Abstract
Background
Cannabinoid type-1 receptors (CB1Rs) are expressed in primary sensory neurones, but their role in pain modulation remains unclear.
Methods
We produced Pirt-CB1R conditional knockout (cKO) mice to delete CB1Rs in primary sensory neurones selectively, and used behavioural, pharmacological, and electrophysiological approaches to examine the influence of peripheral CB1R signalling on nociceptive and inflammatory pain.
Results
Conditional knockout of Pirt-CB1R did not alter mechanical or heat nociceptive thresholds, complete Freund adjuvant-induced inflammation, or heat hyperalgesia in vivo. The intrinsic membrane properties of small-diameter dorsal root ganglion neurones were also comparable between cKO and wild-type mice. Systemic administration of CB-13, a peripherally restricted CB1/CB2R dual agonist (5 mg kg−1), inhibited nociceptive pain and complete Freund adjuvant-induced inflammatory pain. These effects of CB-13 were diminished in Pirt-CB1R cKO mice. In small-diameter neurones from wild-type mice, CB-13 concentration-dependently inhibited high-voltage activated calcium current (HVA-ICa) and induced a rightward shift of the channel open probability curve. The effects of CB-13 were significantly attenuated by AM6545 (a CB1R antagonist) and Pirt-CB1R cKO.
Conclusion
CB1R signalling in primary sensory neurones did not inhibit nociceptive or inflammatory pain, or the intrinsic excitability of nociceptive neurones. However, peripheral CB1Rs are important for the analgesic effects of systemically administered CB-13. In addition, HVA-ICa inhibition appears to be a key ionic mechanism for CB-13-induced pain inhibition. Thus, peripherally restricted CB1R agonists could have utility for pain treatment.
Keywords: cannabinoid receptor, conditional knockout, dorsal root ganglion, high-voltage activated calcium current, inflammation, pain
Editor's key points.
-
•
Cannabinoids have been proposed as clinical analgesics, and cannabinoid type-1 receptors (CB1Rs) are expressed in primary sensory neurones, but their role in pain modulation remains unclear.
-
•
Selective knockout of CB1Rs in primary sensory neurones did not alter mechanical or thermal nocifensive responses or intrinsic membrane properties, but reduced analgesia by the peripherally restricted CB1R/CB2R dual agonist CB-13.
-
•
CB-13 concentration-dependently inhibited calcium currents in small-diameter dorsal root ganglion neurones, which provides a potential mechanism for its analgesic effects.
-
•
Peripherally restricted CB1R agonists provide a translational approach to non-opioid analgesia without central cannabinoid side-effects.
Primary sensory neurones of the dorsal root ganglion (DRG) represent the major conduit through which peripheral sensory information, including noxious inputs, is transmitted to the spinal cord.1,2 About 20% of adults across the USA and Europe suffer from chronic pain,3,4 which leads to hundreds of billions of dollars in economic and societal costs.5,6 Pharmacologic treatments that target mu-opioid receptors (MORs) have an unfavourable risk/benefit balance and significant limitations owing to central adverse effects.7,8 Hence, interest has been growing in finding effective non-opioid pharmacologic targets for pain management.
The endocannabinoid system is a promising pharmacological target for new pain management strategies9; in particular, development of peripherally acting cannabinoid receptor agonists10 may circumvent negative central effects and increase therapeutic viability. The cannabinoid type-1 receptor (CB1R) is highly expressed in neurones of pain-related regions in the central and peripheral nervous systems,11 including in the DRG.12,13 Yet, the roles of peripheral CB1R signalling in various pain conditions are only partially known, and previous findings have been contradictory. Several studies have shown a tonic peripheral endocannabinergic inhibition of nociceptive pain and inflammatory pain,13,14 whereas others support a pro-nociceptive influence or no influence at all.15,16 To ascertain whether endogenous CB1R signalling in DRG neurones tonically modulates nociceptive pain, inflammatory pain, and intrinsic excitability of DRG neurones, we developed Pirt-CB1R conditional knockout (cKO) mice for animal behavioural, pharmacological, and electrophysiological investigations. In Pirt-CB1R cKO mice, CB1R expression is selectively deleted in most DRG and trigeminal ganglion neurones because Cre recombinase is controlled by the Pirt promoter, which is expressed exclusively in >80% of primary sensory neurones, but not in any other cell type.17
In addition to endocannabinoids, synthesised CB1R agonists inhibit small-diameter DRG neurones that are mostly nociceptive.18 CB-13 is a potent CB1/CB2R dual agonist with limited brain penetration that may attenuate neuropathic mechanical hypersensitivity.10,19 The ionic mechanisms of exogenous CB1R agonist-induced neuronal inhibition may involve various ion channels and receptors, including high-voltage activated (HVA) calcium channels, TRPV1, acid-sensing ion channels, and inward-rectifying potassium channels.20, 21, 22, 23 Yet, much of the current evidence was gathered from recordings in heterologous systems rather than in native DRG neurones. Accordingly, we examined the effects of systemic CB-13 administration on nociceptive and inflammatory pain and determined the contribution of CB1R and ionic mechanisms in DRG neurones to CB-13-induced pain inhibition.
Methods
Animals
Molecular experiments used 3–6-month-old wild-type (WT) mice (C57BL/6J; The Jackson Laboratory, Bar Harbor, ME, USA) and Pirt-Cre+/–;CB1fl/fl (Pirt-CB1R cKO, or CB1R cKO) mice. Behavioural experiments were conducted with adult CB1fl/fl (CB1fl/fl WT), CB1R cKO, and Pirt-Cre+/–;Oprmfl/fl (MOR cKO) mice. DRG for electrophysiological tests were harvested from CB1fl/fl WT and CB1R cKO mice (3–5 weeks old) of both sexes. Experiments were approved by the Institutional Animal Care and Use Committee, consistent with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Analysis of genomic DNA
Polymerase chain reaction (PCR) was used to detect the presence of CB1fl/fl and Pirt-Cre+/– alleles in genomic DNA, using the following primer sequences: CB1R: 5′-GCTGTCTCTGGTCCTCTTAAA-3′ (forward) and 5′-GGTGTCACCTCTGAAAACAGA-3′ (reverse); Pirt-Cre: 5′-ATCCGTAACCTGGATAGTGAA-3′ (Cre forward), 5′-CAACTTTGTGGTACCCGAAG-3′ (Pirt forward), and 5′-TCCCTGGGACTCATGATGCT-3′ (Pirt reverse).
Conditional knockout of CB1R
Cre/LoxP recombination was used to generate Pirt-CB1R cKO mice in which CB1Rs were deleted specifically from most of primary sensory neurones. Pirt-Cre mice were provided by Xinzhong Dong (Johns Hopkins University, Baltimore, MD, USA), and CB1fl/fl mice by George Kunos (NIH, Bethesda, MD, USA) and Elisabeth Glowatzki (Johns Hopkins University). Each mouse line was backcrossed to the C57BL/6J background. Owing to the disruption of the Pirt gene in homozygous Pirt-Cre+/+ mice, which has been shown to alter TRPV1 functionality,17 Pirt-Cre+/– mice were crossed with CB1fl/fl mice to generate the CB1R cKO mice (Fig. 1a). Pirt-Cre+/– mice have no known phenotype that affects receptor function or interpretation of behavioural results, and have been used to generate other cKO mice used in studies of pain behaviour.24,25 The control WT mice expressed only CB1fl/fl. Pirt-MOR cKO mice, in which MORs were deleted specifically from primary sensory neurones, were similarly generated to be used in pharmacologic studies to examine whether efficacy of pain inhibition by systemic CB-13 is influenced by endogenous MOR signalling in DRG neurones.
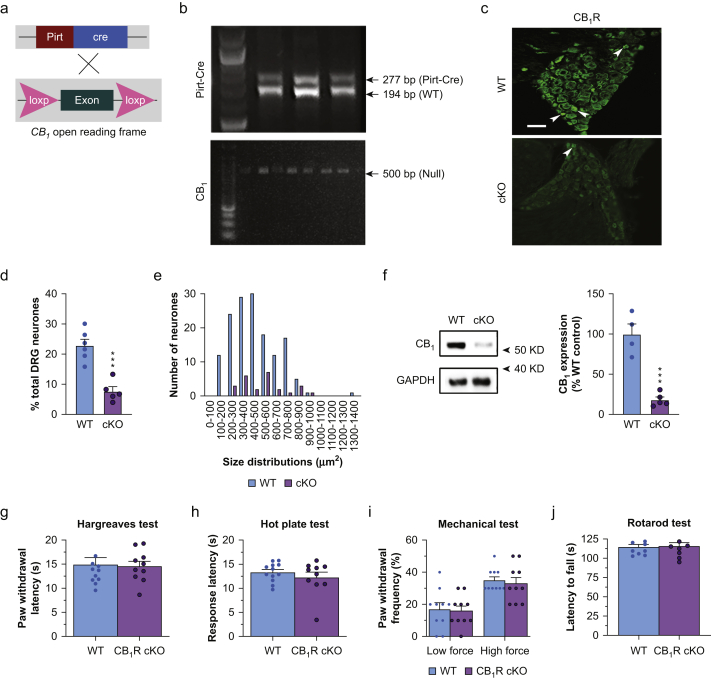
Fig 1.
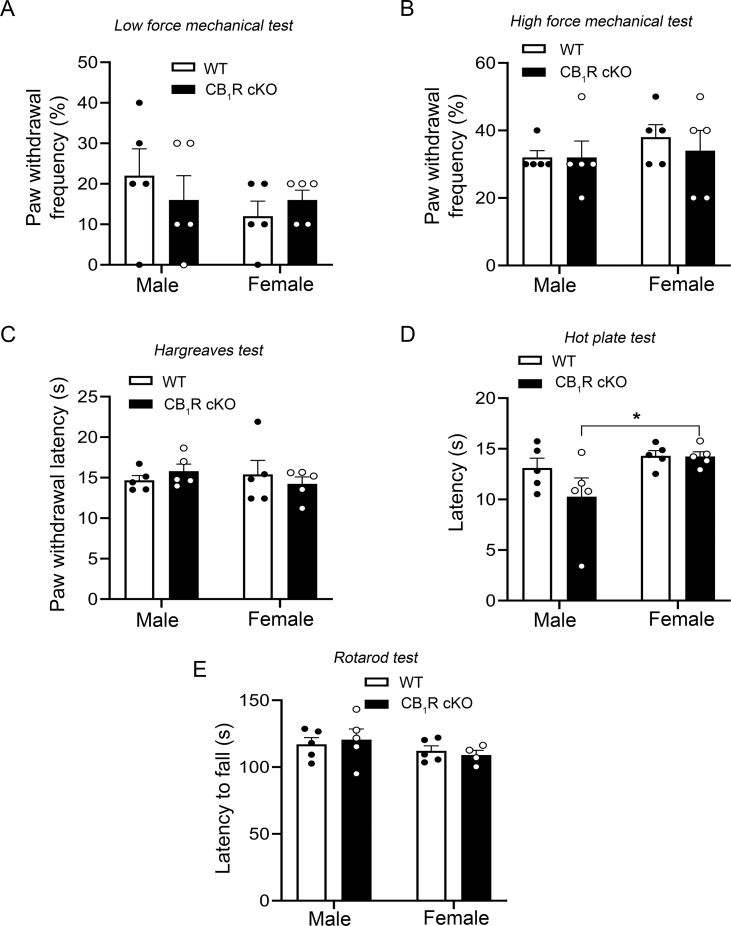
Pirt-Cre conditional knockout (cKO) of CB1R in dorsal root ganglion (DRG) neurones does not affect nociceptive thresholds or locomotor function. (a) Strategy for generating CB1R cKO mice. Pirt-Cre+/– mice and CB1fl/fl mice were intercrossed to delete CB1 expression exclusively in most primary sensory neurones. (b) Representative images of ethidium bromide-stained agarose gels show PCR genotyping of Pirt-Cre (top) and CB1 (bottom) from tail snip tissue samples. The upper band (277 bp) in the Pirt-Cre panel indicates the Pirt-Cre+ allele, and the lower band (194 bp) indicates the WT allele (Pirt gene without Cre [Pirt-Cre–]). The single band (500 bp) in the CB1 panel indicates the null gene (floxed allele [ΔCB1fl/fl]). (c) Representative images of immunofluorescence-stained DRG sections. A subset of neurones were stained with the CB1R antibody in WT mice but the staining was much less in CB1R cKO mice. Scale bar: 50 μm. (d) Quantification of CB1R-positive neurones in the L4–L6 DRGs of each group (∗∗∗P=0.0003, unpaired t-test, n=5–6 mice/group). (e) Size distribution of CB1R-positive neurones, which are predominantly small-diameter (area ≤600 μm2) in WT mice. (f) Left panel, representative Western blot images of CB1R protein expression in the DRGs of WT and CB1R cKO mice. Right panel, quantification of CB1R protein levels (∗∗∗P=0.0002, unpaired t-test, n=4–5 mice/group). (g, h) CB1R cKO and WT mice displayed similar withdrawal latency of the hind paw in response to radiant heat stimulus (g; P=0.85, Welch's t-test, n=10/group, five/sex in each group) and comparable response latencies in the hot plate test (h; P=0.42, Welch's t-test). (i) CB1R cKO and WT mice displayed similar paw withdrawal frequency (F1,36=0.023, P=0.88, two-way anova) to low force (0.07 g; P>0.99) and high force (0.4 g; P>0.99, Bonferroni's post-test) mechanical stimulation with von Frey monofilaments. (j) Latency to fall during the rotarod test was comparable between WT and CB1R cKO mice (P=0.89, Welch's t-test). Data are shown as mean (standard error of the mean). CB1R, cannabinoid type-1 receptor; cKO, conditional knockout; PCR, polymerase chain reaction; WT, wild-type; anova, analysis of variance.
Immunohistochemistry
Cryosections of lumbar DRGs (4% paraformaldehyde fixed) were immunostained with rabbit anti-CB1R antibody (1:200; 10006590; Cayman, Ann Arbor, MI, USA), with Alexa 488-conjugated goat antibody to rabbit (A-11008; Thermo Fisher Scientific, Waltham, MA, USA) as the secondary antibody (1:100). Raw confocal (TIFF) images (LSM 700; Zeiss, White Plains, NY, USA) were analysed with Fiji (NIH). The total number of neurones in each section was determined by counting both labelled and unlabelled cell bodies. Positively stained neurones had clear stomata and an increase in fluorescence intensity ≥30% of background. To quantify the neuronal cross-sectional area of DRG neurones, cells were identified by morphology with a clearly defined, dark, condensed nucleolus. Positively stained cells were chosen for cross-sectional area measurement. The somata of the labelled cells were traced manually with the Fiji ‘Freehand selection’ tool and the areas were measured. Tissues from different groups were processed together.
Western immunoblotting
Mouse DRGs were harvested and the tissues were lysed in radioimmunoprecipitation assay (RIPA) buffer (Sigma, St. Louis, MO, USA) containing protease/phosphatase inhibitor cocktail (Cell Signaling Technology, Boston, MA, USA). A standard bicinchoninic acid protein assay (Thermo Fisher Scientific) was used to determine protein concentration of RIPA lysates. Samples (20 μg) were separated on a 4–12% Bis-Tris Plus gel (Thermo Fisher Scientific) and then transferred onto a polyvinylidene difluoride membrane (Thermo Fisher Scientific). Immunoreactivity was detected by enhanced chemiluminescence (ECL; Bio-Rad, Hercules, CA, USA) after incubating the membranes with the indicated primary antibody (4°C, overnight). We used primary antibodies against CB1 receptor (1:1000; Cayman) and CB2 receptor (1:1000; Cayman). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:100 000; Millipore, Darmstadt, Germany) was used as an internal control for protein loading. ImageJ (ImageJ 1.46r) was used to quantify the intensity of immunoreactive bands of interest on autoradiograms.
Inflammatory pain model
Under isoflurane (Sigma, 2%, mixed in 2.5 L min−1 oxygen) anaesthesia, 20 μl of 1 mg ml−1 complete Freund's adjuvant (CFA; Sigma-Aldrich, St. Louis, MO, USA) was injected into the intra-plantar region of the left hind paw. Paw oedema and heat hypersensitivity were assessed at 24 and 72 h after injection. The dorsoventral paw thickness was measured with a digital calliper at the metatarsophalangeal border.
Behavioural pain tests
Before behavioural testing, animals were subjected to a 30-min acclimatisation period inside the testing room with the experimenter, to minimise the effects of the testing environment. All testing occurred between 09:00 and 17:00. Animals were randomised to treatment groups, and investigators were blinded to treatment. Sample sizes were determined based on similar protocols in previous studies.24,26
Mechanical hypersensitivity was evaluated by measuring paw withdrawal frequency (PWF) to von Frey filament stimulation.27 Briefly, low-force (0.07 g) and high-force (0.45 g) von Frey monofilaments (Stoelting Co., Wood Dale, IL, USA) were applied to the mid-plantar area of each hind paw 10 times (with 1–2 s intervals). Vertical elevation of the paw, licking, or both were considered positive withdrawal responses.
Heat nociception was examined in the hot plate test (55°C; Ugo Basile, Varese, Italy), and response latencies were recorded. Nocifensive behaviours included forepaw or hind paw withdrawal or licking, stamping, leaning posture, and jumping. The Hargreaves test was also used to assess heat hypersensitivity. Animals were placed in Plexiglas chambers positioned on a heated glass floor (30°C). Radiant heat was applied to the plantar surface of the hind paw, and paw withdrawal latency (PWL) was measured using an automatic plantar stimulator meter (IITC model 390; IITC Life Sciences, Woodland Hills, CA, USA). Both procedures were repeated three times (5 min intervals) and data were averaged for analysis. A cut-off time of 30 s was used to prevent sensitisation and tissue damage. Behavioural results were analysed together after initial sex comparisons of nociceptive thresholds showed that there were no significant differences in the tests that would be used in subsequent pain model/drug testing (Supplementary Fig. S2).
Motor function evaluation
Locomotor activity was evaluated with open field tests. After a 30-min acclimatisation period inside the testing room, one mouse per trial was placed in the corner of a Plexiglass arena (50 × 50 × 38 cm) equipped with a video tracking system (SMART 3; Panlab Harvard Apparatus; Barcelona, Spain). Mice were then allowed to freely explore for 10 min. The distance that the mice travelled was tracked and recorded by the SMART 3 software. The testing arena was cleaned with 70% ethanol before and after each trial. To assess the effects of CB-13 administration on locomotive activity, after the mice were acclimated to the testing room, CB-13 (5 mg kg−1, s.c.) or vehicle was administered between the shoulders under isoflurane anaesthesia. Mice were then allowed to recover from anaesthesia in their home cage for 30 min before open field testing.
Motor coordination, ataxia, and equilibrium were evaluated with rotarod tests (Ugo Basile). Before training or testing sessions, animals were first acclimatised to the testing room for 30 min. For training sessions (three times, 10 min intervals), mice were placed in separate lanes on a rod rotating at 5 rpm for 60 s. Animals unable to remain on the rod for 60 s were excluded from further study. For testing, animals were placed on a rod rotating at 4 rpm and then accelerated to 40 rpm over 300 s. The trial began at the start of acceleration and ended when the animal fell from the rod. Fall latencies were recorded, and the average of three trials was used for analysis. To assess the effects of CB-13 administration on motor coordination, mice were first acclimatised to the testing room for 30 min before both the training and testing sessions. Training sessions were run as above. For testing sessions, under isofluorane anaesthesia, CB-13 (5 mg kg−1, s.c.) or vehicle was administered between the shoulders. Mice were then allowed to recover from anaesthesia in their home cage for 30 min before rotarod testing.
Dorsal root ganglion neuronal culture
Bilateral L4–L6 DRGs were collected from deeply anaesthetised (3% isoflurane) WT and CB1R cKO mice, and placed into fresh, ice-cold DH10 media consisting of Dulbecco's modified Eagle's medium (DMEM)/F-12 supplemented with 10% fetal bovine serum (no dimethyl sulfoxide [DMSO]) and 1% penicillin/streptomycin (∼1.5 ml per animal) and washed in Hanks balanced salt solution (HBSS; Gibco, Dublin, Ireland). Then, an enzyme solution containing 3.55 mg ml−1 dispase (Worthington Biochemicals, Lakewood, NJ, USA) and 1.65 mg ml−1 collagenase type I (Gibco) in HBSS without Ca2+ and Mg2+ was applied. Cells were incubated at 37°C for 45–60 min with gentle shaking/rotation every 10 min. After trituration, the cell suspension was strained through a 40 μm mesh (Sigma-Aldrich) and centrifuged (5 min, 4°C, 58 g). Cells were resuspended in DH10 medium containing nerve growth factor (NGF; 20 ng ml−1; Upstate Biotechnology, Lake Placid, NY, USA) and glial cell line derived neurotrophic factor (GDNF; 50 ng ml−1; Upstate Biotechnology) then plated on coverslips freshly coated with poly-d-lysine (100 μg ml−1; Biomedical Technologies) and laminin (100 μg ml−1). Cultures were incubated at 37°C in 5% CO2 for ∼48 h before electrophysiological recordings.
Whole-cell patch-clamp recording
Patch-clamp electrodes were constructed from single-filament borosilicate glass (1.5 mm o.d., 0.84 mm i.d.; World Precision Instruments, Sarasota, FL, USA; impedance: 2–4 MΩ) and formed seal resistances ≥1 GΩ. All recordings were obtained at room temperature. All solution/drug perfusions were delivered at a rate of 1–2 ml min−1.
For intrinsic excitability recordings under current clamp conditions, neurones were perfused with an oxygenated solution of (in mM) 140 NaCl, 4 KCl, 2 MgCl2, 2 CaCl2, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 10 glucose (pH=7.4 adjusted with NaOH; ∼305–310 mOsm) adjusted with sucrose, measured by an osmometer (Vapro 5600; Wescor, Logan, UT, USA). Junction potential differences between internal and external solutions (15.7 mV; junction potential calculator; Clampex software, Molecular Devices, San Jose, CA, USA) were corrected for. The internal solution was composed of (in mM) 135 K-gluconate, 10 KCl, 10 HEPES, 2 Na2ATP, 0.4 Na2GTP, and 1 MgCl2 (pH=7.4 with KOH; ∼300–305 mOsm).
For HVA-ICa recording under voltage-clamp conditions, neurones were perfused with an oxygenated solution consisting of (in mM) 130 N-methyl-d-glucamine chloride (NMDG-Cl; solution of 130 mM NMDG pH balanced to 7.4 with HCl), 5 BaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH=7.4 adjusted with 1 M NMDG; ∼310–315 mOsm adjusted with sucrose). Junction potential differences between the internal and external solutions were not adjusted. The internal solution was composed of (in mM) 140 TEA-Cl, 10 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1 MgCl2, 10 HEPES, 0.5 Na2GTP, and 3 Na2ATP (pH=7.4 with 1 M NMDG; ∼300–305 mOsm).
All recordings were filtered at 4 kHz with a –3 dB, four-pole, low-pass Bessel filter, sampled at a rate of 20 kHz, and stored on a personal computer (Dell) using pClamp 11 and a digitiser (Digidata 1550B; Molecular Devices). Currents were digitally filtered offline by using a low-pass Gaussian filter with a –3 dB cut-off set to 2 kHz (Clampfit software; pClamp 11, Molecular Devices).
Intrinsic excitability
After whole-cell configuration was established, a 5 min equilibration period was allowed before the spontaneous activity of the neurone was recorded for 2 min from Vrest. Rheobase was measured by injecting a series of square-wave current steps via the patch electrode (500 ms, 10 pA steps; starting at –40 pA) until a single action potential (AP) was generated. AP threshold (mV), AP amplitude (mV), AP half-width (ms), and input resistance (MΩ) were then measured. AP trains in response to a prolonged current application via the patch electrode (1×, 2×, and 3× rheobase intensity; 1 s) were then recorded. Both the number of APs and the instantaneous frequency of APs (Inst. Freq [Hz]=mean interspike interval−1 [ms]) generated by each stimulus intensity were computed.
High voltage-activated calcium current recording
Under whole-cell configuration, series resistance was maintained at <20 MΩ under voltage-clamp conditions. For examination of HVA-ICa current–voltage (I–V) relationships and channel open probabilities after a 5 min equilibration period, 25 ms depolarising square wave voltage steps were delivered via the patch electrode (–70 to +40 mV, 10 mV step).28,29
Protocols used to evoke low-voltage-activated (LVA) and HVA currents were based on prior studies.28,29 Briefly, with neurones held at –80 mV, once every 10 s a +40 mV pulse was applied via the patch electrode for 20 ms to activate LVA Ca2+ channels. The holding voltage was then set to –60 mV for 20 ms followed by a +50 mV step for 20 ms to evoke HVA-ICa. After recording 1 min of baseline HVA-ICa, CB-13 (1–10 nM), vehicle, CB-13 (10 nM) + AM6545 (10 nM), or CB-13 (10 nM) + AM630 (100 nM) was delivered by bath application using a perfusion system (VC-6; Warner Instruments, Hamden, CT, USA) for 2 min, followed by 15 min of washout.
Analysis of channel open probabilities
The current–voltage relationship (I–V curve) was determined by plotting normalised peak HVA-ICa amplitudes at each test voltage (–70 to +40 mV). The voltage dependency of channel open probabilities was determined by plotting normalised tail currents as a function of test voltages, which were then fitted with a Boltzmann equation for channel open probabilities:
where P(V) represents the channel open probability as a function of membrane potential; Pmin and Pmax are the minimum and maximum open probabilities, respectively; Vhalf is the voltage at 50% maximum current; and k is the default slope value.
Drugs
CB-13 (1-naphthalenyl[4-(pentyloxy)-1-naphthalenyl]methanone; Tocris, Bristol, UK), a dual agonist of CB1R (EC50=6.1 nM) and CB2R (EC50=27.9 nM), was diluted with DMSO followed by normal saline, and injected subcutaneously (5 mg kg−1) or diluted with NMDG-Cl solution for electrophysiological testing (1–10 nM). AM6545 (5-[4-(4-cyano-1-butyn-1-yl)phenyl]-1-(2,4-dichlorophenyl)-N-(1,1-dioxido-4-thiomorpholinyl)-4-methyl-1H-pyrazole-3-carboxamide; Sigma), a high-affinity CB1R antagonist (Ki=3.1 nM), and AM630 (6-iodo-2-methyl-1-2-(4-morpholinyl)ethyl-1H-indol-3-yl(4-methoxyphenyl)methanone; Sigma), a CB2R inverse agonist (Ki=31.2 nM), were dissolved in DMSO to stock concentration, and in NMDG-Cl solution for electrophysiological tests (AM6545: 10 nM; AM630: 100 nM). All drug solutions were freshly prepared before use, and concentrations used were determined by their EC50 based on preliminary concentration–effect analysis and previous studies.26
Statistical analysis
Data were subjected to normality tests (Shapiro–Wilk or Kolmogorov–Smirnov). Behavioural data with naïve animals were compared across genotypes with Welch's t-test or Mann–Whitney U-test. The time course of drug and pain model effects was assessed using repeated-measures or mixed-model two-way analysis of variance (anova), with Bonferroni, or Holm–Sidak post hoc tests. The Greenhouse–Geisser correction was used to adjust for lack of sphericity in the repeated-measures anova.
Current-clamp data were compared using unpaired t-tests. Data from voltage-clamp experiments involving drug/antagonist tests were compared using two-way repeated measures or mixed-model anova with Holm–Sidak tests for multiple comparisons. Prism 9.0 (GraphPad Inc., San Diego, CA, USA) was used for statistical analyses. All tests were two-tailed, with statistical significance set at P<0.05. Data are presented as mean (standard error of the mean [sem]).
Results
Nociceptive thresholds or locomotor function not affected by knockout of CB1R in primary sensory neurones
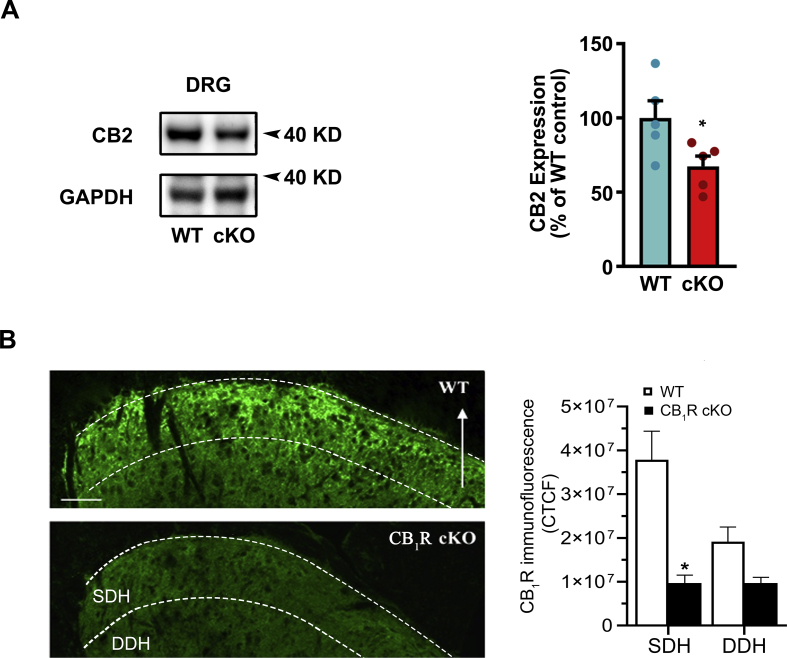
We tested whether endogenous CB1R signalling in DRG neurones tonically inhibits nociceptive sensitivity or affects locomotor function. Through Pirt-Cre/LoxP-mediated recombination, CB1R expression was conditionally knocked out in a majority of DRG neurones in CB1R cKO mice (Fig. 1a and b). The proportions of CB1R-positive neurones (% of total neurones counted) were significantly reduced compared with those in WT mice (Fig. 1c and d; t9=5.64, P=0.0003, unpaired t-test). Size distribution analysis showed that CB1Rs were expressed predominantly in small- and medium-diameter (≤600 μm2) neurones in WT mice (Fig. 1e). CB1 immunoreactivity of the central terminals within the spinal superficial dorsal horn (SDH) was also reduced in CB1R cKO mice compared with WT (Supplementary Fig. S1b; t6=4.16, ∗P=0.01, Bonferroni's post-test).
By immunoblotting, CB1 protein (50 kDa) levels were reduced in the DRG of CB1R cKO mice (Fig. 1f; t7=6.87, ∗∗∗P=0.0002, unpaired t-test). In addition, CB2 (40 kDa) protein levels were also reduced in CB1R cKO mice (Supplementary Fig. S1a; t8=2.41, ∗P=0.04, unpaired t-test).
In behavioural studies, naive WT and CB1R cKO mice responded to radiant heat stimulation with similar paw withdrawal latencies (Fig. 1g; t19=0.193, P=0.85, Welch's t-test), and exhibited similar response latencies in the hot plate test (Fig. 1h; t13=0.835, P=0.42, Welch's t-test). Paw withdrawal frequencies to punctate mechanical stimulation were also comparable between the two groups (Fig. 1i, 0.07 g: t36=0.215, P>0.99; 0.4 g: t36=0.429, P>0.99, Bonferroni's post-test). Motor coordination was similar in WT and CB1R cKO mice as evidenced by comparable fall latencies in the rotarod test (Fig. 1j; t13=0.147, P=0.89, Welch's t-test). Overall, there were no significant sex differences in mechanical thresholds or radiant heat thresholds (Supplementary Fig. S2a–c). However, there was a significant sex difference in response latencies to hot plate tests in CB1R cKO mice (Supplementary Fig. S2d; t16=2.55, P=0.04, Holm–Sidak post-test).
No effect of knockout of CB1R on intrinsic membrane properties of small-diameter DRG neurones
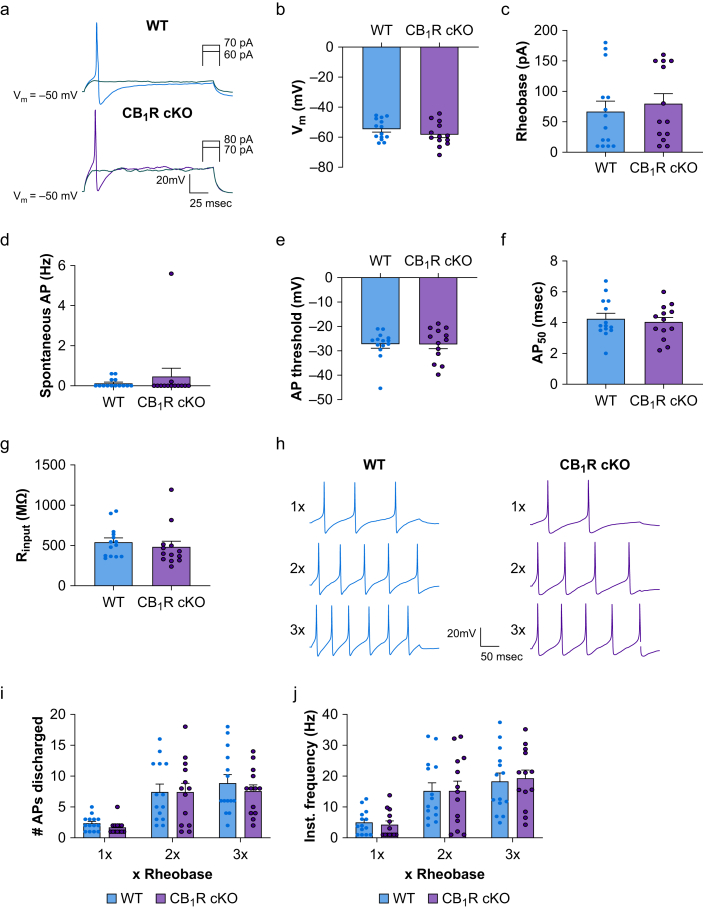
To investigate whether constitutive activation of CB1R modulates DRG neurone excitability, we compared the intrinsic excitability of small-diameter neurones in WT and CB1R cKO mice. Patch-clamp recordings showed no significant difference in resting membrane potential (Fig. 2a and b; t25=1.16, P=0.26; mean: WT, –54.8 [1.8] mV; cKO, –58.1 [2.2] mV), rheobase level (Fig. 2c; t25=0.51, P=0.62; mean: WT, 64.2 [16.8] pA; cKO, 79.2 [17.0] pA), spontaneous AP discharge (Fig. 2d; t25=0.45, P=0.76; mean: WT, 0.13 [0.06] Hz; cKO, 0.45 [0.43] Hz), AP threshold (Fig. 2e; t25=0.035, P=0.97; mean: WT, –27.3 [1.6] mV; cKO, –27.2 [1.9] mV), AP half-width (Fig. 2f; t25=0.52, P=0.61; mean: WT, 4.3 [0.3] ms; cKO, 4.02 [0.31] ms), or input resistance (Fig. 2g; t25=0.72, P=0.48, unpaired t-test; mean: WT, 543 [51] MΩ; cKO, 482 [71] MΩ) between the two genotypes. Additionally, neurones in both groups discharged similar AP trains evoked by increasing stimulation intensities (1–3× rheobase). The number of APs (Fig. 2i and Supplementary Table S1; 1×: t75=0.43, P=0.89; 2×: t75=0.029, P=0.98; 3×: t75=0.86, P=0.78) and the instantaneous frequency of APs (Fig. 2j and Supplementary Table S1; 1×: t75=0.22, P=0.99; 2×: t75=0.001, P=0.99; 3×: t75=0.31, P=0.98) generated by WT and CB1R cKO neurones in response to each stimulation intensity did not differ between the two groups (Supplementary Table S1).
Fig 2.
The intrinsic membrane properties of small-diameter dorsal root ganglion (DRG) neurones are not affected by CB1R conditional knockout. (a) Representative traces of rheobase levels of small-diameter DRG neurones from wild-type (WT) and CB1R cKO mice under current-clamp conditions. The electrophysiological parameters shown in panels b–g did not differ significantly between the WT and CB1R cKO groups (n=13–14 neurones/group, four mice/sex). (b) Resting membrane potentials (P=0.26). (c) Rheobase levels (P=0.62). (d) Rate of spontaneous action potential (AP) discharge (P=0.76). (e) AP threshold (P=0.97). (f) AP half-width (AP50; P=0.61). (G) Input resistance (Rinput; P=0.48). (h) Representative traces of AP trains evoked by current stimulation (1×, 2×, 3× rheobase intensity) of small-diameter neurones from WT and CB1R cKO mice. (i, j) The number of APs generated (1×: P=0.89, 2×: P=0.98, 3×: P=0.78) and the instantaneous frequency (1×: P=0.99, 2×: P=0.99, 3×: P=0.98) of AP trains discharged by DRG neurones did not differ between WT (n=14 neurones) and CB1R cKO mice (n=13 neurones) across stimulation intensities. (b–g): unpaired t-test; (i, j): two-way mixed-model anova. Data are shown as mean (standard error of the mean). CB1R, cannabinoid type-1 receptor; cKO, conditional knockout; anova, analysis of variance.
CB-13 inhibition of nociceptive pain and inflammatory heat hyperalgesia reduced in CB1R conditional knockout mice
Owing to potential functional interactions between CB1Rs and MORs,30,31 significant CB1R-MOR interactions may alter CB-13 efficacy under conditions where MORs are absent in DRG neurones as endogenous MOR signalling and the resulting CB1R interactions would be compromised. Accordingly, we examined systemic CB-13 effects on nociceptive pain and inflammatory heat hyperalgesia in Pirt-MOR cKO, Pirt-CB1R cKO, and WT mice.
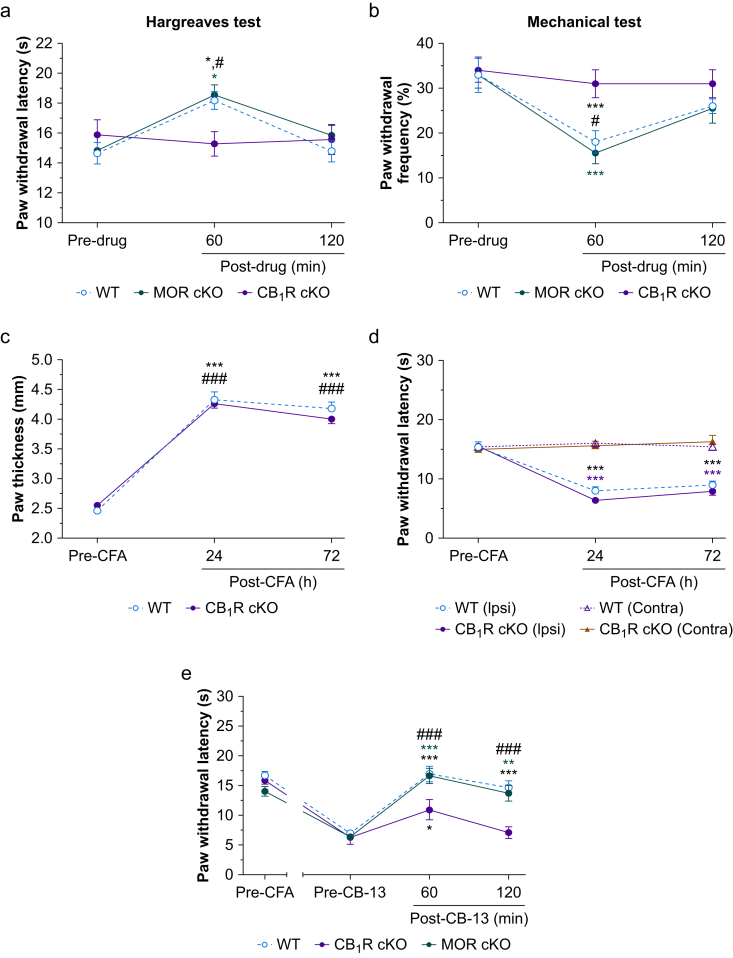
Systemic CB-13 administration (5 mg kg−1; s.c.) increased PWL at 60 min after injection in both WT (Fig. 3a; t52=2.79, P<0.05) and MOR cKO mice (t52=2.78, P<0.05), but not in CB1R cKO mice (t52=0.48, P>0.99, Bonferroni's post-test). CB-13 also decreased PWF in WT (Fig. 3b; t52=3.9, P<0.001) and MOR cKO mice (t52=4.38, P<0.001), but not in CB1R cKO mice (t52=0.78, P=0.88, Bonferroni's post-test). Changes in PWL and PWF after CB-13 treatment were comparable between WT and MOR cKO mice. Thus, genetic deletion of CB1Rs, but not MORs, in primary sensory neurones reduced the antinociceptive effects of CB-13.
Fig 3.
Systemic administration of CB-13 strongly inhibits nociceptive pain and inflammatory heat hypersensitivity in wild-type (WT) mice and in mice lacking peripheral mu-opioid receptors (MOR cKO) but weakly in CB1R cKO mice. (a) Systemic administration of CB-13 (5 mg kg−1, s.c.) significantly increased paw withdrawal latency (PWL) to radiant heat stimulation in both WT and MOR cKO mice at 60 min, but not in CB1R cKO mice (F2,52=5.23, P=0.009, two-way mixed-model anova). ∗P<0.05 vs pre-drug, #P<0.05 vs CB1R cKO, Bonferroni's post-test. (b) CB-13 (5 mg kg–1, s.c.) significantly decreased paw withdrawal frequency (PWF) to high-force (0.4 g) mechanical stimulation at 60 min in both WT and MOR cKO mice, but not in CB1R cKO mice (F2,52=13.89, P<0.001, two-way mixed model anova). ∗∗∗P<0.001 vs pre-drug; #P<0.05 vs CB1R cKO, Bonferroni's post-test (a, b: n=10/group, five/sex in each group). (c) Intraplantar injection of complete Freund's adjuvant (CFA; 20 μl, 1 mg ml−1) similarly increased paw thickness in WT and CB1R cKO mice (F2,114=393.7, P<0.001, two-way mixed model anova). ∗∗∗P<0.001 vs pre-CFA of WT, ###P<0.001 vs pre-CFA of CB1R cKO, n=30 mice/group, Bonferroni's post-test. (d) PWL to radiant heat stimulation was significantly decreased in the ipsilateral hind paw of both groups after intraplantar CFA injection (F6,152=20.26, P<0.001, two-way mixed model anova). ∗∗∗P<0.001 vs pre-CFA, n=20 mice/group, 10/sex, Bonferroni's post-test. (e) Effects of systemic CB-13 (5 mg kg−1, s.c.) on the ipsilateral PWL of mice at 72 h post-CFA (F6,78=6.4, P<0.001, two-way mixed-model anova). WT and CB1R cKO: n=10/group, five/sex; MOR cKO: n=9 mice. ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001 vs pre-drug values; ###P<0.001, WT and MOR cKO vs CB1R cKO, Bonferroni's post-test. Data are shown as mean (standard error of the mean). Ipsi, ipsilateral; Contra, contralateral; anova, analysis of variance.
We further investigated whether peripheral CB1R signalling modulates the development of heat hyperalgesia and the analgesic effect of systemic CB-13 under inflammatory pain conditions. Intra-plantar injection of CFA (20 μL, 1 mg ml−1) caused similar paw thickness increases in WT (Fig. 3c; t29=16.7, P<0.001) and CB1R cKO mice (t29=21.5, P<0.001, Bonferroni's post-test). Ipsilateral PWLs were decreased to a similar level in WT (Fig. 3d; t19=9.5, P<0.001) and CB1R cKO mice (t19=15, P<0.001, Bonferroni's post-test) at 72 h after CFA. Contralateral PWLs were not significantly changed (Fig. 3d). CB-13 (5 mg kg−1; s.c.) increased PWLs in WT (Fig. 3e; t9=8.7, P<0.001) and MOR cKO mice (t9=8.23, P<0.001, Bonferroni's post-test) at both 60 and 120 min after drug injection compared with pre-drug. CB-13 only modestly increased PWL in CB1R cKO mice (t9=3.5, P<0.05, Bonferroni's post-test). Importantly, the increased PWLs in WT and MOR cKO mice at 60 and 120 min after CB-13 injection were greater than in CB1R cKO mice (Fig. 3e; WT: t104=4.0, P<0.001; MOR cKO: t104=3.7, P<0.001, Bonferroni's post-test).
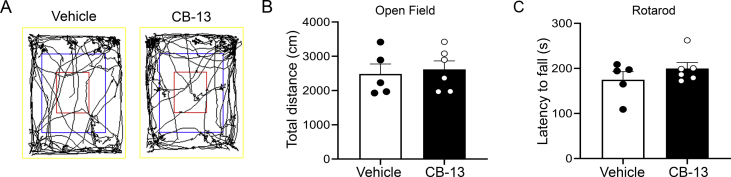
We verified that the CB-13 dose does not affect locomotion or motor coordination (Supplementary Fig. S3). There were no differences in total distance travelled in open field testing (Supplementary Fig. S3b; t9=0.35, P=0.74, unpaired t-test) or mean latency to fall in rotarod tests (Supplementary Fig. S3c; t9=1.14; P=0.28) following either systemic CB-13 (5 mg kg−1, s.c.) administration or vehicle.
CB-13 inhibits HVA-ICa in small-diameter DRG neurones
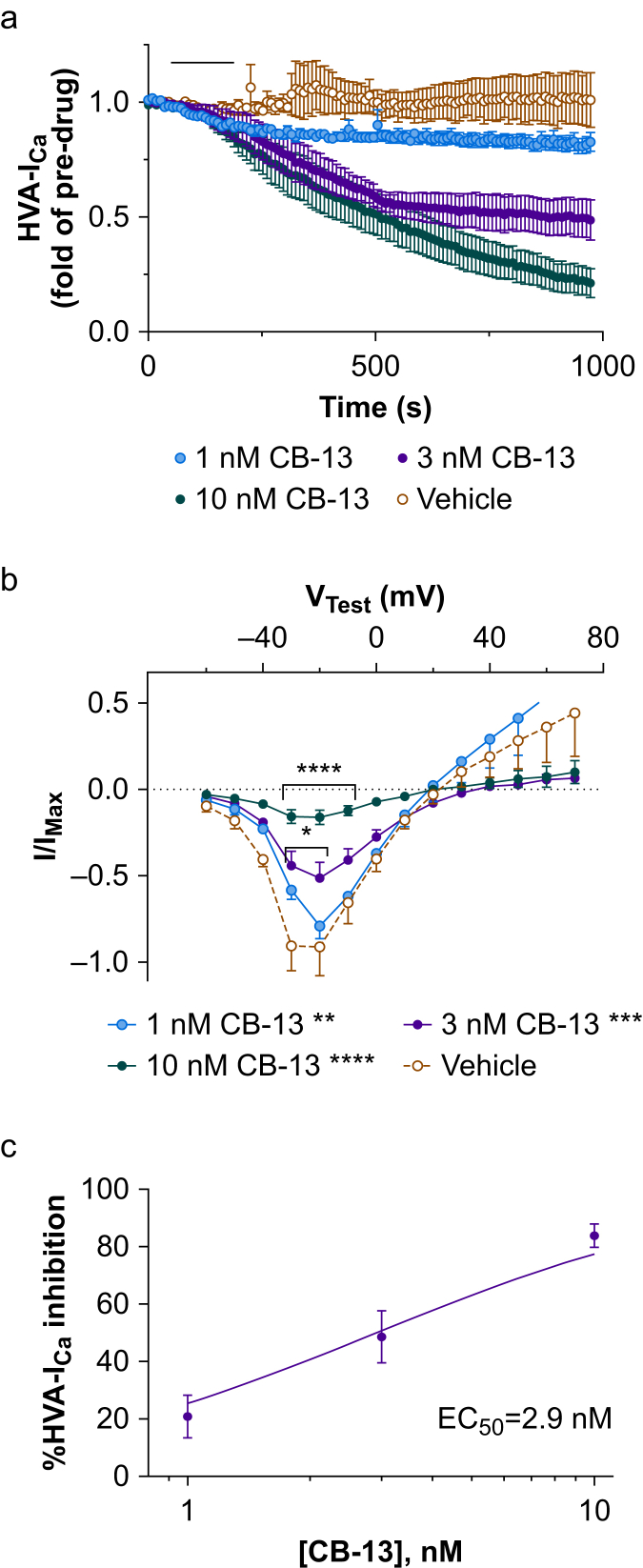
We examined the ionic mechanisms that contribute to pain inhibition by CB-13. Under voltage-clamp conditions, bath application of CB-13 (1, 3, and 10 nM) inhibited HVA-ICa in small-diameter neurones of WT mice (Fig. 4a; F324,1296= 6.42, P<0.0001, two-way mixed-model anova) in a concentration-dependent manner. Increasing CB-13 concentration also progressively decreased HVA-ICa conductance (Fig. 4b; 1 nM: t132=3.38, P<0.01; 3 nM: t132=4.21, P<0.001; 10 nM: t132=9.67, P<0.0001, Holm–Sidak post-tests). Concentration–effect analysis was performed for peak inhibition at each concentration, with an EC50 value of 2.9 nM (Fig. 4c; R2=0.79).
Fig 4.
CB-13 inhibits high-voltage activated calcium currents (HVA-ICa) in small-diameter dorsal root ganglion (DRG) neurones from wild-type (WT) mice. (a) Bath application of CB-13 (horizontal black bar: 1, 3, and 10 nM) dose-dependently inhibited HVA-ICa in cultured small-diameter DRG neurones from WT mice (P<0.0001, two-way mixed-model anova). n=3–5 neurones/group. (b) CB-13 (1–10 nM) also dose-dependently altered the current–voltage (I–V) relationship of HVA-ICa conductance (F30,132=6.2, P<0.0001, two-way mixed model anova) in WT mice. Increasing CB-13 concentration produced progressively larger reductions in HVA-ICa conductance (1 nM: ∗∗P<0.01; 3 nM: ∗∗∗P<0.001; 10 nM: ∗∗∗∗P<0.0001 compared with vehicle treatment, Holm–Sidak post-test). ∗P<0.05, ∗∗∗∗P<0.0001 vs vehicle at corresponding test voltages, Holm–Sidak post-test. (c) Dose–response curve for the inhibitory effect of CB-13 on HVA-ICa (% HVA-ICa inhibition) in WT mice. I/IMax values from VTest=–20 mV at peak of drug inhibition compared with pre-drug values (EC50=2.9 nM). anova, analysis of variance.
CB-13 inhibition of HVA-ICa is attenuated by a CB1R-selective antagonist
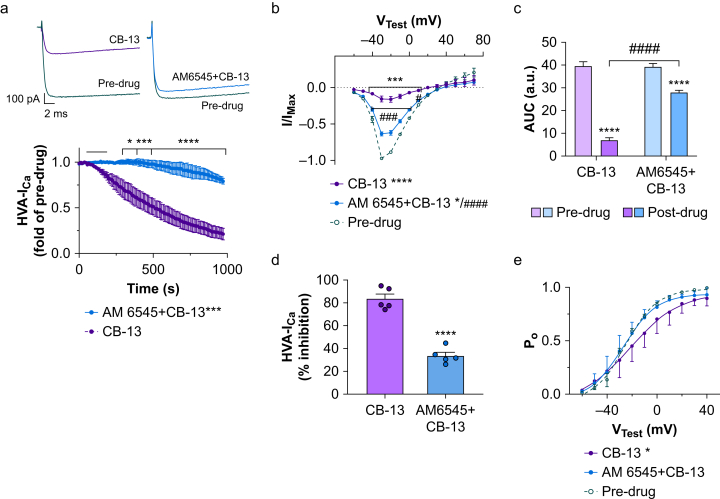
We investigated the involvement of CB1R in inhibition of HVA-ICa by CB-13. CB-13 (10 nM) induced prolonged inhibition of HVA-ICa, but the drug effect was attenuated in the presence of AM6545 (10 nM, a CB1R-selective antagonist, Fig. 5a; t10=5.5, P<0.001, Holm–Sidak post-test).
Fig 5.
AM6545 significantly attenuates the inhibition of high-voltage activated calcium currents (HVA-ICa) by CB-13 in small-diameter dorsal root ganglion (DRG) neurones from wild-type (WT) mice. (a) Upper: representative traces of HVA-ICa in cultured WT DRG neurones under voltage-clamp conditions before and after bath application of 10 nM CB-13 (left) or a combination of 10 nM AM6545 (a selective peripheral CB1R antagonist) and 10 nM CB-13 (right). Lower: time courses of decreased HVA-ICa after bath application (horizontal black bar) of indicated drug (F216,1080=8.19, P<0.0001, two-way repeated measures anova). n=5 neurones/group; ∗P<0.05, ∗∗∗P<0.001, ∗∗∗∗P<0.0001 vs CB-13, Holm–Sidak post-test. (b) The current–voltage (IV) relationship of HVA-ICa conductance before and after drug treatment (F26,247=23.56, P<0.0001, two-way repeated-measures analysis of variance (anova), n=5 neurones/group). CB-13 (∗∗∗∗P<0.0001, Holm–Sidak post-test) and AM6545 + CB-13 (t19=2.51, ∗P<0.05, Holm–Sidak post-test) significantly reduced overall HVA-ICa conductance. AM6545 significantly reduced the CB-13-induced HVA-ICa inhibition (####P<0.0001, Holm–Sidak post-test, mean effect of AM6545 + CB-13 vs CB-13). ∗∗∗P<0.001 pre-drug vs CB-13 at corresponding test voltages; ###P<0.001 for AM6545 + CB-13 vs CB-13 at corresponding test voltages, Holm–Sidak post-tests. (c) The areas under the curve (AUC; from VTest=–70 to +40 mV) of I–V relationship before and after drug treatment (F1,16=55.78, P<0.0001 vs individual pre-drug values, two-way anova, n=5 neurones/group). ∗∗∗∗P<0.0001 vs pre-drug, ####P<0.0001 vs CB-13 post-drug, Holm–Sidak post-test. (d) The overall HVA-ICa inhibition (% reduction from predrug; I/IMax values at VTest=–30 mV) by CB-13 or AM6545 + CB-13 application. The percent HVA-ICa inhibition produced by CB-13 application was significantly decreased in the presence of AM6545 (∗∗∗∗P<0.0001, unpaired t-test, n=5 neurones/group). (e) Analysis of open probability (PO) curves before and after drug treatment (F2,187=3.51, P=0.032, two-way anova, n=5 neurones/group). CB-13 application caused a significant rightward shift of the PO curve (∗P<0.05, Holm–Sidak post-test). Co-application of AM6545 and CB-13 did not affect the PO curve (P>0.05, Holm–Sidak post-test). a.u., arbitrary units. (c, f) Pre-drug data were pooled from both groups. Data are shown as mean (standard error of the mean).
Analysis of the I–V relationship showed that CB-13 reduced mean HVA-ICa conductance (Fig. 5b; t19=9.7, P<0.0001, Holm–Sidak post-test) and that this effect was decreased by co-application of AM6545 (Fig. 5b; t19=6.05, P<0.0001, Holm–Sidak post-test). Total HVA-ICa conductance across all test voltages was calculated by measuring the area under the curve (AUC): CB-13 decreased the AUC from pre-drug values (Fig. 5c; t16=16.21, P<0.0001, Holm–Sidak post-test), and AM6545 attenuated the CB-13 effect (Fig. 5c; t16=10.40, P<0.0001, Holm–Sidak post-test). A comparison of inhibition of HVA-ICa by CB-13 with and without AM6545 is shown in Fig. 5d (t8=9.57, P<0.0001, unpaired t-test).
CB-13 caused a rightward shift of the open probability curve (Fig. 5e; t187=2.59, P<0.05), but this effect was blocked in the presence of AM6545 (t187=1.95, P>0.05, Holm–Sidak post-test).
CB-13 inhibition of HVA-ICa is reduced in CB1R conditional knockout mice
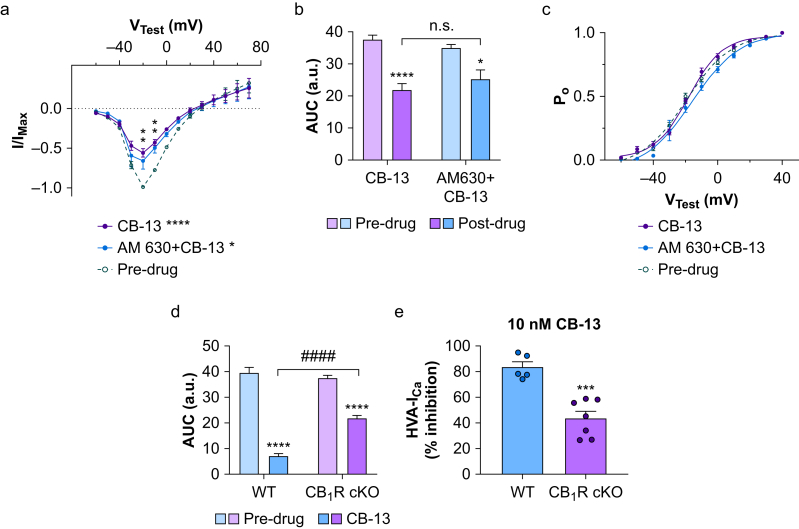
AM6545 did not completely block CB-13-induced inhibition of HVA-ICa, as the remaining HVA-ICa after co-application of AM6545 and CB-13 was still less than the pre-drug value (Fig. 5c). It may be that the concentration of AM6545 was insufficient to block CB1R completely or that CB-13 inhibits HVA-ICa through targets other than CB1R. To further examine the influences of CB1R, and potential CB2R signalling, on HVA-ICa inhibition by CB-13, we tested CB-13 along with AM630 (an inverse CB2R agonist) in small-diameter neurones from CB1R cKO mice. Both I–V relationship (Fig. 6a) and AUC analysis (Fig. 6b) showed that CB-13 (10 nM) reduced HVA-ICa from pre-drug levels in CB1R cKO mice (Fig. 6a; t266=4.48, P<0.0001) without changing the open probability curve (Fig. 6c; t209=1.25, P>0.05, Holm–Sidak post-test). Furthermore, co-application of AM630 did not attenuate CB-13-induced HVA-ICa inhibition (Fig. 6a; t266=0.832, P>0.05, Holm–Sidak post-test). Changes in AUC were used to compare CB-13-induced inhibition between WT and CB1R cKO mice. Although CB-13 reduced the AUC in both genotypes (Fig. 6d; t24=17.6, P<0.0001), the effect was less in the cKO group (Fig. 6d; t24=24, P<0.0001, Holm–Sidak post-test). The percent HVA-ICa inhibition by CB-13 was also lower in cKO than in WT neurones (Fig. 6e; t10=5.37, P=0.0003, unpaired t-test).
Fig 6.
CB-13-induced inhibition of high-voltage activated calcium currents (HVA-ICa) is attenuated in CB1R conditional knockout (cKO) mice. (a) The current–voltage (I–V) relationships of HVA-ICa conductance in small-diameter dorsal root ganglion (DRG) neurones from CB1R cKO mice before and after bath application of CB-13 (10 nM, n=7 neurones) or a combination of AM630 (a CB2R inverse agonist, 10 nM) and CB-13 (10 nM, n=4 neurones, F26,266=1.94, P=0.0052, two-way mixed-model analysis of variance [anova]). CB-13 application significantly reduced HVA-ICa conductance (∗∗∗∗P<0.0001, Holm–Sidak post-test). Co-application of AM630 and CB-13 induced a similar reduction in HVA-ICa (t266=2.82, ∗P<0.05, Holm–Sidak post-test). ∗∗P<0.01, CB-13 alone and AM630 + CB-13 vs pre-drug at corresponding test voltages, Holm–Sidak post-tests. Pre-drug data were pooled from both groups. (b) The area under the curve (AUC; from VTest=–70 to +40 mV) of the HVA-ICa I–V relationship before and after drug treatment (F1,18=43.17, P<0.0001, two-way anova). ∗P<0.05, ∗∗∗∗P<0.0001 vs pre-drug, Holm–Sidak post-test. n.s., not significant. (c) Open probability (Po) curves before and after drug treatment. Application of neither CB-13 nor AM630 + CB-13 altered the Po curve (F20,209=1.32, P=0.17, two-way mixed model anova) in CB1R cKO neurones. (d) Comparisons of AUC (from VTest=70 to +40 mV) for the HVA-ICa I–V relationship before and after CB-13 application in wild-type (WT) and CB1R cKO neurones. CB-13 (10 nM) application still induced a significant reduction of AUC in the CB1R cKO group (F1,24=42.02, P<0.0001, two-way mixed-model anova), but the effect was significantly less than that in WT mice. ∗∗∗∗P<0.0001 vs pre-drug; ####P<0.0001 for comparison of the effect of CB-13 on WT and CB1R cKO neurones, Holm–Sidak post-test. a.u., arbitrary units. (e) Comparison of the overall inhibition of HVA-ICa (% reduction from pre-drug; I/IMax values at VTest=–20 mV) by CB-13 (10 nM) in WT and CB1R cKO groups. The percent HVA-ICa inhibition produced by CB-13 was significantly less in CB1R cKO neurones than in WT neurones (∗∗∗P<0.001, unpaired t-test, n=5 neurones/group).
Discussion
We found that selective deletion of CB1R in most primary sensory neurones does not affect basal nociceptive thresholds, inflammatory heat hyperalgesia, or intrinsic excitability of small-diameter DRG neurones in mice. However, deletion of CB1R abolishes the analgesic effects of CB-13 in naïve and inflammatory states and attenuates inhibition of HVA-ICa by CB-13.
Role of peripheral CB1R signalling in nociception and inflammatory pain
The endocannabinoid system represents an important component of pain modulatory mechanisms.32 Mounting evidence suggests that endocannabinoids are important for pain modulation across multiple species.33, 34, 35, 36 CB1R expression has been localised to DRG neurones in rats, canines, and equines.33, 34, 35 The localisation and function of cannabinoid receptors have also been demonstrated in human sensory neurones.36 Collectively, these findings suggest that new insights into the endocannabinoid system from studies performed in model species could have high translational impact. Yet, evidence regarding the influence of endogenous CB1R signalling on pain has been conflicting.14,16 Global CB1R KO mice showed either unaltered37 or decreased pain sensitivity (hypoalgesia) in hot plate assays and formalin tests.15 Furthermore, functional knockout of CB1R did not affect the duration or severity of mechanical hypersensitivity in nerve-injured mice.38 However, Nav1.8-Cre-driven CB1R cKO mice exhibited increased basal nociceptive sensitivity, exaggerated inflammatory pain, and elevated excitability in nociceptive afferents, suggesting tonic pain inhibition mediated by CB1R signalling in nociceptive DRG neurones.13 In the current study, neither nociceptive thresholds nor inflammatory heat hyperalgesia was altered in Pirt-CB1R cKO mice. After removing an outlier in the cKO group of the hot plate test (Fig. 1h; P<0.05; Grubbs test), the comparison is even more similar. Consistent with the behavioural results, electrophysiological recordings showed similar intrinsic excitability in small-diameter DRG neurones of WT and Pirt-CB1R cKO mice when we compared multiple parameters, including resting membrane potential, rheobase level, AP threshold, rate of spontaneous APs, and evoked AP trains.
Multiple factors that require further investigation may explain the discrepancy between our findings and reports in the literature. Previous studies showed that CB1R mRNA and protein are abundantly expressed in a major subpopulation of nociceptive DRG neurones, but they are also detected in medium- and large-diameter neurones.12,13,39 Consistent with these findings, we found that CB1R immunoreactivity was distributed in all sizes of DRG neurones, but the signal was predominant in small-diameter neurones that signal pain and thermal stimuli. Because Pirt is expressed in >80% of DRG neurones, but not in the CNS or other cell types in the peripheral nervous system,17 CB1R immunoreactivity was substantially decreased across a broad range of DRG neurones in Pirt-CB1R cKO mice. However, it was preserved in large-diameter neurones of Nav1.8-Cre-driven CB1R cKO mice.13 Thus, it is plausible that conditionally deleting CB1R from a subpopulation of nociceptive neurones (with Nav1.8-Cre) and knocking out a much larger population of CB1R using Pirt-Cre may produce different functional changes.
The levels of CB2R protein were also reduced in the DRGs of CB1R cKO mice, likely resulting from a loss of membrane stabilisation of CB2Rs, as G protein-coupled receptors (GPCRs) are known to form heteromers that could modulate the trafficking and post-endocytotic sorting of GPCRs.40,41 Thus, CB1 and CB2 receptors form functional heteromers in the brain, and would likely be subject to altered trafficking or membrane sorting owing to dimerisation.42 We cannot rule out possible compensatory changes in other receptors, ion channels, and intracellular signalling that may occur and differentially affect pain behaviour in different CB1R cKO lines. However, Pirt-Cre has been used to delete or modulate gene expression selectively in primary sensory neurones without inducing notable compensatory changes.24,43
In a previous study,13 the background genetics of the CB1 floxed mice consisted of both C57BL/6J and C57BL/6N. Evaluations of these two strains have revealed various phenotypic differences involving metabolism, immunological function, and neurological function.44 Although it is difficult to attribute the conflicting results to any specific phenotypic difference between the two strains without conducting additional studies, differences in genetic background may introduce variability in pain behaviour tests. In fact, Nav1.8-Cre mice (C57BL/6J background) and Nav1.8-CB1R cKO mice showed comparable nociceptive thresholds,13 consistent with our findings that Cre-dependent deletion of CB1R from DRG neurones does not alter nociceptive thresholds and inflammatory heat hyperalgesia.
CB1R expression was elevated in DRG and in nerve fibres of skin after CFA-induced inflammation.39 Although our findings suggest that this increase does not induce tonic CB1R-mediated pain inhibition, it could increase the efficacy of pain inhibition by exogenous CB1R agonists.39 Our data do not exclude the possibility that peripheral endogenous CB1R signalling controls exaggerated pain in other modalities (e.g. cold, mechanical, ongoing pain) and neurone hyperexcitability after injury. The roles for peripheral CB1R-mediated signalling in endogenous pain modulation warrant additional investigation, especially to determine whether signalling is context-dependent (e.g. nerve injury, insult severity, post-injury time).
Role of peripheral CB1R in pain inhibition by CB-13
CB-13 is a CB1/CB2R dual agonist with limited CNS penetration. In our behavioural study, systemic administration of CB-13 induced heat and mechanical anti-nociception in naïve WT mice, and attenuated heat hyperalgesia in those with CFA-induced inflammation of the hind paw. The drug effect was diminished in CB1R cKO mice, suggesting an indispensable role for CB1R in DRG neurones. These results are in line with previous findings that CB1R-mediated inhibitory mechanisms in DRG neurones are paramount to the analgesic effects of cannabinoids administrated peripherally45,46 or systemically.13
Inhibition of inflammatory heat hypersensitivity remained significant in Pirt-CB1R cKO at 60 min after systemic CB-13 administration. The remaining effect may be attributable to an incomplete deletion of CB1R in all DRG neurones, or CB-13 may activate CB1R expressed in non-neuronal cells (e.g. macrophages, keratinocytes) or other receptors in the peripheral nervous system. Although we cannot completely discount the possibility that some CB-13 may penetrate into the CNS and induce pain inhibition, our results show that acute systemic administration of CB-13 did not alter locomotive activity in open field tests or fall latencies in rotarod tests, which suggests that the dose used in our studies does not have central effects that could alter performance in these assays. Functional interaction between the cannabinoid and opioid systems may participate in pain control.30,31 However, systemic CB-13-induced pain inhibition remained intact in peripheral MOR cKO mice, suggesting that CB-13 effects were not dependent on MORs in DRG neurones.
Ionic mechanisms involved in CB-13-induced pain inhibition
The cannabinoid agonist WIN55,212-2 inhibits HVA-ICa in retinal ganglion cells, an effect that was reduced by the CB1R antagonist SR141716A.20 We show that CB-13 can concentration-dependently inhibit HVA-ICa in small-diameter DRG neurones of WT mice, as indicated by decreased current and a rightward shift of the channel open probability curve. This action was reduced in the presence of CB1R antagonist AM6545 and by CB1R cKO. HVA calcium channels are important for neurotransmitter release and neuronal excitability,47 and inhibition of HVA-ICa contributes to pain inhibition by known analgesics such as morphine and gabapentin.24,48 Accordingly, pain inhibition by CB-13 may be partially mediated by HVA-ICa inhibition, which can reduce neurone excitability and nociceptive afferent output.29,47
The ability of a CB1R antagonist and CB1R cKO to block CB-13-induced HVA-ICa inhibition was only partial. It is conceivable that other targets on DRG neurones also contribute to the drug effect. CB-13 can activate CB2R, which is expressed in both rodent and human DRG neurones and plays a functional role in pain.34,49 Yet the CB2R-biased inverse agonist AM630 did not attenuate HVA-ICa inhibition by CB-13 in CB1R cKO neurones. In addition, CB2R expression was reduced in the DRGs of CB1R cKO mice. Taken together, these findings suggest that compensatory changes in CB2R expression do not underlie HVA inhibition by CB-13 in CB1R cKO neurones. Further studies are needed to determine if CB-13 has off-target effects on other receptors (e.g. opioid receptors, gamma-aminobutyric acid B [GABAB] receptors) that might also inhibit HVA-ICa.24,50,51 Mounting evidence suggests that cannabinoids have both metabotropic and ionotropic receptor targets that contribute to the modulation of pain processing,21 including voltage-gated calcium channels, potassium channels, diverse transient receptor potential channels (e.g. TRPV1), acid-sensing ion channels, and purinergic receptors (e.g. P2X3).21, 22, 23,52 Thus, the complete underlying ionic mechanisms and molecular targets responsible for CB-13-induced neuronal and pain inhibition warrant further investigation.
Conclusions
By a combination of genetic, behavioural, pharmacological, and electrophysiological approaches, we show that CB1R in mouse DRG neurones is essential to mediating the analgesic effects of systemic CB-13, and that HVA-ICa inhibition is a potential ionic mechanism for CB-13-induced analgesia. However, peripheral CB1R-mediated signalling may not tonically inhibit nociceptive thresholds or inflammatory heat hyperalgesia, nor affect the intrinsic excitability of nociceptive DRG neurones in mice. These findings contribute to our understanding of how CB1Rs expressed in primary sensory neurones affect pain and cannabinoid analgesia. CB1R activation in the brain causes myriad side-effects that significantly limit CB1R agonist use, but our study suggests that peripherally acting CB1R agonists may have potential for pain management.
Authors' contributions
Study design: SR, YG, NCF, QH, AB
Experimental design for conditional knockout species: XD
Conduct of most of the experiments: NCF, AB
Conduct of portions of experiments: SH, QH, CZ
Data analysis: NCF, AB, SH, QH, CZ
Discussion of the work: NCF, XD, YG, SR
Directed the project: SR, YG
Drafting of the manuscript: NCF, AB, YG, SR
Manuscript review: SH, QH, CZ
All authors read and approved the final manuscript.
Acknowledgements
The authors thank Claire Gaveriaux-Ruff (University of Strasbourg, France), George Kunos (US National Institutes of Health, Bethesda, MD, USA), and Elisabeth Glowatzki (Johns Hopkins University School of Medicine, Baltimore, MD, USA) for providing the transgenic mice. The authors also thank Claire Levine (Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University) for assistance in editing the manuscript.
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.10.020.
Contributor Information
Yun Guan, Email: yguan1@jhmi.edu.
Srinivasa N. Raja, Email: sraja2@jhmi.edu.
Declarations of interest
SNR is a consultant for Allergan, Averitas Pharma, Bayer, and Lexicon Pharmaceuticals, and has consulted for Aptinyx Inc., Heron Therapeutics, and Insys Therapeutics. YG and SNR are principal and co-investigators in a research grant from Medtronic, Inc. YG received a research award from TissueTech, Inc. The other authors declare no other potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
National Institutes of Health (Bethesda, MD, USA) (NS026363 to SNR; NS110598, NS117761, and NS070814 to YG; NS070201 [NINDS T32] to NCF). This work was facilitated by the Pain Research Core funded by the Blaustein Fund and the Neurosurgery Pain Research Institute at the Johns Hopkins University (Baltimore, MD, USA).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig S1.
Fig S2.
Fig S3.
References
- 1.Naim M.M., Shehab S.A., Todd A.J. Cells in laminae III and IV of the rat spinal cord which possess the neurokinin-1 receptor receive monosynaptic input from myelinated primary afferents. Eur J Neurosci. 1998;10:3012–3019. doi: 10.1111/j.1460-9568.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- 2.Light A.R., Perl E.R. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- 3.Johannes C.B., Le T.K., Zhou X., Johnston J.A., Dworkin R.H. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Fayaz A., Croft P., Langford R.M., Donaldson L.J., Jones G.T. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaskin D.J., Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Breivik H., Eisenberg E., O'Brien T. Openminds. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013;13:1229. doi: 10.1186/1471-2458-13-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregorian R.S., Jr., Gasik A., Kwong W.J., Voeller S., Kavanagh S. Importance of side effects in opioid treatment: a trade-off analysis with patients and physicians. J Pain. 2010;11:1095–1108. doi: 10.1016/j.jpain.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Boudreau D., Von Korff M., Rutter C.M., et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18:1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacher P., Batkai S., Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dziadulewicz E.K., Bevan S.J., Brain C.T., et al. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone: a potent, orally bioavailable human CB1/CB2 dual agonist with antihyperalgesic properties and restricted central nervous system penetration. J Med Chem. 2007;50:3851–3856. doi: 10.1021/jm070317a. [DOI] [PubMed] [Google Scholar]
- 11.Salio C., Fischer J., Franzoni M.F., Conrath M. Pre- and postsynaptic localizations of the CB1 cannabinoid receptor in the dorsal horn of the rat spinal cord. Neuroscience. 2002;110:755–764. doi: 10.1016/s0306-4522(01)00584-x. [DOI] [PubMed] [Google Scholar]
- 12.Mitrirattanakul S., Ramakul N., Guerrero A.V., et al. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. Pain. 2006;126:102–114. doi: 10.1016/j.pain.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal N., Pacher P., Tegeder I., et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson J.D., Aanonsen L., Hargreaves K.M. SR 141716A, a cannabinoid receptor antagonist, produces hyperalgesia in untreated mice. Eur J Pharmacol. 1997;319:R3–R4. doi: 10.1016/s0014-2999(96)00952-1. [DOI] [PubMed] [Google Scholar]
- 15.Zimmer A., Zimmer A.M., Hohmann A.G., Herkenham M., Bonner T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaulieu P., Bisogno T., Punwar S., et al. Role of the endogenous cannabinoid system in the formalin test of persistent pain in the rat. Eur J Pharmacol. 2000;396:85–92. doi: 10.1016/s0014-2999(00)00226-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim A.Y., Tang Z., Liu Q., et al. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 2008;133:475–485. doi: 10.1016/j.cell.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker J.M., Huang S.M. Cannabinoid analgesia. Pharmacol Ther. 2002;95:127–135. doi: 10.1016/s0163-7258(02)00252-8. [DOI] [PubMed] [Google Scholar]
- 19.Berrocoso E., Rey-Brea R., Fernandez-Arevalo M., Mico J.A., Martin-Banderas L. Single oral dose of cannabinoid derivate loaded PLGA nanocarriers relieves neuropathic pain for eleven days. Nanomedicine. 2017;13:2623–2632. doi: 10.1016/j.nano.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Ross R.A., Coutts A.A., McFarlane S.M., et al. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 21.Akopian A.N., Ruparel N.B., Jeske N.A., Patwardhan A., Hargreaves K.M. Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol Sci. 2009;30:79–84. doi: 10.1016/j.tips.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y.Q., Qiu F., Qiu C.Y., et al. Cannabinoids inhibit acid-sensing ion channel currents in rat dorsal root ganglion neurons. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackie K., Lai Y., Westenbroek R., Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barpujari A., Ford N., He S.Q., et al. Role of peripheral sensory neuron mu-opioid receptors in nociceptive, inflammatory, and neuropathic pain. Reg Anesth Pain Med. 2020;45:907–916. doi: 10.1136/rapm-2020-101779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S., Huang Q., He S., et al. Dermorphin [D-Arg2, Lys4] (1–4) amide inhibits below-level heat hypersensitivity in mice after contusive thoracic spinal cord injury. Pain. 2019;160:2710–2723. doi: 10.1097/j.pain.0000000000001671. [DOI] [PubMed] [Google Scholar]
- 26.Tiwari V., Anderson M., Yang F., et al. Peripherally acting mu-opioid receptor agonists attenuate ongoing pain-associated behavior and spontaneous neuronal activity after nerve injury in rats. Anesthesiology. 2018;128:1220–1236. doi: 10.1097/ALN.0000000000002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan Y., Liu Q., Tang Z., Raja S.N., Anderson D.J., Dong X. Mas-related G-protein-coupled receptors inhibit pathological pain in mice. Proc Natl Acad Sci U S A. 2010;107:15933–15938. doi: 10.1073/pnas.1011221107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H., Ikeda S.R. Modulation of ion channels and synaptic transmission by a human sensory neuron-specific G-protein-coupled receptor, SNSR4/mrgX1, heterologously expressed in cultured rat neurons. J Neurosci. 2004;24:5044–5053. doi: 10.1523/JNEUROSCI.0990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z., Tseng P.Y., Tiwari V., et al. Targeting human Mas-related G protein-coupled receptor X1 to inhibit persistent pain. Proc Natl Acad Sci U S A. 2017;114:E1996–E2005. doi: 10.1073/pnas.1615255114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desroches J., Bouchard J.F., Gendron L., Beaulieu P. Involvement of cannabinoid receptors in peripheral and spinal morphine analgesia. Neuroscience. 2014;261:23–42. doi: 10.1016/j.neuroscience.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 31.Mohammadkhani A., Borgland S.L. Cellular and behavioral basis of cannabinioid and opioid interactions: implications for opioid dependence and withdrawal. J Neurosci Res. 2020 doi: 10.1002/jnr.24770. [DOI] [PubMed] [Google Scholar]
- 32.Woodhams S.G., Chapman V., Finn D.P., Hohmann A.G., Neugebauer V. The cannabinoid system and pain. Neuropharmacology. 2017;124:105–120. doi: 10.1016/j.neuropharm.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahluwalia J., Urban L., Capogna M., Bevan S., Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- 34.Chiocchetti R., Galiazzo G., Tagliavia C., et al. Cellular distribution of canonical and putative cannabinoid receptors in canine cervical dorsal root ganglia. Front Vet Sci. 2019;6:313. doi: 10.3389/fvets.2019.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiocchetti R., Rinnovati R., Tagliavia C., et al. Localisation of cannabinoid and cannabinoid-related receptors in the equine dorsal root ganglia. Equine Vet J. 2021;53:549–557. doi: 10.1111/evj.13305. [DOI] [PubMed] [Google Scholar]
- 36.Anand U., Otto W.R., Sanchez-Herrera D., et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 2008;138:667–680. doi: 10.1016/j.pain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Ledent C., Valverde O., Cossu G., et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 38.Wilkerson J.L., Alberti L.B., Kerwin A.A., et al. Peripheral versus central mechanisms of the cannabinoid type 2 receptor agonist AM1710 in a mouse model of neuropathic pain. Brain Behav. 2020;10 doi: 10.1002/brb3.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amaya F., Shimosato G., Kawasaki Y., et al. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain. 2006;124:175–183. doi: 10.1016/j.pain.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Gurevich V.V., Gurevich E.V. GPCR monomers and oligomers: it takes all kinds. Trends Neurosci. 2008;31:74–81. doi: 10.1016/j.tins.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He S.Q., Zhang Z.N., Guan J.S., et al. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Callen L., Moreno E., Barroso-Chinea P., et al. Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J Biol Chem. 2012;287:20851–20865. doi: 10.1074/jbc.M111.335273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao X., Han S., Huang Q., et al. Calcium imaging in population of dorsal root ganglion neurons unravels novel mechanisms of visceral pain sensitization and referred somatic hypersensitivity. Pain. 2021;162:1068–1081. doi: 10.1097/j.pain.0000000000002096. [DOI] [PubMed] [Google Scholar]
- 44.Simon M.M., Greenaway S., White J.K., et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14:R82. doi: 10.1186/gb-2013-14-7-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johanek L.M., Simone D.A. Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. Pain. 2004;109:432–442. doi: 10.1016/j.pain.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Yu X.H., Cao C.Q., Martino G., et al. A peripherally restricted cannabinoid receptor agonist produces robust anti-nociceptive effects in rodent models of inflammatory and neuropathic pain. Pain. 2010;151:337–344. doi: 10.1016/j.pain.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Hoppa M.B., Lana B., Margas W., Dolphin A.C., Ryan T.A. alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature. 2012;486:122–125. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Callaghan B., Haythornthwaite A., Berecki G., Clark R.J., Craik D.J., Adams D.J. Analgesic alpha-conotoxins Vc1.1 and Rg1A inhibit N-type calcium channels in rat sensory neurons via GABAB receptor activation. J Neurosci. 2008;28:10943–10951. doi: 10.1523/JNEUROSCI.3594-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beltramo M., Bernardini N., Bertorelli R., et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 50.Sadeghi M., Carstens B.B., Callaghan B.P., et al. Structure-activity studies reveal the molecular basis for GABAB-receptor mediated inhibition of high voltage-activated calcium channels by alpha-conotoxin Vc1.1. ACS Chem Biol. 2018;13:1577–1587. doi: 10.1021/acschembio.8b00190. [DOI] [PubMed] [Google Scholar]
- 51.Park K.S., Jeong S.W., Cha S.K., et al. Modulation of N-type Ca2+ currents by A1-adenosine receptor activation in male rat pelvic ganglion neurons. J Pharmacol Exp Ther. 2001;299:501–508. [PubMed] [Google Scholar]
- 52.Oliveira-Fusaro M.C.G., Zanoni C.I.S., Dos Santos G.G., et al. Antihyperalgesic effect of CB1 receptor activation involves the modulation of P2X3 receptor in the primary afferent neuron. Eur J Pharmacol. 2017;798:113–121. doi: 10.1016/j.ejphar.2017.01.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.