Abstract
Previously, we demonstrated that extracts of the ripe fruit (rPM) and unripe fruit (uPM) of Prunus mume (Siebold) Siebold & Zucc. and citric acid have a laxative effect, which is at least partially mediated by the increase in fecal parameters as seen in the low-fiber diet-induced constipation model rats. This study aims at investigating the laxative effects of citric acid-enriched aqueous extracts of rPM, uPM, and its active compounds, such as citric acid and malic acid, on loperamide-induced constipation rat models. Animal studies were conducted with loperamide-induced constipation animal models. The results showed that rPM and citric acid, the major organic acid compounds, significantly improved stool parameters (number, weight, and water content of the stools) generated in loperamide-induced constipation rats, without adverse effects of diarrhea. The gastrointestinal (GI) motility was activated fully in the rPM- and citric acid-treated rats than in rats treaded with loperamide alone. In addition, when rPM and citric acid were added to RAW264.7 cells and used to treat loperamide-induced constipation model rats, the secretion of prostaglandin E2 (PGE2) increased significantly in cells and tissue. Furthermore, rPM and citric acid decreased the expression of the aquaporin 3 (AQP3) in the rat colons. Our results demonstrated that rPM and citric acid, the major organic acid compound in rPM, can effectively promote defecation frequency and regulate PGE2 secretion and AQP3 expression in the colon, providing scientific evidence to support the use of rPM as a therapeutic application.
Keywords: aquaporin 3 (AQP3) , constipation , laxative effect , loperamide , prostaglandin E2 (PGE2) , Prunus mume (Siebold) Siebold & Zucc
INTRODUCTION
Constipation is defined as a gastrointestinal (GI) disorder described by a difficult, irregular, or deficient defecation.1 Diagnosis of functional constipation is based on the following Rome III criteria2: (I) there must be two or more of the following occurrences >25% of the time: straining, hard stools, sensation of incomplete evacuation, sensation of anorectal obstruction/blockage, manual maneuvers to facilitate evacuation, or fewer than three defecations/week; (II) loose stools are rarely present without the use of laxatives; and (III) the criteria are insufficient for irritable bowel syndrome. Generally, stool softeners, osmotic agents, bulking agents, and stimulant laxatives are used to treat constipation.3 However, laxatives can cause adverse cardiac effects and artery contraction.4–6
The fruit of Prunus mume (Siebold) Siebold & Zucc. (P. mume), also called Maesil, has been used traditionally to treat intestinal disorders in Korea. The fruit of P. mume ripens in early summer in Korea and is harvested at the unripe stage (green fruit) (uPM). At the ripe stage (rPM), the fruit of P. mume is soft and has a different color than the unripe fruit (green vs. yellow). We previously proposed the use of rPM as therapeutics for the contraction using low-fiber diet-induced constipation model. In addition, citric acid and malic acid in rPM were shown to accelerate the spontaneous contraction (both amplitude and frequency) of isolated rat colons.7
Interestingly, we found that rPM and citric acid were more effective for improving constipation than uPM and malic acid were. However, few studies have evaluated the laxative effects of rPM, and the laxative effects of citric acid are still largely unknown. In this study, the laxative effects of rPM and citric acid were investigated in a rat model of constipation, and the detailed mechanisms were explored. In this study, the laxative effects of uPM, rPM, and the major organic acid compounds were investigated in a loperamide-induced constipation rats, and the mechanisms were explored.
Many studies have reported that constipation was successfully induced by administration of loperamide.8 According to previous study results, after administering morphine to the animals, constipation was induced, and the level of aquaporin 3 (AQP3) increased in the colon.9 In addition, bisacodyl decreases the levels of AQP3 in the intestine.10 Furthermore, bisacodyl increases the secretion of prostaglandin E2 (PGE2) in the colon.10 Hence, the aim of this study was to confirm the preventive and therapeutic effects of uPM and rPM in a loperamide-induced constipation rat model, and the possible mechanisms of uPM and rPM in the rat colon.
MATERIALS AND METHODS
Reagents
Dulbecco's modified eagle medium (DMEM) (Lonza, Basel, Switzerland) and fetal bovine serum (Invitrogen, Inc., Grand Island, NY, USA) were used for the cell culture. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of the extracts and high-performance liquid chromatography analysis of extracts
To ensure the standardization of extracts and reproducibility of efficacy, unripe and ripe fruits of P. mume (Siebold) Siebold & Zucc. harvested by selecting the region (35°04′31.7′′N, 127°42′59.3′′E, Gwangyang City, Jeollanamdo, Korea) and season (June) were used in this study. The seeds of the harvested P. mume were removed immediately, and the pulp was cut into small pieces and then freeze dried. The freeze-dried fruits of P. mume were extracted with 30 volumes of water at 100°C for 4 h three times and powdered by freeze drying.
Usually, 72.2 and 70.7 g of dried extracts were obtained from 100 g of dried unripe and ripe fruits of P. mume, respectively. These steps resulted in the sample labeled uPM and rPM, and the samples were stored at −20°C to avoid compound degradation before use in the experiment. The amounts of citric acid and malic acid in uPM and rPM were analyzed by high-performance liquid chromatography (HPLC), and compared with the standard preparations of citric acid and malic acid prepared according to our previously reported standard method.7
Cell culture and PGE2 content measurement
Murine macrophage RAW264.7 cells were purchased from Korea Cell Line Bank (KCLB 40071; Seoul, Korea) and were grown in DMEM at 37°C in a humidified atmosphere under 5% CO2. The cells were incubated with samples for 30 min. The PGE2 extraction and analysis were performed using an enzyme immunoassay (EIA) kit (Cayman, Ann Arbor, MI, USA) according to the manufacturer's instructions.
Animals
One hundred eighty to 200 g Sprague–Dawley rats were provided by Central Lab Animal, Inc. (Seoul, Korea). The experiment was conducted according to the international guidelines.11 In this study, seven animals per group were employed to minimize the number of animals used. All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of B&Tech Co., Ltd., Korea (Approval number: BT-007-2020, July 9, 2020).
Induction of the loperamide-induced constipation and treatment
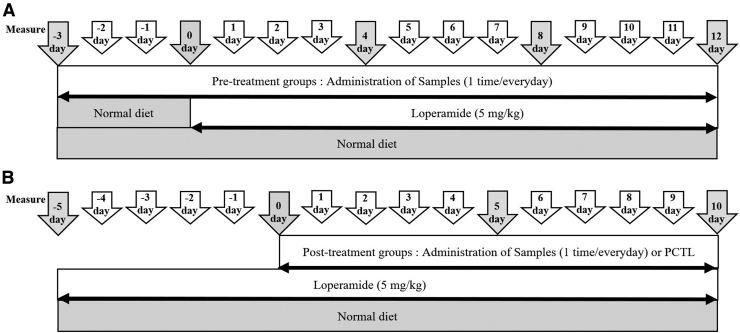
In this study, we have applied two experiments to validate the ability of uPM and rPM to prevent and alleviate constipation (Fig. 1A, B). The experimental groups (n = 7/group) were designed as a control (CTL) group (consumed a regular diet; Purina, Inc., Korea) and a constipation group in which constipation was induced by loperamide.12–16 To test the ability of uPM and rPM (100 and 200 mg/kg) to prevent constipation, the samples were administered uPM and rPM for 3 days before administering loperamide. After pretreatment with uPM and rPM for 3 days, uPM and rPM were administered once daily during the loperamide administration period. uPM and rPM (100 and 200 mg/kg) were dissolved in saline and administered orally 1 h after oral administration of 5 mg/kg of loperamide, daily for 12 days.
FIG. 1.
The scheme of the Prunus mume laxative experiment. The laxative effect of P. mume was assessed. The extracts (100 and 200 mg/kg) and physiological saline solution were administered once a day during the experiment. (A) The prevention of constipation by pretreatment with P. mume 3 days before constipation induced by the loperamide. (B) The therapeutic effect of constipation by post-treatment with P. mume. After 5 days of loperamide administration, three different doses of P. mume and bisacodyl (0.25 mg/kg), as a PCTL, were administered. PCTL, positive control.
To test the therapeutic effects of uPM and rPM (100 and 200 mg/kg) or bisacodyl (0.25 mg/kg), the samples were treated 5 days after loperamide administration. Constipation was induced in rats through the oral administration of 5 mg/kg of loperamide, once a day for 10 continuous days at 1 h before administration of uPM and rPM. The positive control (PCTL) group was orally administered with bisacodyl dissolved in saline once a day during the experiment. When the experimental period was complete, animals were anesthetized with 2.5 mL/kg pentobarbital (i.p.), followed by decapitation.
Measurement of stool parameters
The age (weeks), body weight (g), daily food intake (g), daily water intake (mL), and mass of feces (g) were recorded daily at 9:00 am. The number of feces and total weight of the feces were assessed for each rat during day 1. The stool water content (%) is calculated as follows: Stool water content (%) = [(feces weight before dried−feces weight after dried)/feces weight before dried] × 100.
GI motility test
The charcoal meal excretion test was performed on the last day of the experiment to assess GI motility. Each rat was fed 1 mL of charcoal meal (3% activated charcoal, 0.5% aqueous methylcellulose) as previously described.7 In brief, charcoal meal was orally treated 1 h after sample administration, and the number of black stools in each rat was measured at 2 h intervals for a total of 24 h.
Measurement of the PGE2 level in colons
PGE2 extraction and analysis in the rat colon were performed using a PGE2 assay kit (Cayman, USA). In brief, the rat colon was snap-frozen in liquid nitrogen. Frozen tissue was pulverized to fine powder under dry ice to extract PGE2. The frozen tissue powder (200 mg) was homogenized in 1 mL of phosphate-buffered saline (PBS) (containing 1 mM EDTA; pH 7.4) on ice using an ultrasonic processor. After complete lysis of samples, the supernatant was measured according to manufacturer's instructions.
Protein extraction and immunoblot assays
The rat colon tissues were removed and immediately soaked in ice-cold PBS. The rat colon was snap-frozen in liquid nitrogen. Frozen tissue was pulverized to fine powder under dry ice to extract protein. The frozen tissue powder (100 mg) was homogenized in 1 mL of RIPA buffer on ice. Anti-AQP3 (1:100, ab153694) and anti-β-actin (1:3000, ab8226) antibodies and secondary antibodies (1:10,000) were obtained from Abcam (Cambridge, UK). Immunoreactive protein bands were visualized using a ChemiDoc XRS+ System (Bio-Rad).
Immunohistochemistry and mucin staining
The 5 μm-thick rat colon tissue slices (paraffin embedded) were deparaffinized with xylene and rehydrated with graded ethanol. The slices were then immunostained with the primary antibody anti-AQP3 (1:30, ab153694; Abcam) and biotinylated secondary antibody (goat antirabbit IgG H&L, ab207995; Abcam). Mucin staining was performed using the Alcian blue stain kit (Abcam) according to the manufacturer's protocol. The 5 μm-thick rat colon tissue slices were stained with an Alcian blue solution (pH 2.5). Digital images were acquired by an optical microscope (Olympus, Tokyo, Japan) and software (MetaMorph 6.1 software; Universal Imaging Corp., Dowingtown, PA, USA).
Statistical analysis
Results are presented as the mean and standard deviation from three independent experiments. Data were analyzed by Student's t-test or two-way analysis of variance with GraphPad Prism version 8.0.0 for Windows (GraphPad, Inc., San Diego, CA, USA) software programs. Differences at the P < .05 level were considered statistically significant.
RESULTS
Identification of malic acid and citric acid in extracts of uPM and rPM
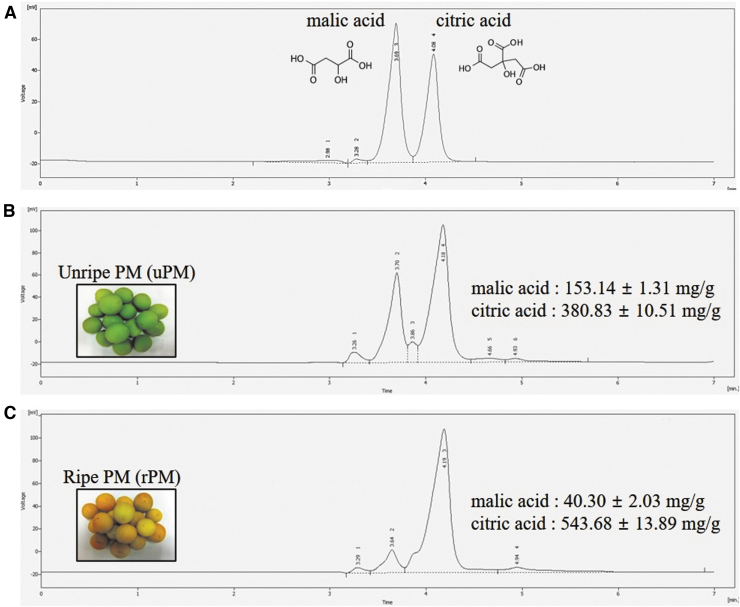
The yields of uPM and rPM after extraction were 72.2 g/100 g and 70.7 g/100 g, respectively. Characterization and identification of natural compounds in P. mume were determined by HPLC. As shown in Figure 2, the identified organic acids were malic acid and citric acid from rPM and uPM. The malic acid concentrations in uPM and rPM were 153.14 ± 1.31 and 40.30 ± 2.03 mg/g, respectively. The citric acid contents of uPM and rPM were 380.83 ± 10.51 and 543.68 ± 13.89 mg/g, respectively. Compared with citric and malic acid, the other organic acid compounds, such as oxalic acid, fumaric acid, and succinic acid, were present at lower concentrations in uPM and rPM (data not shown).
FIG. 2.
HPLC-DAD chromatograms of the major components of standards (A), unripe fruit of P. mume (uPM) extract (B), and ripe fruit of P. mume (rPM) extract (C). The peaks with retention times of 3.8 and 4.2 min were assigned to malic acid and citric acid, respectively. Malic acid and citric acid were identified at a wavelength of 214 nm. The data are represented as the means ± SDs. HPLC-DAD, high-performance liquid chromatography with diode-array detection; SD, standard deviation.
Effect of P. mume administration on feeding behavior in rats with loperamide-induced constipation
To evaluate the effect of P. mume on feeding behavior of constipated rats, we monitored the feeding behaviors of rats with loperamide-induced constipation. The body weight, food intake, and water consumption did not differ significantly between the CTL and the loperamide-induced constipation group during the experiment (Table 1). Furthermore, no significant increase in stool parameters was detected at any of the tested doses of uPM and rPM. Similar results were obtained for the body weights of all groups both before and after constipation was induced. Taken together, these results show that loperamide and P. mume administration did not induce alterations in feeding behavior.
Table 1.
Measurements of the Body Weight, Feed Intake, and Water Intake in Sprague–Dawley Rats with Loperamide-Induced Constipation
| Normal diet group (12 day) | Loperamide group (12 day) | PCTL group (10 day) | Pretreated groups (12 day) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Citric acid |
Malic acid |
rPM 100 |
rPM 200 |
uPM 100 |
uPM 200 |
||||
| Post-treated groups (10 day) | |||||||||
| Citric acid | Malic acid | rPM 100 | rPM 200 | uPM 100 | uPM 200 | ||||
| Body weight (g) | 294.00 ± 7.84 | 287.80 ± 17.11 | 279.00 ± 15.17 | 280.80 ± 23.40 | 299.60 ± 12.97 | 289.20 ± 22.53 | 288.00 ± 22.51 | 286.40 ± 6.35 | 287.60 ± 7.89 |
| 278.00 ± 7.87 | 294.60 ± 15.73 | 283.40 ± 19.78 | 296.20 ± 11.26 | 273.17 ± 21.37 | 295.20 ± 13.03 | ||||
| Feed intake (g) | 21.9 ± 4.31 | 24.06 ± 4.96 | 23.99 ± 8.97 | 22.73 ± 8.99 | 24.17 ± 7.46 | 22.11 ± 8.43 | 23.18 ± 6.28 | 25.14 ± 7.19 | 24.83 ± 9.34 |
| 22.55 ± 6.01 | 23.76 ± 6.91 | 21.07 ± 3.39 | 24.06 ± 4.67 | 24.97 ± 4.90 | 20.30 ± 5.50 | ||||
| Water intake (mL) | 29.40 ± 5.13 | 30.40 ± 5.90 | 28.20 ± 4.15 | 27.60 ± 6.73 | 27.80 ± 2.17 | 30.40 ± 2.61 | 26.80 ± 1.92 | 29.60 ± 4.62 | 28.80 ± 5.40 |
| 30.80 ± 7.01 | 30.00 ± 9.35 | 27.60 ± 3.21 | 32.20 ± 6.91 | 28.00 ± 10.37 | 30.20 ± 9.37 | ||||
Seven rats per group were used for all assessments, and each parameter was assayed in triplicate in each test. The data are reported as the mean ± SD.
SD, standard deviation.
Preventive effects of P. mume pretreatment on loperamide-induced constipation
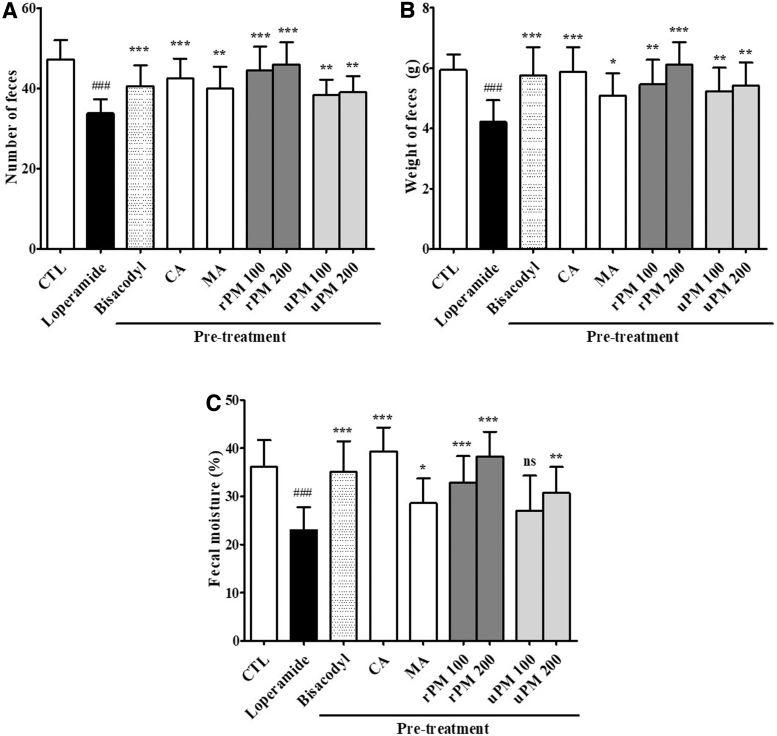
The preventive effects of P. mume pretreatment 3 days before loperamide administration were evaluated. The results showing the preventive effects of P. mume on constipation are shown in Figure 3. Compared with those of the CTL diet, administration of loperamide (12 days) caused a significant decrease in the number (P < .01), weight (P < .001), and moisture content (P < .001) of stools. The 100 and 200 mg/kg uPM-treated groups showed significant increases (P < .01 and P < .01, respectively) in the number of stools compared with that of the loperamide group at 12 days (Fig. 3A). The 100 and 200 mg/kg uPM-treated rats showed significant increases (P < .01 and P < .01, respectively) in the weight of stools compared with that of the loperamide group at days 12 (Fig. 3B).
FIG. 3.
The preventive effects of P. mume on loperamide-induced constipation. At 12 days, the total number (A), weight (B), and water content (C) of stools were measured as described in the materials and methods. The stool water content was calculated using the fresh and dry weights of stools. The stools were collected from seven rats per group, and each parameter was assayed in triplicate. Each bar represents the mean ± SD for seven rats. ###Significant difference at P < .001 compared with that of the CTL group. *Significant difference at P < .05, ** at P < .01 and *** at P < .001 compared with that of the loperamide-induced constipation. CTL, control.
Interestingly, the 100 and 200 mg/kg rPM-treated rats showed significant increases in the number (P < .001 and P < .001, respectively) and weight (P < .01 and P < .001, respectively) of stools compared with that of the loperamide group at 12 days. Moreover, the 100 and 200 mg/kg rPM-treated rats showed a significant increase (P < .001 and P < .001, respectively) in the water content of stools, whereas the 100 mg/kg uPM-treated rats did not show any significant changes compared with that of the loperamide group at 12 days (Fig. 3C).
Furthermore, the malic acid-treated groups showed significant increases in the number (P < .01), weight (P < .05), and water content (P < .05) of stools compared with that of the loperamide group at 12 days. In addition, after the induction of constipation (Day 12), the stool parameters in the citric acid groups were similar to those in the CTL (all P < .001), in which constipation was not induced. Furthermore, rPM and citric acid did not cause diarrhea in the present experiments (data not shown). These results clearly show that pretreatment with rPM and citric acid can prevent constipation.
The laxative effect of P. mume on rats with loperamide-induced constipation
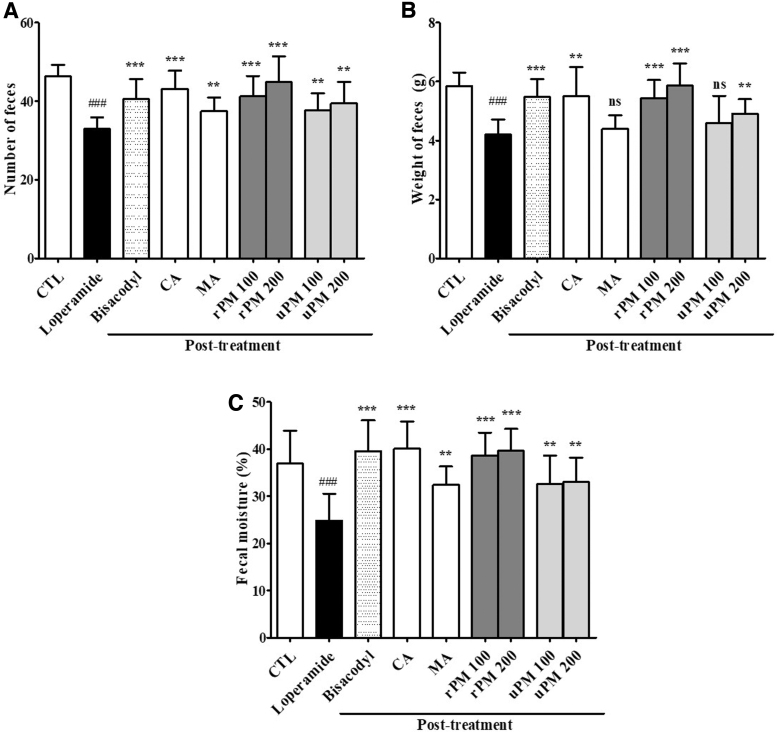
To examine the laxative effect of P. mume on the stool parameters in rats, rats were treated with P. mume (100 and 200 mg/kg) once daily for 10 days. As shown in Figure 4, the number (P < .001), weight (P < .001), and water content (P < .001) of stools were decreased after constipation induction (Day 10) in all groups. While the loperamide-treated group showed significant decreases in the stool parameters, rats administered 100 and 200 mg/kg rPM showed significant increases in the stool number (all P < .001), weight (all P < .001), and water content (all P < .001) starting at 10 days after initiating rPM administration.
FIG. 4.
The laxative effects of P. mume on loperamide-induced constipation. At 10 days, the total number (A), weight (B), and water content (C) of stools were measured as described in the materials and methods. The stool water content was calculated using the fresh and dry weights of stools. Stools were collected from seven rats per group, and each parameter was assayed in triplicate. Each bar represents the mean ± SD for seven rats. ###Significant difference at P < .001 compared with the CTL group. **Significant difference at P < .01 and *** at P < .001 compared with that of the loperamide-induced constipation group.
Furthermore, the oral administration of citric acid for 10 days significantly increased the stool parameters to 10.16 ± 4.33 g (P < .001, number), 1.28 ± 0.06 g (P < .01, weight), and 15.26% ± 8.63% (P < .001, water content) at 50 mg/kg. However, the malic acid-treated groups showed no significant increases (P > .05) in the weight of stools compared with that of the loperamide group at 10 days (Fig. 4B). The 100 mg/kg uPM-treated rats also showed no significant increases (P > .05) in the water content of stools compared with that of the loperamide group. Bisacodyl was used as the PCTL, and the bisacodyl-treated group showed significant increases in the number (P < .001), weight (P < .001), and water content (P < .001) of stools compared with that of the loperamide group at 10 days.
The effect of P. mume on charcoal meal GI motility in rats with loperamide-induced constipation
To evaluate the effects of P. mume on the GI tract, we monitored GI motility in the rats. Changes in GI motility by P. mume treatment are shown in Table 2 (preventative effects; pretreatment protocol) and Table 3 (therapeutic effects; post-treatment protocol). The time required for the excretion of charcoal meal containing stools in the loperamide group (10–24 h) was 4–6 h later than that in the CTL (6–20 h); the number of feces was decreased in the loperamide group compared with the CTL. Table 2 presents the results of the preventative test; the 200 mg/kg uPM-treated rats excreted more feces than those of the loperamide group at 8–10 h, and the 100 and 200 mg/kg rPM-treated rats showed more rapid fecal excretion than those of the loperamide group.
Table 2.
The Preventive Effects of Prunus mume on Gastrointestinal Motility in Rats
| |
Mean number of charcoal-containing stools/2 h (n = 7) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (hours) | 0–2 | 2–4 | 4–6 | 6–8 | 8–10 | 10–12 | 12–14 | 14–16 | 16–18 | 18–20 | 20–22 | 22–24 |
| CTL | — | — | — | 0.8 | 1.0 | 5.6 | 4.0 | 7.6 | 2.0 | 1.2 | — | — |
| Loperamide | —1 | — | — | — | — | 0.6 | 0.8 | 3.6 | 6.0 | 3.0 | 1.7 | 1.5 |
| Bisacodyl | — | — | — | 0.6 | 1.4 | 0.8 | 2.2 | 1.4 | 6.6 | 3.8 | 3.0 | 6.4 |
| CA | — | — | — | 0.4 | 1.0 | 0.4 | 0.2 | 3.0 | 3.0 | 3.6 | 3.2 | — |
| MA | — | — | — | — | — | 1.2 | 2.4 | 6.8 | 6.0 | 3.6 | 5.8 | 3.1 |
| rPM 100 | — | — | — | — | 0.9 | 4.0 | 5.6 | 6.5 | 3.0 | 3.5 | 3.0 | — |
| rPM 200 | — | — | — | 0.4 | 1.2 | 3.4 | 3.6 | 3.0 | 4.2 | 3.6 | — | — |
| uPM 100 | — | — | — | — | — | 0.6 | 0.6 | 6.2 | 3.6 | 3.6 | 4.8 | 3.0 |
| uPM 200 | — | — | — | — | 0.4 | — | 2.4 | 6.6 | 4.8 | 3.8 | 4.6 | 3.0 |
Table 3.
The Therapeutic Effects of P. mume on Gastrointestinal Motility in Rats
| |
Mean number of charcoal-containing stools/2 h (n = 7) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (hours) | 0–2 | 2–4 | 4–6 | 6–8 | 8–10 | 10–12 | 12–14 | 14–16 | 16–18 | 18–20 | 20–22 | 22–24 | |
| CTL | — | — | — | 1.0 | 1.2 | 4.8 | 5.2 | 6.6 | 1.5 | 1.0 | — | — | |
| Loperamide | — | — | — | — | — | 0.4 | 0.8 | 1.6 | 5.8 | 3.4 | 2.6 | 1.2 | |
| PCTL | — | — | — | 0.6 | 1.2 | 1.6 | 2.8 | 2.4 | 4.6 | 2.8 | 2.2 | — | |
| CA | — | — | — | 0.2 | 1.2 | 3.5 | 4.2 | 3.0 | 4.2 | 2.6 | 2.2 | — | |
| MA | — | — | — | — | — | 1.0 | 2.2 | 6.5 | 3.6 | 1.7 | 0.8 | 1.0 | |
| rPM 100 | — | — | — | — | 1.6 | 3.8 | 5.0 | 7.2 | 3.0 | 3.8 | 3.2 | — | |
| rPM 200 | — | — | — | 0.4 | 3.0 | 4.0 | 4.5 | 3.2 | 5.6 | 3.8 | — | — | |
| uPM 100 | — | — | — | — | — | 0.5 | 0.2 | 5.7 | 4.7 | 3.5 | 4.2 | 3.0 | |
| uPM 200 | — | — | — | — | 0.4 | 1.6 | 3.4 | 3.8 | 4.4 | 3.6 | 4.6 | 2.0 | |
PCTL, positive control.
The 50 mg/kg malic acid-treated rats excreted more feces than those of the loperamide group at 10–14 h, and the 50 mg/kg citric acid-treated group showed more rapid fecal excretion (6–8 h) than those of the loperamide group. In addition, a similar result was shown in the therapeutic test (Table 3) on GI motility. Taken together, these results demonstrate that rPM and citric acid treatment can enhance GI motility in the loperamide-induced constipation rat.
The effects of P. mume on the PGE2 concentration in RAW264.7 cells
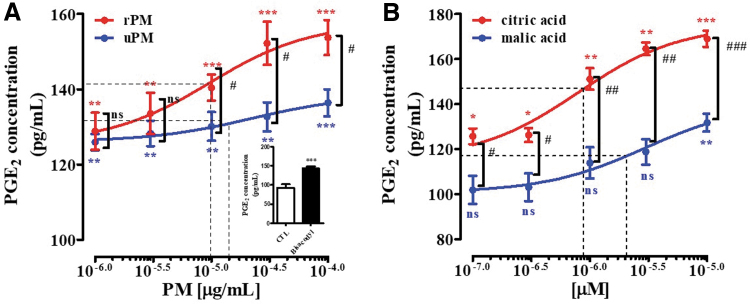
The PGE2 concentrations in the culture medium 30 min after adding bisacodyl (10 μg/mL) to RAW264.7 cells were significantly increased (P < .001) compared with those of the CTL (Fig. 5A, insert). Similarly, compared with the control condition, the addition of uPM (EC50 value: 22.28 ± 0.87 μg/mL) and rPM (EC50 value: 9.65 ± 0.45 μg/mL) caused a significant and dose-dependent increase in the PGE2 concentrations in the culture medium (Fig. 5A).
FIG. 5.
Changes in PGE2 levels in RAW264.7 cells. Thirty minutes after the addition of P. mume (A) and organic acid (B), the supernatant was collected, and the concentrations of PGE2 were measured by using EIA. RAW264.7 cells were treated with bisacodyl (10 μg/mL) and recovered 30 min later (A; insert). Each data point represents the mean ± SD of six experiments. *Significant difference at P < .05, ** at P < .01, and *** at P < .001 compared with that of the CTL group. #, ##, ###indicate P < .05, P < .01, and P < .001, respectively, compared with the malic acid treated group. NS, not significant; EIA, enzyme immunoassay; PGE2, prostaglandin E2.
In addition, the efficiency of rPM in stimulating PGE2 secretion was much higher than that of uPM (P < .05). Similarly, the respective EC50 values were also significantly different among the malic acid (EC50 value: 2.99 ± 0.04 μM) and citric acid (EC50 value: 0.79 ± 0.02 μM) and dose-dependent increase in the PGE2 concentrations in the culture medium (Fig. 5B). In addition, the efficiency of citric acid in stimulating PGE2 secretion was much higher (P < .001) than that of malic acid.
The effects of P. mume on the PGE2 concentration in the colons of rats with loperamide-induced constipation
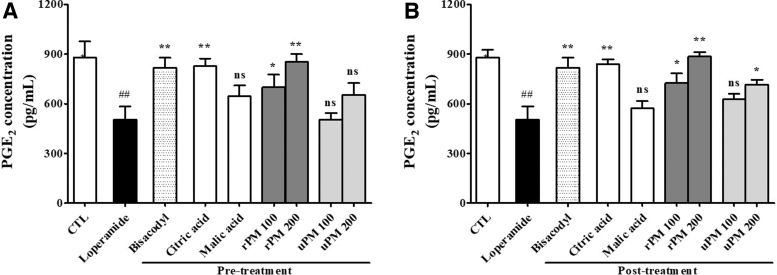
As shown in Figure 6, the PGE2 concentrations in the rat colon were decreased after the induction of constipation with loperamide. The PGE2 concentrations were significantly increased (P < .05 and P < .01, respectively) in the rPM groups pretreated with 100 and 200 mg/kg compared with that of the NCTL (Fig. 6A). The level of PGE2 in the colon was significantly increased (P < .05 and P < .01, respectively) in the groups post-treated with rPM (100 and 200 mg/kg) compared with that of the NCTL (Fig. 6B).
FIG. 6.
Changes in the PGE2 levels in the colon caused by loperamide administration to rats pretreated with samples (A) and post-treated with samples (B). The concentrations of PGE2 were measured by using EIA. Each bar represents the mean ± SD for seven mice. ##Significant difference at ## at P < .01 compared with the CTL group. *Significant difference at P < .05 and ** at P < .01 compared with that of the loperamide-induced constipation group.
Effects of P. mume on the protein expression levels of AQP3 in the colon
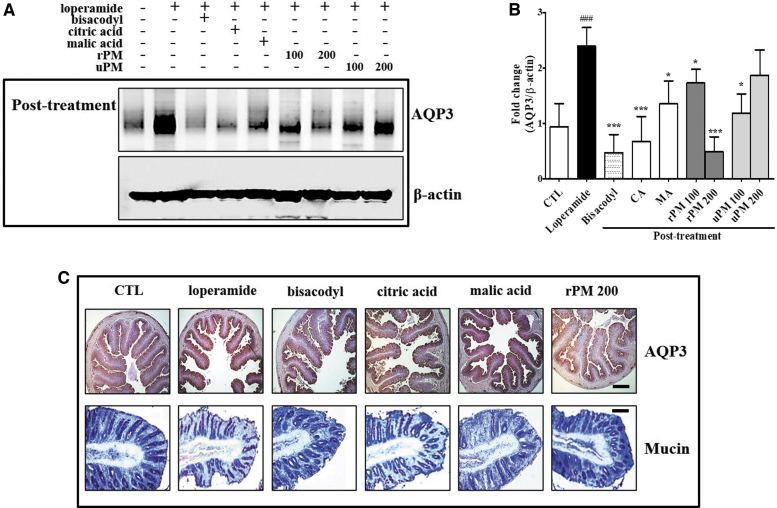
As shown in Figure 7A and B, compared with that of the CTL, loperamide increased the protein level of AQP3, whereas bisacodyl, citric acid, and 200 mg/kg rPM significantly decreased (all P < .001) it from 2.39 ± 0.34 to 0.47 ± 0.33, 0.68 ± 0.45, and 0.49 ± 0.27, respectively. Using immunohistochemistry, we observed changes in AQP3 levels after treatment with citric acid and rPM in the colon of the rat model of loperamide-induced constipation (Fig. 7C). Compared with CTL, the expression of AQP3 was increased in the mucosal epithelial cells of the loperamide-induced constipation model. On the contrary, treatment with bisacodyl, citric acid, and 200 mg/kg rPM attenuated this phenomenon.
FIG. 7.
Detection of AQP3 and mucin levels in the colon. (A) Effects of P. mume on AQP3 expression in the colon as assessed by Western blot analysis. (B) Each value was normalized to β-actin and expressed as the mean ± SD. (C) Immunohistochemical localization of the AQP3 protein in the rat colon, and images were obtained at 4 × magnification. Mucin secreted from the crypt layer cells was stained with Alcian blue, and images were obtained at 10 × magnification. The scale bar indicates 50 μm for AQP3 immunostaining and 10 μm for Alcian blue staining. Each bar represents the mean ± SD (n = 8). *Significant difference at P < .05 and *** at P < .001 compared with the baseline (CTL). AQP3, aquaporin 3.
Effects of P. mume administration on the regulation of mucin secretion in the colon
As shown in Figure 7C, we found that regions secreting mucin were concentrated in the crypts of the mucosal layer of the colon in the CTL. Conversely, lower levels of mucin were observed in the loperamide-treated rats. However, the secretion levels of mucin increased in citric acid- and rPM-treated rats. Furthermore, we detected a similar result in the secretion level of mucin in the bisacodyl-treated rats. These results indicate that the secretion of mucin in the colon was increased by citric acid and rPM similarly to bisacodyl.
DISCUSSION
Medicinal plants have received increased attention as new therapeutics for the treatment of constipation.6 Thus, in this work, the preventive effects and therapeutic laxative effects of P. mume were evaluated in loperamide-induced constipation model rats. The results clearly demonstrated that P. mume has laxative effects, including stool parameters, GI mobility, secretion of PGE2 and mucin, and expression of AQP3 protein. Our results are the first to study that the laxative effects of P. mume are strongly related to the promotion of defecation and colon contraction.
The quantitative assessment of natural compounds is helpful for the proper standardization of natural products due to their various pharmacological effects and potential variation. HPLC fingerprints are useful for qualitative and quantitative analyses of natural product formulations. The HPLC chromatograms shown in Figure 2 show the chromatographic fingerprint of P. mume as well the isolation of citric acid and malic acid, from P. mume. Interestingly, as the P. mume ripened, the content of citric acid increased, and conversely, the content of malic acid decreased. This suggests that there may be differences in the physiological activities of rPM and uPM depending on the maturation stage.
In our previous study, we reported that rPM has a superior effect on alleviating constipation to uPM using a low-fiber diet-induced animal model.7 It was also reported that citric acid promotes peristalsis of the colon, which is superior to malic acid. Therefore, in this study, the effect and mechanism of constipation in animal models using other chemical compounds, such as loperamide, were investigated.
We present herein four principal findings regarding P. mume through cellular and animal studies aimed at explaining the laxative effect of P. mume on loperamide-induced constipation model rats. First, we found that stool parameters were increased in the P. mume-treated rats compared with the loperamide-treated rats. An effective laxative should increase the frequency of defecation, reduce stool retention in the colon lumen, and increase the water content of the stool.9,17 Our results showed that fecal excretion (fecal number, weight, and water content) was significantly increased by the administration of P. mume (Figs. 3 and 4).
Furthermore, after administration of P. mume, a significant increase in GI motility was observed, consistent with the bisacodyl treatment (PCTL), which induced similar inhibitory effects on the loperamide-induced decreases in GI motility (Tables 2 and 3). Taken together, this study demonstrated that P. mume prevented and also alleviated constipation in a rat model of loperamide-induced constipation. Loperamide is commonly used to produce constipation in animals.18–21 Many studies have reported that constipation was successfully induced by administration of 1.5–3 mg/kg loperamide for 3–7 days.12–16 In this study, we used loperamide to induce constipation and observed the constipation in animal models administered with 3 mg/kg loperamide that did not have any other specific problems.
Second, as a result of verifying the laxative effect according to the ripening stage of P. mume, a higher effect was found in the mature rPM than in the immature uPM. In addition, when the laxative effects of citric acid and malic acid were compared, superior effects were found in rats treated with citric acid. Interestingly, when the organic acid content of rPM and uPM was quantified by HPLC, the amount of citric acid in rPM increased and the content of malic acid decreased as the ripening stage progressed.
Taken together, the reason rPM has a superior laxative effect to uPM is that citric acid is the main active compound that alleviates constipation, suggesting that the citric acid content increases as the P. mume matures. In our previous study using an animal model of constipation treated with a low-fiber diet, rPM showed a superior laxative effect to that of uPM.7 In this study, citric acid showed a laxative effect superior to that of malic acid in a low-fiber diet-administered constipated animal model. We verified the laxative effect of rPM for the first time through these studies, and the results suggest that citric acid is an effective candidate compound that can be used to prevent and alleviate constipation.
Third, we observed that rPM and citric acid have similar mechanisms to that of bisacodyl. According to the results of the previous study, after morphine was administered to the animals, constipation was induced, and the expression of AQP3 increased in the intestine, which enhanced water transport.9 However, the AQP3 in the intestine was increased due to the administration of morphine, but the expression of AQP3 decreased with the administration of bisacodyl, thereby reducing water transfer.22 Furthermore, bisacodyl directly activates macrophages and increases the production of PGE2.10 Therefore, the oral administration of bisacodyl to rats increased the stool water content.10,23
Our results also demonstrated significant increases in stool parameters after the administration of bisacodyl in rat models of loperamide-induced constipation (Figs. 3 and 4). In addition, we confirmed through in vitro and in vivo studies that PGE2 levels were increased by rPM and citric acid treatment. PGE2 has also been implicated in several GI pathologies.24–26 Furthermore, when macrophages are activated, the production of PGE2 increases.27,28 Therefore, the effect of rPM and citric acid on the activation of macrophages was examined using RAW264.7 cells (Fig. 5).
The concentrations of PGE2 after the treatment of rPM and citric acid to RAW264.7 cells were dose-dependently increased. The effect of increasing PGE2 secretion by rPM and citric acid treatment demonstrated in RAW264.7 cells was also verified in loperamide-induced constipation model (Fig. 6). Taken together, these results indicate that the expression of AQP3 was reduced by treatment with rPM and citric acid, and the secretion of PGE2 was promoted to increase the amount of water in the feces. Although the exact mechanisms of the correlation between the decreased expression of AQP3 and the secretion of PGE2 are not yet known, it is possible that these results involve endocytosis or the degradation of AQP3.29
Finally, we observed that the secretion of mucin was significantly increased in the constipation rat model when the colons were treated with rPM and citric acid. The decrease in mucin secretion by loperamide treatment is well known through many studies, and it is particularly known to be caused by the decrease in the number of mucus-producing cells.6,30–33 In this study, it was confirmed that the secretion of mucin was decreased in the intestine of the constipation model rats treated with loperamide. However, in the group administered rPM and citric acid, the decreased amount of mucin secretion increased.
In the group administered bisacodyl, the same result of increased mucin secretion was observed. The gel-forming mucins synthesized by the goblet cells were present in larger quantities in the crypt than in the colon.34,35 Among the gel-forming mucins, mucin 2 (Muc2) is the best characterized mucin of the intestine.34,36 The process of synthesizing Muc2 is very complex.34,37,38 Therefore, further research is needed to elucidate the cause of the increased Muc2 secretion by treatment with citric acid and rPM.
Since the cytotoxicity of rPM is one factor involved in intestinal inflammation or epithelial cell response, cytotoxicity has been reported in various cells by many researchers. They demonstrated noncytotoxicity in the range of up to 380 μg/mL of P. mume in a variety of cells.39–41 Moreover, in a study evaluating the cytotoxicity of citric acid in various cells, cytotoxicity was not observed as a result of treatment up to a concentration of 60%.42,43 Although this experiment did not study cytotoxicity, no cytotoxicity is expected according to the results of these previous reports. In addition, we plan to study the cytotoxicity of PMs by isolating intestinal immune cells and epithelial cells in the next study.
Although the clinical application of the findings obtained in loperamide-induced animal models has some limitations, it is widely used in the development and evaluation of novel laxatives.44,45 Therefore, it is necessary to develop a new constipation model through the study of diversification of the phenotype of constipation through genetic or chemical-induced constipation models, and to conduct pharmacological evaluations using these constipation animal models.
The results of our study have proven through animal experiments that rPM, especially citric acid, is effective for improving the symptoms of constipation. Therefore, for the first time, our results provide evidence for ameliorating and preventing symptoms of constipation by rPM in an animal model of loperamide-induced constipation. The results justify the use of rPM as a laxative in natural herbal product. In conclusion, these results scientifically support the use of P. mume as a natural product-derived laxative without causing diarrhea and tolerance.
In conclusion, these findings scientifically support the value of citric acid-enriched rPM as a therapeutic laxative without causing diarrhea and tolerance.
ACKNOWLEDGMENT
We sincerely appreciate other colleagues in our laboratory for their help and effort in this study.
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
FUNDING INFORMATION
This research was supported by a Korea Innovation Foundation (INNIPOLIS) grant funded by the Korean government (Ministry of Science and ICT) through a science and technology project that opens the future of the region (grant number: 2021-DD-UP0380). The funding body did not play a role in the study design, performance, data collection and analysis, decision to publish, or preparation/writing of the article.
REFERENCES
- 1. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC: Functional bowel disorders. Gastroenterology 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 2. Mostafa R: Rome III: The functional gastrointestinal disorders, third edition, 2006. World J Gastroenterol 2008;14:2124–215. [Google Scholar]
- 3. Johnson DA: Treating chronic constipation: How should we interpret the recommendations? Clin Drug Investig 2006;26:547–557. [DOI] [PubMed] [Google Scholar]
- 4. Lembo A, Camilleri M: Chronic constipation. N Engl J Med 2003;349:1360–1368. [DOI] [PubMed] [Google Scholar]
- 5. Busti AJ, Murillo JR Jr, Cryer B: Tegaserod-induced myocardial infarction: Case report and hypothesis. Pharmacotherapy 2004;24:526–531. [DOI] [PubMed] [Google Scholar]
- 6. Kim JE, Lee YJ, Kwak MH, Ko J, Hong JT, Hwang DY: Aqueous extracts of Liriope platyphylla induced significant laxative effects on loperamide-induced constipation of SD rats. BMC Complement Altern Med 2013;13:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Na JR, Oh KN, Park SU, et al. : The laxative effects of Maesil (Prunus mume Siebold & Zucc.) on constipation induced by a low-fiber diet in a rat model. Int J Food Sci Nutr 2013;64:333–345. [DOI] [PubMed] [Google Scholar]
- 8. Kakino M, Tazawa S, Maruyama H, et al. : Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Complement Altern Med 2010;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kon R, Ikarashi N, Hayakawa A, et al. : Morphine-induced constipation develops with increased aquaporin-3 expression in the colon via increased serotonin secretion. Toxicol Sci 2015;145:337–347. [DOI] [PubMed] [Google Scholar]
- 10. Ikarashi N, Baba K, Ushiki T, et al. : The laxative effect of bisacodyl is attributable to decreased aquaporin-3 expression in the colon induced by increased PGE2 secretion from macrophages. Am J Physiol Gastrointest Liver Physiol 2011;301:G887–895. [DOI] [PubMed] [Google Scholar]
- 11. The European Parliament and the Council of the European Union: Directive 2010/63/EU of the European parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Off J Eur Union 2010;276:33–79. [Google Scholar]
- 12. Wintola OA, Sunmonu TO, Afolayan AJ: The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol 2010;10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee HY, Kim JH, Jeung HW, et al. : Effects of Ficus carica paste on loperamide-induced constipation in rats. Food Chem Toxicol 2012;50:895–902. [DOI] [PubMed] [Google Scholar]
- 14. Méité S, Bahi C, Yéo D, Datté JY, Djaman JA, N'guessan DJ: Laxative activities of Mareya micrantha (Benth.) Müll. Arg. (Euphorbiaceae) leaf aqueous extract in rats. BMC Complement Altern Med 2010;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang ZH, Yu HJ, Pan A, et al. : Cellular mechanisms underlying the laxative effect of flavonol naringenin on rat constipation model. PLoS One 2008;3:e3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bustos D, Ogawa K, Pons S, Soriano E, Bandi JC, Bustos Fernández L: Effect of loperamide and bisacodyl on intestinal transit time, fecal weight and short chain fatty acid excretion in the rat. Acta Gastroenterol Latinoam 1991;21:3–9. [PubMed] [Google Scholar]
- 17. Lim JM, Kim YD, Song CH, et al. : Laxative effects of triple fermented barley extracts (FBe) on loperamide (LP)-induced constipation in rats. BMC Complement Altern Med 2019;19:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hughes S Higgs NB, Turnberg LA: Loperamide has antisecretory activity in the human jejunum in vivo. Gut 1984;25:931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sohji Y, Kawashima K, Shimizu M: Pharmacological studies of loperamide, an anti-diarrheal agent. II. Effects on peristalsis of the small intestine and colon in guinea pigs (author's transl). Nihon Yakurigaku Zasshi 1978;74:155–163. [DOI] [PubMed] [Google Scholar]
- 20. Schemann M: Control of gastrointestinal motility by the “gut brain”-the enteric nervous system. J Pediatr Gastroenterol Nutr 2005;41:S4–6. [DOI] [PubMed] [Google Scholar]
- 21. Foxx-Orenstein AE, Grider JR: Regulation of colonic propulsion by enteric excitatory and inhibitory neurotransmitters. Am J Physiol 1996;271:G433–437. [DOI] [PubMed] [Google Scholar]
- 22. Marr N Bichet DG, Hoefs S, Savelkoul PJ, et al. : Cell-biologic and functional analyses of five new Aquaporin-2 missense mutations that cause recessive nephrogenic diabetes insipidus. J Am Soc Nephrol 2002;13:2267–2277. [DOI] [PubMed] [Google Scholar]
- 23. Leng-Peschlow E: Effects of sennosides A + B and bisacodyl on rat large intestine. Pharmacology 1989;38:310–318. [DOI] [PubMed] [Google Scholar]
- 24. Beubler E, Kollar G, Saria A, Bukhave K, Rask-Madsen J: Involvement of 5-hydroxytryptamine, prostaglandin E2, and cyclic adenosine monophosphate in cholera toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology 1989;96:368–376. [DOI] [PubMed] [Google Scholar]
- 25. Resta-Lenert S, Barrett KE: Enteroinvasive bacteria alter barrier and transport properties of human intestinal epithelium: Role of iNOS and COX-2. Gastroenterology 2002;122:1070–1087. [DOI] [PubMed] [Google Scholar]
- 26. Cong P, Pricolo V, Biancani P, Behar J: Abnormalities of prostaglandins and cyclooxygenase enzymes in female patients with slow-transit constipation. Gastroenterology 2007;133:445–453. [DOI] [PubMed] [Google Scholar]
- 27. Haskó G, Szabó C, Németh ZH, Kvetan V, Pastores SM, Vizi ES: Adenosine receptor agonists differentially regulate IL-10, TNF-α, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol 1996;157:4634–4640. [PubMed] [Google Scholar]
- 28. Lee JY, Cho BJ, Park TW, et al. : Dibenzylbutyrolactone lignans from Forsythia koreana fruits attenuate lipopolysaccharide-induced inducible nitric oxide synthetase and cyclooxygenase-2 expressions through activation of nuclear factor-κb and mitogen-activated protein kinase in RAW264.7 cells. Biol Pharm Bull 2010;33:1847–1853. [DOI] [PubMed] [Google Scholar]
- 29. Zhu D, Chen C, Bai L, Kong L, Luo J: Downregulation of aquaporin 3 mediated the laxative effect in the rat colon by a purified resin glycoside fraction from pharbitis semen. Evid Based Complement Alternat Med 2019;13:2019:9406342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jang SH, Yang DK: Correction: The combination of Cassia obtusifolia L. and Foeniculum vulgare M. exhibits a laxative effect on loperamide-induced constipation of rats. PLoS One 2018;13:e0202259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan S, Yue YZ, Wang XP, et al. : Aqueous extracts of Herba Cistanche promoted intestinal motility in loperamide-induced constipation rats by ameliorating the interstitial cells of cajal. Evid Based Complement Alternat Med 2017;2017:6236904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi JS, Kim JW, Cho HR, et al. : Laxative effects of fermented rice extract in rats with loperamide-induced constipation. Exp Ther Med 2014;8:1847–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hajji N, Wannes D, Jabri MA, et al. : Purgative/laxative actions of Globularia alypum aqueous extract on gastrointestinal-physiological function and against loperamide-induced constipation coupled to oxidative stress and inflammation in rats. Neurogastroenterol Motil 2020;32:e13858. [DOI] [PubMed] [Google Scholar]
- 34. Johansson ME, Hansson GC: Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 2016;16:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Birchenough GM, Johansson ME, Gustafsson JK, Bergström JH, Hansson GC: New developments in goblet cell mucus secretion and function. Mucosal Immunol 2015;8:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johansson ME, Larsson JM, Hansson GC: The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A 2011;108 Suppl 1:4659–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johansson ME, Ambort D, Pelaseyed T, et al. : Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci 2011;68:3635–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paone P, Cani PD: Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020;69:2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu S-F, Chen Y-H, Lin C-L, et al. : Qualitative and quantitative analyses of the anti-allergic constituent of commercial Prunus mume products in Taiwan. J Food Drug Anal 2011;19:66–72. [Google Scholar]
- 40. Pi K, Lee K: Prunus mume extract exerts antioxidant activities and suppressive effect of melanogenesis under the stimulation by alpha-melanocyte stimulating hormone in B16-F10 melanoma cells. Biosci Biotechnol Biochem 2017;81:1883–1890. [DOI] [PubMed] [Google Scholar]
- 41. Kono R, Nakamura M, Nomura S, et al. : Biological and epidemiological evidence of anti-allergic effects of traditional Japanese food ume (Prunus mume). Sci Rep 2018;8:11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. An T-E-B, Kim D-C: In vitro cytotoxicity, skin regeneration, anti-wrinkle, whitening and in vivo skin moisturizing effects of Oncheongeum. J Korean Obstet Gynecol 2016;29:14–34. [Google Scholar]
- 43. Khoswanto C, Arijani E, Soesilawati P: Cytotoxicity test of 40, 50 and 60% citric acid as dentin conditioner by using MTT assay on culture cell line. Dental J (Majalah Kedokteran Gigi) 2008;41:103–106. [Google Scholar]
- 44. Neri F, Cavallari G, Tsivian M, et al. : Effect of colic vein ligature in rats with loperamide-induced constipation. J Biomed Biotechnol 2012;2012:896162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T: Decreased colonic mucus in rats with loperamide-induced constipation. Comp Biochem Physiol A Mol Integr Physiol 2000;126:203–212. [DOI] [PubMed] [Google Scholar]