Abstract
Context
An increase in maternal insulin resistance (IR) during pregnancy is essential for normal fetal growth. The mechanisms underlying this adaptation are poorly understood. Placental factors are believed to instigate and maintain these changes, as IR decreases shortly after delivery. Methylation of placental gene loci that are common targets for miRNAs are associated with maternal IR.
Objective
We hypothesized that placental miRNAs targeting methylated loci are associated with maternal IR during late pregnancy.
Methods
We collected placentas from 132 elective cesarean sections and fasting blood samples at delivery to estimate maternal homeostasis model assessment of insulin resistance (HOMA-IR). Placental miRNA expression was measured via whole genome small-RNA sequencing in a subset of 40 placentas selected by maternal pre-gravid body mass index (BMI) and neonatal adiposity. Five miRNAs correlated with maternal HOMA-IR and previously identified as targeting methylated genes were selected for validation in all 132 placenta samples via RT-qPCR. Multiple regression adjusted for relevant clinical variables.
Results
Median maternal age was 27.5 years, with median pre-pregnancy BMI of 24.7 kg/m2, and median HOMA-IR of 2.9. Among the 5 selected miRNA, maternal HOMA-IR correlated with the placental expression of miRNA-371b-3p (r = 0.25; P = 0.008) and miRNA-3940-3p (r = 0.32; P = 0.0004) across the 132 individuals. After adjustment for confounding variables, placental miRNA-3940-3p expression remained significantly associated with HOMA-IR (β = 0.16; P = 0.03).
Conclusion
Placental miRNA-3940-3p was associated with maternal IR at delivery. This placental miRNA may have an autocrine or paracrine effect—regulating placental genes involved in modulating maternal IR.
Keywords: placenta, miRNA, insulin resistance, HOMA-IR, miRNA-3940-3p
During pregnancy, peripheral insulin resistance increases 40% to 50% by late gestation (1-3). This increase results in higher maternal circulating lipids and amino acids (4), providing glucose and additional nutrients for placental transfer to the fetus, and contributing to fetal growth (5). Such maternal-placental crosstalk is believed to play a key role in maternal metabolic adaptations to pregnancy.
The placenta serves as the main interface between the mother and the fetus (6-8). As maternal insulin resistance resolves in the immediate postpartum period (9), it is believed that the placenta is the main mediator of gestational insulin resistance. Hormones, adipokines, and cytokines synthesized and released by the placenta—such as progesterone, estradiol, human placental lactogen, tumor necrosis factor (TNF)α, and leptin—have been studied as potential mediators of pregnancy-induced insulin resistance. However, none of these molecules have been robustly associated with changes in maternal insulin resistance in well-powered human studies (10-12).
Hivert et al recently examined 430 mother-infant dyads, correlating insulin sensitivity using 75 g oral glucose tolerance test at ~26 weeks’ gestation and placental DNA methylation at term (13), and identified 188 CpG sites (178 unique genes) where placental DNA methylation was associated with insulin sensitivity (reciprocal to insulin resistance) as indicated by the Matsuda index (14). Interestingly, placental differentially methylated loci were enriched in targets for miRNAs (13), which are known to posttranscriptionally regulate mRNA degradation or translational repression and play important roles in developmental and pathological processes (15-17). These data suggested that miRNA might play a role in pregnancy-induced insulin resistance (13). We therefore surmised that placental miRNA play a role in the establishment and maintenance of maternal insulin resistance. In this study, our aim was to assess if placental miRNAs that were linked to placental DNA methylation sites were associated with maternal IR during late pregnancy.
Methods
Samples were collected from healthy women who were recruited at 37 to 41 weeks of pregnancy and delivered by elective cesarean delivery following uncomplicated pregnancies at Metro Health Medical Center (Cleveland, OH). We selected samples from women with obesity (pre-gravid body mass index [BMI] > 30 kg/m2; n = 62) and without obesity (pre-gravid BMI < 25; n = 70). Subjects with fetal anomalies, multiple gestations, preeclampsia, diabetes (preexisting and gestational), or other comorbid disease were excluded. Maternal blood was obtained after a 9- to 10-hour fast after being admitted in labor and delivery. A 5-mL aliquot was drawn into EDTA tubes, centrifuged, and the plasma was kept at −20 °C.
Placental tissue biopsies were collected immediately after delivery from below the placental basal plate, while avoiding any macroscopic lesions. Multiple villous samples were further dissected into small pieces, blotted for removal of blood and separately snap frozen in liquid nitrogen within 5 minutes of biopsy.
The study was conducted according to the guidelines in the Declaration of Helsinki. Written and informed consent was obtained prior to participation, and the analyses presented here were approved by the Institutional Review Board of Tufts Medical Center (IRB #12842) and Metro Health Medical Center (IRB #1300650).
Maternal Metabolic Assessments
Using maternal blood samples collected at the time of admission before scheduled cesarean section, plasma glucose was assessed by the glucose oxidase method. Plasma insulin was measured using an enzyme-linked immunosorbent assay (EMD Millipore Corp, Burlington, MA). Insulin resistance was estimated using the homeostatic model assessment of insulin resistance (HOMA-IR) as a ratio of fasting plasma insulin (microunits per milliliter) to fasting plasma glucose ([millimoles per liter]/22.5) (18).
Placental and Maternal Plasma RNA Isolation
Total placental RNA was extracted by homogenization of ~50 mg placental biopsy tissue in TRIzol reagent (Invitrogen, Carlsbad, CA), following the manufacturer’s guidelines. RNA quantification and integrity were measured using an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). All samples had an RNA integrity number >7. Total RNA was isolated from 200 µL of maternal plasma by using miRNeasy Serum/Plasma (Qiagen, Germantown, MD) according to manufacturer instructions.
Placental miRNA Expression Analysis by miRNA Sequencing
A subset of 40 placentas was used for whole genome miRNA sequencing (demographic information is presented in Supplementary Table 1 (19)). Gel isolation of small RNA, library construction, and sequencing were conducted by Genome Quebec (Montreal, Quebec, Canada). After removing the adapters, all sequencing libraries were aligned to the GRCh38 human reference genome using Bowtie aligner (20). Aligned reads were intersected with mature miRNA coordinates on the basis of miRBase (v21) (http://www.mirbase.org/). We normalized libraries using the median of ratios normalization method implemented in the R package DESeq (21). The negative binomial test implemented in DESeq2 was used to identify differentially expressed miRNAs.
Using the placental miRNA expression data generated via small-RNA sequencing, we tested associations between maternal insulin resistance (HOMA-IR) in late pregnancy and placental miRNA expression. We cross-referenced the 135 miRNAs associated with HOMA-IR in late pregnancy in our sample set (P value ≤ 0.1) with the TargetScan list of n = 126 miRNAs from Hivert et al that were predicted to target genes annotated to placenta DNA methylation loci associated with maternal insulin sensitivity (Matsuda index) at 26 weeks gestation (Gen3G prospective cohort) (13) (Supplementary Table 2 (19)). Details about the Gen3G cohort including recruitment, phenotypic measures during pregnancy, and placental methylation methods have been published (13, 22).
Placental cDNA Synthesis and Quantitative Polymerase Chain Reaction
We performed reverse transcriptase and quantitative polymerase chain reaction (RT-qPCR) on 132 placenta samples with the miRScript PCR system (Cat#218161 Qiagen) and QuantStudio 7 Pro Real Time PCR system according to the manufacturer’s protocols. Dissociation curves were run on all reactions, and samples were normalized to the expression of the geometric mean between 2 housekeeping genes (SNOR95, SNOR68) as an endogenous control. Fold increase relative to a calibrator sample was determined by using the 2-ΔΔCt method (23). Primer information is included in Supplementary Table 3 (19).
To assess circulating levels of candidate miRNAs, we performed RT-qPCR on 30 maternal plasma samples collected at the time of scheduled cesarean section and corresponding to above placenta samples. We used miScript Single Cell qPCR kit (Cat#331053 Qiagen) and QuantStudio 7 Pro Real Time PCR system according to the manufacturer’s protocol. Dissociation curves were run on all reactions, and samples were normalized to the expression of the geometric mean between 2 housekeeping genes (hsa-mir-23a-3p and hsa-mir-425-5p) as endogenous control. Fold increase relative to a calibrator sample was determined by using the 2-ΔΔCt method.
Statistical and Pathway Analysis
All data were presented as mean ± SD. Results of the miRNA quantification were expressed in arbitrary units and normalized by log-2 transformation before analysis. Differences between women with and without obesity were analyzed using Student’s t test.
We calculated Pearson correlations to test associations between maternal insulin resistance (HOMA-IR) in late pregnancy and placental miRNA expression using small-RNA sequencing data (N = 40). We used a P value cutoff ≤ 0.1 for this preliminary step. With 40 samples, if the true Pearson correlation coefficient was 0.4 (or −0.4), applying 2-sided Z test to Fisher’s transformation of the correlation, we could achieve a power of 0.8 at the significance level of 0.10. We generated simple and multiple linear regression models to adjust for potential confounders between selected placental miRNA measured via RT-qPCR and maternal insulin resistance (HOMA-IR). We ran 3 models for each placental miRNA. In each model the dependent variable was maternal HOMA-IR. Model 1 adjusted for gestational age at delivery. Model 2 additionally adjusted for maternal ethnicity, maternal age, and parity. Lastly, Model 3 additionally adjusted for maternal pre-pregnancy BMI. Residuals from models were checked for conformance to assumptions of normality and homeostasis. Models were tested for multicollinearity by scoring the variance inflation factor. All continuous variables included in linear regressions were tested for normality and, if necessary, normalized with log 2 transformation. Statistical analyses were performed with R studio (Boston, MA) version 3.6.2 (24). P values ≤ 0.05 were considered significant.
We performed cell signaling pathway analysis for gene targets of miRNAs associated with maternal HOMA-IR in late pregnancy in our main analyses of 132 participants. The TargetScan (v7.2) (25) tool, and DIANA-TarBase v8 (26) were employed for exploring miRNA target sites in human mRNA. For TargetScan the accumulative weighted context++score (CS) cutoff was set to −0.9, with the most effective canonical site types in mRNA in the seed region, which includes 8mer, 7mer-m8, and 7mer-A1 sites. We did this in order to minimize false positive associations between miRNA and their targets (27, 28). DIANA-TarBase genes were chosen based on experimentally validated genes using PAR-CLIP. Following identification of gene targets, we tested the targets for enrichment in biological pathways with Enrichr (29, 30) web platform using Reactome cell signaling pathway 2020 database version 75 (31, 32), and KEGG 2019 Human.
Results
Identification of miRNA Associated With Maternal IR and Cross-Reference Analysis
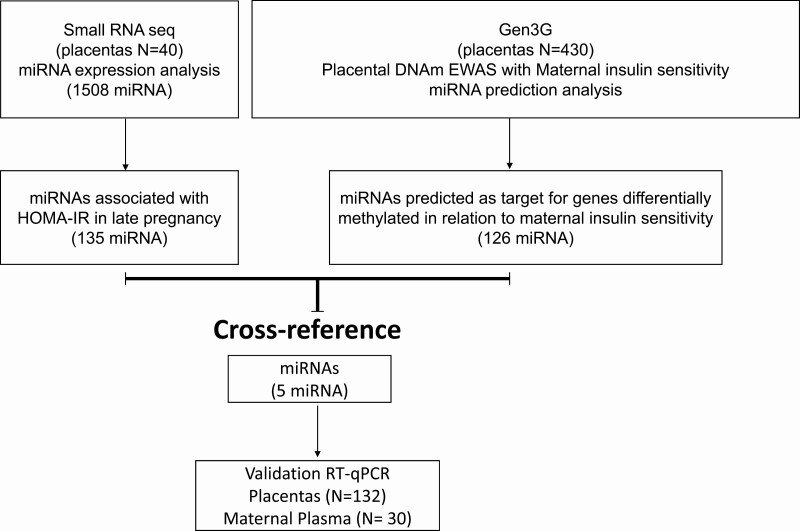
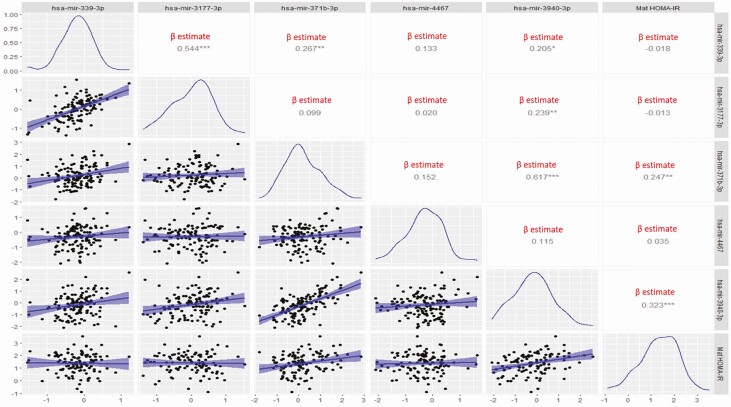
The demographic data are summarized in (Table 1). The experimental design and results are summarized in the flow chart in (Fig. 1). Using whole genome miRNA sequencing, we detected 1508 miRNAs expressed in 40 healthy term placentas (GEO accession GSE169523). Of these, 135 miRNAs were associated with maternal HOMA-IR in late pregnancy (P < 0.1). On the basis of the reported placental epigenome-wide association study (EWAS) in Gen3G, we had previously identified 188 CpG sites (178 unique genes—nearest gene selected at each site) at which placenta DNA methylation was significantly associated with maternal insulin sensitivity (Matsuda index; reciprocal of IR) at 24 to 30 weeks (13). The prior Gen3G analyses had shown that these 178 genes were enriched as miRNA targets (n = 126 miRNAs according to TargetScan; enrichment P < 0.1; Supplementary Table 2 (19)). We then cross-referenced the list of 135 miRNAs associated with maternal HOMA-IR in our dataset with the 126 miRNAs targeting genes identified in placenta EWAS of maternal insulin sensitivity in Gen3G (see Fig. 1). We identified 5 miRNAs that were common to both datasets (hsa-mir-339-3p, hsa-mir-3177-3p, hsa-mir-4467, hsa-mir-3940-3p, and hsa-mir-371b-3p), and used RT-qPCR to validate the placental expression of these 5 miRNAs in our 132 mother-newborn pairs. Among these 5 miRNAs we found that hsa-mir-3940-3p (r = 0.32; P value = 0.0004) and hsa-mir-371b-3p (r = 0.25; P = 0.008) were significantly correlated with maternal HOMA-IR at delivery in our full sample of 132 placentas (Fig. 2). Moreover, both miRNAs had a higher placental expression in women with BMI ≥ 30 kg/m2 compared to women with BMI < 25 kg/m2: hsa-mir-3940-3p (P = 4.09e-05), and hsa-mir-371b-3p (P = 3.13e-06).
Table 1.
Maternal and neonatal characteristics of study population
| Maternal characteristics (N = 132) | Mean ± SD or N/N |
|---|---|
| Maternal age (y) | 28.5 ± 6.0 |
| Race (AA/Cauc/Hisp) | 44/70/18 |
| Parity (previous live births) | 1.6 ± 1.0 |
| Pre-pregnancy BMI (kg/m2) | 27.9 ± 6.3 |
| Fasting glucose (mg/dL) | 75.6 ± 9.0 |
| Insulin (µU/mL) | 15.6 ± 7.4 |
| HOMA-IR | 2.9 ± 1.6 |
| Smoking (yes/no) | 27/105 |
| Neonatal Characteristics | |
| Gestational age at delivery (wk) | 38.8 ± 0.5 |
| Sex (M/F) | 78/54 |
| Birth weight (kg) | 3.3 ± 0.5 |
Data are mean ± SD unless otherwise indicated.
Abbreviations: AA/Cauc/Hisp, African American/Caucasian/Hispanic; HOMA-IR, homeostatic model assessment of insulin resistance.
Figure 1.
Flow diagram summarizing the miRNA selection, cross-reference, and validation strategy.
Figure 2.
Scatterplots displaying the simple linear regression between each of the miRNAs and maternal insulin resistance (HOMA-IR) in late pregnancy. The blue histograms show the normalized curves of each variable. In the right upper corner squares show the β estimate (significant β estimates marked with an *). * P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
We performed 3 multilinear regression models to assess the impact of potential confounders on the relationship between placental miRNAs and maternal HOMA-IR at delivery (Table 2). In model 1, we found that hsa-miR-3940-3p and hsa-miR-371b-3p were significantly associated with maternal HOMA-IR (β = 0.27, SE = 0.07; P = 0.0005 and β = 0.23, SE = 0.08; P = 0.008 respectively). After adjusting for gestational age, ethnicity, maternal age, and parity (Model 2), hsa-miR-3940-3p and hsa-miR-371b-3p remained statistically associated with maternal HOMA-IR in late pregnancy with similar strengths of association (β = 0.27, SE = 0.08; P = 0.0007), (β = 0.22, SE = 0.09; P = 0.01). However, after further adjusting for maternal pre-pregnancy BMI (Model 3), the association between hsa-miR-371b-3p and maternal HOMA-IR was nonsignificant, and the strength of association between hsa-miR-3940-3p and maternal HOMA-IR was attenuated but remained significant (β = 0.17, SE = 0.08; P = 0.03). We did not find significant associations for the other 3 miRNA species with maternal HOMA-IR at delivery.
Table 2.
Multiple linear regression for the association between maternal HOMA-IR in late pregnancy and placental expression of selected miRNA
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| miRNA | β | SE | P value | β | SE | P value | β | SE | P value |
| hsa-miR-339-3p | -0.03 | 0.17 | 0.84 | -0.02 | 0.17 | 0.88 | 0.06 | 0.15 | 0.67 |
| hsa-miR-3177-3p | -0.03 | 0.13 | 0.85 | 0.04 | 0.14 | 0.76 | 0.20 | 0.13 | 0.13 |
| hsa-miR-4467 | 0.04 | 0.11 | 0.73 | 0.008 | 0.11 | 0.93 | -0.08 | 0.10 | 0.40 |
| hsa-miR-3940-3p | 0.27 | 0.07 | 0.0005 | 0.27 | 0.08 | 0.0007 | 0.17 | 0.08 | 0.03 |
| hsa-miR-371b-3p | 0.23 | 0.08 | 0.008 | 0.22 | 0.09 | 0.01 | 0.09 | 0.09 | 0.29 |
Model 1: adjusted for gestational age.
Model 2: adjusted with model 1 + ethnicity, maternal age, and parity.
Model 3: adjusted with model 2 + maternal pre-pregnancy BMI.
Lastly, we found that hsa-mir-3940-3p was detectable in plasma of 37% of the women, while hsa-mir-371b-3p was detected in plasma of 87% of the mothers at delivery. We did not find significant associations between plasma levels of these 2 miRNA and maternal HOMA-IR at delivery (data not shown).
Pathway Analysis
Using TargetScan v.7.2 and employing the cumulative weighted context++score cutoff level of −0.9 we retrieved 2 putative gene targets for hsa-miR-3940-3p (VWA1, and GHRH), which have 1 conserved site in both 8mer and 7mer-A1 of the seed region; and 1 gene target for hsa-miR-371b-3p (CYB561D1). Using DIANA-TarBase we found 14 validated genes targets of hsa-mir-3940-3p (POU2F1, ENO1, CYB5R3, GLCE, ANKRD52, CDCA7, FAM177A1, RAD54L2, ORMDL3, FLII, CEP63, FAN1, LTA, and BIRC5). However, for hsa-mir-371b-3p there were no validated genes in the DIANA-TarBase database.
Using Reactome for cell signaling enrichment analysis, we found 13 out of 16 gene identifiers for hsa-mir-3940-3p (FLII, FAM177A1, and CDCA7 were not found in Reactome). The 17 pathways identified for target genes of hsa-mir-3940-3p with nominal significance enrichment (P < 0.05) are shown in Table 3. The most significant pathways were “Interleukin-4 and Interleukin-13 signaling” False Discovery Rate (FDR) ≤ 0.0001; “Signaling by Interleukins,” FDR = 0.005; and “Cytokine Signaling in immune system,” FDR = 0.01. In the KEGG Pathway analysis 3 hits were nominally significant; “Type I diabetes mellitus (P = 0.03)”; “Amino sugar and nucleotide sugar metabolism (P = 0.03)”; and “Glycosaminoglycan biosynthesis (P = 0.03)”; however, the FDR was 0.1 for all 3 pathways.
Table 3.
Hsa-mir-3940-3p cell signaling pathway (Reactome 2020 database)
| Pathway name | Entities P value | FDR | Genes |
|---|---|---|---|
| Interleukin-4 and interleukin-13 signaling | <0.0001 | <0.0001 | POU2F1;BIRC5;FAN1 |
| Signaling by interleukins | 0.0001 | 0.005 | POU2F1;BIRC5;FAN1 |
| Cytokine signaling in immune system | 0.0003 | 0.010 | POU2F1;LTA;BIRC5;FAN1 |
| TP53 regulates transcription of several additional cell death genes whose specific roles in p53-dependent apoptosis remain uncertain | 0.0006 | 0.015 | BIRC5 |
| Immune system | 0.0008 | 0.016 | POU2F1;CYB5R3;ORMDL3;LTA;BIRC5;ANKRD52;FAN1 |
| Manipulation of host energy metabolism | 0.003 | 0.061 | ENO1 |
| TP53 regulates transcription of cell death genes | 0.005 | 0.071 | BIRC5 |
| TYSND1 cleaves peroxisomal proteins | 0.010 | 0.123 | ANKRD52 |
| TNF receptor superfamily (TNFSF) members mediating non-canonical NF-kB pathway | 0.021 | 0.133 | LTA |
| Neutrophil degranulation | 0.022 | 0.133 | CYB5R3;ORMDL3;ANKRD52 |
| Mitotic prometaphase | 0.029 | 0.133 | BIRC5;CEP63 |
| Vitamin C (ascorbate) metabolism | 0.033 | 0.133 | CYB5R3 |
| RNA polymerase III transcription initiation from type 3 promoter | 0.035 | 0.133 | POU2F1 |
| TNFs bind their physiological receptors | 0.038 | 0.133 | LTA |
| RNA polymerase III transcription initiation | 0.045 | 0.133 | POU2F1 |
| Beta-oxidation of very long chain fatty acids | 0.047 | 0.133 | ANKRD52 |
| Synthesis of IP3 and IP4 in the cytosol | 0.049 | 0.133 | POU2F1 |
Abbreviation: FDR, false discovery rate
Discussion
We demonstrated that placental expression of 2 miRNAs—miR-3940-3p and miR-371b-3p—were positively correlated with maternal insulin resistance in late pregnancy. These 2 miRNAs target genes at loci where placental DNAm was associated with maternal insulin sensitivity (reciprocal of IR) as previously reported by Hivert et al (13). These data suggest placental miRNAs may be involved in metabolic adaptations to pregnancy, potentially in synergism with placental DNA methylation.
Placental expression of miR-3940-3p positively correlated with maternal HOMA-IR following adjustment for multiple confounding variables including pre-pregnancy BMI. Menon et al reported that miR-3940-3p was one of the exosomal miRNAs that changed the most in maternal circulation during pregnancy (33). Consistent with our finding that miR-3940-3p was only detectable in 37% of maternal plasma samples at the time of delivery, Menon et al reported that miR-3940-3p circulating levels were lowest at 41 weeks of gestation. Any endocrine function of this miRNA is likely to occur in mid-pregnancy, when circulating levels are maximal (33). As circulating levels of miR-3940-3p at delivery were very low, we speculate that it may also play a paracrine or autocrine role within the placenta in late pregnancy. Hivert et al predicted miR-3940-3p would bind to CHRNA4—1 of the 5 loci where placental DNA methylation may modulate pregnancy-associated increased insulin resistance based on Mendelian randomization analyses (13). Though the function of this miRNA is poorly understood, our pathway analyses identified several cell signaling pathways enriched for validated miR-3940-3p gene targets. Among these the interleukin 4 and 13 signaling pathway is the most significantly overrepresented. One of the mechanisms of signal transduction activated by IL-4 and IL-13 leads to insulin receptor substrate family (IRS1-4) activation, which propagate downstream insulin signaling, regulate protein synthesis, glucose uptake, and gluconeogenesis (34). These findings suggest potential mechanisms by which placental miR-3940-3p may impact maternal insulin resistance.
The correlation between maternal HOMA-IR and miR-371b-3p was primarily driven by its association with maternal pre-pregnancy BMI. Hsa-mir-371b-3p is part of 6 mature miRNAs of the miR-371-3 cluster. Its expression is almost exclusively restricted to a few extraembryonic tissues, including the placenta (35). The expression of the 371-3 cluster is associated with cell proliferation and invasion (36), but it may also inhibit dysregulated proliferation through cell cycle arrest (37). Though miR-371b-3p’s biological effects are poorly understood, based on its elevated expression in placentas of women with obesity, a potential role in trophoblast growth should be examined in future studies.
Key strengths of our study include: (1) the use of 2 large independent prospective cohorts to cross-reference and select placental miRNAs that may be involved in regulating maternal insulin resistance; (2) cutting-edge tools to analyze miRNA data; and (3) the validation of miRNA sequencing results by RT-qPCR in a larger number of samples. A limitation of our study was the use of HOMA-IR at the time of delivery. HOMA-IR is more reflective of changes in hepatic insulin resistance, and it may be limited in detecting changes in peripheral insulin resistance with advancing gestation, the time in which women are the most insulin resistant (38). However, Gen3G data using mid-pregnancy Matsuda index (previously validated against clamps) supports the plausibility that placental miR-3940-3p plays a role in maternal glucose homeostasis during pregnancy. The initial selection of miRNA for further analysis by cross-referencing may have been limited by differences in gestational time-points of glucose homeostasis measures between cohorts, and a sample size of 40, which limited our power to detect smaller associations. Target prediction pathway analysis is an agnostic way of studying miRNA-mRNA interactions; we also used a lenient total weighted context ++score of −0.9 which may have increased the likelihood of false positive targets. Detailed mechanistic studies, though beyond the scope of this manuscript, will be necessary to determine the mechanism of action of these miRNAs in the placenta.
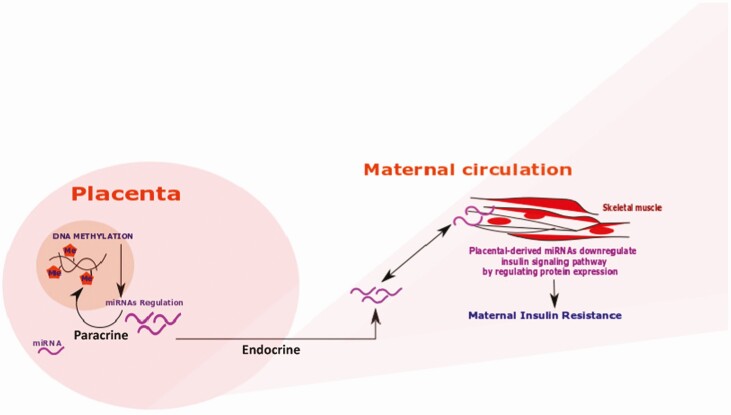
In conclusion, we demonstrated that placental miR-3940-3p expression is associated with maternal HOMA-IR in late pregnancy. Altogether, our data support a role for placental miRNA in maternal metabolic adaptations to pregnancy as proposed in our conceptual model (Fig. 3).
Figure 3.
Proposed conceptual model. Placenta: miRNAs can work in a paracrine way affecting cell signaling in neighboring cells as well as regulating DNA methylation in the cell of origin. Maternal circulation: Placental miRNAs can be released in an endocrine fashion, into the maternal circulation where they can be integrated into distant target cells (skeletal muscle cells) and affect the insulin signaling pathway, leading to maternal insulin resistance.
Acknowledgments
We would like to thank Dr. Mary Haghiac and Ms. Judi Minium for contributing to recruitment and sample and data collection. We thank the women who participated in our study.
Financial Support: This study was funded by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R01HD091735.
Author Contributions: All authors have reviewed and approved the final manuscript, contributed to the design of the study and interpretation of the data. M-F.H. and P.O-G. conceived of the design of the study and contributed to the data analysis. F.L.A., J.K., W.B., T.K.T and T.C. performed the experiments and participated in the data analysis. F.L.A., M-F.H. and P.O-G. drafted the manuscript, and P.C., Y.S., and T.C. critically reviewed the data and the manuscript.
Glossary
Abbreviations
- BMI
body mass index
- FDR
false discovery rate
- HOMA-IR
homeostatic model assessment of insulin resistance
- RT-qPCR
quantitative reverse transcription–polymerase chain reaction
- TNF
tumor necrosis factor
Contributor Information
Fernanda Alvarado-Flores, Mother Infant Research Institute, Tufts Medical Center, Boston, MA 02111, USA.
Tomoko Kaneko-Tarui, Mother Infant Research Institute, Tufts Medical Center, Boston, MA 02111, USA.
William Beyer, Mother Infant Research Institute, Tufts Medical Center, Boston, MA 02111, USA.
Jacqueline Katz, Mother Infant Research Institute, Tufts Medical Center, Boston, MA 02111, USA.
Tianjiao Chu, Magee Womens Research Institute, Pittsburgh, PA 15213, USA.
Patrick Catalano, Mother Infant Research Institute, Tufts Medical Center, Boston, MA 02111, USA.
Yoel Sadovsky, Magee Womens Research Institute, Pittsburgh, PA 15213, USA.
Marie-France Hivert, Department of Population Medicine, Harvard Medical School; Harvard Pilgrim Health Care Institute, Boston, MA 02115, USA; Diabetes Unit, Massachusetts General Hospital, Boston, MA 02114, USA.
Perrie O’Tierney-Ginn, Mother Infant Research Institute, Tufts Medical Center, Boston, MA 02111, USA.
Additional Information
Disclosures: The authors have no conflicts of interest to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in (19).
References
- 1. Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180(4):903-916. [DOI] [PubMed] [Google Scholar]
- 2. Ryan EA, O’Sullivan MJ, Skyler JS. Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes. 1985;34(4):380-389. [DOI] [PubMed] [Google Scholar]
- 3. Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165(6 Pt 1):1667-1672. [DOI] [PubMed] [Google Scholar]
- 4. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. doi:10.1136/bmj.j1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Catalano PM, Kirwan JP. Maternal factors that determine neonatal size and body fat. Curr Diab Rep. 2001;1(1):71-77. [DOI] [PubMed] [Google Scholar]
- 6. Paquette AG, Chu T, Wu X, Wang K, Price ND, Sadovsky Y. Distinct communication patterns of trophoblastic miRNA among the maternal-placental-fetal compartments. Placenta. 2018;72-73:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burton GJ, Jauniaux E. What is the placenta? Am J Obstet Gynecol 2015;213(4, Supplement):S6.e1-S6.e4. [DOI] [PubMed] [Google Scholar]
- 8. Karahoda R, Abad C, Horackova H, et al. Dynamics of tryptophan metabolic pathways in human placenta and placental-derived cells: effect of gestation age and trophoblast differentiation. Front Cell Dev Biol. 2020;8:574034. doi: 10.3389/fcell.2020.574034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waters TP, Kim SY, Sharma AJ, et al. Longitudinal changes in glucose metabolism in women with gestational diabetes, from late pregnancy to the postpartum period. Diabetologia. 2020;63(2):385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirwan JP, Hauguel-De Mouzon S, Lepercq J, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51(7):2207-2213. [DOI] [PubMed] [Google Scholar]
- 11. McIntyre HD, Chang AM, Callaway LK, et al. ; Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group . Hormonal and metabolic factors associated with variations in insulin sensitivity in human pregnancy. Diabetes Care. 2010;33(2):356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cseh K, Baranyi E, Melczer Z, Kaszás E, Palik E, Winkler G. Plasma adiponectin and pregnancy-induced insulin resistance. Diabetes Care. 2004;27(1):274-275. [DOI] [PubMed] [Google Scholar]
- 13. Hivert MF, Cardenas A, Allard C, et al. Interplay of placental DNA methylation and maternal insulin sensitivity in pregnancy. Diabetes. 2020;69(3):484-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. [DOI] [PubMed] [Google Scholar]
- 15. Nigi L, Grieco GE, Ventriglia G, et al. MicroRNAs as regulators of insulin signaling: research updates and potential therapeutic perspectives in type 2 diabetes. Int J Mol Sci 2018;19(12):3705. doi: 10.3390/ijms19123705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glaich O, Parikh S, Bell RE, et al. DNA methylation directs microRNA biogenesis in mammalian cells. Nat Commun. 2019;10(1):5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mouillet JF, Ouyang Y, Coyne CB, Sadovsky Y. MicroRNAs in placental health and disease. Am J Obstet Gynecol. 2015;213(4 Suppl):S163-S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lassance L, Haghiac M, Minium J, Catalano P, Hauguel-de Mouzon S. Obesity-induced down-regulation of the mitochondrial translocator protein (TSPO) impairs placental steroid production. J Clin Endocrinol Metab. 2015;100(1):E11-E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Tierney-Ginn PF, Alvarado F. Placental miR-3940-3p is associated with maternal insulin resistance in late pregnancy [dataset]. Harvard Dataverse. Posted March 26, 2021. doi: 10.7910/DVN/SSSL2D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guillemette L, Allard C, Lacroix M, et al. Genetics of Glucose regulation in Gestation and Growth (Gen3G): a prospective prebirth cohort of mother-child pairs in Sherbrooke, Canada. BMJ Open. 2016;6(2). doi: 10.1136/bmjopen-2015-010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-408. [DOI] [PubMed] [Google Scholar]
- 24. RStudio Team. RStudio: Integrated Development for R. Boston, MA: RStudio, PBC.;2020. http://www.rstudio.com/ [Google Scholar]
- 25. Agarwal V, Bell GW, Nam J-W, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015;4. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karagkouni D, Paraskevopoulou MD, Chatzopoulos S, et al. DIANA-TarBase v8: a decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 2018;46(D1):D239-D245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riffo-Campos ÁL, Riquelme I, Brebi-Mieville P. Tools for sequence-based miRNA target prediction: what to choose? Int J Mol Sci 2016;17(12). doi: 10.3390/ijms17121987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benna C, Rajendran S, Rastrelli M, Mocellin S. miRNA deregulation targets specific pathways in leiomyosarcoma development: an in silico analysis. J Transl Med. 2019;17. doi: 10.1186/s12967-019-1907-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuleshov MV, Jones MR, Rouillard AD, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90-W97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool | BMC Bioinformatics | Full Text. [DOI] [PMC free article] [PubMed]
- 31. Fabregat A, Korninger F, Viteri G, et al. Reactome graph database: efficient access to complex pathway data. Plos Comput Biol. 2018;14(1):e1005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jassal B, Matthews L, Viteri G, et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020;48(D1):D498-D503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Menon R, Debnath C, Lai A, et al. ; Garbhini Study Team . Circulating exosomal miRNA profile during term and preterm birth pregnancies: a longitudinal study. Endocrinology. 2019;160(2):249-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300(5625):1527-1528. [DOI] [PubMed] [Google Scholar]
- 35. Gottlieb A, Flor I, Nimzyk R, et al. The expression of miRNA encoded by C19MC and miR-371-3 strongly varies among individual placentas but does not differ between spontaneous and induced abortions. Protoplasma. 2021;258(1):209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang H, Zhao Y, Chen T, Liu G, He N, Hu H. MiR-371 promotes proliferation and metastasis in hepatocellular carcinoma by targeting PTEN. BMB Rep. 2019;52(5):312-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langroudi L, Jamshidi-Adegani F, Shafiee A, et al. MiR-371-373 cluster acts as a tumor-suppressor-miR and promotes cell cycle arrest in unrestricted somatic stem cells. Tumour Biol. 2015;36(10):7765-7774. [DOI] [PubMed] [Google Scholar]
- 38. Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care. 2001;24(9):1602-1607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in (19).