Highlights
-
•
Both high dose and low dose TSEBT achieve high response rates.
-
•
No statiscal difference between two dose regimens was found in this study.
-
•
We propose to use low dose TSEBT in all patients with boost to residual disease if indicated.
-
•
High dose regimens should be reserved for patients where it is not feasible to boost too many lesions.
Keywords: Total skin electron beam therapy, Mycosis fungoides, Radiotherapy, Total skin irradiation, Cutaneous T-cell lymphoma
Abstract
Purpose
Total skin electron beam therapy (TSEBT) is used mostly in the treatment of cutaneous T cell lymphoma. In this study we describe the results of TSEBT applied in the Netherlands using two different schedules, a conventional dose schedule of 35 Gy and a low-dose schedule of 12 Gy. We aimed to evaluate the treatment results in and compare treatment outcomes between the two treatment groups and to further define indications for both doses.
Methods
In the LUMC, Leiden, we performed a retrospective analysis of 51 patients treated with TSEBT between January 2008 and December 2018, with follow-up untill December 2019. Thirty one patients were treated with 35 Gy and twenty with 12 Gy. The dose was chosen based on the severity of skin involvement. Outcome measures were time to meaningful progression, survival, response rate and toxicity.
Results
Time to meaningful progression was 5.1 months with no significant differences between dose groups (P = 0.77). Overall survival was 27.4 months. Both time to progression and survival were significantly better for T2 vs T3 stage. Overall response rate was 80.4 %. Both dose groups showed improvement of symptoms. Treatment was generally well tolerated.
Conclusions
Both high-dose and low-dose TSEBT offer similar results for TMP and OS. It remains unclear which patients benefit most from a high-dose schedule. We propose to use the low-dose schedule as a standard for TSEBT and use supplementary boosts or escalation to high-dose treatment for patients unresponsive to the low-dose schedule.
Introduction
Mycosis Fungoides (MF) and Sezary Syndrome are the most common cutaneous T cell lymphomas. MF is characterized by a variety of skin lesions such as patches, plaques, tumors and erythroderma, either localized or widespread. There may be involvement of lymph nodes or visceral organs at presentation or later on in the disease course although this occurs rarely. Sezary Syndrome is a distinctive disease with blood involvement of the malignant T-cells of the skin. Both diseases are staged by the TNMB classification [1]. Both type and number of skin lesions as well as the TNMB classification are indicators of prognosis [2], [3], [4]. Specific subtypes of MF such as folliculotropic or transformed MF and Sezary Syndrome have a worse prognosis compared to classical MF [5], [6], [7]. Several studies show the impact of different histopathological factors that affect prognosis such as CD30 and KI-67 [6], [8]. The possible therapeutic implications of these findings are yet undefined. MF can be treated by a variety of therapy modalities depending on disease stage. Treatment can either be local, such as topical corticosteroids and local radiotherapy, or directed to the complete body or skin by systemic medication, PUVA or total skin electron beam therapy (TSEBT) [9]. Allogenic hematopoietic stem cell transplantation is the only curative treatment option in MF. In the transplantation setting TSEBT is often used before conditioning to minimize disease burden in the skin since skin lesions tend to be less responsive to chemotherapy regimens [10].
Radiation has been used in cutaneous lymphoma since the beginning of the twentieth century and immediately showed impressive results [11]. In 1940, Trump suggested using accelerated electrons and in 1952 the first patient was treated with TSEBT [12], [13]. Further refinements in the technique of total skin irradiation were made in Stanford [14]. Good outcomes with conventional dose TSEBT have been published with median time to progression ranging from 11 up to 102 months [15], [16], [17], [18]. More recently there has been a peak of interest in TSEBT with lower doses to reduce treatment time and toxicity and allow for retreatment schedules [18], [19], [20], [21]. TSEBT at our department is reserved for patients with progression after primary treatment with superficial skin-directed therapies or with the tumor burden of the skin estimated too extensive for such therapies. However, published reports, as reviewed by Chowdhary, document a wide variety of stages of disease and number of previous treatments at the time of TSEBT, highlighting the heterogeneity of the patient population receiving TSEBT [18]. A clear guideline for how to select patients for either high-dose or low-dose therapy is lacking. The goals of this study are to describe the results of TSEBT at our center, to evaluate whether there are predictive factors for time to progression and overall survival and to describe the selection of patients for either high dose or low dose treatment. Based on the comparison of our results with the literature treatment recommendations were formulated to select patients for either schedule.
Methods and materials
Study subjects
In The Netherlands treatment expertise of cutaneous lymphoma is centered at Leiden University Medical Center (LUMC) and all TSEBT’s in the Netherlands are performed at the LUMC radiotherapy department. Consecutive patients treated with TSEBT for T-cell lymphoma between January 1st 2008 and December 31th 2018 were included in our database. Follow up data up until December 31th 2019 were retrospectively extracted. We excluded patients that had a diagnosis other than T-cell lymphoma (N = 5), did not have adequate follow up data (N = 3), were treated with a prior TSEBT before 2008 (N = 5), received a personalized dose (N = 2), were treated with TSEBT as part of a conditioning regimen for allogenic stem cell transplantation (N = 4) or were treated with total skin technique on much smaller skin surface areas (e.g. total head irradiation, N = 15).
Treatment selection
Patients with multiple skin tumors of considerable thickness received a high dose TSEBT. In doing so gradual remission of thick tumors during the treatment period allows the dose to reach the deeper parts of the lesions. Low-dose treatment was given to patients with generally lower disease stages with more superficial skin involvement but unresponsive to other types of treatment such as PUVA. Photo 1 and 2 clarify these selection criteria based on skin lesion burden. Both patients would classify as stage IIB/T3 although there are clear differences in skin involvement. Twice only a half body TSEBT was given when the upper part of the body was free of disease. Since clear criteria for treatment selection are lacking, the decision is made by consensus within the multidisciplinary team consisting of the radiation oncologists and dermatologists involved in TSI treatment at our center.
Irradiation technique
TSEBT was administered by the dual fixed-angle six-field technique originally developed at Stanford University and currently used by>80% of the radiation facilities that perform TSEBT [14]. This technique consists of anterior and posterior dual fields combined with four oblique fields to improve dose uniformity. All fields given with gantry 69° and 111° to respectively irradiate the lower and upper half of the patient. To minimize the treated volume and selectively treat the skin up to 1 cm depth 4 MeV electrons are applied. Supplementary 4 MeV electron fields were used to compensate for underdosage of the TSEBT, routinely to the perineal region, vertex, soles, epaulets and if needed also on inframammary or infra-abdominal folds. Moreover, if indicated, tumor specific boost fields were sometimes used. The eye lenses were routinely shielded unless there was peri-ocular tumor localization. In cases of serious toxicity during treatment additional shielding was applied, mostly to feet and ankles, or supplementary fields were omitted (e.g. soles). Both high-dose and low-dose treatment were given in 2 fractions per week, supplementary fields were given once a week. High dose treatment was given in 20 fractions of 1,75 Gy per fraction, supplemental doses were 25 Gy in 10 fractions. With the high dose schedule 3 out of 6 standing positions per day were treated, alternating between anterior/posterior oblique fields on Monday and posterior/anterior oblique fields on Thursday. Irradiating 3 out of 6 positions each fraction results in a somewhat less homogeneous fraction dose per day, however, it allows for more patient comfort and is easier from a logistical point of view [22]. For the low dose treatment (6 fractions of 2 Gy with supplemental doses of 7.5 Gy in 3 fractions) all six positions were irradiated each fraction, giving a more homogeneous fraction dose. Dosimetric analysis of the irradiation delivered is performed as described in the recently published Code of Practice and recommendations for Total Body Irradiation and Total Skin Irradiation by the Netherlands Commission on Radiation Dosimetry (NCS) [23].
Data collected
The following data were extracted from the medical records. With regard to patient characteristics: age; gender; date of diagnosis, histological subtype and immunohistochemistry; disease stage at time of TSEBT [1]; comorbidities; time from diagnosis to TSEBT; previous treatments and medication history. Furthermore treatment parameters: treatment completion (completion of prescribed dose); radiotherapy dose including data on boost location and dose; treatment adjustments and reason (e.g. due to toxicity) and end date of treatment. With regard to outcomes: clinical response; symptoms before and after treatment; adjuvant treatment after TSEBT; subsequent treatment lines including date of treatment; acute toxicity (radiation dermatitis comprising erythema, edema and desquamation, and alopecia and as stated above derived indications of toxicity such as analgetics use and addition of shielding during therapy); long term toxicity (skin pigmentation changes, hyperkeratosis, hypohidrosis, fibrosis); date and site of progression and survival. Symptoms prior to and after TSEBT were graded by the researcher based on retrospective examination of the patient chart.
End points
The primary end point was determined as time to meaningful progression (TMP), defined as the time from end of TSEBT to subsequent use of either irradiation (mostly local but including subsequent TSEBT) or other total-skin equivalent therapy such as PUVA, phototherapy or systemic therapy. Secondary end points were response rate, treatment effect on symptoms and acute and long term toxicity and overall survival. The aforementioned patient and disease characteristics were used to determine predictive factors that influenced our end points. Response rates for the tumor as well as symptoms, were scored within 6 months after start of TSEBT. Complete remission (CR) was defined as a complete disappearance of all lesions, partial remission (PR) as > 50% improvement, stable disease (SD) as no or minimal improvement and progressive disease (PD) as the occurrence of new lesions during or shortly after TSEBT. PD might occur in the presence of otherwise good response of the skin. Acute toxicity was scored during treatment till 3 months after treatment.
Statistics
Statistical analyses were performed with IBM SPSS Statistics for Windows, Version 25.0. (Armonk, NY: IBM Corp. Released 2017). Baseline-, tumor and treatment characteristics were analysed with χ2 tests and the Mann-Whitney U test. Survival analysis for overall survival and TMP was performed with Cox regression analysis. Differences were considered statistically significant if P < 0.05.
Ethical review
The protocol for our study was submitted for review to the LUMC ethics committee (G20.023, March 5, 2020) and was deemed not necessary to be subjected to further review under the Medical Research Involving Human Subjects Act (WMO).
Results
Clinical characteristics and follow-up data
A total of 51 patients were included in the study of whom 31 (60.8%) received high-dose TSEBT and 20 (39.2%) low-dose TSEBT. Most patients were diagnosed with MF (N = 46), but a small number of patients were treated for Sezary Syndrome (N = 2) or another cutaneous manifestation of T-cell lymphoma (N = 3). At the time of the analysis 14 (27.5%) patients were alive and 37 (72.5%) patients had died. Median age was 68 years (range 35–90). Follow-up was calculated from start of TSEBT and ranged from 1 month to 11 years with a median of 2.4 years. Most patients were male (70.6%) and were treated while the disease was in tumor stage (T-stage 3) (N = 34; 70.8%). Respectively 64.7% and 70.6% of patients received local radiotherapy and PUVA before TSEBT. There was no difference in number of treatment types between the high-dose and low-dose patients, but there was a trend for a longer median time from diagnosis till start of TSEBT for the low-dose group (37.8 months vs 14.3 months). Patient and disease characteristics as well as data on treatment before TSEBT are further described in Table 1 and in the supplementary file.
Table 1.
Patient en tumor characteristics.
| Low-dose TSEBT | High-dose TSEBT | Overall | |
|---|---|---|---|
| No. of patients | 20 | 31 | 51 |
| Median age (Years, range) | 69 (57-90) | 68 (35-78) | 69 (35-90) |
| Mean follow-up (Months, SD) | 31.4 (22.8) | 27.6 (37.0) | 29.1 (32.0) |
| seX (Male, %) | 14 (70.0) | 22 (71.0) | 36 (70.6) |
| Diagnosis (%) | |||
| Mycosis Fungoides | 19 (95.0) | 27 (87.1) | 46 (90.2) |
| Sezary | 0 (0.0) | 2 (6.5) | 2 (3.9) |
| Other CTCL | 1 (5.0) | 2 (6.5) | 3 (5.9) |
| Folliculotropic (Yes, %) | 13 (68.4) | 14 (48.3) | 27 (56.3) |
| Missing | 1 | 2 | 3 |
| Blastic transformation (Yes, %) | 10 (55.6) | 15 (53.6) | 25 (54.3) |
| Missing | 2 | 3 | 5 |
| T-Stage prior to TSEBT (%) | |||
| T2 | 7 (36.8) | 5 (17.2) | 12 (25.0) |
| T3 | 12 (63.2) | 22 (75.9) | 34 (70.8) |
| T4 | 0 (0.0) | 2 (6.9%) | 2 (4.2) |
| Missing | 1 | 2 | 3 |
| Time of diagnosis till TSEBT | |||
| Mean (Months, SD) | 57.3 (62.8) | 36.7 (47.7) | 44.8 (54.5) |
| MEDIAN (Months, range) | 37.8 (2.3 - 272.7) | 14.3 (1.6 - 195.5) | 26.2 (1.6 - 272.7) |
Treatment delivery
Several patients did not complete the high-dose treatment resulting in a mean given dose of 30.0 Gy in the high dose group. The first patient in the low-dose treatment group received 10 Gy, resulting in a mean given dose in this patient group of 11.8 Gy. One patient with T3 disease started with low dose TSEBT, did not respond and proceeded to a full dose treatment, this patient was analysed in the high dose group. In both treatment groups several patients received a local boost. Maintenance treatment was given to 41.9% and 45.0% respectively of patients of the high-dose and low-dose treatment arms. This mostly consisted of systemic treatment (mainly continuation of pre-TSEBT therapy such as interferon and methotrexate) or topical corticosteroids and topical chemotherapy.
Time to meaningful progression and overall survival
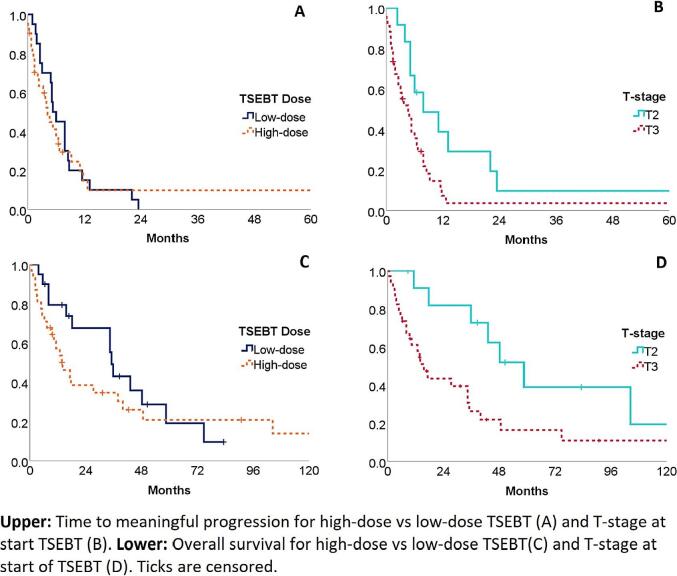
Overall median time to meaningful progression (TMP) was 5.1 months, slightly shorter for patients receiving high-dose TSEBT (4.1) compared to low-dose TSEBT (5.3), although not statistically significant. Median overall survival (OS) was 27.4 months with substantially shorter survival for the high-dose TSEBT group (14.0 months) compared to the low-dose group (35.2 months). This difference was not significant however, also not in the subgroup analysis performed for MF patients only. Disease T-stage did significantly influence both TMP (7.8 vs. 4.6 months T2 vs T3, p = 0.030) and OS (58.6 vs 15.6 months T2 vs T3 p = 0.017), regardless of treatment dose. Cox regression analysis however, did not show a dose effect after correcting for T-stage. Analysis of histologic subtype on TMP and OS also showed no significant differences. Ki-67 was reported sparsely in the pathology reports. Survival times for both time to meaningful progression and overall survival are shown in Table 2. Kaplan-Meier curves are shown in Fig. 1.
Table 2.
Survival data
| Median TMP in months (95% CI) | Sig. | Median OS in months (95% CI) | Sig. | |
|---|---|---|---|---|
| Total | 5.1 (4.1 – 6.0) | 27.4 (8.7 – 46.1) | ||
| By Treatment | ||||
| High-dose | 4.1 (3.3 – 7.3) | .77 | 14.0 (6.7 – 21.3)) | .335 |
| Low-dose | 5.3 (2.6 – 5.6) | 35.2 (32.5 – 37.8) | ||
| By T-Stage* | ||||
| T2 | 7.8 (0.3 – 15.4) | .030 | 58.6 (37.6 – 79.7) | .017 |
| T3 | 4.6 (4.1-6.5) | 15.6 (14.5 – 54.5) |
TMP: time to meaningful progression. OS: overall survival. Analysis by T-stage only consisted of MF patients. *Two patients with the diagnosis Sezary Syndrome had T4-disease and were excluded. Three patients with other T-cell lymphoma were excluded due to different staging method.
Fig. 1.
TMP and OS. Upper: Time to meaningful progression for high-dose vs low-dose TSEBT (a) and T-stage at Start TSEBT (b). Lower: Overall survival for high-dose vs low-dose TSEBT (c) and T-stage at Start of OSEBT (D). Ticks are censored.
Response to TSEBT
Most patients responded well to the TSEBT, 13 (25.5%) reached a complete response (CR) and 28 (54.9%) a partial response (PR). Remission status did not differ between patients receiving a high-dose treatment and low-dose treatment, but differed significantly between disease stage, all progressive patients had T3 disease (p = 0.007). For nine patients the first clinical evaluation after treatment showed progressive disease (PD), i.e. new lesions or few progressive lesions with usually otherwise good response of the rest of the affected skin. Six of these patients experienced progression of part of their lesions during the irradiation, three patients after initial response during treatment. Best recorded responses for each patient after TSEBT are reported in Table 3.
Table 3.
Overall response rate.
| N. | CR (%) | PR (%) | SD (%) | PD (%) | |
|---|---|---|---|---|---|
| Overall | 51 | 13 (25.5) | 28 (54.9) | 1 (2.0) | 9 (17.6) |
| By Treatment | |||||
| High-dose | 31 | 7 (22.6) | 17 (54.8) | 0 (0.0) | 7 (22.6) |
| Low-dose | 20 | 6 (30.0) | 11 (55.0) | 1 (5.0) | 2 (10.0) |
| By T-Stage at TSEBT* | |||||
| T2 | 12 | 7 (58.3) | 4 (33.3) | 1 (8.3) | 0 (0.0) |
| T3 | 34 | 5 (14.7) | 21 (61.8) | 0 (0.0) | 8 (23.5) |
| T4 | 2 | 0 (0.0) | 2 (100.0) | 0 (0.0) | 0 (0.0) |
CR: Complete response (no skin lesions at evaluation). PR: partial response (≥50%–99% clearance of skin disease). SD: stable disease (<50% clearance of skin disease). PD: progressive disease (any new lesions). *Three patients with other T-cell lymphoma were excluded from T-stage analysis due to different staging method.
Symptom resolution
For 40 patients one or more symptoms before TSEBT were reported. Both pruritis and pain were reported equally in both treatment groups. Ulcerative tumors were significantly more present in the high-dose group (N = 12, 38.7%) compared to the low-dose group (N = 2, 10.0%) (p = 0.018). In both groups the majority of patients responded with symptom relieve after TSEBT, except both patients with ulcerative lesions in the low-dose group which showed no improvement (Table 4).
Table 4.
Symptoms and response.
| High-dose | Low-dose | Sig. | |
|---|---|---|---|
| No. patients with Pruritis (%) | |||
| Total | 17 (54.8) | 14 (70.0) | 0.275 |
| Improvement after TSEBT | 11 (64.7) | 9 (64.3) | |
| No. patients with Pain (%) | |||
| Total | 11 (35.5) | 6 (30.0) | 0.684 |
| Improvement after TSEBT | 6 (54.5) | 3 (50.0) | |
| No. patients with Ulcer (%) | |||
| Total | 12 (38.7) | 2 (10.0) | 0.018 |
| Improvement after TSEBT | 8 (66.7) | 0 (0.0) | |
| Overall Respons after TSEBT (%) | |||
| No symtpom response | 7 (26.9) | 4 (28.6) | 1.000 |
| Respone of ≥ 1 symptom(s) | 19 (73.1) | 10 (71.4) |
Toxicity
In our analysis we limited ourselves to review acute effects of radiation dermatitis and (mostly reversible) alopecia. Within 3 months after starting TSEBT 39.6% (N = 19) of patients experienced grade 1–2 radiation dermatitis. Rates of alopecia were sparsely reported but was seen in at least 10 patients. In the high-dose group more grade 3 to 4 dermatitis was reported. There was however no statistically significant difference between the two dose arms. One patient died shortly after completing high dose TSEBT due to sepsis secondary to ulcera. Data on late radiation toxicity were too sparsely reported for thorough analysis, but included several reports of xerosis cutis (N = 8), skin ulceration (N = 2), pigmentation changes (N = 10), hyperkeratosis (N = 1) and hypohydrosis (N = 1).
Discussion
In this paper, we described our results of two different schedules of TSEBT used over the past 11 years. As far as we know, this is the first study reporting a series of consecutive patients treated with either high or low dose based on the disease load. Time to meaningful progression (TMP) after TSEBT was on average 5 months and was not influenced by the radiation dose regimen. Both groups experienced a significant improvement of symptoms and an 80% overall remission rate with a trend for less toxicity in the low-dose group. All patients were heavily pretreated before commencing to TSEBT.
We implemented our low dose schedule in 2009 after previous discussions at the CTCL conference in 2008 and the subsequent email discussion on starting an international trial using a dose of 10–12 Gy with which several centers were piloting [24]. The international trial never took place and unfortunately all groups eventually choose a slightly different approach [19], [21], [25]. Rather than fully replacing the high dose schedule with a lower dose we decided to choose either schedule based on the pattern of skin involvement of an individual patient. The selection for dose is done subjectively by our clinicians based on individual patient disease course, where we try to judge the likelihood of sufficiency of 12 Gy in 6 fractions with 4 MeV electrons for a patients disease burden. It is known that 8 Gy in 2 fractions can give a good and lasting tumor response in MF [26]. For localized radiotherapy the energy of the electrons used is based on a measurement of the thickness of the tumor, ranging from 4 MeV to sometimes 15 MeV for tumors > 4 cm. The beam quality for TSEBT in the LUMC is 4 MeV, therefore treating at maximum 1 cm below the surface area. In case of multiple thicker lesions we therefore choose to give the conventional TSEBT dose, reasoning that regression of the lesions during treatment allowed for penetration of electrons to deeper parts of the tumor later in the treatment course. It is however not clear if all lesions in the same patient need this high-dose, as progression mostly appears to be limited to several lesions rather than all. If the disease burden was more superficial 12 Gy in 6 fractions of 2 Gy was given with an occasional boost to one or two thicker tumors. Others choose instead to boost all thicker tumors separately prior to TSEBT as well as employing different treatment schedules for low dose treatments (8 × 1,5 Gy, 10 × 1 Gy, 12 × 1 Gy) [19], [21].
Other institutions have reported TSEBT results both for high dose and low dose schedules. From these papers however it is deduced that the schedules were not used simultaneously in clinical practice, neither by randomization nor based on clinical decision making. Only Georgakopoulos et al randomized 14 patients and found similar good results of both schedules [27].
As expected, TMP is better for T2 than for T3 patients. It was not associated with the dose regimen when the different dose regimens within the same T-stage were analysed. This lack of difference can be partly explained by the small numbers of patients within both treatment groups and the heterogeneity with the group of T3 patients as is illustrated in Photo 1. Furthermore, a higher percentage of patients in the high-dose group had ulcerative skin lesions at start of treatment, denoting a worse skin involvement compared the low-dose group.
Photo 1.
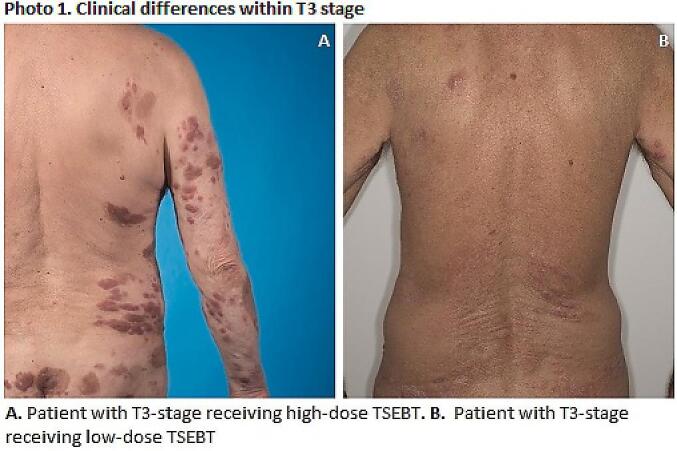
Clinical differences within T3 Stage. A. Patient with T3-stage receiving high-dose TSEBT. B. Patient with T3-stage receiving low-dose TSEBT.
Time to (meaningful) progression was approximately 6 months which is lower than the 12.2–16.3 and 11.3–102 months seen by others for low-dose [20], [21], [25], [28] and high-dose TSEBT [15], [16], [17], [18] respectively.
Patient receiving high dose treatment did have a shorter median TMP compared to patients receiving low dose. This may partly be explained by more patients with T3 disease in this group, as well as due to the heterogeneity as discussed above.
We choose TMP as our primary endpoint. Comparison with other institutions is difficult due to heterogeneity in patients included with regard to disease stage, prior and adjuvant treatments. Also (definition of) endpoints vary between papers. TMP is somewhat, but not fully comparable to Duration of Clinical Benefit (DCOB) as used by Hoppe et al [25]. DCOB however includes any progressive disease as defined by Olsen et al. in the endpoint but not a subsequent local irradiation field [1]. TMP, with meaningful defined as the need for substantial sequential treatment, is a useful outcome measure for retrospective evaluation because new treatments are well documented in the patient files. Due to these differences comparison of endpoints is hardly possible. As the primary goal of TSEBT is to achieve a more manageable disease status rather than cure, changes in Modified Severity-Weighted Assessment Tool (mSWAT) scores probably better reflect the true benefit of TSEBT, as is shown in the prospective study from Morris et al [21]. A local radiotherapy field given 6 months after TSEBT denotes meaningful progression in our definition but might still coexist with a major clinical benefit of the TSEBT.
Overall survival of our patients (median 27 months, 50 % at 2 years) is also lower than reported by others [15], [16], [18], [21]. This is likely due to the use of TSEBT at later stages in the disease course compared to others. From personal communications we know that treatment choices in CTCL vary between countries with a rather conservative approach in the Netherlands, applying TSEBT after all other options are used. In one paper TSEBT is even used as first line therapy [15]. A more objective measure for this is the number of previous treatments before TSEBT. Again this is impossible to compare between papers due to either lack of reporting or summarizing treatments of the same type instead of counting them separately. Interval from initial diagnosis to TSEBT could also be an indicator of differences between institutions in the use of this treatment modality. A median of 26.2 months (1.6–282.7) from diagnosis to TSEBT was found compared to 4–21 months in other reports [15], [19], [28]. Not all papers however mention this parameter [16], [18], [25], [21]. Remission rates were adequate in our study at over 80 % with 25.5 % of patients reaching CR. This is comparable to other papers when taking the higher disease stage of our patients into account [29]. It should be noted that the 20% of patients with PD by our definition still had a good remission on the largest surface area of the skin. Also symptom relieve was impressive and comparable to others, underscoring the fact that the main goal of TSEBT treatment is achieved.
No patient or tumor characteristic other than T-stage that influenced disease course was identified. As expected, TSEBT dose did not influence overall survival. Our study shows that for both low and high dose groups TSEBT gives a clinical benefit. It appears to be possible to divide our patients in distinctive groups based on subjective clinical decision through joint evaluation by the dermatologist and radiation-oncologist appears to be possible. However, from our data nor from literature is it clear if patients currently treated with low-dose regimens would experience longer remissions and symptom relieve if treated with a high-dose. The opposite is also true, high dose patients might achieve similar results with a low dose schedule supplemented with boosts to thicker and ulcerative tumors. Low-dose TSEBT has advantages both for patients and department logistics. As shown in our study it offers similar symptom relieve, with the exception of ulcerative disease. With the same reduction in disease burden and symptoms it might be preferable to use several low-dose treatments in the course of a patients disease if necessary as opposed to one high-dose series. A rigorous implementation of mSWAT scoring at every patient visit will help in better documenting disease status prior to and after treatment and determine response duration. A major limitation of the study is its retrospective non-randomized design which could create bias in the data. For example, more patients with T2 stage were present in the low dose group and more patients with T3/T4 stage in the high dose group. Despite adjustment for T stage in the analysis, remaining bias cannot be excluded. We should be careful to draw to strong conclusions on this study
Conclusions
We call on the international community to start using a uniform dose schedule and objective indications for TSEBT. Consensus on endpoints and prospective data collection including quality of life parameters would be beneficial to compare results between study groups and institutions. Low-dose TSEBT has several benefits (reduction in patient visits, retreatment capabilities and lower toxicity profile) as opposed to high dose TSEBT. In this study we have shown no statiscal difference between two dose regimens. Apparent differences both within our results as well as compared with other published studies is likely to be explained by confounding by indication. Therefore, based on current results, we intend to change our practice and start using low dose TSEBT in all patients with supplemental radiotherapy to residual disease shortly after finishing TSEBT. The high dose regimen should be reserved for patients where it is not feasible to boost too many lesions.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2021.12.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Olsen E.A. Evaluation, diagnosis, and staging of cutaneous lymphoma. Dermatol Clin. 2015;33(4):643–654. doi: 10.1016/j.det.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Quaglino P., Pimpinelli N., Berti E., Calzavara-Pinton P., Alfonso Lombardo G., Rupoli S., et al. Time course, clinical pathways, and long-term hazards risk trends of disease progression in patients with classic mycosis fungoides: a multicenter, retrospective follow-up study from the Italian Group of Cutaneous Lymphomas. Cancer. 2012;118(23):5830–5839. doi: 10.1002/cncr.27627. [DOI] [PubMed] [Google Scholar]
- 3.Agar N.S., Wedgeworth E., Crichton S., et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730–4739. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- 4.de Coninck E.C., Kim Y.H., Varghese A., Hoppe R.T. Clinical characteristics and outcome of patients with extracutaneous mycosis fungoides. J Clin Oncol. 2001;19(3):779–784. doi: 10.1200/JCO.2001.19.3.779. [DOI] [PubMed] [Google Scholar]
- 5.Smoller B.R., Santucci M., Wood G.S., Whittaker S.J. Histopathology and genetics of cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 2003;17(6):1277–1311. doi: 10.1016/s0889-8588(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 6.Benner M.F., Jansen P.M., Vermeer M.H., Willemze R. Prognostic factors in transformed mycosis fungoides: a retrospective analysis of 100 cases. Blood. 2012;119(7):1643–1649. doi: 10.1182/blood-2011-08-376319. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita T, Abbade LP, Marques ME, Marques SA. Mycosis fungoides and Sezary syndrome: clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. Nov-Dec 2012;87(6):817-28; quiz 829-30. doi:10.1590/s0365-05962012000600001. [DOI] [PMC free article] [PubMed]
- 8.Edinger J.T., Clark B.Z., Pucevich B.E., Geskin L.J., Swerdlow S.H. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33(12):1860–1868. doi: 10.1097/PAS.0b013e3181bf677d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trautinger F., Eder J., Assaf C., et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome – Update 2017. Eur J Cancer. 2017;77:57–74. doi: 10.1016/j.ejca.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Duvic M., Donato M., Dabaja B., et al. Total skin electron beam and non-myeloablative allogeneic hematopoietic stem-cell transplantation in advanced mycosis fungoides and Sezary syndrome. J Clin Oncol. 2010;28(14):2365–2372. doi: 10.1200/JCO.2009.25.8301. [DOI] [PubMed] [Google Scholar]
- 11.Hoppe R.T. Mycosis fungoides: radiation therapy. Dermatol Ther. 2003;16(4):347–354. doi: 10.1111/j.1396-0296.2003.01647.x. [DOI] [PubMed] [Google Scholar]
- 12.Trump J.G., Wright K.A., Evans W.W., et al. High energy electrons for the treatment of extensive superficial malignant lesions. Am J Roentgenol Radium Ther Nucl Med. 1953;69(4):623–629. [PubMed] [Google Scholar]
- 13.Malcolm A., Bagshaw H.M.S., Farber E.M., Kaplan H.S. Electron beam therapy of mycosis fungoides. Calif Med. 1961;95(5):292–297. [PMC free article] [PubMed] [Google Scholar]
- 14.Karzmark C.J., Loevinger R., Steele R.E., Weissbluth M. A technique for large-field, superficial electron therapy. Radiology. 1960;74(4):633–644. doi: 10.1148/74.4.633. [DOI] [PubMed] [Google Scholar]
- 15.Navi D., Riaz N., Levin Y.S., Sullivan N.C., Kim Y.H., Hoppe R.T. The Stanford University experience with conventional-dose, total skin electron-beam therapy in the treatment of generalized patch or plaque (T2) and tumor (T3) mycosis fungoides. Arch Dermatol. 2011;147(5):561–567. doi: 10.1001/archdermatol.2011.98. [DOI] [PubMed] [Google Scholar]
- 16.Morris S.L., McGovern M., Bayne S., Wain M., Child F., Whittaker S. Results of a 5-week schedule of modern total skin electron beam radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(5):936–941. doi: 10.1016/j.ijrobp.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 17.Heumann T.R., Esiashvili N., Parker S., Switchenko J.M., Dhabbaan A., Goodman M., et al. Total skin electron therapy for cutaneous T-cell lymphoma using a modern dual-field rotational technique. Int J Radiat Oncol Biol Phys. 2015;92(1):183–191. doi: 10.1016/j.ijrobp.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhary M., Song A., Zaorsky N.G., Shi W. Total skin electron beam therapy in mycosis fungoides—a shift towards lower dose? Chin Clin Oncol. 2019;8(1) doi: 10.21037/cco.2018.09.02. 9-9. [DOI] [PubMed] [Google Scholar]
- 19.Kamstrup M.R., Gniadecki R., Iversen L., Skov L., Petersen P.M., Loft A., et al. Low-dose (10-Gy) total skin electron beam therapy for cutaneous T-cell lymphoma: an open clinical study and pooled data analysis. Int J Radiat Oncol Biol Phys. 2015;92(1):138–143. doi: 10.1016/j.ijrobp.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 20.Kroeger K., Elsayad K., Moustakis C., Haverkamp U., Eich H.T. Low-dose total skin electron beam therapy for cutaneous lymphoma: Minimal risk of acute toxicitiesNiedrigdosis-Ganzhautelektronenbestrahlung bei Patienten mit kutanen Lymphomen: Minimales Risiko für akute Toxizitäten. Strahlenther Onkol. 2017;193(12):1024–1030. doi: 10.1007/s00066-017-1188-8. [DOI] [PubMed] [Google Scholar]
- 21.Morris S., Scarisbrick J., Frew J., Irwin C., Grieve R., Humber C., et al. The results of low-dose total skin electron beam radiation therapy (TSEB) in Patients with mycosis fungoides from the uk cutaneous lymphoma group. Int J Radiat Oncol Biol Phys. 2017;99(3):627–633. doi: 10.1016/j.ijrobp.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 22.Hoppe R.T., Fuks Z., Bagshaw M.A. Radiation therapy in the management of cutaneous T-cell lymphomas. Cancer Treat Rep. 1979;63(4):625–632. [PubMed] [Google Scholar]
- 23.Murrer L, Van der Hulst HP, Jansen W, et al. NCS Report 34: Code of Practice and Recommendations for Total Body Irradiation and Total Skin Irradiation. 2021.
- 24.Kamstrup M.R., Specht L., Skovgaard G.L., Gniadecki R. A prospective, open-label study of low-dose total skin electron beam therapy in mycosis fungoides. Int J Radiat Oncol Biol Phys. 2008;71(4):1204–1207. doi: 10.1016/j.ijrobp.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 25.Hoppe R.T., Harrison C., Tavallaee M., Bashey S., Sundram U., Li S., et al. Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: results of a pooled analysis from 3 phase-II clinical trials. J Am Acad Dermatol. 2015;72(2):286–292. doi: 10.1016/j.jaad.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Neelis K.J., Schimmel E.C., Vermeer M.H., Senff N.J., Willemze R., Noordijk E.M. Low-dose palliative radiotherapy for cutaneous B- and T-cell lymphomas. Int J Radiat Oncol Biol Phys. 2009;74(1):154–158. doi: 10.1016/j.ijrobp.2008.06.1918. [DOI] [PubMed] [Google Scholar]
- 27.Georgakopoulos I, Papadavid E, Platoni K, et al. Clinical application of Total Skin Electron Beam (TSEB) therapy for the management of T cell cutaneous lymphomas. The evolving role of low dose (12Gy) treatment schedule. Clin Transl Radiat Oncol. Feb 2019;15:26-30. doi:10.1016/j.ctro.2018.12.002. [DOI] [PMC free article] [PubMed]
- 28.Lindahl L.M., Kamstrup M.R., Petersen P.M., Wirén J., Fenger-Grøn M., Gniadecki R., et al. Total skin electron beam therapy for cutaneous T-cell lymphoma: a nationwide cohort study from Denmark. Acta Oncol. 2011;50(8):1199–1205. doi: 10.3109/0284186X.2011.585999. [DOI] [PubMed] [Google Scholar]
- 29.Chowdhary M., Chhabra A.M., Kharod S., Marwaha G. Total skin electron beam therapy in the treatment of mycosis fungoides: a review of conventional and low-dose regimens. Clin Lymphoma Myeloma Leuk. 2016;16(12):662–671. doi: 10.1016/j.clml.2016.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.