation for acute gastroenteritis in young children, Australia 1993-96. Med J Aust. 1998;169:252–256. doi: 10.5694/j.1326-5377.1998.tb140248.x. [DOI] [PubMed] [Google Scholar]
- 5.Coulson B S, Unicomb L E, Pitson G A, Bishop R F. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol. 1987;25:509–515. doi: 10.1128/jcm.25.3.509-515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyall-Smith M L, Holmes I H. Sequence homology between human and animal rotavirus serotype-specific glycoproteins. Nucleic Acids Res. 1984;12:3973–3982. doi: 10.1093/nar/12.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estes M K. Rotaviruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Field's virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lipincott-Raven; 1996. pp. 1625–1655. [Google Scholar]
- 8.Gault E, Chikhi-Brachet R, Delon S, Schnepf N, Albiges L, Grimprel E, Girardet J-P, Begue P, Garbarg-Chenon A. Distribution of human rotavirus G types circulating in Paris, France, during the 1997–1998 epidemic: high prevalence of type G4. J Clin Microbiol. 1999;37:2373–2375. doi: 10.1128/jcm.37.7.2373-2375.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentsch J R, Woods P A, Ramachandran M, Das B K, Leite J P, Alfieri A, Kumar R, Bhan M K, Glass R I. Review of G and P typing results from a global collection of rotavirus strains: implications for vaccine development. J Infect Dis. 1996;174(Suppl. 1):S30–S36. doi: 10.1093/infdis/174.supplement_1.s30. [DOI] [PubMed] [Google Scholar]
- 10.Gentsch J R, Glass R I, Woods P, Gouvea V, Gorziglia M, Flores J, Das B K, Bhan M K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gouvea V, Glass R I, Woods P, Taniguchi K, Clark H F, Forrester B, Fang Z-Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouvea V, Santos N. Rotavirus serotype G5: an emerging cause of epidemic childhood diarrhea. Vaccine. 1999;17:1291–1292. doi: 10.1016/s0264-410x(98)00378-8. [DOI] [PubMed] [Google Scholar]
- 13.José M V, Bobadilla J R, Bishop R F. Oscillatory fluctuations in the incidence of rotavirus infections by serotypes 1, 2, 3 and 4. J Diarrhoeal Dis Res. 1996;14:194–200. [PubMed] [Google Scholar]
- 14.Kaga E, Nakagomi O. Recurrent inoculation of single nonstructural gene substitution reassortants among human rotaviruses with a short RNA pattern. Arch Virol. 1994;134:63–71. doi: 10.1007/BF01538817. [DOI] [PubMed] [Google Scholar]
- 15.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Field's virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lipincott-Raven; 1996. pp. 1657–1708. [Google Scholar]
- 16.Kilbourne E D. An explanation of the interpandemic antigenic mutability of influenza viruses. J Infect Dis. 1973;128:668–670. doi: 10.1093/infdis/128.5.668. [DOI] [PubMed] [Google Scholar]
- 17.Leite J P G, Alfieri A A, Woods P A, Glass R I, Gentsch J R. Rotavirus G and P types circulating in Brazil: characterization by RT-PCR, probe hybridization, and sequence analysis. Arch Virol. 1996;141:2365–2374. doi: 10.1007/BF01718637. [DOI] [PubMed] [Google Scholar]
- 18.Masendycz P J, Palombo E A, Gorrell R J, Bishop R F. Comparison of enzyme immunoassay, PCR and type-specific cDNA probe techniques for identification of group A rotavirus gene 4 types (P types) J Clin Microbiol. 1997;35:3104–3108. doi: 10.1128/jcm.35.12.3104-3108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakata S, Gatheru Z, Ukae S, Adachi N, Kobayashi N, Honma S, Muli J, Ogaja P, Nyangao J, Kiplagat E, Tukei P M, Chiba S. Epidemiological study of the G serotype distribution of group A rotaviruses in Kenya from 1991 to 1994. J Med Virol. 1999;58:296–303. doi: 10.1002/(sici)1096-9071(199907)58:3<296::aid-jmv17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Noel J S, Beards G M, Cubitt W D. Epidemiological survey of human rotavirus serotypes and electropherotypes in young children admitted to two children's hospitals in northeast London from 1984 to 1990. J Clin Microbiol. 1991;29:2213–2219. doi: 10.1128/jcm.29.10.2213-2219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Mahony J, Foley B, Morgan S, Morgan J G, Hill C. VP4 and VP7 genotyping of rotavirus samples recovered from infected children in Ireland over a 3-year period. J Clin Microbiol. 1999;37:1699–1703. doi: 10.1128/jcm.37.6.1699-1703.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Ryan M L, Mamani N, Avendano L F, Cohen J, Pena A, Villarroel J, Chavez A, Valdivieso F, Matson D O. Molecular epidemiology of human rotaviruses in Santiago, Chile. Pediatr Infect Dis J. 1997;16:305–311. doi: 10.1097/00006454-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Palombo E A. Genetic and antigenic diversity of human rotaviruses: potential impact on the success of candidate vaccines. FEMS Microbiol Lett. 1999;181:1–8. doi: 10.1111/j.1574-6968.1999.tb08819.x. [DOI] [PubMed] [Google Scholar]
- 24.Palombo E A, Bishop R F. Genetic and antigenic characterization of a serotype G6 human rotavirus isolated in Melbourne. J Med Virol. 1995;47:348–354. doi: 10.1002/jmv.1890470410. [DOI] [PubMed] [Google Scholar]
- 25.Palombo E A, Bugg H C, Masendycz P J, Coulson B S, Barnes G L, Bishop R F. Multiple-gene rotavirus reassortants responsible for an outbreak of gastroenteritis in central and northern Australia. J Gen Virol. 1996;77:1223–1227. doi: 10.1099/0022-1317-77-6-1223. [DOI] [PubMed] [Google Scholar]
- 26.Palombo E A, Clark R, Bishop R F. Characterisation of a “European-like” serotype G8 human rotavirus isolated in Australia. J Med Virol. 2000;60:56–62. [PubMed] [Google Scholar]
- 27.Palombo E A, Masendycz P J, Bugg H C, Bogdanovic N, Barnes G L, Bishop R F. Emergence of serotype G9 human rotaviruses in Australia. J Clin Microbiol. 2000;38:1305–1306. doi: 10.1128/jcm.38.3.1305-1306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parashar U D, Bresee J S, Gentsch J R, Glass R I. Rotavirus. Emerg Infect Dis. 1998;4:561–570. doi: 10.3201/eid0404.980406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramachandran M, Gentsch J R, Parashar U D, Jim S, Woods P A, Holmes J L, Kirkwood C D, Bishop R F, Greenberg H B, Urasawa S, Gerna G, Coulson B S, Taniguchi K, Bresee J S, Glass R I the National Strain Surveillance System Collaborating Laboratories. Detection and characterization of novel rotavirus strains in the United States. J Clin Microbiol. 1998;36:3223–3229. doi: 10.1128/jcm.36.11.3223-3229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos N, Lima R C C, Pereira C F A, Gouvea V. Detection of rotavirus types G8 and G10 among Brazilian children with diarrhea. J Clin Microbiol. 1998;36:2727–2729. doi: 10.1128/jcm.36.9.2727-2729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu M, Shimizu Y, Kodama Y. Effects of ambient temperatures on induction of transmissible gastroenteritis in feeder pigs. Infect Immun. 1978;21:747–752. doi: 10.1128/iai.21.3.747-752.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torok T J, Kilgore P E, Clarke M J, Holman R C, Bresee J S, Glass R I the National Respiratory and Enteric Virus Surveillance System Collaborating Laboratories. Visualizing geographic and temporal trends in rotavirus activity in the United States, 1991 to 1993. Pediatr Infect Dis J. 1997;16:941–946. doi: 10.1097/00006454-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Unicomb L E, Podder G, Gentsch J R, Woods P A, Hasan K Z, Faruque A S G, Albert M J, Glass R I. Evidence of high-frequency genomic reassortment of group A rotavirus strains in Bangladesh: emergence of type G9 in 1995. J Clin Microbiol. 1999;37:1885–1891. doi: 10.1128/jcm.37.6.1885-1891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urasawa S, Urasawa T, Taniguchi K, Wakasugi F, Kobayashi N, Chiba S, Sakurada N, Morita M, Morita O, Tokieta M, Kawamoto H, Minekawa Y, Otseto M. Survey of human rotavirus serotypes in different locations in Japan by enzyme-linked immunosorbent assay with monoclonal antibodies. J Infect Dis. 1989;160:44–51. doi: 10.1093/infdis/160.1.44. [DOI] [PubMed] [Google Scholar]
- 35.Woods P A, Gentsch J, Gouvea V, Mata L, Simhon A, Santosham M, Bai Z-S, Urasawa S, Glass R I. Distribution of serotypes of human rotavirus in different populations. J Clin Microbiol. 1992;30:781–785. doi: 10.1128/jcm.30.4.781-785.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]