Abstract
Studying the composition of a certain food is not enough to predict its health benefits. Research over the past decades has decisively strengthened the notion that any putative health benefit is best related to the fraction of compounds transferred from ingested foods into the body since the absorption may be incomplete after oral consumption. In other words, the bioavailability of food components is crucial information. Therefore, a variety of in vitro models have been developed to predict their bioaccessibility and bioavailability in the most diverse food matrices and food products. These models can also be applied to study the impact of several endogenous or exogenous factors on the bioaccessibility and bioavailability of nutrients and bioactive compounds, guiding nutrition and food scientists, technologists, and engineers towards the development of strategies to optimize the positive impact of the diet on well-being and quality of life. While bioavailability is ideally examined in human volunteers, in vitro digestion methods, as well as intestinal absorption and microphysiological models, simulate human physiological conditions. Additionally, in vitro methods are alternatives to offset ethical, economical, and experimental limitations associated with in vivo studies conducted either with individuals or animals. This graphical review draws parallels between in vitro models mimicking digestion processes, uptake, absorption, metabolism, and distribution of dietary compounds and human physiology.
Keywords: In vitro digestion, Nutrient absorption, Transepithelial transport, Nutrient distribution, Microphysiological systems, Intestinal cell models
Highlights
-
•
Bioavailability determination in humans is considered the “gold standard”.
-
•
Static, semi-dynamic, and dynamic in vitro digestion simulate physiological conditions.
-
•
Bioaccessibility is the fraction of compounds that become accessible for absorption.
-
•
Changes in the gastrointestinal tract occur since the gestational period.
-
•
Multi-organs-on-a-chip can simulate the systemic distribution and organ interactions.
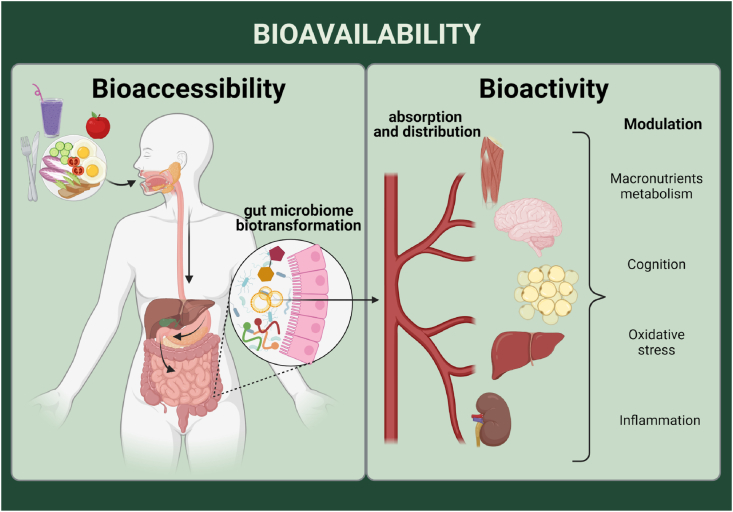
Figure 1.Background and definitions. Bioaccessibility measures the proportion of a compound consumed in a meal that is released from the food matrix during digestion in the luminal content and is accessible for absorption in the small intestine or biotransformed by the gut microbiota. Bioactivity represents the activity of the absorbed compounds or their metabolites in the metabolic pathways, resulting in biological effects on the body. Lastly, bioavailability refers to the amount of compounds that completes the route passing through the digestive tract, is absorbed, and reaches the target tissues in the intact or metabolized form to perform its bioactivity or to be stored. Created with BioRender.com.
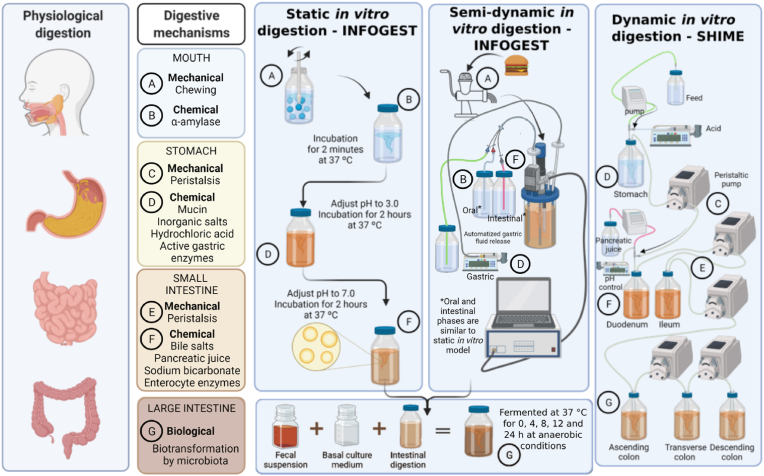
Figure 2.Comparison between physiological human digestion and in vitro digestion (IVD) methods. Diverse IVD models are described in the literature, with variable degrees of complexity, automation, and experimental conditions for digestion. To harmonize these conditions and facilitate the result comparison throughout the scientific community, the INFOGEST network (an international network of excellence on the fate of food in the gastrointestinal tract) has proposed a standardized, consensus protocol for both static and semi-dynamic simulated digestion, largely adopted by research groups worldwide and represented in this figure (Minekus et al., 2014; Brodkorb et al., 2019; Mulet-Cabero et al., 2020). These methods simulate the physiological conditions of the oral, gastric, and small intestinal phases of digestion. In the static method, these steps are all carried out or set up by the analyst, whereas in the semi-dynamic method the gastric phase is dynamic, with the pH variation occurring gradually by using a pump and an automatic titrator (Brodkorb et al., 2019; Mulet-Cabero et al., 2020). The digestion process starts with the oral phase that comprises mechanical (chewing) and chemical (α-amylase activity) steps. A and B - The chewing in static and semi-dynamic methods is represented by the maceration of food and the chemical step by the enzyme addition. C and D - In the gastric phase, simulated fluid containing inorganic salts, pepsin, and gastric lipase is added, and the pH is adjusted to 3. The digestive mixture is incubated at 37 °C under shaking to simulate the peristalsis. E and F - The pH is adjusted to 7, and pancreatic enzymes and bile are added, the digestive mixture is incubated at 37 °C under shaking. The chyme obtained after completion of the simulated duodenal phase can be used to estimate the bioaccessibility of compounds of interest, their uptake and transport in cellular models, or for further bioactivity assays. Moreover, it is possible to proceed with the simulation of G - colonic fermentation, in which fecal material from healthy donors is added as a source of gut microbiota (Mosele et al., 2016). Besides the static and semi-dynamic methods, in vitro digestion can be conducted by using dynamic models in either mono- or multi-compartmental configurations that take advantage of the automatized regulation of the flux of simulated digestive fluids, enzymes, and pH adjustments (Dupont et al., 2019). The schematic representation of the Simulator of the Human Intestinal Microbial Ecosystem from Ghent University, Belgium (SHIME®) is shown in the figure as an example of a dynamic model that includes the colonic fermentation stage. This system allows the inoculation of the microbiota from specific population groups adapted to different conditions and monitoring specific colon regions (Verhoeckx et al., 2015). Although the dynamic models better replicate the in vivo digestion, the need for specific instrumentation, their complexity, and the associated costs limit their use by many research groups (Brodkorb et al., 2019). Created with BioRender.com.
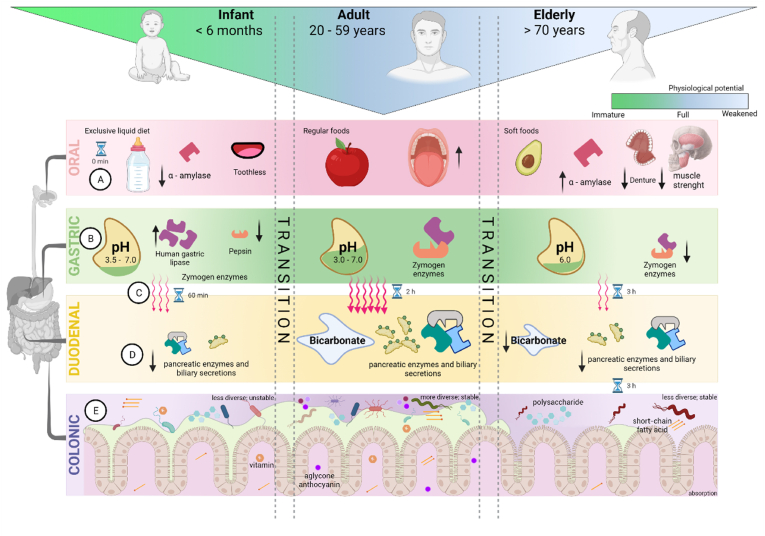
Figure 3.Changes in gastrointestinal function throughout the lifespan to be considered when developing human population-specific IVD models. The gastrointestinal tract of an individual gradually changes since the gestational period but three main life stages with specific characteristics can be defined: early age, adult, and elderly (Bourlieu et al., 2014; Shani-Levi et al., 2017). For infants, the oral phase (A) of digestion is almost negligible due to the exclusive liquid diet (breast milk or similar) of very short residence time, but also due to the characteristic low enzyme activity of salivary α-amylase (Bourlieu et al., 2014). In adults, the oral phase is complete, and during aging, there is a reduction in salivary flow and an increase in α-amylase content (Shani-Levi et al., 2017), combined with difficulty chewing due to weakened dentition (Nomura et al., 2020) and reduced muscle strength (Shani-Levi et al., 2017). The gastric phase (B) of infants is characterized by the high pH, low pepsin activity, and slow gastric fluid secretion (Bourlieu et al., 2014), while human gastric lipase is already fully active (Shani-Levi et al., 2017). The release rate of gastric secretion in adults decreases gradually with aging (Shani-Levi et al., 2017); thus, the stomach pH of elderlies is elevated. Gastric emptying (C) is modulated by food characteristics and related to gastrointestinal motility, which develops from the first weeks of life (Shani-Levi et al., 2017), and is diminished in older people, extending the emptying time (Blechman and Gelb, 1999). The contribution of the duodenal phase (D) is minimal in infants due to the immature pancreatic and biliary secretagogues in this stage of life (Bourlieu et al., 2014). The gut bacteriota (GB) undergoes ecological changes over age (E) that directly impact the bioaccessibility and bioactivity, mainly of functional compounds (Danneskiold-Samsøe et al., 2018). In neonates, GB is less diverse and quite unstable; in adulthood, GB is stable and shows greater diversity; while in senescence, we observe low diversity and stability of GB (DeJong et al.2020). The ecological diversity addresses a bacterial signature for each life stage generating specific metabolites (short-chain fatty acids, aglycone phenolic compounds, and vitamins) to be absorbed, so nutrients and non-nutrients can undergo divergent biotransformation and end routes (Danneskiold- Samsøe et al., 2018). Created with BioRender.com.
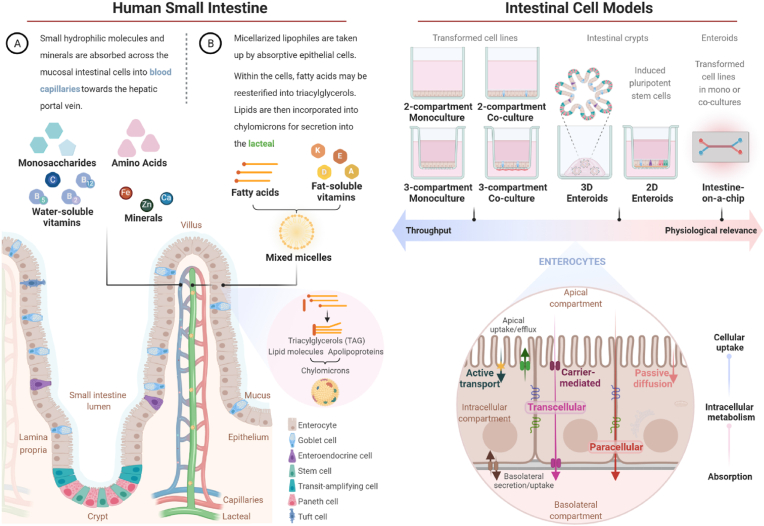
Figure 4.Intestinal uptake, metabolism, and absorption of nutrients in vivo and intestinal cell models of varying degrees of complexity, potential throughput, and physiological relevance. After food digestion, water-soluble (A) and micellarized fat-soluble (B) nutrients that are bioaccessible in the intestinal lumen diffuse through the mucin layer for uptake across the apical membrane of enterocytes, absorptive epithelial cells lining the mucosa of the small intestine. Once within the cells, nutrients may be utilized, metabolized, effluxed back into the lumen, and a portion of these compounds is transported across the basolateral membrane of enterocytes for absorption. Amino acids, monosaccharides, minerals, water-soluble vitamins, and their metabolites are absorbed into blood capillaries to be transported to the liver, whereas fat-soluble molecules are incorporated into chylomicrons and secreted into lacteals, central lymphatic vessels of villi (Wielen et al., 2017; Ko et al., 2020; Koepsell, 2020). Intestinal cell culture models vary in the extent to which they recapitulate the in vivo conditions. They range from technically simpler two- and three-compartment systems seeded with a monolayer of mono or co-cultured transformed intestinal cells through the novel 2D and 3D self-organizing enteroids, generated from crypt-derived or induced pluripotent stem cells, and gut chip devices containing either transformed cells or enteroids grown under dynamic conditions (Rodrigues and Failla, 2021). In the schematic, monolayers of enterocyte-like Caco-2 cells, a transformed cell line originating from a human colonic adenocarcinoma and established as the most widely used intestinal cell model, are represented in monocultures or co-cultured with the also transformed goblet-like HT-29 cells. Monolayers of intestinal cells can be grown adhered to the bottom of culture vessels, in a two-compartment configuration, or on semi-permeable membrane inserts to generate three-compartment (apical, cellular, and basolateral) systems. The access to the basolateral region of the monolayer allows the study of the absorption of dietary compounds. In contrast to the classical cultures of transformed cells adhered to static, flat surfaces, enteroids and gut chips better recapitulate the morphology and the dynamic microenvironment of the human intestine, respectively, and are expected to provide results with improved predictive reliability (Rodrigues and Failla, 2021). In vitro, the compounds and their digested products are primarily delivered to the apical compartment of the cellular model in mixtures of cell culture medium with either soluble or micellar fractions obtained from the chyme upon completion of in vitro digestion, as well as with extracts or solutions containing compounds of interest, or artificial micelles produced in the laboratory. As in vivo, nutrients are apically taken up by absorptive enterocytes through active or passive transport mechanisms and intact or metabolized transported to the basolateral compartment. Nonetheless, differentiated human Caco-2 parenteral cells and clones such as TC7 can differ from normal enterocytes in the expression of some transmembrane transporters and enzymes (Sambuy et al., 2005). It is important to note that the uptake into the cellular compartment of these systems does not necessarily mean that the compound of interest is absorbed, which requires basolateral transport. Besides passive and active transcellular transport mechanisms, absorption of some nutrients and ions can occur through paracellular diffusion. Created with BioRender.com.
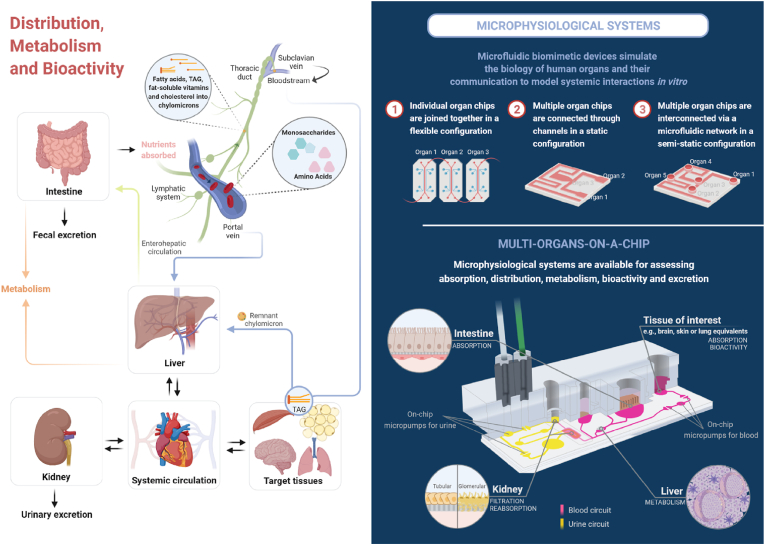
Figure 5.Overview of nutrient distribution between systemic circulation and tissues after intestinal absorption, in vivo and microphysiological systems. Absorbed hydrophilic molecules and ions are transported freely or associated with transporters in the bloodstream directly to the liver. In contrast, lymph carries chylomicrons containing lipophilic compounds, moving upwards through the lymphatic system before eventually entering the bloodstream at the connection of the thoracic duct with the left subclavian vein, bypassing the liver. Chylomicrons flow through the venous system delivering triacylglycerides from diet to peripheral tissues, being converted by lipoprotein lipase into chylomicron remnants that are finally taken up by the liver. This central organ regulates the metabolic homeostasis of all ingested nutrients, storing and distributing these compounds and their phase I or phase II metabolites appropriately (Ghanemi et al., 2018). The cargo of cholesterol and fat-soluble vitamins is incorporated into VLDL (Very Low-Density Lipoprotein) particles for secretion into the systemic circulation to be delivered to target tissues, whereas a portion of these compounds may be effluxed to bile (enterohepatic pathway) (Ko et al., 2020). From the bloodstream, nutrients or conjugated products reach target tissues and can be either utilized to exert their bioactivity or stored to be mobilized upon necessity, and later, they are excreted by the kidneys in the urine. Compounds from consumed foods that are either not absorbed by the small intestinal epithelium, effluxed back to the lumen, or excreted via the bile into the duodenum may be transferred to the large intestine to be excreted into feces as gut microbiota end-products or unconjugated forms (Van der Wielen et al., 2017; Danneskiold-Samsøe et al., 2019). Nonetheless, some non-absorbable compounds in the small intestine are biotransformed by the host colonic microbiota to metabolites that are possibly used in situ or absorbed from the colon epithelial cells (Danneskiold-Samsøe et al., 2019). The coupling of different organ chips on multi-organs-on-a-chip platforms or microphysiological systems (MPS), simulating the interface and communication among barrier and parenchymal tissues and the systemic circulation, has provided new opportunities to study not only the absorption but also the distribution, metabolism, and bioactivity of dietary compounds in vitro. It is important to emphasize that MPS is a new tool in the initial development stage and available only for some specific research groups. MPS may be established through (1) the combination of individual chips, each one modeling a distinct organ, via capillary tubing into a flexible platform (Herland et al., 2020); (2) the connection of multiple organs into a static microfluidic board of permanent configuration with a fluid stream (Kimura et al., 2015), and (3) a semi-static, reconfigurable platform, consisting of a single plate in which individual and possibly pre cultured organ chips are accommodated and connected via microfluidic channels (Maschmeyer et al., 2015; Edington et al., 2018). One such example of the latter approach is illustrated in the lower panel with the schematic representation of the Humimic Chip4 (TissUse GmbH), a commercially available MPS that enables the integration of up to four different organs represented herein by the intestine, liver, and kidney, in addition to another tissue of interest (Marx et al., 2020) [https://www.tissuse.com/en/humimic/chips]. Organ chambers indicated with zoom may support monolayer cell cultures to organotypic models, including organoids and spheroids. They are linked via a microfluidic network with built-in micropumps and composed of two separated circuits simulating the circulatory (in pink) and urinary (in yellow) systems. These circuits, in their turn, are connected through the tubular reabsorption and glomerular filtration units of the kidney chip that consist of renal tubule cells and podocytes, respectively, both illustrated in the enlarged detail. Similar platforms replicating key aspects of the intestinal barrier, metabolic activity, blood circulation, and the dynamic molecular transport against gradients are being developed by different research groups as particularly useful tools for the determination of ADME (absorption, distribution, metabolism, and excretion) profiles of compounds of interest. Whereas applications of these innovative models to address bioavailability questions are still concentrated in pharmaceutical research and development, we highlight that there are great possibilities for their utilization to advance investigations in the context of Nutrition and Food Science. Created with BioRender.com. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
CRediT authorship contribution statement
Daniele Bobrowski Rodrigues: Conceptualization, Writing – original draft. Marcella Camargo Marques: Conceptualization, Writing – original draft. Adriele Hacke: Conceptualization, Writing – original draft. Paulo Sérgio Loubet Filho: Conceptualization, Writing – original draft. Cinthia Baú Betim Cazarin: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. Lilian Regina Barros Mariutti: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. AH thanks CNPq for the PhD Scholarship.
References
- Blechman M.B., Gelb A.M. Aging and gastrointestinal physiology. Clin. Geriatr. Med. 1999;15(3):429–438. [PubMed] [Google Scholar]
- Bourlieu C., Ménard O., Bouzerzour K., Mandalari G., Macierzanka A., Mackie A.R., et al. Specificity of infant digestive conditions: some clues for developing relevant in vitro models. Crit. Rev. Food Sci. Nutr. 2014;54(11):1427–1457. doi: 10.1080/10408398.2011.640757. [DOI] [PubMed] [Google Scholar]
- Brodkorb A., Egger L., Alminger M., Alvito P., Assunção R., Ballance S., et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019;14(4):991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- Danneskiold-Samsøe N.B., Dias de Freitas Queiroz Barros H., Santos R., Bicas J.L., Cazarin C.B.B., Madsen L., et al. Interplay between food and gut microbiota in health and disease. Food Res. Int. 2019;115:23–31. doi: 10.1016/j.foodres.2018.07.043. [DOI] [PubMed] [Google Scholar]
- DeJong E.N., Surette M.G., Bowdish D.M.E. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe. 2020;28(2):180–189. doi: 10.1016/j.chom.2020.07.013. [DOI] [PubMed] [Google Scholar]
- Dupont D., Alric M., Blanquet-Diot S., Bornhorst G., Cueva C., Deglaire A., et al. Can dynamic in vitro digestion systems mimic the physiological reality? Crit. Rev. Food Sci. Nutr. 2019;59(10):1546–1562. doi: 10.1080/10408398.2017.1421900. [DOI] [PubMed] [Google Scholar]
- Edington C.D., Chen W.L.K., Geishecker E., Kassis T., Soenksen L.R., Bhushan B.M., et al. Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci. Rep. 2018;8(1):4530. doi: 10.1038/s41598-018-22749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanemi A., Yoshioka M., St-Amand J. Broken energy homeostasis and obesity pathogenesis: the surrounding concepts. J. Clin. Med. 2018;7(11):453. doi: 10.3390/jcm7110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herland A., Maoz B.M., Das D., Somayaji M.R., Prantil-Baun R., Novak R., et al. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat Biomed Eng. 2020;4(4):421–436. doi: 10.1038/s41551-019-0498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Ikeda T., Nakayama H., Sakai Y., Fujii T. An on-chip small intestine-liver model for pharmacokinetic studies. J. Lab. Autom. 2015;20(3):265–273. doi: 10.1177/2211068214557812. [DOI] [PubMed] [Google Scholar]
- Ko C.-W., Qu J., Black D.D., Tso P. Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17(3):169–183. doi: 10.1038/s41575-019-0250-7. [DOI] [PubMed] [Google Scholar]
- Koepsell H. Glucose transporters in the small intestine in health and disease. Pflügers Archiv. 2020;472(9):1207–1248. doi: 10.1007/s00424-020-02439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx U., Dehne E.-M., Hasenberg T. Patent Application Publication; 2020. Novel Microfluidic Devices Implementing Blood and Urine Circuits for Emulating Homeostatic Microphysiological System. US 2020/0392441 A1. [Google Scholar]
- Maschmeyer I., Lorenz A.K., Schimek K., Hasenberg T., Ramme A.P., Hübner J., et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015;15(12):2688–2699. doi: 10.1039/c5lc00392j. [DOI] [PubMed] [Google Scholar]
- Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., et al. A standardised static in vitro digestion method suitable for food - an international consensus. Food Funct. 2014;5(6):1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- Mosele J.I., Macià A., Romero M.P., Motilva M.J. Stability and metabolism of Arbutus unedo bioactive compounds (phenolics and antioxidants) under in vitro digestion and colonic fermentation. Food Chem. 2016;201:120–130. doi: 10.1016/j.foodchem.2016.01.076. [DOI] [PubMed] [Google Scholar]
- Mulet-Cabero A.-I., Egger L., Portmann R., Ménard O., Marze S., Minekus M., et al. A standardised semi-dynamic in vitro digestion method suitable for food – an international consensus. Food Funct. 2020;11(2):1702–1720. doi: 10.1039/c9fo01293a. [DOI] [PubMed] [Google Scholar]
- Nomura Y., Kakuta E., Okada A., Otsuka R., Shimada M., Tomizawa Y., et al. Effects of self-assessed chewing ability, tooth loss and serum albumin on mortality in 80-year-old individuals: a 20-year follow-up study. BMC Oral Health. 2020;20(1):122. doi: 10.1186/s12903-020-01113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues D.B., Failla M.L. Intestinal cell models for investigating the uptake, metabolism, and absorption of dietary nutrients and bioactive compounds. Curr. Opin. Food Sci. 2021;41:169–179. [Google Scholar]
- Sambuy Y., De Angelis I., Ranaldi G., Scarino M.L., Stammati A., Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005;21(1):1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- Shani-Levi C., Alvito P., Andrés A., Assunção R., Barberá R., Blanquet-Diot S., et al. Extending in vitro digestion models to specific human populations: perspectives, practical tools, and bio-relevant information. Trends Food Sci. Technol. 2017;60:52–63. [Google Scholar]
- van der Wielen N., Moughan P.J., Mensink M. Amino acid absorption in the large intestine of humans and porcine models. J. Nutr. 2017;147(8):1493–1498. doi: 10.3945/jn.117.248187. [DOI] [PubMed] [Google Scholar]
- Verhoeckx K., Cotter P., López-Expósito I., Kleivel C., Lea T., Mackie A., et al. Springer Open; London: 2015. The Impact of Food Bio-Actives on Gut Health: in Vitro and Ex-Vivo Models. [PubMed] [Google Scholar]