Abstract
Background: Pre-operative administration of combined oral antibiotic agents and mechanical bowel preparation has been demonstrated to improve post-operative outcomes after elective colectomy, however, many patients do not receive combined preparation. Patient and procedural determinants of combined preparation receipt remain understudied.
Patients and Methods: All patients undergoing elective colectomy within the 2018 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) Participant Use File and Targeted Colectomy datasets were included. Univariable and multivariable logistic regression analyses were performed to identify factors associated with receipt of combined preparation.
Results: A total of 21,889 patients were included, of whom 13,848 (63.2%) received combined preparation pre-operatively. Patients who received combined preparation tended to be younger, male, of white race, and of non-Hispanic ethnicity (all p < 0.05). After multivariable adjustment, male gender, body mass index (BMI) 30–39 kg/m2, independent functional status, and laparoscopic and robotic surgical approaches were associated with receipt of combined preparation (all p < 0.05), whereas Asian race, hypertension, disseminated cancer, and inflammatory bowel disease were associated with omission of combined preparation (all p < 0.05).
Conclusions: Patients with risk factors for infectious complications—including a poor functional status, comorbid conditions, and undergoing an open procedure—are less likely to receive combined preparation before elective colectomy. Similarly, female and Asian patients are less likely to receive combined preparation, emphasizing the need for equitable administration of combined preparation.
Keywords: anastomotic leak, colectomy, colorectal surgery, mechanical bowel preparation, surgical site infection
The efficacy of administration of oral antibiotic agents with neomycin and erythromycin in addition to mechanical bowel preparation prior to elective colectomy was first demonstrated by Nichols et al. [1,2] in 1973 and subsequently confirmed by multiple other randomized clinical trials [3,4]. Given the more recent uptake of minimally invasive colon resection techniques, a number of observational reports have since corroborated these findings in the contemporary era, demonstrating a reduction in the incidence of surgical site infection, organ/space infection, and anastomotic leakage with the use of combined preparation (CP) [5–7]. As such, the American Society of Colon and Rectal Surgeons as well as the Society of American Gastrointestinal and Endoscopic Surgeons recommend the routine use of CP before elective colectomy [8,9].
Nonetheless, use of CP in practice remains controversial, with many citing a lack of clinical benefit as well as concerns related to an increased risk of post-operative Clostridioides difficile infection [10], although this risk has been largely refuted [6,11–13]. Accordingly, many patients do not receive CP and the reasons for omission of CP remain understudied [14,15]. Therefore, the purpose of this study was to explore patient and procedural factors associated with receipt of CP before elective colectomy among a national cohort of U.S. patients. In this way, this work sought to identify patient populations who may be at risk of not receiving CP so that targeted strategies may be developed to ensure the equitable administration of CP.
Patients and Methods
Patient population
The 2018 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) Participant Use File and Targeted Colectomy datasets were merged and all patients who underwent elective colon resection identified [16]. The ACS NSQIP datasets include detailed demographic and clinical information from participating U.S. hospitals and have been extensively characterized [17,18].
Patients who underwent an emergent or non-elective colectomy were excluded from analysis. Similarly, patients diagnosed with an obstruction or ileus (as identified by an ACS NSQIP-defined indication for surgery of “colon cancer with obstruction” or “volvulus” or International Classification of Diseases, 10th Revision (ICD-10) codes K43.0, K43.1, K43.3, K43.4, K43.6, K50.012, K50.112, K50.812, K50.912, K51.012, K51.812, K50.912, K51.012, K51.312, K51.412, K51.512, K51.812, K51.912, K56.0, K56.1, K56.2, K56.41, K56.5, K56.50, K56.51, K56.52, K56.6, K56.60, K56.600, K56.601, K56.609, K56.69, K56.690, K56.691, K56.699, or K56.7) or assigned an American Society of Anesthesiologists (ASA) physical status class 5 also excluded. Those patients with missing demographic or clinical characteristics data, with the exception of race and ethnicity, were additionally excluded.
Variable specification
Demographic variables included age, gender, race, and ethnicity. Within ACS NSQIP, race and ethnicity may be self-reported by the patient or assigned by institutional personnel, according to internal practices. Clinical variables included ACS NSQIP-defined comorbidities, such as hypertension requiring medication, diabetes mellitus, and obesity as defined by body mass index (BMI) [19]. Additional procedural variables included the surgical approach, defined as open, laparoscopic, or robotic, as well as the indication for surgery, defined by malignancy, diverticular disease, benign polyp, inflammatory bowel disease, or other diagnoses (e.g., enterocolitis and bleeding).
Combined preparation was designated as patient receipt of both pre-operative oral antibiotic agents and mechanical bowel preparation, as previously described [5–7]. Patients who received oral antibiotic agents alone or mechanical bowel preparation alone were categorized as not having received CP. Within ACS NSQIP, partial receipt of mechanical bowel preparation as well as enemas or suppositories alone are considered not to meet the criteria for receipt of mechanical bowel preparation. Importantly, ACS NSQIP does not capture which patients were prescribed CP, so receipt of CP in this study reflects patients who were both prescribed and successfully completed CP pre-operatively. Patients with unknown receipt of oral antibiotic agents or mechanical bowel preparation were excluded from analysis.
Statistical analysis
Continuous and categorical variables were summarized as medians with interquartile ranges and frequencies with percentages, respectively. On unadjusted analysis, univariable associations of patient and procedural factors with receipt of CP were compared using Wilcoxon rank-sum and χ2 tests, as appropriate. Subsequently, adjusted associations of patient and procedural factors with receipt of CP were explored using forward stepwise multivariable logistic regression. Factors with a p < 0.20 on univariable analysis were sequentially entered into the logistic regression model. Variables with a p < 0.10 upon model entry and p < 0.20 maintained throughout the stepwise introduction of variables were included in the final model.
A two-sided p < 0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute). The University of Virginia Institutional Review Board has deemed analyses of ACS NSQIP data to not be Human Subjects Research.
Results
Unadjusted results
A total of 21,889 patients undergoing elective colectomy were included, of whom 13,848 (63.2%) received and 8,041 (36.7%) did not receive CP pre-operatively. Patients who received CP tended to be younger, male, of white race, and of non-Hispanic ethnicity (all p < 0.05; Table 1). Although patients who received CP exhibited a higher BMI (p < 0.001), they also demonstrated a lower ASA classification (p < 0.001), were more likely to have an independent functional status (p < 0.001), and had fewer comorbid conditions such as hypertension, diabetes mellitus, and disseminated cancer (all p < 0.01).
Table 1.
Patient and Procedural Factors by Receipt of Combined Preparation
| Combined preparation omitted (n = 8,041) | Combined preparation received (n = 13,848) | p | |
|---|---|---|---|
| Age, y, median (IQR) | 64.0 (53.0–73.0) | 62.0 (52.0–71.0) | < 0.001 |
| Gender | 0.03 | ||
| Male | 3727 (46.3) | 6634 (47.9) | |
| Female | 4314 (53.7) | 7214 (52.1) | |
| Race | < 0.001 | ||
| White | 4887 (60.8) | 10723 (77.4) | |
| Black | 582 (7.2) | 1201 (8.7) | |
| Asian | 397 (4.9) | 414 (3.0) | |
| Other | 32 (0.4) | 137 (1.0) | |
| Unknown/not reported | 2143 (26.7) | 1373 (9.9) | |
| Ethnicity | < 0.001 | ||
| Non-Hispanic | 5689 (70.8) | 12068 (87.2) | |
| Hispanic | 405 (5.0) | 778 (5.6) | |
| Unknown/not reported | 1947 (24.2) | 1002 (7.2) | |
| Hypertension | 3898 (48.5) | 6402 (46.2) | 0.001 |
| Diabetes mellitus | 1360 (16.9) | 2113 (15.3) | 0.001 |
| BMI, kg/m2, median (IQR) | 27.9 (24.2-32.1) | 28.3 (24.5-32.7) | < 0.001 |
| BMI category | < 0.001 | ||
| <18.5 kg/m2 | 183 (2.3) | 272 (2.0) | |
| 18.5–29 kg/m2 | 4987 (62.0) | 8198 (59.2) | |
| 30–39 kg/m2 | 2368 (29.5) | 4499 (32.5) | |
| ≥ 40 kg/m2 | 503 (6.3) | 879 (6.4) | |
| Congestive heart failure | 52 (0.7) | 67 (0.5) | 0.11 |
| Dyspnea | 545 (6.8) | 867 (6.3) | 0.13 |
| Chronic obstructive pulmonary disease | 351 (4.4) | 583 (4.2) | 0.58 |
| Current smoker | 1225 (15.2) | 2028 (14.6) | 0.24 |
| Dialysis | 40 (0.5) | 56 (0.4) | 0.32 |
| Disseminated cancer | 501 (6.2) | 649 (4.7) | < 0.001 |
| Independent functional status | 7898 (98.2) | 13699 (98.9) | < 0.001 |
| Open wound/infection | 85 (1.1) | 139 (1.0) | 0.71 |
| Chronic steroid use | 580 (7.2) | 957 (6.9) | 0.40 |
| >10% weight loss | 266 (3.3) | 437 (3.2) | 0.54 |
| Inpatient procedure | 7920 (98.5) | 13739 (99.2) | < 0.001 |
| ASA physical status classification | < 0.001 | ||
| ASA 1 | 151 (1.9) | 243 (1.8) | |
| ASA 2 | 3304 (41.1) | 6383 (46.1) | |
| ASA 3 | 4171 (51.9) | 6828 (49.3) | |
| ASA 4 | 415 (5.2) | 394 (2.9) | |
| Surgical approach | < 0.001 | ||
| Open | 1821 (22.7) | 2144 (15.5) | |
| Laparoscopic | 5223 (65.0) | 9054 (65.4) | |
| Robotic | 997 (12.4) | 2650 (19.1) | |
| Indication for surgery | < 0.001 | ||
| Malignancy | 3981 (49.5) | 6615 (47.8) | |
| Diverticular disease | 1607 (20.0) | 3485 (25.2) | |
| Benign polyp | 783 (9.7) | 1432 (10.3) | |
| Inflammatory bowel disease | 474 (5.9) | 771 (5.6) | |
| Othera | 1196 (14.9) | 1545 (11.2) |
IQR = interquartile range; BMI = body mass index; ASA = American Society of Anesthesiologists.
Enterocolitis, bleeding, and other diagnoses.
Surgical approaches also differed between groups, with fewer open procedures and more robotic procedures among those receiving CP (p < 0.001; Table 1). Additionally, patients who received CP were more likely to undergo surgery for diverticular disease and less likely to undergo surgery for other conditions such as enterocolitis and bleeding (p < 0.001).
Adjusted results
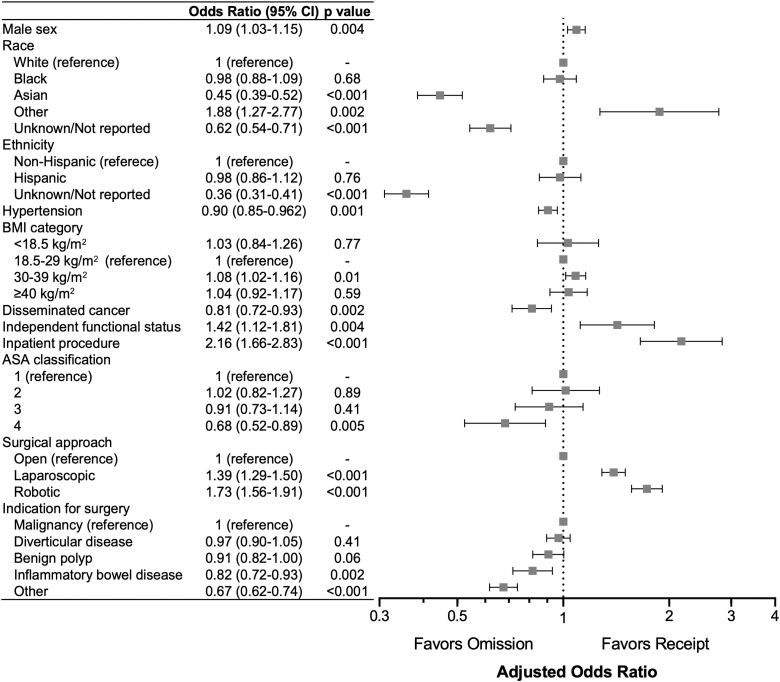
After multivariable adjustment, male gender (odds ratio [OR], 1.09; p = 0.004), BMI 30–39 kg/m2 (OR, 1.08; p = 0.01), independent functional status (OR, 1.42; p = 0.004), inpatient procedure class (OR, 2.16; p < 0.001), and laparoscopic (OR, 1.39; p < 0.001) and robotic (OR, 1.73; p < 0.001) approaches were all significantly associated with receipt of CP (Fig. 1). Conversely, Asian race (OR, 0.45; p < 0.001), hypertension (OR, 0.90; p = 0.001), disseminated cancer (OR, 0.81; p = 0.002), ASA class 4 (OR, 0.68; p = 0.005), and a surgical indication of inflammatory bowel disease (OR, 0.82; p = 0.002) or other conditions (e.g., enterocolitis and bleeding; OR, 0.68; p < 0.001) were all significantly associated with omission of CP. Similarly, unknown or not reported race and ethnicity were also associated with omission of CP (OR, 0.62; p < 0.001 and OR, 0.36; p < 0.001, respectively).
FIG. 1.
Patient and procedural factors associated with receipt of combined preparation. CI = confidence interval; BMI = body mass index; ASA = American Society of Anesthesiologists.
Discussion
In this cross-sectional analysis of patients undergoing elective colectomy in 2018, both patient and procedural factors were found to be independently associated with receipt of CP. Increasing BMI, a laparoscopic or robotic approach, and an independent functional status were associated with receipt of CP. Conversely, greater comorbidity burden, including hypertension and disseminated cancer, and diagnoses such as inflammatory bowel disease, enterocolitis, and bleeding were associated with omission of CP. Of particular concern were the gender and racial disparities observed in this study, with patients of female gender and Asian race independently less likely to receive CP. Together, these findings highlight the multifactorial nature of determining which patients may be able to tolerate and benefit from CP. Furthermore, these results suggest that the administration of CP may be inequitable among certain populations, such as women and Asian patients.
The patient clinical characteristics independently associated with CP receipt in this study confirm many surgeons' beliefs about which patients will benefit from CP prior to elective surgery. Although many statistically significant associations found in this study may have represented small, clinically insignificant differences between groups, a poor functional status and an increasing number of comorbidities are consistent with a patient's reduced ability to tolerate CP. Intake of mechanical bowel preparation frequently causes nausea, vomiting, and abdominal pain [20], making completion of CP more difficult in patients with reduced physiologic reserve. The adverse effects caused by ingestion of mechanical bowel preparation may help explain why in this study patients with inflammatory bowel disease, who often already struggle with abdominal pain and poor oral intake, were less likely to receive CP. The omission of CP in patients with inflammatory bowel disease is unfortunate given their increased risk for surgical site infection [21], which CP has been demonstrated to prevent [22,23]. Similarly, patient comorbidities such as hypertension, obesity, and disseminated cancer—all of which may increase a patient's risk for a post-operative infectious complication—were also predictive of not receiving CP.
It is possible that surgeons may be hesitant to prescribe CP in patients with an increasing comorbidity burden, because osmotically acting bowel preparation agents such as sodium phosphate and polyethylene glycol can cause detrimental electrolyte imbalances and fluid shifts [24–26]. As patients are increasingly optimized in the pre-operative period through exercise and nutritional pre-habilitation programs [27,28], their ability to tolerate CP may also improve. Nevertheless, it is important to acknowledge that patients who are frail or have certain comorbid conditions are at elevated risk of both omitting CP and having an infectious complication. Failing to administer CP in these higher risk patients may represent a contributing factor in the development of infectious complications.
It is similarly important to recognize that choice of surgical approach may influence patient receipt of CP. Understanding that mechanical bowel preparation administration is generally felt to improve bowel handling through a reduction in stool burden [20], it is unsurprising that new and technically challenging laparoscopic and robotic-assisted approaches were associated with receipt of CP in this study. Surgeons may be more likely to prescribe CP for patients undergoing minimally invasive procedures, where mobilization and anastomosis of the colon is often more difficult than with more traditional open approaches. Regardless of the cause, the association between a laparoscopic or robotic approach and receipt of CP has broader implications. Namely, taken together with the previously discussed finding that patients with fewer comorbidities and greater physiologic reserve are more likely to receive CP, previously-reported observational associations of CP with improved clinical outcomes [5–7] may be confounded by the use of minimally invasive approaches among healthier, more physiologically robust patients. Nevertheless, it is worth recalling that the effects of CP on reduced post-operative complications have been demonstrated in several randomized clinical trials [1–3] and as such is recommended by multiple surgical societies [8,9].
Despite the evidence supporting CP administration before elective colectomy, in practice its use remains controversial [29,30]. In particular, a possible increased risk of post-operative Clostridioides difficile infection caused by the effect of oral antibiotic agents on normal bowel flora has been suggested [10]. However, a number of more robust studies have disproven this suggestion with the administration of CP being consistently associated with a decreased risk of Clostridioides difficile infection [6,11–13]. Nonetheless, routine use of CP is generally consistent across geographic regions and practice settings, with approximately 80% of colorectal surgeons using CP in 2018 [15]. As such, the gender and racial disparities in receipt of CP observed in this work are alarming and likely multifactorial in etiology. Of note, these results are consistent with a previous international audit of European colorectal patients, where men were more likely to receive CP [14]. Although residual confounding may explain why women and Asian patients were independently less likely to receive CP, it is also possible that implicit biases among surgeons, language or cultural barriers, and patient socioeconomic factors may impact CP administration. Future investigations are needed to determine the reasons for surgeon- or patient-driven omission of CP.
This study has several important limitations. First, this analysis was limited to data regarding patient receipt of CP, which were obtained through review of the medical record by trained and audited Surgical Clinical Reviewers [31]. As with all ACS NSQIP variables, patient completion of mechanical bowel preparation and oral antibiotic agents are retrospectively collected data and therefore may be incomplete. For example, patients who were prescribed but did not fully complete CP may be erroneously coded as having received CP if there is no documentation of the patient's failure to complete CP. Given this, more granular data on patients who are being offered, prescribed, and ultimately complete CP would clarify these findings.
Similarly, further examination of patient preferences or limitations in obtaining or completing CP prior to surgery is needed. Although gender and racial disparities in CP receipt were identified in this study, other unmeasured factors such as patient insurance status or language barriers may partially explain these differences. Second, a substantial amount of missing data on race and ethnicity is present in ACS NSQIP data and may not missing at random. Supporting this possibility is the finding that patients with unknown/not reported race or ethnicity were independently less likely to receive CP, suggesting that the institutional processes involved in reliably collecting patient data on race and ethnicity may be reflective of the processes involved in consistently administering CP. Further supporting this idea is the finding that patients with a documented race other than white, black, or Asian were more likely to receive CP. Although institutional data is not provided in ACS NSQIP, detailed collection of race and ethnicity as well as the consistent administration of CP may represent overall institutional quality of care. Finally, although 2018 ACS NSQIP colectomy data is derived from almost 350 hospitals across the United States, these findings may not be generalizable to other hospitals or patients outside the United States.
Conclusions
In conclusion, this national analysis of CP administration among patients undergoing elective colon resection identified a number of pre-operative factors associated with CP receipt. In particular, patients with greater functional status, fewer comorbid conditions, and those undergoing minimally invasive surgery tended to receive CP. Conversely, women and Asian patients were less likely to undergo CP. These findings should prompt further investigation into organizational barriers, provider prescribing practices, and patient factors to ensure the benefits of CP before elective colectomy can be equitably felt by all patients.
Acknowledgments
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
This work was presented at the 2021 Society of American Gastrointestinal and Endoscopic Surgeons Annual Meeting (August 31–September 3, 2021, Las Vegas, Nevada).
Authors' Contributions
Dr. Kane helped with study concept and design; acquisition, analysis, or interpretation of data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content; final approval of the version to be published; and is in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Dr. Lynch helped with acquisition, analysis, or interpretation of data; critical revision of the manuscript for important intellectual content; final approval of the version to be published; and is in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Dr. Hassinger helped with acquisition, analysis, or interpretation of data; critical revision of the manuscript for important intellectual content; final approval of the version to be published; and is in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Dr. Hoang helped with acquisition, analysis, or interpretation of data; critical revision of the manuscript for important intellectual content; final approval of the version to be published; and is in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Dr. Friel helped with acquisition, analysis, or interpretation of data; critical revision of the manuscript for important intellectual content; final approval of the version to be published; and is in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Dr. Hedrick helped with study concept and design; acquisition, analysis, or interpretation of data; critical revision of the manuscript for important intellectual content; final approval of the version to be published; and is in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding Information
Research reported in this article was supported by awards T32CA163177 (Dr. Kane) and T32HL007849 (Dr. Lynch) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Author Disclosure Statement
All authors declare that they have no competing interests.
References
- 1. Nichols RL, Broido P, Condon RE, et al. Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann Surg 1973;178:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nichols RL, Schumer W, Nyhus LM. Technique of preoperative bowel sterilisation. Lancet 1973;2:735. [DOI] [PubMed] [Google Scholar]
- 3. Clarke JS, Condon RE, Bartlett JG, et al. Preoperative oral antibiotics reduce septic complications of colon operations: Results of prospective, randomized, double-blind clinical study. Ann Surg 1977;186:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matheson DM, Arabi Y, Baxter-Smith D, et al. Randomized multicentre trial of oral bowel preparation and antimicrobials for elective colorectal operations. Br J Surg 1978;65:597–600. [DOI] [PubMed] [Google Scholar]
- 5. Kiran RP, Murray AC, Chiuzan C, et al. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg 2015;262:416–425. [DOI] [PubMed] [Google Scholar]
- 6. Klinger AL, Green H, Monlezun DJ, et al. The role of bowel preparation in colorectal surgery: Results of the 2012–2015 ACS-NSQIP data. Ann Surg 2019;269:671–677. [DOI] [PubMed] [Google Scholar]
- 7. Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: An analysis of colectomy-targeted ACS NSQIP. Ann Surg 2015;262:331–337. [DOI] [PubMed] [Google Scholar]
- 8. Migaly J, Bafford AC, Francone TD, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the use of bowel preparation in elective colon and rectal surgery. Dis Colon Rectum 2019;62:3–8. [DOI] [PubMed] [Google Scholar]
- 9. Carmichael JC, Keller DS, Baldini G, et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 2017;60:761–784. [DOI] [PubMed] [Google Scholar]
- 10. Wren SM, Ahmed N, Jamal A, Safadi BY. Preoperative oral antibiotics in colorectal surgery increase the rate of Clostridium difficile colitis. Arch Surg 2005;140:752–756. [DOI] [PubMed] [Google Scholar]
- 11. Mangieri CW, Ling JA, Modlin DM, et al. Utilization of combination bowel preparation (CBP) is protective against the development of post-operative Clostridium difficile infection (CDI), decreases septic complications, and provides a survival benefit. Surg Endosc 2021;35:928–933. [DOI] [PubMed] [Google Scholar]
- 12. Kim EK, Sheetz KH, Bonn J, et al. A statewide colectomy experience: The role of full bowel preparation in preventing surgical site infection. Ann Surg 2014;259:310–314. [DOI] [PubMed] [Google Scholar]
- 13. Al-Mazrou AM, Hyde LZ, Suradkar K, Kiran RP. Effect of Inclusion of oral antibiotics with mechanical bowel preparation on the risk of Clostridium difficile infection after colectomy. J Gastrointest Surg 2018;22:1968–1975. [DOI] [PubMed] [Google Scholar]
- 14. The 2017 European Society of Coloproctology (ESCP) collaborating group. Association of mechanical bowel preparation with oral antibiotics and anastomotic leak following left sided colorectal resection: An international, multi-centre, prospective audit. Colorectal Dis 2018;20(Suppl 6):15–32. [DOI] [PubMed] [Google Scholar]
- 15. McChesney SL, Zelhart MD, Green RL, Nichols RL. Current U.S. pre-operative bowel preparation trends: A 2018 Survey of the American Society of Colon and Rectal Surgeons Members. Surg Infect 2020;21:1–8. [DOI] [PubMed] [Google Scholar]
- 16. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2018 ACS NSQIP Targeted Participant Use Data File (PUF). 2019. www.facs.org/-/media/files/quality-programs/nsqip/pt_nsqip_puf_userguide_2018.ashx (Last accessed October 3, 2021).
- 17. Cohen ME, Dimick JB, Bilimoria KY, et al. Risk adjustment in the American College of Surgeons National Surgical Quality Improvement Program: a comparison of logistic versus hierarchical modeling. J Am Coll Surg 2009;209687–693. [DOI] [PubMed] [Google Scholar]
- 18. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: Patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg 2013;217:336–346. [DOI] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention. About Adult BMI 2020. www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html (Last accessed October 3, 2021).
- 20. Bucher P, Gervaz P, Soravia C, et al. Randomized clinical trial of mechanical bowel preparation versus no preparation before elective left-sided colorectal surgery. Br J Surg 2005;92:409–414. [DOI] [PubMed] [Google Scholar]
- 21. Cima RR, Bergquist JR, Hanson KT, et al. Outcomes are local: Patient, disease, and procedure-specific risk factors for colorectal surgical site infections from a single institution. J Gastrointest Surg 2017;21:1142–1152. [DOI] [PubMed] [Google Scholar]
- 22. Uchino M, Ikeuchi H, Bando T, et al. Efficacy of preoperative oral antibiotic prophylaxis for the prevention of surgical site infections in patients with Crohn disease: A randomized controlled trial. Ann Surg 2019;269:420–426. [DOI] [PubMed] [Google Scholar]
- 23. Oshima T, Takesue Y, Ikeuchi H, et al. Preoperative oral antibiotics and intravenous antimicrobial prophylaxis reduce the incidence of surgical site infections in patients with ulcerative colitis undergoing IPAA. Dis Colon Rectum 2013;56:1149–1155. [DOI] [PubMed] [Google Scholar]
- 24. Nyberg C, Hendel J, Nielsen OH. The safety of osmotically acting cathartics in colonic cleansing. Nat Rev Gastroenterol Hepatol 2010;7:557–564. [DOI] [PubMed] [Google Scholar]
- 25. Kumar AS, Kelleher DC, Sigle GW. Bowel preparation before elective surgery. Clin Colon Rectal Surg 2013;26:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shapira Z, Feldman L, Lavy R, et al. Bowel preparation: Comparing metabolic and electrolyte changes when using sodium phosphate/polyethylene glycol. Int J Surg 2010;8:356–358. [DOI] [PubMed] [Google Scholar]
- 27. Bruns ERJ, Argillander TE, Van Den Heuvel B, et al. Oral Nutrition as a form of pre-operative enhancement in patients undergoing surgery for colorectal cancer: A systematic review. Surg Infect 2018;19:1–10. [DOI] [PubMed] [Google Scholar]
- 28. Minnella EM, Awasthi R, Loiselle SE, et al. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: A randomized clinical trial. JAMA Surg 2018;153:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Basson MD. Oral antibiotics for colon surgery: The questions remain the same, as do the answers. JAMA Surg 2018;153:121–122. [DOI] [PubMed] [Google Scholar]
- 30. Rovera F, Dionigi G, Boni L, et al. Mechanical bowel preparation for colorectal surgery. Surg Infect 2006;7(Suppl 2):S61–63. [DOI] [PubMed] [Google Scholar]
- 31. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg 2010;210:6–16. [DOI] [PubMed] [Google Scholar]