Abstract
After the expiration of trastuzumab data exclusivity, biosimilar drugs were approved by regulatory agencies; among them, CT-P6 which was approved for the treatment of HER2-positive early- and advanced-breast cancer (BC) in 2018. Yet, reference trastuzumab (RTZ) is often combined with pertuzumab in early BC (EBC) patients treated with chemotherapy as it significantly improves the pathological complete response rate. Unfortunately, scarce preclinical and clinical data exists about the combination of CT-P6, pertuzumab and chemotherapy. Therefore, our aim was to study in vitro and in a retrospective cohort of EBC patients, whether CT-P6 was equivalent to RTZ when combined with pertuzumab with or without taxanes. In BT-474 and SKBR3 HER2+ cells we found that CT-P6 alone or in combination with pertuzumab had the same negative effect on cell proliferation, colony formation and HER2 downregulation as well as downstream activation, as RTZ. Adding paclitaxel to these treatments increased their effectivity to a similar extent. In HER2 1+ neuregulin-secreting MB-MDA-175 cells, combinations of CT-P6 or RTZ with pertuzumab were also effective, and mainly dependent on HER3:HER2 heterodimerization. In a retrospective cohort of 44 EBC HER2+ patients treated with neoadjuvant RTZ or CT-P6 in combination with pertuzumab and chemotherapy, we found no differences in efficacy or in adverse events. Moreover, the costs of CT-P6-based treatments were reduced by 1474.07 €/patient. All together we provide pre-clinical and clinical evidence of the equivalence of CT-P6 in combination with pertuzumab and chemotherapy and suggest studying these combinations also in HER2 low/negative BC patients.
Keywords: HER2-Positive breast cancer, Biosimilar, Trastuzumab, Pertuzumab, Taxanes, CT-P6
Abbreviations: RTZ, reference trastuzumab; ICO, Institut Català d’Oncologia (Catalan Institute of Oncology)
Highlights
-
•
CT-P6 or RTZ + pertuzumab + paclitaxel are equivalent treatments in HER2+ BC cells.
-
•
Equivalent efficacy and safety of CT-P6 or RTZ in HER2+ EBC patients.
-
•
Using CT-P6 represents important savings for the health system.
-
•
HER2 low BC is a promising scenario for new anti-HER2 therapies.
1. Introduction
In approximately 20% of all breast cancers (BC) the human epidermal growth factor receptor 2 gene (HER2) is amplified, resulting in a constitutive activation of the tyrosine kinase domain of the transmembrane receptor. HER2 activation occurs after dimerization with other members of the HER family including HER1, HER3 and HER4 [1]. HER2:HER3 dimers are the most oncogenic as they activate two key pathways that regulate cell survival and growth: the mitogen-activated protein kinase (MAPK) and the phosphoinositide 3-kinase (PI3K) pathways [2,3].
According to the guidelines of the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP), tumor HER2 positivity is defined by HER2 protein overexpression measured by immunohistochemistry (IHC3+) or by the presence of six or more HER2 copies or a HER2/CEP17 ratio of 2.0 or greater as measured by fluorescent in-situ hybridization (FISH) [4,5]. This molecular alteration results in a more aggressive type of BC that had a poor prognosis until the development and introduction of targeted therapies. The first of them was trastuzumab, a recombinant humanized monoclonal antibody that binds subdomain IV of the HER2 extracellular domain and inhibits ligand independent HER2 signaling [6]. The development of trastuzumab changed the natural history of the HER2-positive BC. In early stage patients (EBC), the addition of trastuzumab to standard chemotherapy significantly improved event-free survival and overall survival [[7], [8], [9]]. Data from the neoadjuvant setting fostered the approval of trastuzumab for EBC treatment [10].
Considering this success, other therapies targeting HER2 were developed to enhance the effect of trastuzumab. One of them is pertuzumab, another humanized monoclonal antibody that in this case, binds the subdomain II of the HER2 extracellular domain and blocks its ligand-dependent heterodimerization with HER1, HER3, and HER4 [11]. Because trastuzumab and pertuzumab have complementary mechanisms of action, together they provide a dual blockade of HER2-driven signaling [12]. In EBC patients, the addition of pertuzumab to chemotherapy and trastuzumab significantly improved the pathological complete response rate (pCR) [11,[13], [14], [15], [16], [17]]. According to THE APHINITY trial, pertuzumab significantly improved the rates of invasive-disease–free survival among patients with HER2-positive, operable BC when it was added to trastuzumab and chemotherapy [18].
As these therapies are usually quite expensive, the development of biosimilar medicines has been shown to be a straightforward strategy for reducing public healthcare costs and increasing their accessibility [19]. A biosimilar is a biological medicine highly similar to another already approved (the ‘reference medicine’) in terms of structure, biological activity, and efficacy, as well as safety and immunogenicity profile [20].
So far, the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have approved several trastuzumab biosimilars. One of them is CT-P6, whose approval was based on preclinical [21,22] and clinical studies in which the biosimilar was compared to the reference trastuzumab (RTZ, Herceptin®). A phase III trial compared CT-P6 to the RTZ in patients with operable EBC in combination with docetaxel followed by FEC (fluorouracil, epirubicin, and cyclophosphamide) showing that CT-P6 had equivalent efficacy to RTZ and that adverse events were also similar [23]. As this study was designed before the introduction of pertuzumab in combination with trastuzumab in the neoadjuvant setting, scarce data about the efficacy of the combination of CT-P6, chemotherapy and pertuzumab exists. Because this combination is currently used in the clinical practice, in the present study we aimed to demonstrate that CT-P6 (Herzuma®) and RTZ had the same efficacy in combination with pertuzumab and chemotherapy, both in vitro and in a retrospective cohort of EBC patients. Additionally, we studied the associated cost savings for the health care system.
2. Material and methods
2.1. Patient cohort
A single-center retrospective study was carried out in 44 patients diagnosed with HER2-positive EBC who received neoadjuvant treatment based on chemotherapy, pertuzumab and trastuzumab, either CT-P6 (n = 20) o RTZ (n = 24). Almost all patients (95.5%) were treated with four cycles of doxorubicin plus cyclophosphamide followed by 12 administrations of weekly paclitaxel or nab-paclitaxel with concomitant pertuzumab plus trastuzumab, either CT-P6 o RTZ, before surgery. Only two patients were treated with a neoadjuvant schedule without anthracyclines, one in each arm of therapy (Table 1). Patients within RTZ and CT-P6 groups were treated from year 2017–2019 and from year 2019–2020, respectively. To avoid selection bias, we chose all the patients treated with neoadjuvant treatment in the period immediately before and after the approval of the biosimilar in our center.
Table 1.
Characteristics of the patients.
| CT-P6 (n = 20) | RTZ (n = 24) | P∗ | |
|---|---|---|---|
| Age (years) | |||
| Mean | 48 (4.9) | 60 (11.2) | 0.001 |
| Weight (kg) | |||
| Mean | 68.4 (11.0) | 68.2 (10.3) | 0.951 |
| Clinical stage | 0.298 | ||
| I | 0 (0%) | 1 (4%) | |
| IIa | 13 (65%) | 9 (38%) | |
| IIb | 5 (25%) | 10 (42%) | |
| IIIa | 1 (5%) | 3 (12%) | |
| IIIb | 1 (5%) | 0 (0%) | |
| IIIc | 0 (0%) | 1 (4%) | |
| IV | 0 (0%) | 0 (0%) | |
| Nodal status | 0.263 | ||
| Positive | 10 (50%) | 16 (67%) | |
| Negative | 10 (50%) | 8 (33%) | |
| Hormone status | 0.223 | ||
| Estrogen receptor | |||
| Positive | 14 (70%) | 13 (54%) | |
| Negative | 6 (30%) | 11 (46%) | |
| Progesterone receptor | 0.146 | ||
| Positive | 12 (60%) | 19 (79%) | |
| Negative | 8 (40%) | 5 (21%) | |
| Grade | 0.363 | ||
| G1 | 1 (5%) | 0 (0%) | |
| G2 | 5 (25%) | 8 (42%) | |
| G3 | 14 (70%) | 11 (58%) | |
| Ki67 (%) | 0.974 | ||
| Median | 40 (18–70) | 40 (10–70) | |
| LVEF (%) | |||
| Median | 66 (57–77) | 65 (56–78) | 0.270 |
| Neoadjuvant chemotherapy | 0.873 | ||
| AC-THP | 19 (95%) | 23 (96%) | |
| TCHP | 1 (5%) | 1 (4%) | |
Data are mean (SD), median (range), or n (%). LVEF = left ventricular ejection fraction. AC-THP = doxorubicin + cyclophosphamide followed by paclitaxel or nab-paclitaxel + trastuzumab + pertuzumab. TCHP = docetaxel + carboplatin + trastuzumab + pertuzumab. ∗p-value from Chi-Square test or Student's t-test; p-value under 0.05 is considered as statistical significant.
All patients were evaluated by our center's BC committee from 2017 to 2020. The study was conducted in accordance with the ethics principles of the Declaration of Helsinki and approved by the Research and Ethics Committee of the Institut Català d’Oncologia (ICO) at Hospital Germans Trias i Pujol. All patients provided written informed consent. Demographic, clinical and biological data as well as toxicity parameters according to Common Terminology Criteria for Adverse Events (CTCAE, version 4.0) were collected for all study participants and are resumed in Table 1.
At the time of diagnosis, patients were subject to a computed tomography (CT) scan, a bone scan and a blood analysis for evaluation of disease extension; only patients without distant metastasis were treated with neoadjuvant schedules. Clinical response was evaluated following standard procedures [24]. Tumor stage was classified according to TNM classification of the Union International Cancer Control [25]. Clinical data were obtained from review of electronic medical records. Safety endpoints were the prevalence and severity of two major adverse events (infusion-related reactions and diarrhea) and cardiotoxicity, as assessed by mean change from baseline to endpoint assessment in left ventricular ejection fraction (LVEF). LVEF was assessed with multi-gated acquisition scan (MUGA) at baseline, after cycle 4 of neoadjuvant treatment and at the end of neoadjuvant period. We defined a significant LVEF decrease as a decrease of ten ejection fraction points from the baseline value and a decrease of an absolute value of less than 50%. Costs information of administered treatments were obtained from electronic records, considering number of vials used and drug acquisition prices (€) of the ICO. For this analysis, only anticancer drugs were considered excluding costs of antiemetics or other support treatments such as granulocyte colony-stimulating factor or iron infusions.
2.2. Cell lines and reagents
Human HER2+ (SKBR3, BT-474) and HER2-low (MDA-MB-175) BC cell lines (American Type Culture Collection) were grown as monolayer in DMEM (Life Technologies) (SKBR3, BT-474) or RPMI1640 (MDA-MB-175) (Invitrogen, Life Technologies) medium supplemented with 10% of heat-inactivated FBS (Reactiva), 2 mmol/L l-glutamine (Sigma), 10 mmol/L HEPES (Sigma), 1% penicillin/streptomycin (basic medium). All cell lines were cultured in a 5% CO2 humidified atmosphere at 37 °C. RTZ (Herceptin 150 mg; Roche), CT-P6 (Herzuma 150 mg; CellTrion), pertuzumab (Perjeta 420 mg; Roche) and paclitaxel 6 mg/mL (Accord) were supplied by the ICO Pharmacy Unit and were stored at room temperature or at 4 °C as indicated by manufacturers.
2.3. Immunohistochemistry
Cell lines were harvested and centrifuged to obtain a pellet that was washed with PBS and fixed in 10% neutral buffered formalin at room temperature and paraffin-embedded to obtain a block. Sections of approximately 4 μm thick were cut and put on glass slides. IHC staining was performed using the Ventana iVIEW DAB Detection Kit and the anti-HER2/neu (4B5) rabbit monoclonal antibody (Roche Diagnostics). We used the Scoring Conventions for the Interpretation of PATHWAY HER2 BC [26]. Images were obtained with a Leica DMI6000B microscope.
2.4. In vitro functional analysis
In vitro proliferation/cytotoxicity assay in response to RTZ, CT-P6, pertuzumab and paclitaxel was assessed by the 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT) method. Briefly, 8 × 103 (BT-474), 2 × 103 (SKBR3) and 1 × 104 (MDA-MB-175) cells/well were plated in 96-well plates. After 24 h, increasing concentrations of each drug alone, or in combinations as indicated below, were added. Medium with drugs was fully removed and refreshed every 3 days. After 7 (BT-474 and SKBR3) or 12 (MDA-MB-175) days of treatment, 100 μL of MTT mix (80% RPMI 1640 medium, 10% FBS, 10% MTT) was added to each well and the plates were incubated for 4 h. Note that the long times used in the case of the MDA-MB-175 cells were due to the high doubling time this cell line has (112.26 h). The resulting formazan product was dissolved with a solubilization solution (10% SDS in 0.01 M HCl) and the absorbance at wavelength of 590 nm was measured on a SPECTROstar Nano equipment (BMG Labtech). Median effect lines analysis was conducted to determine the effect on proliferation and cytotoxicity activity of assayed drugs in each case. The synergistic effect of RTZ + pertuzumab and CT-P6 + pertuzumab without or with paclitaxel (0.1 nM) was assessed by calculating the Combination Index (CI; CI < 1 indicates synergy, CI = 0 addivism, CI > 1 antagonism) as described by Chou and Talalay [27] using the Compusyn Software (Combosyn Inc.).
For colony-formation assays 2 × 103 (BT-474 and MDA-MB-175) and 5 × 102 cells/well (SKBR3) were seeded in 6-well plates (in two replicates) in basic medium. After 24 h cells were treated with RTZ and CT-P6 alone or in combination with pertuzumab and paclitaxel for 20 (SKBR3) or 25 (BT-474 and MDA-MB-175) days. Medium with treatments was fully removed and refreshed every 3 days. After 20–25 days cells were washed with PBS 1X, fixed with a methanol/acetic acid (3:1) solution and stained with crystal violet (0,5%) for 10 min. Quantification of colony number was assessed using ImageJ software. To evaluate the status of HER2 signaling and its modulation in response to treatments, HER2+ and HER2-low BC cells were seeded at 60% of confluence and incubated for 24 h in basic medium. RTZ or CT-P6 alone and in combination with pertuzumab were then added to the medium and the cells were treated for 48 h. Plates were placed on ice, washed with cold PBS, and recovered for analysis by Western blot.
2.5. Western blot assays
We followed standard methods already described by our group [28]. Antibodies targeting phospho-HER2 (6B12), HER2 (D8F12), phospho-HER3 (21D3), HER3 (D22C5), phospho-AKT (D9E), AKT (C67E7), phospho-ERK1/2, ERK1/2 (137F5) were from Cell Signaling and GAPDH (G9545) and αTubulin (B512) were from Sigma. GAPDH and αTubulin were used as a protein-loading controls. Membranes were incubated with IRDye rabbit (1:7500) for 1 h protected from the light. Membranes were scanned by using an Odyssey Imaging System and analysed with Odyssey v2.0 software (LICOR Biosciences).
2.6. Statistical analysis
A comprehensive cohort description analysis based on demographic, clinical and biological data was performed. Categorical variables were summarized through frequencies and percentages, and quantitative variables using means and standard errors or medians and interquartile ranges. Overall response rate (ORR) was reported as the proportion of patients who have a partial (PR) or complete response (CR) to therapy. Differences in the complete pCR and in the main adverse effects were evaluated using the Chi-square test (χ2) or the Student's t-test. Differences in efficacy and safety endpoints between groups were assessed by χ2 or independent t-test. All analyses were performed with the Statistical Package for the Social Sciences (SPSS) software (IBM SPSS Statistics for Macintosh, Version 23.0. Armonk, NY: IBM Corp.). In in vitro clonogenicity assays statistical differences were calculated using a two-tailed Student t-test. p < 0.05 was considered significant.
3. Results
3.1. Retrospective clinical study
Baseline characteristics were similar between the two groups except for age, as patients in the CT-P6 group were younger (Table 1). Thus, non-statistically significant differences were found in the average of estrogen receptor positivity, clinical stage IIa and IIb, and the average of nodal positivity. Almost the same proportion of patients achieved a pCR in the CT-P6 (65.0%; 95% CI 44.1–85.9) and in the RTZ groups (66.7%; 95% CI 48.2–85.8) (p = 0.908). Neither difference was observed in clinical CR rates between the CT-P6 (55-0%; 95% CI 33.2–76.8) and the RTZ groups (66.7%; 95% CI 48.2–85.8) (p = 0.429) (Table 2).
Table 2.
Clinical response and pathological complete response analysis.
| CT-P6 (n = 20) | RTZ (n = 24) | P∗ | |
|---|---|---|---|
| Clinical complete response | 11 (55%; 33.2–76.8) | 16 (66.7%; 48.2–85.8) | 0.429 |
| Pathological complete response | 13 (65%; 44.1–85.9) | 16 (66.7%; 48.2–85.8) | 0.908 |
∗p-value from Chi-Square test; p-value under 0.05 is considered as statistical significant.
Data are n (%; 95% CI).
The proportion of adverse events was similar between groups (Table 3). Grade 3 toxicity was infrequent, and in all cases was related to diarrhea (two cases in the CT-P6 group and one case in the RTZ group). In these three cases, the doses of paclitaxel were reduced to 80% but not those of trastuzumab (CT-P6 or RTZ), pertuzumab or anthracyclines. There were no treatment interruptions. We did not observe differences in LVEF values between groups, either at baseline, or after cycle 4 and at the end of the neoadjuvant period (Table 3). The overall worst value was 60.5 in the CT-P6 and 57.5 in the RTZ groups. (Table 3).
Table 3.
Summary of adverse events.
| CT-P6 (n = 20) | RTZ (n = 24) | P∗ | |
|---|---|---|---|
| Infusion-related reactions | 1 (5%) | 1 (4.2%) | 0.895 |
| Diarrhea | 0.731 | ||
| Grade 1 | 7 (35%) | 10 (41%) | |
| Grade 2 | 6 (30%) | 5 (20%) | |
| Grade 3 | 2 (10%) | 1 (4.2%) | |
| LVEF | |||
| Baseline | 66 (57–77) | 65 (56–78) | 0.270 |
| After cycle 4 | 64 (52–72) | 60.5 (54–75) | 0.973 |
| End of neoadjuvant period | 61.5 (47–75) | 63 (45–75) | 0.510 |
| Overall worst value | 60.5 (47–68) | 57.5 (45–73) | 0.954 |
| Decrease of ≥10 points from baseline | 8 (44.4%) | 6 (28.6%) | 0.303 |
| <50 points | 2 (10%) | 2 (8.3%) | 0.848 |
| <50 points and decrease of ≥10 points | 2 (10%) | 1 (4.2%) | 0.445 |
Data are median (range) or n (%). LVEF = left ventricular ejection fraction. ∗p-value from Chi-Square test or Student's t-test; p-value under 0.05 is considered as statistical significant.
Finally, the cost per patient treated in the CT-P6 group was 20,465.91 € and 21,939.98 € in the RTZ group, thus the decremental cost with trastuzumab biosimilar was of 1474.07 €/patient.
3.2. In vitro study
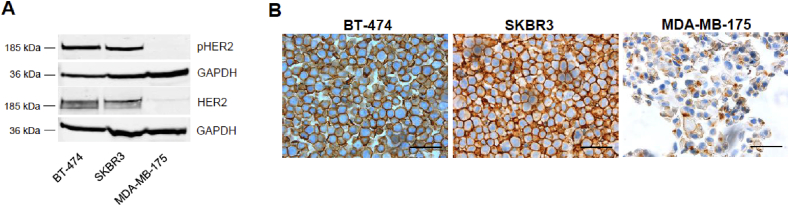
To characterize the expression of HER2 as well as the downstream pathway activation in our cell models, we used two well-known HER2+ cell lines, the BT-474 and the SKBR3; the MDA-MB-175 cell line was used as negative control. BT-474 and SKBR3 cells, expressed higher levels of HER2 (+3) as compared to the MDA-MB-175 (+1) as previously reported (Fig. 1A and B); HER2 staining was mainly observed in the cell plasma membrane (Fig. 1B). Accordingly, HER2 (3+) cells lines showed high levels of phospho-HER2, indicating that this pathway is activated in an autocrine manner in these cells.
Fig. 1.
Characterization of HER2 + breast cancer cells. (A) Total and phosphorylated HER2 levels in indicated breast cancer cell lines. GAPDH was used as a protein-loading control. (B) HER2 immunocytochemistry of indicated breast cancer cell lines. Scale bar, 50 μm. Magnification, 40×.
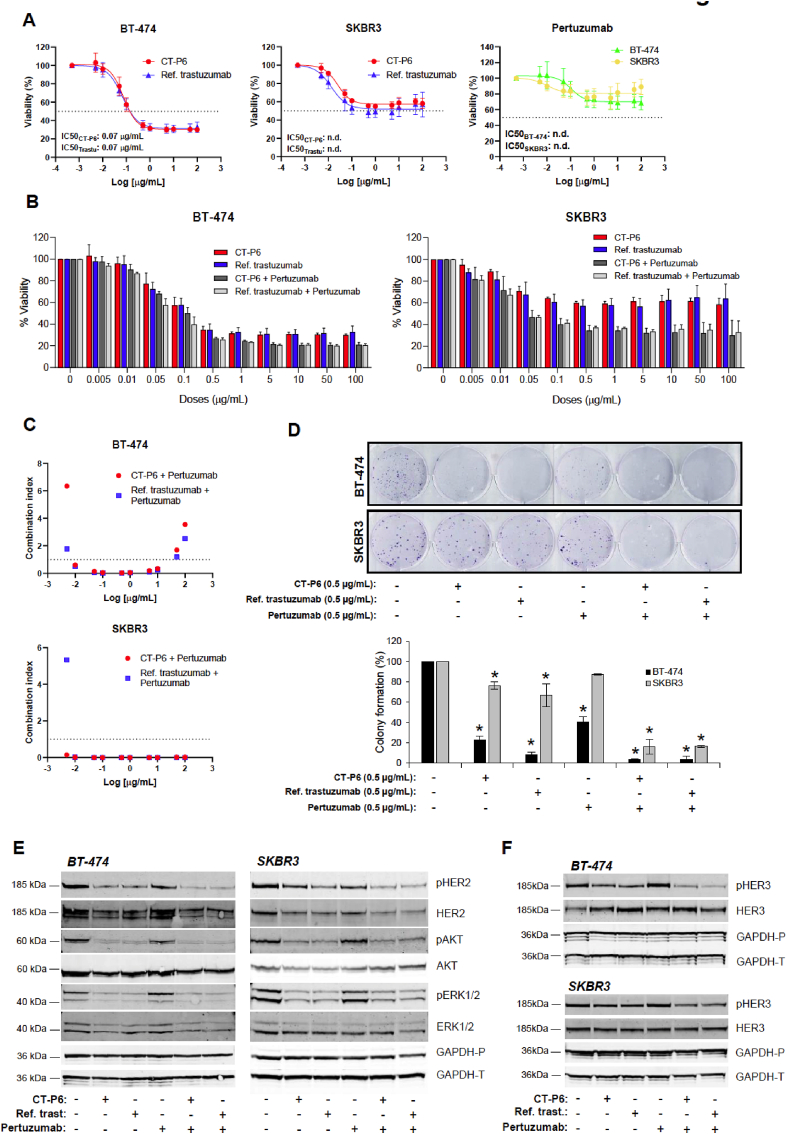
To study the relevance of HER2-mediated signaling pathway and to compare the effect on cell proliferation of RTZ and CT-P6, cell lines were exposed to increasing doses of RTZ, CT-P6 or pertuzumab (Fig. 2A). HER2+ cell lines were sensitive to RTZ and CT-P6 treatment and less sensitive to pertuzumab, as single agents. As expected, RTZ and CT-P6 showed similar effect on proliferation inhibition, demonstrated by the overlapping dose-response curves and the identical IC50 values of both drugs (Fig. 2A). Concomitant combinations of RTZ or CT-P6 with pertuzumab were highly synergistic and comparable, especially in the SKBR3 cells (Fig. 2B and C). In line with this, the number of colonies formed was significantly reduced in response to treatment with RTZ or CT-P6; however, this reduction was increased to approximately 90–95% when each one was combined with pertuzumab (Fig. 2D).
Fig. 2.
Effect of CT-P6 and RTZ (Ref. trastuzumab) in combination with pertuzumab in HER2 + BC cell proliferation, colony formation and HER2 signaling pathway. (A) Effect of CT-P6, RTZ or pertuzumab on proliferation (% mean ± SD) of the indicated HER2-positive cell lines. (B) Effect of CT-P6 and RTZ and in combination with pertuzumab on proliferation (% mean ± SD) of the indicated HER2-positive cell lines. (C) Graphic representation of the combination index (CI) of the indicated drug combinations. CI < 1 indicates synergistic effect; CI = 1 additive effect and CI > 1 antagonism of combined drugs. (D) Effect of CT-P6 (0.5 μg/mL) and RTZ (0.5 μg/mL) and in combination with pertuzumab (0.5 μg/mL) on colony formation of the indicated HER2-positive cell lines. Bar plot representing the percentage (mean ± SD) of colonies after 20–25 days of treatments with indicated drugs in indicated cell lines. (E) Effect of CT-P6 and RTZ and in combination with pertuzumab on HER2, AKT and ERK1/2 phosphorylation in the indicated HER2-positive cell lines. (F) Effect of CT-P6 and RTZ and in combination with pertuzumab on HER3 phosphorylation in the indicated HER2-positive cell lines. GAPDH was used as a protein-loading control. ∗indicates significant differences (t-test; p < 0.05). GAPDH-P and GAPDH-T indicate protein-loading control of gels used for determining phosphorylated and total proteins, respectively.
To examine whether these observations were due to similar mechanisms of action, cell lines were treated with RTZ and CT-P6 alone or in combination with pertuzumab and levels of phospho-proteins downstream of HER2+ were checked by Western blot. Constitutive pathway activation was demonstrated in HER2+ cell lines by high basal levels of phospho-HER2 (pHER2), phospho-AKT (pAKT) and phospho-ERK1/2 (pERK 1/2) (Fig. 1, Fig. 2E). Both drugs inhibited HER2, AKT and ERK1/2 phosphorylation to a similar degree when administered alone in HER2+ cells. According to our previous results, stronger reduction was observed when they were combined with pertuzumab. (Fig. 2E). We also observed that RTZ or CT-P6 alone or in combination with pertuzumab caused a reduction of total HER2 levels (Fig. 2E), suggesting an alternative mechanism of action based on irreversibly binding of anti-HER2 antibodies to the receptor eventually causing its degradation.
Interestingly, HER3 phosphorylation was marginally inhibited in response to RTZ or CT-P6, while this inhibition was more significant when these drugs were administered in combination with pertuzumab (Fig. 2F). These observations indicated that HER2+ cells are highly dependent on HER2:HER2 homodimerization and less dependent on HER2:HER3 heterodimerization.
Additionally, we wanted to gain further insight by studying the HER2-low cell line MDA-MB-175. Although these cells do not fit the clinical criterium to be treated with anti-HER2 drugs, they have been described to secrete neuregulin-1 in an autocrine manner [29] and therefore, they are a good model for assaying treatments with pertuzumab. We confirmed the low expression patterns of HER2 (Fig. 1A and B); however, HER2-low showed higher activation of HER3 as compared to the HER2+ cells (Fig. S1A) probably due to their ability to secrete neuregulin-1 as commented above. Neuregulin is the main ligand with affinity to HER3 promoting HER3 heterodimerization with other HER family receptors [3]. In the MDA-MB-175 cell line, treatments with RTZ or CT-P6 showed similar effect on cell proliferation as with the HER2+ cells although they were less sensitive to these drugs (Fig. S1B). However, they were shown to be highly sensitive to pertuzumab compared with HER2+ cells (Fig. S1B) causing the combination of RTZ or CT-P6 with pertuzumab to be highly synergistic (Fig. S1C). In fact, HER2, HER3 and ERK1/2 phosphorylation were not affected in response to RTZ or CT-P6 alone, while they were highly inhibited when these drugs were administered in combination with pertuzumab (Fig. S1D-E). So, in contrast to HER2+ cells, HER2-low cells are highly dependent on HER2:HER3 heterodimerization and less dependent on HER2:HER2 homodimerization.
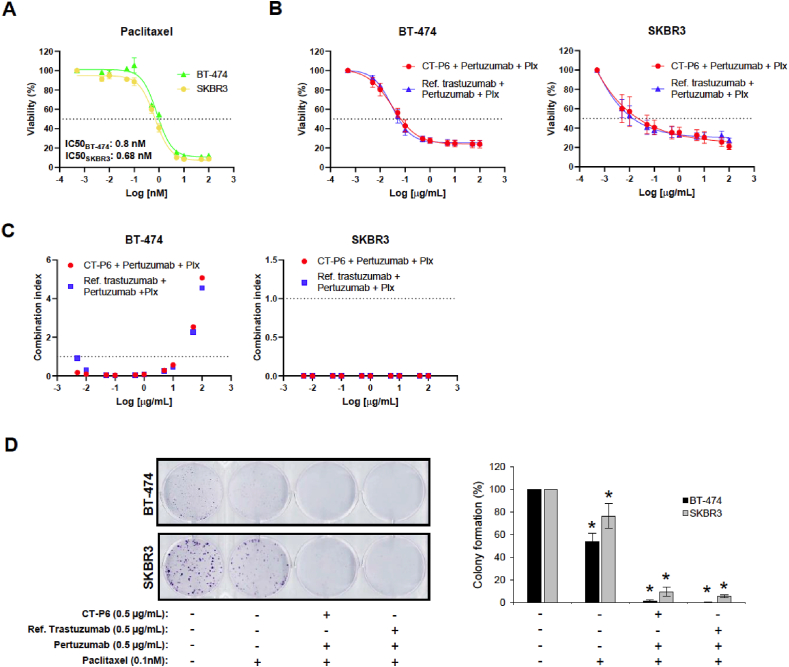
Finally, to mimic the standard of care of neoadjuvant treatment in HER2+ EBC patients, HER2+ cells were treated with CT-P6 or RTZ in combination with pertuzumab and paclitaxel. Our results demonstrated that even in combination with doses of paclitaxel below the corresponding IC50 of each cell line, dual anti-HER2 therapy with either CT-P6 or RTZ and pertuzumab, has the same synergistic effect inhibiting proliferation and colony-forming capacity (Fig. 3A–D). These results are in agreement with those found in the retrospective clinical study.
Fig. 3.
CT-P6 andRTZ (Ref. trastuzumab) effect in combination with pertuzumab and paclitaxel (Ptx) in HER2 + BC cell proliferation and colony formation. (A) Effect of paclitaxel on proliferation (% mean ± SD) of the indicated HER2-positive cell lines. (B) Effect of CT-P6 (0.5 μg/mL) and RTZ (0.5 μg/mL) and in combination with pertuzumab (0.5 μg/mL) and paclitaxel (0.1 nM) on proliferation (% mean ± SD) of the indicated HER2-positive cell lines. (C) Graphic representation of the combination index (CI) of the indicated drug combinations. (D) Effect of CT-P6 (0.5 μg/mL) and RTZ (0.5 μg/mL) in combination with pertuzumab (0.5 μg/mL) and paclitaxel (0.1 nM) on colony formation of the indicated HER2-positive cell lines.
4. Discussion
This work constitutes one of the few real-world studies showing that biosimilar CT-P6 combined with pertuzumab has similar effect and safety profile as RTZ combined with pertuzumab in patients with HER2-positive EBC. Our results are supported by preclinical evidence demonstrating that RTZ and CT-P6 share mechanisms of action consisting of blocking cell proliferation and colony formation through the inhibition of HER2-mediated AKT and ERK1/2, as single agents and in combination with pertuzumab. Importantly, we show for the first time that this is also the case when adding paclitaxel, supporting the use of CT-P6 in the day-to-day clinical settings.
Even though the clinical trial that established the equivalence of CT-P6 and RTZ did not include combinations with pertuzumab [23], after approval of dual HER2-targeted blockade with RTZ and pertuzumab in the neoadjuvant setting, most patients at our institution were treated with CT-P6 instead of RTZ. Our study supports this decision and adds new data to the few already available [30], confirming that CT-P6 is at least as effective as RTZ when administered as part of dual-targeted therapy with pertuzumab. Specifically, we observed the same pCR rate in HER2-positive BC patients treated with either RTZ or CT-P6. The high rate of pCR (65% in CT-P6 and 66.7% in RTZ arms) was consistent with the fact that 95.5% patients were treated with regimens containing anthracyclines and taxanes. In our cohort, patients were comparable according to different baseline characteristics with the exception of mean age which was younger in the CT-P6 group.
Importantly, we also compared the safety profile of both drugs finding no differences between CT-P6 and RTZ, at least in infusion-related reactions, diarrhea of any grade and cardiotoxicity. This is important as the use of RTZ has historically been associated with an increased risk of cardiotoxicity [6]. In our study, neither CT-P6- nor RTZ-associated serious cardiac toxic profiles were reported which is in agreement with the low cardiotoxicity observed in EBC patients treated with RTZ or CT-P6 in the neoadjuvant [13,23] and adjuvant [13,22] settings. One of the main limitations of this real-world study is that it had a retrospective design and a relatively small sample size. Therefore, attention must be paid to its intrinsic limitations, to avoid a misleading and potentially harmful use of its derived-results. However, sharing our data and experience can be useful for other groups as they incorporate more representative evidence from patients in real life.
Our in vitro studies showed that CT-P6 and RTZ alone or in dual HER2 blockade setting had similar effects on reducing phosphorylation of HER2 and their downstream effectors AKT and ERK1/2 in HER2+ BC cell lines, which is in agreement with previous observations [21,31]. We also report that HER3 signaling residually contributes to promoting proliferation of HER2+ BC cells, probably through the heterodimerization with HER2. Additionally, we observed a downregulation of HER2 expression in response to CT-P6 alone or in combination with pertuzumab, which also occurs in response to RTZ according to our findings and other studies [21]. These observations suggest that CT-P6 promotes HER2 internalization and degradation by enhancing the activity of tyrosine kinase-ubiquitin ligase c-Cbl as described in HER2-overexpressing BC cells in response to RTZ [32,33]. It is well known that RTZ induces antibody-dependent cellular cytotoxicity (ADCC) in HER2-expressing BC cells [34,35]. In the present work, we have not addressed this issue; nevertheless, other authors have previously shown that it may also occur in response to CT-P6 as described for HER2+ gastric cancer cells [21].
So far, the equivalence of CT-P6 and RTZ when administered in combination with pertuzumab and taxanes, which is the standard treatment in HER2-positive patients, was poorly explored in vitro. Our work demonstrates that in vitro dual HER2 blockade using either CT-P6 or RTZ plus paclitaxel is more effective than dual HER2 blockade, which adds further preclinical evidence and supports the introduction of CT-P6 in combinations with pertuzumab and chemotherapy as the standard of care neoadjuvant treatment of HER2+ EBC patients.
Furthermore, we observed that HER2-low BC cells are highly sensitive to dual HER2 blockade mostly due to the action of pertuzumab inhibiting the heterodimerization of HER2 with HER3, which leads to a downregulation of HER2 expression and an inhibition of HER2, HER3, AKT and ERK phosphorylation. These in vitro results are in contrast to data reported in clinical trials using trastuzumab, pertuzumab, margetuximab or TDM1 in HER2-low BC patients [[36], [37], [38], [39]] however, in a phase Ib trial testing the combination of pertuzumab, paclitaxel and the anti-HER3 mAb lumretuzumab in advanced BC patients with HER3 and HER2-low expressing tumors, a clinical benefit was reported which is in line with our results. Unfortunately an unacceptable toxicity profile prompted discontinuation of this study [40]. Additionally, there are current preclinical and clinical evidence demonstrating that HER2 blockade by novel antibody-drug conjugates (ADCs) [41,42] and bispecific antibodies [43] have anticancer activity in HER2-low BC tumors, prompting further clinical exploration in this setting.
Biosimilars have been developed with the objective of reducing drug costs. The average starting price of biosimilars is calculated to be 20–30% lower than reference products, and in addition, biosimilars bring competition to these markets that were previously exclusive, as well as other confidential discounts [40]. In our study, treatments with CT-P6 in the neoadjuvant setting were on average 1474.07 € per patient cheaper than with RTZ. This may be considered a modest saving, but it should be noted that the number of neoadjuvant cycles administered is shorter as compared to other settings. The ICO is a Catalan public enterprise comprising 4 different cancer centres in Catalonia (including ours) and the referral institution of more than 4 million people. All ICO centres have a common pharmacy and a common prescription guide. CT-P6 is currently approved by the Pharmacy and Therapeutics Committee of the ICO in all available indications and settings of use (neoadjuvant, adjuvant and metastatic). For this reason, our exercise helps us to understand the savings that the introduction of the biosimilar has supposed to our institution, corresponding to global savings of 1.67 millions € (46.3% reduction). Savings generated with biosimilars contribute to efficiency and sustainability of health care systems, increasing access to expensive drug treatments and benefiting patients in other disease areas [[44], [45], [46], [47]].
5. Conclusions
To combine CT-P6 with pertuzumab and paclitaxel is as safe and efficacious as doing so with RTZ; however, using CT-P6 represents important savings for the health system as compared to RTZ. Our results, with the main limitation of coming from a retrospective design and a relatively small sample size, suggest that BC patients with HER2-low tumors may benefit from such combinations.
Funding
This work was supported by a grant from KERN Pharma Biologics (no grant number applies) which had no role in the design of this study, its execution, collection, analysis and interpretation of the data, in the writing of the report or in the decision to submit the article for publication.
Dr. Martinez-Balibrea's research group is supported by grant 2017-SGR-1705 from the Department d’Innovació, Universitats i Empresa, Generalitat de Catalunya.
Author contributions statement
AB-P and MT had substantial contributions to the conception and design, acquisition and interpretation of in vitro experiments and data. AF-D had substantial contribution to patient data extraction and interpretation of clinical data in the context of the literature. CI has substantial contribution in cost evaluation, literature research and interpretation of data in the context of the literature. SB and AM-C analysed and interpreted the data in the context of the literature. MM and EM-B had substantial contributions to the conception of the work, literature research, interpretation of data and directing and organising the manuscript preparation. MM, EM-B, AF-D and AB-P had substantial contribution in writing the final manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
AB-P, MT, AF-D, CI, SB, and AM-C declare no conflict of interest. EM-B declare research funding from Kern Pharma Biologics. CI declares consulting or advisory activities for Roche. MM declares consulting or advisory role for Novartis, Pfizer, and Roche; Research funding from Roche, Eisai, AstraZeneca and Kern Pharma Biologics and travel expenses from Roche.
Acknowledgements
We want to particularly acknowledge the patients and the IGTP-HUGTP Biobank integrated in the Spanish National Biobank Network of Instituto de Salud Carlos III (PT13/0010/0009) and Tumor Bank Network of Catalonia for its collaboration.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.01.007.
Contributor Information
Mireia Margelí, Email: mmargeli@iconcologia.net.
Eva Martínez-Balibrea, Email: embalibrea@iconcologia.net.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Balselga J., Swain S.M. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer [Internet] 2009 Jul;9(7) doi: 10.1038/nrc2656. https://pubmed.ncbi.nlm.nih.gov/19536107/ [cited 2021 Jul 20] 463–75. Available from: [DOI] [PubMed] [Google Scholar]
- 2.Hynes N.E., Lane H.A. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005 May;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 3.Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer (Oxf Engl : 1990) [Internet] 2001;37(SUPPL. 4):3–8. doi: 10.1016/s0959-8049(01)00230-1. https://pubmed.ncbi.nlm.nih.gov/11597398/ [cited 2021 Jul 20] Available from: [DOI] [PubMed] [Google Scholar]
- 4.Wolf A.C., Elizabeth M.H., Hicks D.G., Dowsett M., McSane L.M., Allison K.H., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol : Off J Am Soc Clin Oncol [Internet] 2013 Nov 1;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. https://pubmed.ncbi.nlm.nih.gov/24101045/ [cited 2021 Jul 20] Available from: [DOI] [PubMed] [Google Scholar]
- 5.Wolff A.C., Elizabeth Hale Hammond M., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol [Internet] 2018 Jul 10;36(20) doi: 10.1200/JCO.2018.77.8738. https://pubmed.ncbi.nlm.nih.gov/29846122/ [cited 2021 Apr 8] 2105–22. Available from: [DOI] [PubMed] [Google Scholar]
- 6.Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Panton V., Bajamonde A., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New Engl J Med [Internet] 2001 Mar 15;344(11) doi: 10.1056/NEJM200103153441101. https://pubmed.ncbi.nlm.nih.gov/11248153/ [cited 2021 Jul 20] 783–92. Available from: [DOI] [PubMed] [Google Scholar]
- 7.Piccart-Gebhart M.J., Procter M., Leyland-Jones B., Goldhirsch A., Untch M., Smith I., et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. New Engl J Med [Internet] 2005 Oct 20;353(16) doi: 10.1056/NEJMoa052306. https://pubmed.ncbi.nlm.nih.gov/16236737/ [cited 2021 Jul 20] 1659–72. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Joensuu H., Kellokumpu-Lehtinene P.L., Bono P., Alanko T., Kataja V., Aaola R., et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. New Engl J Med [Internet] 2006 Feb 23;354(8) doi: 10.1056/NEJMoa053028. https://pubmed.ncbi.nlm.nih.gov/16495393/ [cited 2021 Jul 20] 809–20. Available from: [DOI] [PubMed] [Google Scholar]
- 9.Romonr E.H., Perez E.A., Bryant J., Suman V.J., Geyer C.E., Davidson N.E., et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. New Engl J Med [Internet] 2005 Oct 20;353(16) doi: 10.1056/NEJMoa052122. https://pubmed.ncbi.nlm.nih.gov/16236738/ [cited 2021 Jul 20] 1673–84. Available from: [DOI] [PubMed] [Google Scholar]
- 10.Gianni L., Eiermann W., Semiglazov V., Lluch A., Tjulandin S., Zambetti M., et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol [Internet] 2014;15(6) doi: 10.1016/S1470-2045(14)70080-4. https://pubmed.ncbi.nlm.nih.gov/24657003/ [cited 2021 Jul 20] 640–7. Available from: [DOI] [PubMed] [Google Scholar]
- 11.Baselga J., Cortes J., Sung-Bae K., Im S.A., Hegg E., Im Y.H., et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. New Engl J Med [Internet] 2012 Jan 12;366(2) doi: 10.1056/NEJMoa1113216. https://pubmed.ncbi.nlm.nih.gov/22149875/ [cited 2021 Jul 20] 109–19. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheuer W., Friess T., Burtscher H., Bossenmaier B., Endl J., Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res [Internet] 2009 Dec 15;69(24) doi: 10.1158/0008-5472.CAN-08-4597. https://pubmed.ncbi.nlm.nih.gov/19934333/ [cited 2021 Jul 20] 9330–6. Available from: [DOI] [PubMed] [Google Scholar]
- 13.Gianni L., Pienkowski T., Im Y.H., Roman L., Tseng L.M., Liu M.C., et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol [Internet] 2012 Jan;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. https://pubmed.ncbi.nlm.nih.gov/22153890/ [cited 2021 Jul 20] Available from: [DOI] [PubMed] [Google Scholar]
- 14.Gianni L., Pienkowski T., Im Y.H., Tseng L.M., Liu M.C., Lluch A., et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol [Internet] 2016 Jun 1;17(6):791–800. doi: 10.1016/S1470-2045(16)00163-7. https://pubmed.ncbi.nlm.nih.gov/27179402/ [cited 2021 Jul 20] Available from: [DOI] [PubMed] [Google Scholar]
- 15.Schneeweiss A., Chia S., Harvey V., Eniu A., Hegg R., et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol : Off J Eur Soc Med Oncol [Internet] 2013 Sep;24(9) doi: 10.1093/annonc/mdt182. https://pubmed.ncbi.nlm.nih.gov/23704196/ [cited 2021 Jul 20] 2278–84. Available from: [DOI] [PubMed] [Google Scholar]
- 16.Swaim S.M., Ewe M.S., Viale G., Delaloge S., Ferrero J.-M., Verill M., et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol : Off J Eur Soc Med Oncol [Internet] 2018 Mar 1;29(3) doi: 10.1093/annonc/mdx773. https://pubmed.ncbi.nlm.nih.gov/29253081/ [cited 2021 Jul 20] 646–53. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurvitz S.A., Martin M., Symmans W.F., Jung K.H., Huang C.S., Thompson A.M., et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol [Internet] 2018 Jan 1;19(1) doi: 10.1016/S1470-2045(17)30716-7. https://pubmed.ncbi.nlm.nih.gov/29175149/ [cited 2021 Jul 20] 115–26. Available from: [DOI] [PubMed] [Google Scholar]
- 18.von Minckwitz G., Procter M., de Azambruja E., Zardavas D., Benynes M., Viale G., et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. New Engl J Med [Internet] 2017 Jul 13;377(2) doi: 10.1056/NEJMoa1703643. https://pubmed.ncbi.nlm.nih.gov/28581356/ [cited 2021 Jul 20] 122–31. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lammers P., Criscitiello C., Curigliano G., Jacobs I. Pharmaceuticals; Basel, Switzerland: 2014 Sep 17. Barriers to the use of trastuzumab for HER2+ breast cancer and the potential impact of biosimilars: a physician survey in the United States and emerging markets.https://pubmed.ncbi.nlm.nih.gov/25232798/ [Internet] [cited 2021 Jul 20];7(9):943–53. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biosimilar medicines . cited 2021 Jul 20. Overview | European medicines agency [internet]https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview Available from: [Google Scholar]
- 21.Jeong S.A., Choi J.M., Park J.M., Lee J.Y., Lee S.J., Lee S.Y., et al. Mechanism of action of the trastuzumab biosimilar CT-P6. Expet Opin Biol Ther [Internet] 2019 Oct 3;19(10) doi: 10.1080/14712598.2019.1554052. https://pubmed.ncbi.nlm.nih.gov/30541352/ [cited 2021 Jul 20] 1085–95. Available from: [DOI] [PubMed] [Google Scholar]
- 22.Esteva F.J., Baranu Y.V., Baryash V., Manikhas A., Moiseyenko V., Dzagnidze G., et al. Efficacy and safety of CT-P6 versus reference trastuzumab in HER2-positive early breast cancer: updated results of a randomised phase 3 trial. Cancer Chemother Pharmacol [Internet] 2019 Oct 19;84(4) doi: 10.1007/s00280-019-03920-4. https://pubmed.ncbi.nlm.nih.gov/31428820/ [cited 2021 Jul 20] 839–47. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stebbing J., Baranu Y., Baryash V., Manikhas A., Moiseyenko V., Dzagnidze G., et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: a randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol [Internet] 2017 Jul 1;18(7) doi: 10.1016/S1470-2045(17)30434-5. https://pubmed.ncbi.nlm.nih.gov/28592386/ [cited 2021 Jul 20] 917–28. Available from: [DOI] [PubMed] [Google Scholar]
- 24.Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmak N., et al. Lancet; London, England): 2014. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis.https://pubmed.ncbi.nlm.nih.gov/24529560/ [Internet] [cited 2021 Jul 20];384(9938):164–72. Available from: [DOI] [PubMed] [Google Scholar]
- 25.O'Sullivan B., Brieeleu J., Byrd D., Bosman F., Kehoe S., Kossary C., et al. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol [Internet] 2017 Jul 1;18(7) doi: 10.1016/S1470-2045(17)30438-2. https://pubmed.ncbi.nlm.nih.gov/28677562/ [cited 2021 Jul 20] 849–51. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf A.C., Hammond M.E.H., Allison K.H., Harvey B.E., Mangu P.B., Barlett J.S., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol : Off J Am Soc Clin Oncol [Internet] 2018 Jul 10;36(20) doi: 10.1200/JCO.2018.77.8738. https://pubmed.ncbi.nlm.nih.gov/29846122/ [cited 2021 Jul 20] 2105–22. Available from: [DOI] [PubMed] [Google Scholar]
- 27.Chou T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res [Internet] 2010 Jan 15;70(2) doi: 10.1158/0008-5472.CAN-09-1947. https://pubmed.ncbi.nlm.nih.gov/20068163/ [cited 2021 Jul 20] 440–6. Available from: [DOI] [PubMed] [Google Scholar]
- 28.Ruiz de Porras V., Bystrup S., Cabrero-de Las Heras S., Musulen E., Palomero L., Alonso M.H., et al. Tumor expression of cyclin-dependent kinase 5 (Cdk 5) is a prognostic biomarker and predicts outcome of oxaliplatin-treated metastatic colorectal cancer patients. Cancers [Internet] 2019 Oct 1;11(10) doi: 10.3390/cancers11101540. https://pubmed.ncbi.nlm.nih.gov/31614664/ [cited 2021 Sep 30] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaefer G., Fitzpatrick V.D., Sliwkopwski M.X. Gamma-heregulin: a novel heregulin isoform that is an autocrine growth factor for the human breast cancer cell line, MDA-MB-175. Oncogene [Internet] 1997;15(12) doi: 10.1038/sj.onc.1201317. https://pubmed.ncbi.nlm.nih.gov/9333014/ [cited 2021 Jul 20] 1385–94. Available from: [DOI] [PubMed] [Google Scholar]
- 30.Bae S.J., Kim J.H., Ahn S.G., Jeung H.-C., Sohn J., Kim G.M., et al. Real-world clinical outcomes of biosimilar trastuzumab (CT-P6) in HER2-positive early-stage and metastatic breast cancer. Front Oncol. 2021 Jun 4:2110. doi: 10.3389/fonc.2021.689587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin M., Holmes F.A., Ejlertsen B., Delaloge S., Moy B., Iwata H., et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12) doi: 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 32.Lvkowitz G., Oved S., Klapper L.N., Harari D., Lavi S., Sela M., et al. c-Cbl is a suppressor of the neu oncogene. J Biol Chem [Internet] 2000 Nov 10;275(45) doi: 10.1074/jbc.M002661200. https://pubmed.ncbi.nlm.nih.gov/10940298/ [cited 2021 Jul 20] 35532–9. Available from: [DOI] [PubMed] [Google Scholar]
- 33.Valabrega G., Montemurro F., Sarotto I., Petrelli A., Rubini P., Tacchetti C., et al. TGFalpha expression impairs Trastuzumab-induced HER2 downregulation. Oncogene [Internet] 2005 Apr 21;24(18) doi: 10.1038/sj.onc.1208478. https://pubmed.ncbi.nlm.nih.gov/15735715/ [cited 2021 Jul 20] 3002–10. Available from: [DOI] [PubMed] [Google Scholar]
- 34.Shi Y., Fan X., Deng H., Brezski R.J., Rycyzyn M., Jordan R.E., et al. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcγ receptors on macrophages. J Immunol (Baltim Md : 1950) [Internet] 2015 May 1;194(9) doi: 10.4049/jimmunol.1402891. https://pubmed.ncbi.nlm.nih.gov/25795760/ [cited 2021 Jul 20] 4379–86. Available from: [DOI] [PubMed] [Google Scholar]
- 35.Maadi H., Nami B., Tong J., Li G., Wang Z. The effects of trastuzumab on HER2-mediated cell signaling in CHO cells expressing human HER2. BMC Cancer [Internet] 2018 Mar 1;18(1) doi: 10.1186/s12885-018-4143-x. https://pubmed.ncbi.nlm.nih.gov/29490608/ [cited 2021 Jul 20] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fehrenbacher L., Cecchini R.S., Geyer C.E., Rastogi P., Costantino J.P., Atkins J.N., et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol : Off J Am Soc Clin Oncol [Internet] 2020 Feb 10;38(5) doi: 10.1200/JCO.19.01455. https://pubmed.ncbi.nlm.nih.gov/31821109/ [cited 2022 Jan 12] 444–53. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gianni L., Lladó A., Bianchi G., Cortes J., Kellokumpu-Lehtinen P.L., Cameron D.A., et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol : Off J Am Soc Clin Oncol [Internet] 2010 Mar 1;28(7) doi: 10.1200/JCO.2009.24.1661. https://pubmed.ncbi.nlm.nih.gov/20124183/ [cited 2022 Jan 12] 1131–7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pegram M.D., Tan-Chiu E., Miller K., Rugo H.S., Yardley D.A., Liv S., et al. A single-arm, open-label, phase 2 study of MGAH22 (margetuximab) [fc-optimized chimeric anti-HER2 monoclonal antibody (mAb)] in patients with relapsed or refractory advanced breast cancer whose tumors express HER2 at the 2+ level by immunohistochemistry and lack evidence of HER2 gene amplification by FISH. 2014 May 20;32(15_suppl) https://doiorg/101200/jco20143215_suppltps671 TPS671–TPS671. [Google Scholar]
- 39.Burris H.A., Rugo H.S., Vukelja S.J., Vogel C.L., Borson R.A., Limentani S., et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol : Off J Am Soc Clin Oncol [Internet] 2011 Feb 1;29(4):398–405. doi: 10.1200/JCO.2010.29.5865. https://pubmed.ncbi.nlm.nih.gov/21172893/ [cited 2022 Jan 12] Available from: [DOI] [PubMed] [Google Scholar]
- 40.Schneeweiss A., Park-Simon T.W., Albanell J., Lassen U., Cortés J., Dieras V., et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Investig New Drugs [Internet] 2018 Oct 1;36(5) doi: 10.1007/s10637-018-0562-4. https://pubmed.ncbi.nlm.nih.gov/29349598/ [cited 2022 Jan 12] 848–59. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modi S., Saura C., Yamashita T., Park Y.H., Kim S.-B., Tamura K., et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. New Engl J Med [Internet] 2020 Feb 13;382(7) doi: 10.1056/NEJMoa1914510. https://pubmed.ncbi.nlm.nih.gov/31825192/ [cited 2021 Jul 20] 610–21. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banerji U., van Herpen C.M.L., Saura C., Thistlethwaite F., Lord S., Moreno V., et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol [Internet] 2019 Aug 1;20(8) doi: 10.1016/S1470-2045(19)30328-6. https://pubmed.ncbi.nlm.nih.gov/31257177/ [cited 2021 Jul 20] 1124–35. Available from: [DOI] [PubMed] [Google Scholar]
- 43.Sedykh S.E., Prinz V.V., Buneva V.N., Nevinsky G.A. Drug Design, Development and Therapy [Internet]; 2018 Jan 22. Bispecific antibodies: design, therapy, perspectives; pp. 12–195. [cited 2021 Jul 20] Available from:/pmc/articles/PMC5784585/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biosimilars in the United States 2020–2024 . cited 2021 Oct 1. IQVIA [internet]https://www.iqvia.com/insights/the-iqvia-institute/reports/biosimilars-in-the-united-states-2020-2024 Available from: [Google Scholar]
- 45.Backwell K., Gligorov J., Jacobs I., Twelves C. The global need for a trastuzumab biosimilar for patients with HER2-positive breast cancer. Clin Breast Cancer [Internet] 2018 Apr 1;18(2):95–113. doi: 10.1016/j.clbc.2018.01.006. https://pubmed.ncbi.nlm.nih.gov/29525430/ [cited 2021 Oct 1] Available from: [DOI] [PubMed] [Google Scholar]
- 46.Cesarec A., Likic R. Budget impact analysis of biosimilar trastuzumab for the treatment of breast cancer in Croatia. Appl Health Econ Health Pol [Internet] 2017 Apr 1;15(2) doi: 10.1007/s40258-016-0285-7. https://pubmed.ncbi.nlm.nih.gov/27730538/ [cited 2021 Oct 1] 277–86. Available from: [DOI] [PubMed] [Google Scholar]
- 47.Povero M., Pradelli L. Funding innovation thanks to anti-TNF-α biosimilars uptake: the economic impact in Italy. Farmecon Health Econ Therapeut Pathw. 2020 May 7;21(1) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.