Abstract
Rationale
Ventilator-associated event (VAE) surveillance provides an objective means to measure and compare complications that develop during mechanical ventilation by identifying patients with sustained increases in ventilator settings after a period of stable or decreasing ventilator settings. The impact of the coronavirus disease (COVID-19) pandemic on VAE rates and characteristics is unknown.
Objectives
To compare the incidence, causes, and outcomes of VAE during the COVID-19 pandemic year versus prepandemic years and among ventilated patients with and without COVID-19.
Methods
In this retrospective cohort study of mechanically ventilated adults at four academic and community hospitals in Massachusetts, we compared VAE incidence rates between March 1 and August 31 for each year from 2017 to 2020 (corresponding to the time frame of the pandemic first wave in 2020) and among COVID-19–positive and COVID-19–negative patients in 2020. The medical records of 200 randomly selected patients with VAEs in 2020 (100 with COVID-19 and 100 without COVID-19) were analyzed to compare conditions precipitating VAEs in patients with versus without COVID-19.
Results
VAEs per 100 episodes of mechanical ventilation were more common in 2020 than in prior years (11.2 vs. 6.7; P < 0.01) but the rate of VAEs per 1,000 ventilator-days was similar (14.2 vs. 12.7; P = 0.08). VAEs were more frequent in COVID-19–positive patients than in COVID-19–negative patients in 2020 (29.0 vs. 7.1 per 100 ventilator episodes [P < 0.01] and 17.2 vs. 12.2 per 1,000 ventilator-days [P < 0.01]). Compared with patients without COVID-19 with VAEs, patients with COVID-19 and VAEs had similar rates of infection-related ventilator-associated complications, longer median durations of mechanical ventilation (22 vs. 14 d; P < 0.01), and similar in-hospital mortality (30% vs. 38%; P = 0.15). Progressive acute respiratory distress syndrome (ARDS) accounted for 53% of VAEs in patients with COVID-19, whereas it accounted for 14% of VAEs among patients without COVID-19.
Conclusions
VAE rates per 100 episodes of mechanical ventilation and per 1,000 ventilator-days were higher among COVID-19–positive patients than among COVID-19–negative patients. Over 50% of VAEs in patients with COVID-19 were caused by progressive ARDS, whereas less than 15% of VAEs in patients without COVID-19 were caused by progressive ARDS. These findings provide insight into the natural history of COVID-19 in ventilated patients and may inform targeted strategies to mitigate complications in this population.
Keywords: COVID-19, SARS-CoV-2, ventilator-associated event, ventilator-associated complication, mechanical ventilation
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral pandemic has increased the global burden of patients requiring mechanical ventilation. Between 5% and 20% of patients hospitalized with coronavirus disease (COVID-19) require ICU-level of care, and many of these patients require mechanical ventilation (1–6). Mortality rates for those who require mechanical ventilation have ranged from as high as 90% early in the pandemic to 30–50% in more recent cohorts (2, 3, 5, 7–13).
Little is known, however, about the incidence and causes of pulmonary complications in patients with COVID-19 who require mechanical ventilation. There remains debate about whether COVID-19 causes novel mechanisms of lung injury, leading to new clinical phenotypes of pneumonia and/or acute respiratory distress syndrome (ARDS) (14–17). There is also concern that providers may be limiting some aspects of care to minimize their exposure to potentially contagious patients (e.g., bronchoscopy for pulmonary hygiene) and/or to preserve personal protective equipment and that less frequent bedside care may put some patients at increased risk for ventilator-associated complications (VACs) (18). Patients with COVID-19 who require invasive ventilation also tend to have long courses of mechanical ventilation (median of 9–16 d), putting them at increased risk for complications (6, 9, 10, 13, 19).
Quality improvement programs have traditionally benchmarked care for ventilated patients by tracking ventilator-associated pneumonia (VAP) rates. VAP surveillance, however, has been problematic because of the subjectivity and lack of specificity of VAP definitions. The risk of VAP misdiagnosis may be accentuated in COVID-19 because fever, hypoxemia, and parenchymal infiltrates can all be caused by COVID-19 pneumonia and secondary ARDS in addition to VAP (20). In addition, VAP surveillance only captures one particular complication of mechanical ventilation rather than the fuller array of complications that can occur in ventilated patients (21, 22).
The Centers for Disease Control and Prevention (CDC) developed ventilator-associated event (VAE) surveillance as an alternative to VAP surveillance to provide a more objective and broader measure of the potential complications of mechanical ventilation (23–27). VAEs are defined by ⩾2 days of sustained increases in ventilator settings (rise in positive end-expiratory pressure [PEEP] of ⩾3 cm H2O and/or rise in the absolute fraction of inspired oxygen of ⩾20%) after ⩾2 days of stable or decreasing ventilator settings (28). Prior studies suggest that most VAEs are caused by VAP, pulmonary edema, atelectasis, and ARDS and that conditions with VAEs are associated with higher mortality rates and longer episodes of mechanical ventilation than similar conditions without VAEs (24–26, 29).
Given the ongoing burden of COVID-19, the high number of patients with COVID-19 requiring mechanical ventilation, and their risk for ventilator-associated harm, we sought to characterize and compare the incidence, causes, and outcomes of VAEs among all ventilated patients during the pandemic versus the prepandemic years and specifically among patients with and without COVID-19 during the initial pandemic surge.
Methods
Data Source and Definitions
We retrospectively identified all adult patients requiring mechanical ventilation in four hospitals in eastern Massachusetts from 2017 to 2020, including two academic (Brigham and Women’s Hospital and Massachusetts General Hospital) and two community hospitals (Faulkner Hospital and Newton-Wellesley Hospital). We limited our cohort to episodes of mechanical ventilation that began between March 1 and August 31 in each year to correspond to the time frame of the initial pandemic surge in Massachusetts in 2020. We identified VAEs by using data extracted from the hospitals’ electronic health record systems and by applying CDC VAE criteria (28). VAE definitions are nested: the subset of VAEs that are potentially infections are called “infection-related VACs” (IVACs), and the subset of IVACs that are potentially pneumonia are called “possible VAP” (PVAP). “IVAC-plus” refers to the count of IVACs that includes PVAPs (whereas “IVAC-alone” is IVAC excluding PVAPs). Ventilator-days and episodes of mechanical ventilation included all patients who spent a portion of a calendar day on mechanical ventilation, which we ascertained by identifying patients on the ventilator at either the start or end of each calendar day.
Patient age, sex, date of hospital admission, start and stop dates of mechanical ventilation, VAE dates, and mortality during hospitalization were also obtained from the electronic health record. Patients on extracorporeal membrane oxygenation were excluded as per the CDC’s VAE criteria. We derived patients’ comorbidities from diagnosis and diagnosis-related-group codes by using the method of Elixhauser and colleagues (30). Patient race was identified from self-reported data within the medical record; instances of a reported race of “other” were manually reviewed and corrected if race was documented elsewhere in the medical record. Patients were considered COVID-19–positive if they had received a positive SARS-COV-2 nucleic acid amplification test result from any site (nasopharyngeal, oropharyngeal, sputum/endotracheal) within 3 weeks of the onset of mechanical ventilation. The study was approved by the Mass General Brigham Institutional Review Board.
Comparison of VAE Rates in the Pandemic versus Prepandemic Years
We calculated the incidence of VAEs per 100 episodes of mechanical ventilation and per 1,000 ventilator-days for each year from 2017 to 2020 for the months of March through August. VAE incidence rates were calculated for each calendar month as the number of events per 100 episodes of mechanical ventilation and as the number of events per 1,000 ventilator-days. We included multiple VAEs per episode of mechanical ventilation for determining rates if their start dates were ⩾14 days apart, as per CDC VAE criteria.
Comparison of VAE Causes in COVID-19–Positive versus COVID-19–Negative Patients
We reviewed the medical records of 200 randomly selected patients with VAEs during the period of March to August 2020 (100 with COVID-19 and 100 without COVID-19) to determine their reasons for intubation and the conditions precipitating VAEs. Clinical notes, vital signs, laboratory values, and radiologic results were all reviewed to determine the primary etiology for PEEP and/or fraction of inspired oxygen being increased. Eligible reasons for intubation and precipitating conditions were based on the prior work by Kerlin and colleagues (31) with modifications made for COVID-19. VAP and aspiration were grouped together as one precipitating condition, given the overlap in the two processes and difficulty distinguishing between them. All 200 records were reviewed by one intensivist (J.W.); a random subset of 20 charts were also reviewed by a second intensivist (C.R.) to assess interrater reliability for the reason for intubation and the clinical event leading to the VAE. Early VAEs were defined as events occurring ⩽7 days after the onset of mechanical ventilation, and late VAEs were defined as those occurring after 7 days.
Statistical Analysis
Patients’ demographics and characteristics were summarized by using standard descriptive statistics. We reported means and standard deviations for normally distributed continuous variables and compared them by using a Student’s t test. We reported medians and interquartile ranges for non–normally distributed continuous variables and compared them by using the Wilcoxon rank-sum test. Categorical variables were compared by using a Fisher exact test. We compared VAE rates during the pandemic year (2020) versus the prepandemic years (2017–2019) by using two-sample tests of proportions. An α level of 0.05 was used for determining statistical significance. Interobserver agreements for intubation diagnoses and clinical events leading to VAEs were reported as the percent agreement and were measured by using Krippendorff’s α (K-α) (32). All other statistical analyses were performed by using R version 4.0.2 (R Foundation for Statistical Computing) (33).
Sensitivity Analyses
We performed two sensitivity analyses to better discern if causes for VAEs differed between patients with COVID-19 and patients without COVID-19 because a disproportionate fraction of those without COVID-19 with VAEs were intubated for surgery or for altered mental status rather than for respiratory failure. For the first sensitivity analysis, we compared characteristics and outcomes of VAEs in patients with COVID-19 with prepandemic patients in medical ICUs alone. For the second sensitivity analysis, we restricted the population without COVID-19 to patients intubated for respiratory failure as per medical record review.
Results
Study Population
There were 11,502 patients initiated on mechanical ventilation between each March through August of 2017–2020, including 13,270 episodes of mechanical ventilation, 79,423 ventilator-days, and 1,048 VAEs. The numbers of ventilated patients, ventilator episodes, ventilator-days, and VAEs per year are shown in Table 1. Patients’ demographics, comorbidities, durations of mechanical ventilation, and in-hospital mortality rates stratified by the presence versus the absence of VAEs, the study time period (pandemic vs. prepandemic), and COVID-19 status are shown in Table 2.
Table 1.
Ventilator episodes, ventilator-days, and VAEs by year
| Ventilated Patients | Ventilator Episodes | Ventilator-Days | VAE-Positive | |
|---|---|---|---|---|
| 2017 | 2,820 | 3,283 | 17,712 | 201 |
| 2018 | 2,752 | 3,179 | 16,246 | 192 |
| 2019 | 2,825 | 3,249 | 17,382 | 257 |
| 2020 | 3,105 | 3,559 | 28,083 | 398 |
| COVID-19–positive | 628 | 661 | 11,178 | 192 |
| COVID-19–negative | 2,477 | 2,898 | 16,905 | 206 |
Definition of abbreviations: COVID-19 = coronavirus disease; VAE = ventilator-associated event.
Table 2.
Demographics and characteristics of study patients stratified by VAE, study period, and COVID-19 status
| VAE-Positive |
VAE-Negative |
|||||
|---|---|---|---|---|---|---|
| Pandemic |
Prepandemic (n = 616) |
Pandemic |
Prepandemic (n = 9,095) |
|||
| COVID-19– Negative (n = 194) |
COVID-19– Positive (n = 172) |
COVID-19– Negative (n = 2,704) |
COVID-19– Positive (n = 489) |
|||
| Age, yr, mean (SD) | 58.5 (16) | 59.7 (14) | 60.2 (15) | 60.0 (16.4) | 61.6 (16) | 61.4 (16) |
| Sex, Male, n (%) | 117 (60) | 123 (72) | 367 (60) | 1,651 (61) | 300 (61) | 5,597 (62) |
| Racial group, n (%) | ||||||
| White | 127 (65) | 65 (38) | 448 (73) | 1,936 (72) | 162 (33) | 6,923 (76) |
| Black | 19 (10) | 28 (16) | 53 (9) | 236 (9) | 90 (18) | 676 (7) |
| Asian | 10 (5) | 5 (3) | 24 (4) | 94 (3) | 22 (4) | 284 (3) |
| Hispanic/Latino | 21 (11) | 55 (32) | 42 (7) | 213 (8) | 156 (32) | 588 (6) |
| Other | 6 (3) | 8 (5) | 17 (3) | 65 (2) | 22 (4) | 163 (2) |
| No response | 11 (6) | 11 (6) | 32 (5) | 160 (6) | 37 (8) | 461 (5) |
| Hypertension, n (%) | 122 (63) | 117 (68) | 414 (67) | 1,747 (65) | 324 (66) | 6,170 (68) |
| Diabetes, n (%) | 54 (28) | 93 (54) | 179 (29) | 814 (30) | 229 (47) | 2,627 |
| Elixhauser score, mean (SD) | 6.3 (2) | 5.8 (2) | 6.4 (2) | 5.7 (2) | 5.3 (2) | 5.4 (3) |
| Duration of MV, d, median (IQR) | 14 (17) | 22 (19) | 14 (14) | 3 (3) | 11 (13) | 2 (3) |
| Time to a VAE from initiation of MV, d, median (IQR) | 4 (6) | 5 (9) | 4 (5) | N/A | N/A | N/A |
| In-hospital mortality, n (%) | 73 (38) | 52 (30) | 261 (42) | 641 (24) | 158 (32) | 2,151 (24) |
Definition of abbreviations: COVID-19 = coronavirus disease; IQR = interquartile range; MV = mechanical ventilation; N/A = not applicable; SD = standard deviation; VAE = ventilator-associated event.
N is equal to the number of distinct ventilator episodes for each subgroup. For ventilator episodes with multiple VAEs, only the first VAE is included for analysis.
VAE Rates and Subtypes in the Pandemic Period versus Prepandemic Years
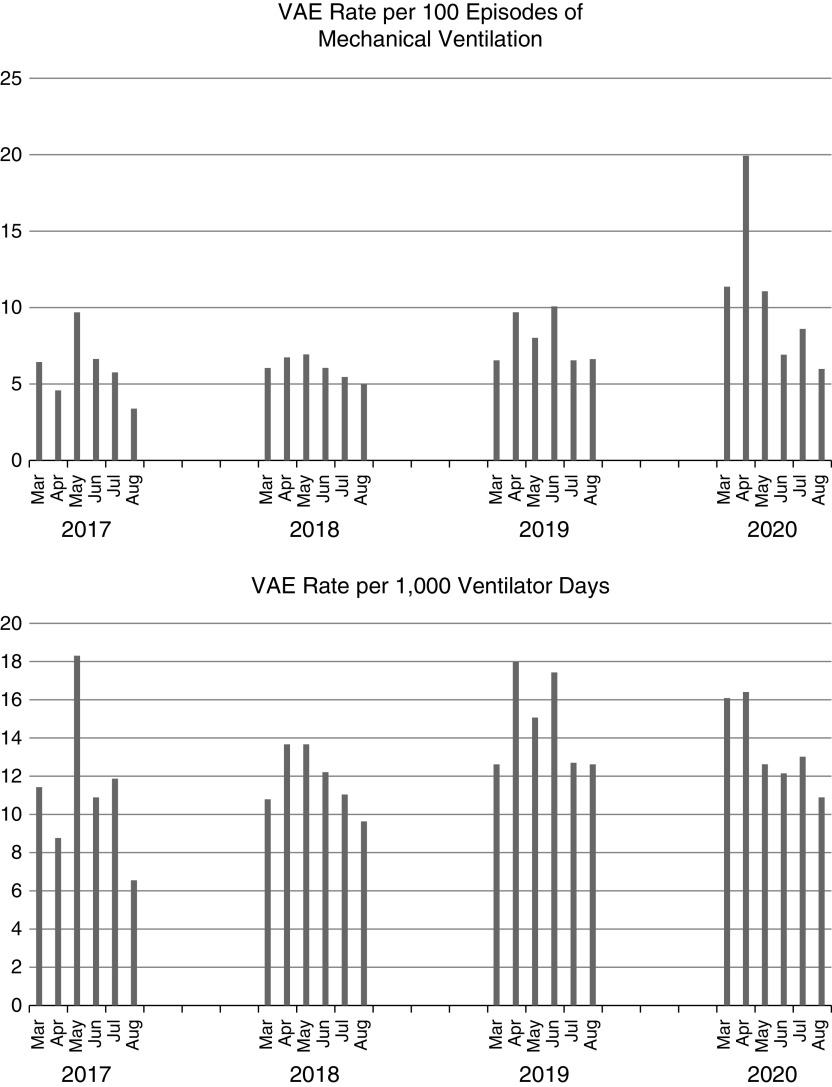
The VAE rate per 100 episodes of mechanical ventilation and VAE rate per 1,000 ventilator-days by month are shown in Figure 1. The rate of VAEs per 100 episodes of mechanical ventilation was higher in 2020 than in prior years (11.2 vs. 6.7; P < 0.01), but the rate of VAEs per 1,000 ventilator-days was similar (14.2 vs. 12.7; P = 0.08). The percentages of noninfectious VAEs (VAC-alone) and potentially infectious VAEs (IVAC-plus) were similar in 2020 as compared with other years (VAC-alone, 51.9% in 2020 vs. 58.3% in other years [P = 0.12]; IVAC-plus, 48.1% in 2020 vs. 41.7% in other years [P = 0.12]).
Figure 1.
Ventilator-associated event rate per 100 episodes of mechanical ventilation and per 1,000 ventilator-days by month from 2017 to 2020. VAE = ventilator-associated event.
Outcomes of Patients with VAEs in the Pandemic Period versus Prepandemic Years
The durations and outcomes of mechanical ventilation among patients with VAEs were compared between the pandemic (2020) and prepandemic (2017–2019) periods. The median duration of mechanical ventilation among patients with VAEs was longer during the pandemic than during prepandemic period (18 d vs. 14 d; P < 0.01). The median interval from initiation of mechanical ventilation to a VAE was similar between the two time periods (5 d vs. 4; P = 0.28). Unadjusted mortality was lower among patients with VAEs during the pandemic period than during the prepandemic period (34% vs. 42%; P = 0.01).
Rates and Characteristics of Pandemic Period VAEs in COVID-19–Positive versus COVID-19–Negative Patients
A total of 3,105 patients initiated mechanical ventilation (628 with COVID-19 and 2,477 without COVID-19) during the period from March to August of 2020. These patients underwent 3,559 episodes of mechanical ventilation (661 in patients with COVID-19 and 2,898 in patients without COVID-19) and developed 398 VAEs (192 in patients with COVID-19 and 206 in patients without COVID-19). Of these, 29 patients (17 with COVID-19 and 12 without COVID-19) had an episode of ventilation with two VAEs, and 3 patients had an episode of ventilation with three VAEs. Patients with COVID-19 had higher rates of VAEs per 100 episodes of mechanical ventilation (29.0 vs. 7.1; P < 0.01) and per 1,000 ventilator-days (17.2 vs. 12.2; P < 0.01) than did COVID-19–negative patients during 2020. VAE rates among patients with COVID-19 were similar in academic and community hospitals (see Table E1 in the online supplement).
Demographics, baseline characteristics, and comorbidities stratified by COVID-19 status are shown in Table 2. The mean age (59.7 vs. 58.5; P = 0.46) was similar and the Elixhauser score was lower (5.8 vs. 6.3; P = 0.03) in patients with COVID-19 with VAEs than in patients without COVID-19 with VAEs, but male sex (72% vs. 60%; P = 0.03), Hispanic/Latino and Black racial groups (P < 0.01), and diabetes (54% vs. 28%; P < 0.01) were more common among those with COVID-19. There was no significant association between COVID-19 status and the VAE subtype (P = 0.32), with similar distributions of VAC (55% vs. 49%), IVAC (30% vs. 33%), and PVAP (15% vs. 18%) among patients with COVID-19 compared with those without COVID-19. Patients with COVID-19 with VAEs were ventilated for longer than ventilated patients with COVID-19 without VAEs (median, 22 d vs. 11 d; P < 0.01) but had similar in-hospital mortality rates (30% vs. 32%; P = 0.64).
Outcomes of Pandemic Period VAEs in COVID-19–Positive versus COVID-19–Negative Patients
The median duration of mechanical ventilation was longer among patients with COVID-19 and VAEs than among patients without COVID-19 with VAEs (22 d vs. 14 d; P < 0.01), the time to a VAE was longer (5 d vs. 4 d; P < 0.01), and in-hospital mortality was similar (30% vs. 38%; P = 0.15).
Reasons for Intubation and Pulmonary Events Leading to VAEs among COVID-19–Positive versus COVID-19– Negative Patients during the Pandemic Period
Agreement between the two physician reviewers was good for both the reasons for intubation (80% agreement; K-α, 0.73) and the triggers for VAEs (95% agreement, K-α, 0.93). Table 3 shows the primary clinical diagnoses leading to intubation. Among patients with COVID-19, 91 out of 100 were intubated for COVID-19 pneumonia. The most frequent reasons for intubation among patients without COVID-19 were surgery (27%) and airway protection (25%).
Table 3.
Diagnosis leading to intubation among patients with VAEs during the pandemic period stratified by COVID-19 status
| COVID-19–Negative (n = 100) |
COVID-19–Positive (n = 100) |
|
|---|---|---|
| Surgery | 27 | 3 |
| AMS/airway protection | 25 | 3 |
| Pneumonia | ||
| CAP | 8 | 0 |
| HAP | 5 | 0 |
| Viral | 0 | 91 |
| Cardiac arrest | 9 | 1 |
| Non–COVID-19 ARDS | 8 | 0 |
| Other/unknown | 8 | 1 |
| Pulmonary edema | 8 | 1 |
| Asthma/COPD | 2 | 0 |
Definition of abbreviations: AMS = altered mental status; ARDS = acute respiratory distress syndrome; CAP = community-acquired pneumonia; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease; HAP = hospital-acquired pneumonia; VAEs = ventilator-associated events.
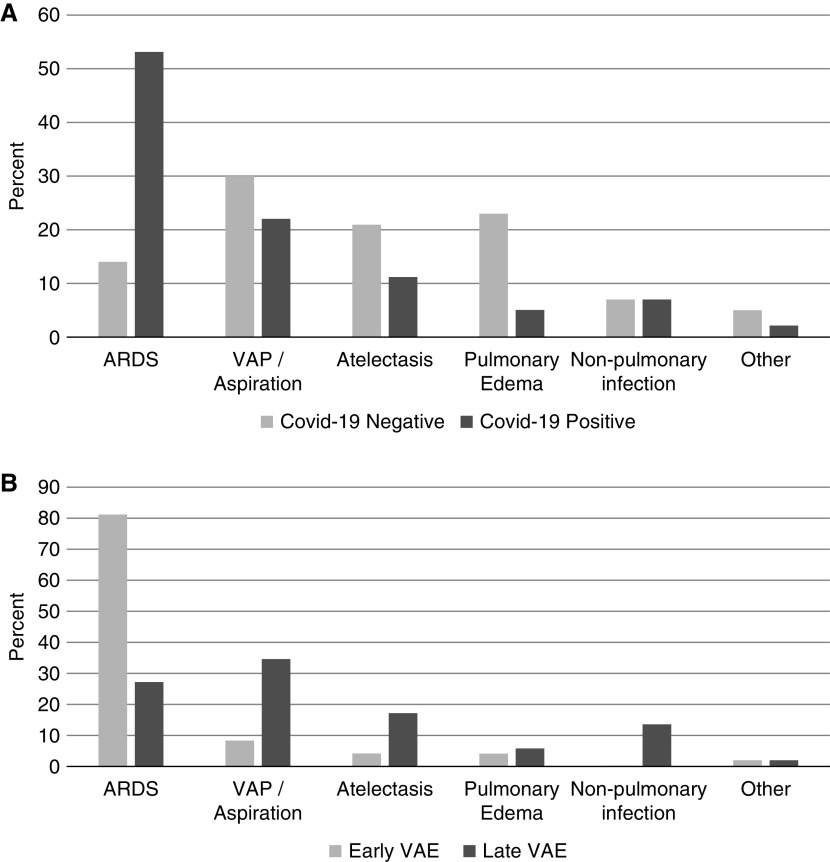
The distribution of clinical events that triggered VAEs in patients with versus without COVID-19 are shown in Figure 2A. ARDS (53%) and VAP (22%) were the most common events leading to VAEs among patients with COVID-19. By contrast, VAP (30%), pulmonary edema (23%), and atelectasis (21%) were the most common events leading to VAEs among patients without COVID-19 (ARDS was the precipitant in only 14%). Of the 100 VAEs in patients with COVID-19, 48 occurred within 7 days of intubation (Figure 2B). Most VAEs within 7 days of intubation were caused by progressive ARDS (81%), whereas those occurring after Day 7 were primarily caused by VAP (35%), ARDS (27%), atelectasis (17%), and extrapulmonary infections (13%).
Figure 2.
Clinical events leading to pandemic period ventilator-associated events (VAEs) stratified by (A) COVID-19 status and (B) early versus late VAEs. ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease; VAP = ventilator-associated pneumonia.
Sensitivity Analyses
Sensitivity analyses were designed to increase comparability between non–COVID-19–related VAEs, both before and during the pandemic, and COVID-19–related VAEs. In the first sensitivity analysis, we restricted our analysis to medical ICU patients alone. In the prepandemic period, there were 158 VAEs in dedicated medical ICUs at the two academic medical centers. The ventilator-associated length of stay (15 d vs. 14 d) and the time from intubation to a VAE (4 d each) were similar for medical ICU patients with VAEs during the prepandemic period and COVID-19 period. In-hospital mortality rates were higher (63% vs. 42%) (see Table E2 in the online supplement).
For the second sensitivity analysis, we focused on patients intubated for respiratory failure alone as per medical record review. Of the 100 patients without COVID-19 who experienced a VAE during the pandemic, 52 were intubated for surgery or an altered mental status. Among the remaining 48 (with intubation for pneumonia, cardiac arrest, non–COVID-19 ARDS, pulmonary edema, asthma/chronic obstructive pulmonary disease, and unknown reasons), the duration of mechanical ventilation was shorter (11 d vs. 14 d), the time to a VAE was similar (4 d each), and the in-hospital mortality rate was higher (50% vs. 38%) compared with the larger group without COVID-19 who experienced VAEs during the pandemic (see Table E2 in the online supplement). Among patients without COVID-19 with VAEs, both before and during the pandemic, there was a higher percentage of oncologic diagnoses than among patients with COVID-19 with VAEs.
Discussion
We report on how the incidence, causes, and outcomes of VAEs in four hospitals were affected by the COVID-19 pandemic. We found an increase in the incidence of VAEs per 100 episodes of mechanical ventilation in 2020 compared with similar time periods in prior years but found no change in the incidence per 1,000 ventilator-days, suggesting that higher VAE rates in 2020 may in part be a reflection of the fact that patients with COVID-19 typically require mechanical ventilation for longer periods than patients without COVID-19. The rate of VAEs was higher among patients with COVID-19 than among most patients without COVID-19. Most VAEs in patients with COVID-19, however, were caused by ARDS and pneumonia, whereas those in patients without COVID-19 were caused by pneumonia, pulmonary edema, and atelectasis.
COVID-19–positive patients with VAEs remained on mechanical ventilation for much longer than COVID-19–negative patients with VAEs. This may reflect a predilection to intubate patients with escalating oxygen requirements early in the course of their illness during the early months of the pandemic, coupled with the limited use of intubation-sparing modalities (high-flow nasal cannula, bilevel positive airway pressure) for fear of generating aerosols. This may have both extended these patients’ duration of mechanical ventilation and increased their risk for VAEs. It is also possible that ARDS in COVID-19 may be associated with a distinct pathobiology that leads to more severe and sustained disease than does ARDS in patients without COVID-19 (19). For example, although there are shared pathologic findings of diffuse alveolar damage between COVID-19 and H1N1 influenza–induced ARDS, COVID-19 lung specimens are notable for more marked endothelial inflammation and pulmonary capillary microthrombi (14, 34).
Despite the longer duration of mechanical ventilation among patients with COVID-19 with VAEs than among patients without COVID-19 with VAEs, in-hospital mortality rates were similar. Patients with COVID-19 with respiratory failure and ARDS often require prolonged mechanical ventilation, but a certain fraction of these patients, particularly those who were previously healthy, go on to recover. Patients without COVID-19 with VAEs in our cohort were more likely to have cancer and other comorbidities associated with progression and death rather than with prolonged ventilation and recovery.
Patients with COVID-19 with VAEs were more likely to be Black or Hispanic and to have diabetes than patients without COVID-19 with VAEs. The overrepresentation of people of color mirrors prior investigations that have found that COVID-19 disproportionately affects Black and Hispanic people (8, 35–38) and that members of these groups with COVID-19 tend to have worse outcomes than other groups (9, 39, 40).
The finding that most VAEs in patients with COVID-19 were attributable to ARDS, particularly during the first week of illness, is a distinctive finding relative to the usual causes of VAEs. Historically, most VAEs have been due to pneumonia, pulmonary edema, and atelectasis (41, 42). It is possible that the higher rate of ARDS-triggered VAEs in patients with COVID-19, particularly early after intubation, reflects a weakness of VAE definitions as they pertain to COVID-19, insofar as VAE criteria may be being triggered by the natural progression of COVID-19 ARDS rather than by a distinct and superimposed complication. The requirement for 2 days of stable oxygenation before VAEs can be considered a possibility in patients should mitigate this potential weakness, but VAE criteria could still be triggered by patients undergoing a stuttering progression of ARDS. Patients with COVID-19 on mechanical ventilation may also be more susceptible to ventilator-induced lung injury, or there may be an interaction between some corollary aspects of care, such as blood transfusions or deep sedation, that contribute to clinical deterioration after a period of stability. Alternatively, the emphasis on conservative fluid management in patients with COVID-19 may shift the epidemiology of VAE triggers away from pulmonary edema toward other causes.
Among patients with COVID-19, those with VAEs were ventilated for longer periods than those without VAEs. Surprisingly, however, we found that the in-hospital mortality rates were similar for patients with COVID-19 with and without VAEs. The high mortality rate in patients with COVID-19 without VAEs may be due to steadily progressive ARDS, without periods of stability or improving oxygen, and subsequent death.
Strengths and Limitations
Strengths of our study include the use of VAE criteria as an objective and consistent means of measuring complications across time and between hospitals, the large number of mechanical ventilation episodes evaluated, and the use of detailed chart reviews to determine the clinical events precipitating VAEs. Limitations of our study include the restriction of the analysis to the first wave of the pandemic in a single region of the country, which may limit generalizability to other regions and to more recent management patterns for COVID-19. In particular, it is unclear whether and how advances in treatment, including deferring intubation whenever possible and prescribing corticosteroids, remdesivir, and tocilizumab, will affect the incidence and outcomes of VAEs in patients with COVID-19. One might speculate, for example, that the increased use of high-flow nasal cannula treatment and noninvasive ventilation after the initial wave of the pandemic may decrease VAE rates among COVID-19–positive patients and that those who went on to require intubation might have had more traditional causes for VAEs than early progressive ARDS. This is an important topic for future research. Our analyses of reasons for intubation and causes of VAEs were done by a small number of reviewers and may have been colored by their subjective judgments. We were able to demonstrate, however, a very high level of agreement between observers, and clinical insight into VAE triggers can only reliably be gathered through manual review. A potential concern of VAE surveillance is that a VAE may be triggered by events that do not represent clinical deterioration. Among the 200 chart reviews, however, we were able to identify clinical reasons for the PEEP or fraction of inspired oxygen increase that triggered each VAE. We did not attempt to determine whether the longer periods of mechanical ventilation and differential mortality in COVID-19–positive versus COVID-19–negative patients with VAEs were due to COVID-19 itself or if they better reflected the kinds of patients who developed COVID-19. A more granular accounting of comorbidities would help differentiate this, although we were able to show that patients without COVID-19 with VAEs were more likely to have cancer than those with COVID-19. Our purpose, however, was not to determine the attributable morbidity and mortality of COVID-19 but rather to describe at a population level how COVID-19 has affected VAE incidence, causes, and outcomes.
Conclusions
In conclusion, we found that VAEs were more frequent on a per-episode basis during the pandemic period than during prior years but that the rates per 1,000 ventilator-days were similar, reflecting the longer duration of mechanical ventilation in patients with COVID-19. Patients with COVID-19 had a higher rate of VAEs than patients without COVID-19. Progressive ARDS accounted for over half of VAEs in patients with COVID-19, whereas pulmonary edema, atelectasis, and pneumonia were the most common triggers in patients without COVID-19. These findings provide insight into the natural history of COVID-19 in ventilated patients and may inform strategies to mitigate complications in this population.
Footnotes
Supported by the Centers for Disease Control and Prevention (6U54CK000484-04-02) and by the National Heart, Lung, and Blood Institute of the National Institutes of Health (T32HL007633). The Centers for Disease Control and Prevention and National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions: J.W. performed all statistical analyses and primary drafting of the manuscript. All authors were involved in primary study design, results interpretation, critical review, and final approval of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. COVID-19 in critically ill patients in the Seattle region: case series. N Engl J Med . 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA . 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 3. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA . 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72-314 cases from the Chinese Center for Disease Control and Prevention. JAMA . 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5. Wunsch H. Mechanical ventilation in COVID-19: interpreting the current epidemiology. Am J Respir Crit Care Med . 2020;202:1–4. doi: 10.1164/rccm.202004-1385ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gamberini L, Tonetti T, Spadaro S, Zani G, Mazzoli CA, Capozzi C, et al. ICU-RER COVID-19 Collaboration Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: multicenter observational study in fifteen Italian ICUs. J Intensive Care . 2020;8:80. doi: 10.1186/s40560-020-00499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. King CS, Sahjwani D, Brown AW, Feroz S, Cameron P, Osborn E, et al. Outcomes of mechanically ventilated patients with COVID-19 associated respiratory failure. PLoS One . 2020;15:e0242651. doi: 10.1371/journal.pone.0242651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet . 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Twigg HL, III, Khan SH, Perkins AJ, Roberts S, Sears C, Rahman O, et al. Mortality rates in a diverse cohort of mechanically ventilated patients with novel coronavirus in the urban Midwest. Crit Care Explor . 2020;2:e0187. doi: 10.1097/CCE.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roedl K, Jarczak D, Thasler L, Bachmann M, Schulte F, Bein B, et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: a multicentric study in Germany. Aust Crit Care . 2021;34:167–175. doi: 10.1016/j.aucc.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fried MW, Crawford JM, Mospan AR, Watkins SE, Munoz B, Zink RC, et al. Patient characteristics and outcomes of 11 721 patients with coronavirus disease 2019 (COVID-19) hospitalized across the United States. Clin Infect Dis . 2021;72:e558–e565. doi: 10.1093/cid/ciaa1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. COVID-19 Lombardy ICU Network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA . 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anesi GL, Jablonski J, Harhay MO, Atkins JH, Bajaj J, Baston C, et al. Characteristics, outcomes, and trends of patients with COVID-19-related critical illness at a learning health system in the United States. Ann Intern Med . 2021;174:613–621. doi: 10.7326/M20-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med . 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med . 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tobin MJ. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med . 2020;201:1319–1320. doi: 10.1164/rccm.202004-1076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med . 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Immunization and Respiratory Diseases (NCIRD) Atlanta, GA: Centers for Disease Control and Prevention; 2020. Recommended infection prevention and control (IPC) practices when caring for a patient with suspected or confirmed SARS-CoV-2 infection.https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html [Google Scholar]
- 19.Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LDJ, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study Lancet Respir Med 20219139–148.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. François B, Laterre PF, Luyt CE, Chastre J. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit Care . 2020;24:289. doi: 10.1186/s13054-020-03013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control . 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 22. Klompas M. Interobserver variability in ventilator-associated pneumonia surveillance. Am J Infect Control . 2010;38:237–239. doi: 10.1016/j.ajic.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 23. Magill SS, Klompas M, Balk R, Burns SM, Deutschman CS, Diekema D, et al. Developing a new, national approach to surveillance for ventilator-associated events. Am J Crit Care . 2013;22:469–473. doi: 10.4037/ajcc2013893. [DOI] [PubMed] [Google Scholar]
- 24. Klompas M, Khan Y, Kleinman K, Evans RS, Lloyd JF, Stevenson K, et al. CDC Prevention Epicenters Program Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS One . 2011;6:e18062. doi: 10.1371/journal.pone.0018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klompas M, Magill S, Robicsek A, Strymish JM, Kleinman K, Evans RS, et al. CDC Prevention Epicenters Program Objective surveillance definitions for ventilator-associated pneumonia. Crit Care Med . 2012;40:3154–3161. doi: 10.1097/CCM.0b013e318260c6d9. [DOI] [PubMed] [Google Scholar]
- 26. Skrupky LP, McConnell K, Dallas J, Kollef MH. A comparison of ventilator-associated pneumonia rates as identified according to the National Healthcare Safety Network and American College of Chest Physicians criteria. Crit Care Med . 2012;40:281–284. doi: 10.1097/CCM.0b013e31822d7913. [DOI] [PubMed] [Google Scholar]
- 27. Muscedere J, Sinuff T, Heyland DK, Dodek PM, Keenan SP, Wood G, et al. Canadian Critical Care Trials Group The clinical impact and preventability of ventilator-associated conditions in critically ill patients who are mechanically ventilated. Chest . 2013;144:1453–1460. doi: 10.1378/chest.13-0853. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention National Healthcare Safety Network. Atlanta, GA: Centers for Disease Control and Prevention; 2021. https://www.cdc.gov/nhsn/PDFs/pscManual/10-VAE_FINAL.pdf [Google Scholar]
- 29.Klompas M.Complications of mechanical ventilation: the CDC’s new surveillance paradigm N Engl J Med 20133681472–1475.. [DOI] [PubMed] [Google Scholar]
- 30. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care . 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 31. Kerlin MP, Trick WE, Anderson DJ, Babcock HM, Lautenbach E, Gueret R, et al. Interrater reliability of surveillance for ventilator-associated events and pneumonia. Infect Control Hosp Epidemiol . 2017;38:172–178. doi: 10.1017/ice.2016.262. [DOI] [PubMed] [Google Scholar]
- 32.Freelon D. Chapel Hill, NC: Deen Freelon; 2010. http://dfreelon.org/utils/recalfront/recal2/ [Google Scholar]
- 33.R Core Team Vienna, Austria: R Foundation for Statistical Computing; 2020https://www.R-project.org/. [Google Scholar]
- 34.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study Lancet Infect Dis 2020201135–1140.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ . 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sim BLH, Chidambaram SK, Wong XC, Pathmanathan MD, Peariasamy KM, Hor CP, et al. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: a nationwide observational study. Lancet Reg Health West Pac . 2020;4:100055. doi: 10.1016/j.lanwpc.2020.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu J, Wang Y. The clinical characteristics and risk factors of severe COVID-19. Gerontology . 2021;67:255–266. doi: 10.1159/000513400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coronavirus Disease 2019 (COVID-19)-associated Hospitalization Surveillance Network (COVID-NET). Atlanta, GA: Centers for Disease Control and Prevention; 2020https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html [Google Scholar]
- 40.Centers for Disease Control and Prevention Atlanta, GA: Centers for Disease Control and Prevention; 2021https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html. [Google Scholar]
- 41. Bouadma L, Sonneville R, Garrouste-Orgeas M, Darmon M, Souweine B, Voiriot G, et al. OUTCOMEREA Study Group Ventilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit Care Med . 2015;43:1798–1806. doi: 10.1097/CCM.0000000000001091. [DOI] [PubMed] [Google Scholar]
- 42. Klein Klouwenberg PM, van Mourik MS, Ong DS, Horn J, Schultz MJ, Cremer OL, et al. MARS Consortium Electronic implementation of a novel surveillance paradigm for ventilator-associated events: feasibility and validation. Am J Respir Crit Care Med . 2014;189:947–955. doi: 10.1164/rccm.201307-1376OC. [DOI] [PubMed] [Google Scholar]