Abstract
Molecular fingerprinting with the IS6110 insertion sequence is useful for tracking transmission of Mycobacterium tuberculosis within a population or confirming specimen contamination in the laboratory or through instrumentation. Secondary typing with other molecular methods yields additional information as to the relatedness of strains with similar IS6110 fingerprints. Isolated, relatively rare, random events within the M. tuberculosis genome alter molecular fingerprinting patterns with any of the methods; therefore, strains which are different by two or more typing methods are usually not considered to be closely related. In this report, we describe two strains of M. tuberculosis, obtained from the same bronchoscope 2 days apart, that demonstrated unique molecular fingerprinting patterns by two different typing methods. They were closely linked through the bronchoscope by a traditional epidemiologic investigation. Genetic analysis of the two strains revealed that a single event, the transposition of an IS6110 insertion sequence in one of the strains, accounted for both the differences in the IS6110 pattern and the apparent deletion of a spacer in the spoligotype. This finding shows that a single event can change the molecular fingerprint of a strain in two different molecular typing systems, and thus, molecular typing cannot be the only means used to track transmission of this organism through a population. Traditional epidemiologic techniques are a necessary complement to molecular fingerprinting so that radical changes within the fingerprint pattern can be identified.
Molecular fingerprinting of Mycobacterium tuberculosis with the IS6110 insertion sequence has been used since the early 1990s (9, 27, 31, 35) in epidemiologic investigations to identify transmission between individuals. Both the number and the sizes of the restriction fragments containing the insertion sequences in the genome are used to determine fingerprint patterns. However, because of ongoing random genetic mutations, the molecular fingerprints are subject to change. Thus, changes will occur in strains that are closely related and good epidemiological links will be found between strains that have some differences in fingerprint patterns. Some changes in IS6110 banding patterns have been noted for isolates from cultures collected more than 90 days apart from the same individual (39). Several other typing methods based on different variable regions within the M. tuberculosis genome have been developed to be used as independent typing methods or to be used in combination with each other or with IS6110 restriction fragment length polymorphism (RFLP) analysis to further determine the relatedness of M. tuberculosis strains. With time, the random nature of these variable genetic systems results in ongoing changes, regardless of the typing method used. Strains that are different by two or more typing methods are usually not considered to be closely related. Linkages between patients infected with such strains are not typically made using conventional epidemiology.
The use of inadequately cleaned fiber-optic bronchoscopes have resulted in both transmission of M. tuberculosis (1, 29, 30, 37, 38) and false-positive cultures from the bronchial washings that did not cause detectable infection in the second patient (7, 13, 22, 32, 34). The propensity of fiber-optic bronchoscopes to harbor mycobacteria is due to both inherent difficulties in disinfecting the instrument (7, 33, 38) and resistance of acid-fast organisms to standard disinfecting procedures (2, 12, 28). Due to frequent reports of laboratory contamination (4, 6, 8, 10, 13, 14, 26), a high degree of suspicion should be raised when a few colonies of M. tuberculosis are cultured from clinical specimens when the patient does not exhibit signs or symptoms of tuberculosis disease.
In this report, we describe two strains of M. tuberculosis, obtained from the same bronchoscope 2 days apart, that demonstrated unique molecular fingerprinting patterns by two different typing methods. A traditional epidemiologic investigation uncovered apparent contamination of the second culture specimen from the bronchoscope. Molecular investigation of the two M. tuberculosis strains revealed that an IS6110 transposition had occurred in the second strain, resulting in an additional band. Interestingly, the second strain also had a loss of one spacer on spoligotyping. Although molecular fingerprinting alone would not have connected these two strains, the fingerprinting information coupled with data from a traditional epidemiologic investigation and the streptomycin resistance revealed that these strains were definitely closely related.
MATERIALS AND METHODS
Primary AFB cultures.
The specimens were processed with equal amounts of 4% NaOH and planted on two tubes of Lowenstein-Jensen medium and two tubes of Middlebrook 7H11 agar. The smear-positive specimen was also cultured on a 7H10 plate and in 7H9 broth enriched with 0.2% egg yolk. The broth from the acid-fast bacillus (AFB) smear-positive specimen was probed on day 8 for M. tuberculosis utilizing AccuProbe (Gen-Probe, San Diego, Calif.). Colonies were counted on the solid media after growth. Identification of both cultures was confirmed with high-performance liquid chromatography. M. tuberculosis organisms isolated from both patients were then sent to the Southeastern Tuberculosis Genotyping Laboratory for molecular typing.
Molecular typing of strains.
IS6110 RFLP typing was performed using standard techniques (14, 23, 35). Additionally, two PCR-based molecular typing methods were used to subtype the two strains. Spoligotyping, which is based on detecting the presence or absence of 43 spacers found between the 36-bp direct repeats of M. tuberculosis, was done using membranes from Isogen Biosciences BV, Maarssen, The Netherlands, using previously described methods (3, 20). Size determinations of seven variable number tandem repeat (VNTR) regions in the two strains were performed using previously reported methods (19, 24), and the size of each was compared to the known size of each tandem repeat from strain H37Rv. All DNA sequencing was done at the Sequencing Core Facility at The University of Alabama at Birmingham by using the ABI PRISM model 377 version 3.3. PCR products were cleaned for sequencing using the standard Qiagen method. The following primers were used in this study: DR7 (5′-TCGGACAGCATCTCCCCGGGCGGG), U16B7 (5′-CATCATCAGCAGGCATTGTTA) (15), INS1 (5′-CGTGAGGGCATCGAGGTGGC), designed to hybridize to IS6110, and DRa (5′-GGTTTTGGGTCTGACGAC-3′, biotinylated at the 5′ end) and DRb (5′-CCGAGAGGGGACGGAAAC), designed to amplify sequences adjacent to the insertion element.
RESULTS
Traditional epidemiologic investigation.
Patient no. 2 was a 56-year-old white male with carcinoma of the lung who underwent fiber-optic bronchoscopy because of presumed lung infection. Bronchoalveolar lavage was performed and the specimen was sent for acid-fast staining and mycobacterial culture. The specimen was AFB smear negative but the culture grew five colonies of M. tuberculosis on Lowenstein-Jensen media. The strain was noted to be streptomycin resistant. The patient did not appear clinically to have tuberculosis disease, and cross-contamination of the bronchoscopic specimen was suspected. The only other M. tuberculosis-positive specimen that had been in the laboratory at that time was a bronchoscope specimen from patient no. 1, who had known smear-positive tuberculosis disease that was streptomycin resistant. The bronchoscopy specimens were processed in the laboratory on different days and were not manipulated on a common day; therefore, contamination within the laboratory was unlikely. A search for other possible mechanisms of contamination revealed that the bronchoscope had last been used 2 days earlier to collect a smear-positive specimen from patient no. 1. The two patients were never in the same location within the hospital and were never in areas that shared ventilation. They did not name each other as contacts and they had no contacts in common. The two M. tuberculosis isolates were the only isolates cultured in the hospital laboratory over the entire year.
Molecular investigation of strains.
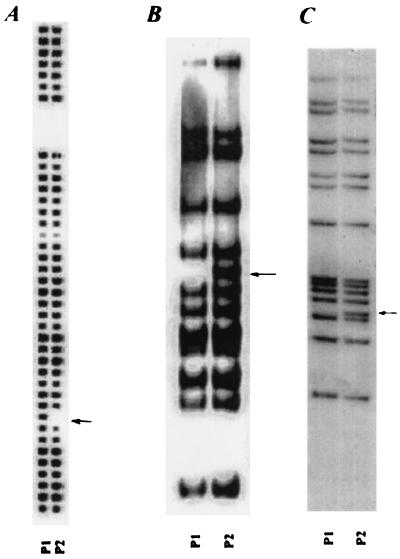
Because patient no. 2's positive culture was thought to be the result of contamination, spoligotyping was initially performed on both M. tuberculosis strains since results can be obtained with the PCR-based method very quickly. The results were equivocal, with a single spacer missing from the isolate from patient no. 2 (Fig. 1A). The isolates were then typed using the standard IS6110 methodology. Although both strains had 15 IS6110 fragments that matched, the isolate from patient no. 2 had an additional IS6110 fragment at 2.5 kb (Fig. 1B). The additional band was also visible after SacI digestion (Fig. 1C). Molecular typing with VNTR showed matching profiles of 6,3,3,3,2,2,2 repeats in the VNTR loci as reported by Frothingham et al. (19). Both strains were found to be streptomycin resistant.
FIG. 1.
Molecular typing of the strains. (A) Spoligotype image of isolates from patient no. 1 (P1) and patient no. 2 (P2). The arrow indicates spacer 9, which was missing in the P2 isolate. (B) IS6110 RFLP fragment digested with PvuII. An arrow indicates the additional 2.5-kb band in the P2 isolate. (C) IS6110 RFLP fragment digested with SacI. The additional band is indicated by the arrow.
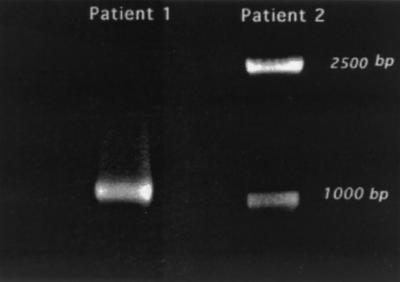
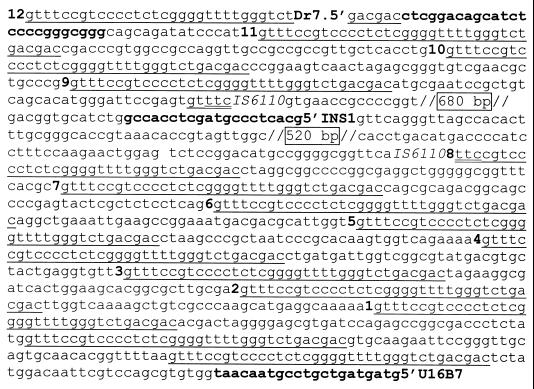
The strong link between the two patients provided by the discovery of the common bronchoscope found by traditional epidemiologic investigation prompted further molecular investigation. Differences in the spoligotyping pattern were further investigated by PCR amplification of the direct repeat region containing spacer 9 using primers described by Fang et al. (16). Although a deletion of one spacer was expected, the results were surprising in that patient no. 2's isolate had two amplicons, 1.0 and 2.5 kb, while patient no. 1 had only one 1.0-kb amplicon (Fig. 2). The three amplicons were purified by excising the bands from agarose and were sequenced utilizing the primers which had been used to amplify them. The 2.5-kb band was found to have an IS6110 insertion in direct repeat 8 that was 5 bp (2 bp and the 3-bp repeat) from spacer 9 (Fig. 3). The 1-kb band from patient no. 1's isolate was found to be the wild-type direct repeats, as expected. The 1-kb band from patient no. 2's isolate was found to be a spurious band that required only primer DR7 for amplification. This band was cloned and sequenced using universal primers from the vector plasmid. The band mapped to bp 3,003,000 to 3,004,000 (Rv3536 and Rv3537) on the H37Rv chromosome (11). Southern blottings using this fragment as a probe revealed a single 3.4-kb PvuII fragment from a panel of M. tuberculosis strains, including both patients' isolates as well as seven other clinical isolates and the reference strain MTB14323 (35), thus confirming this to be a spurious band amplified with only one primer.
FIG. 2.
PCR products of isolates from patients no. 1 and 2 obtained with a forward primer corresponding to spacer 12 (DR7) and a reverse primer corresponding to a sequence downstream of spacer 1 (U16B7). The predicted size of the band from H37Rv was 1009 bp, which matches the size of the band of the isolate from patient 1.
FIG. 3.
Sequence indicating a portion of the direct repeat region and the IS6110 insertion site of the 2.5-kb PCR fragment from patient no. 2's isolate. Primers used are indicated by bold type, with the name in bold on the 5′ end. Direct repeats are indicated by a single underline, with the spacer number indicated 3′ of the direct repeat just before that number's spacer. The three duplicated nucleotides are indicated by a double underline. The boxed numbers indicate the IS6110 sequences that were deleted.
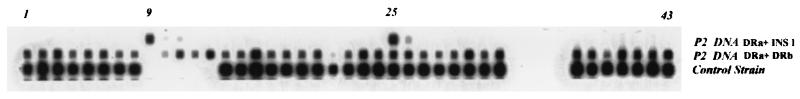
To determine if spacer 9 in patient no. 2's strain could be amplified and detected, the spoligotyping membrane and primer DRa (the labeled primer used in spoligotyping) were used with the IS6110-specific primer INS1, which is directed toward the left end of IS6110 (Fig. 3). Figure 4 shows that with these primers, both spacers (9 and 25) adjacent to the two IS6110 insertions in the direct repeat region were amplified and detected on the spoligotyping membrane. Spacer 9 was also amplified from the 2.5-kb PCR product from patient no. 2's isolate by using other IS6110-specific primers (data not shown).
FIG. 4.
A common spoligotype (bottom) compared with spoligotypes of patient no. 2's isolate. The spoligotype of patient no. 2's strain using DRa and DRb showed a deletion of spacer 9; using only INS1 (an IS6110-specific primer directed toward the right end of the insertion sequence) and DRa (which is biotin labeled and hybridizes to the direct repeat region with the 3′ end toward the lower numbered spacer) showed that the IS6110 adjacent spacers 9 and 25 were amplified.
DISCUSSION
In this report, we describe two strains of M. tuberculosis, obtained from the same bronchoscope 2 days apart, that demonstrated unique molecular fingerprinting patterns by the two most commonly used typing methods. The differences identified in both the IS6110 fingerprint and the spoligotype were shown to be the result of a single transposition event. Ordinarily, when two strains of tuberculosis differ by two typing methods, it is not thought that the strains are closely related. However, as this report shows, molecular fingerprints of organisms can change suddenly, by both IS6110 fingerprinting and spoligotyping, as a result of a single event. Therefore, traditional epidemiology is a necessary complement to molecular tracking of organisms within populations.
There is overwhelming evidence that the two isolates described in this report originated from patient no. 1. Both of the isolates were streptomycin resistant and all 15 of the PvuII and SacI bands from the patient no. 2 isolate matched those of the strain from patient no. 1, which had 16 bands. The patterns from VNTR were identical. The specimens were not processed in the laboratory on the same day, suggesting that contamination must have occurred elsewhere. The bronchoscope, which was used for both patients, was the likely source of contamination.
There are two possible explanations as to how this transposition event could result in the seemingly sudden change in both IS6110 and spoligotyping over the 2 days that the organism was viable within the bronchoscope. The first possibility is that the transposed strain was a rare constituent of the M. tuberculosis population in patient no. 1. During patient no. 1's bronchoscopy, at least one of the transposed organisms remained within the bronchoscope. The transposed colonies were removed during patient no. 2's bronchoscopy. A second possibility is that the adverse conditions in the sublethal glutaraldehyde disinfectant solution used on the bronchoscope stimulated the transpositional event in at least one of the organisms that was subsequently cultured. Only five colonies of M. tuberculosis were cultured from the bronchoscope, and the one chosen for subculture had the transposition.
There are three described “hot spots” for IS6110 insertion in the M. tuberculosis chromosome. The most common region for IS6110 insertion is within the direct repeat region. The most common insertion site is in the direct repeat between spacer 24 and 25. A second insertion within other direct repeats (5, 17, 18, 21, 36) is not uncommon. Nearly all IS6110 insertions are between open reading frames, and multiple insertions in both 3′ and 5′ orientations have been described for a 537-bp region within the origin of replication of M. tuberculosis (25). Similarly, at least six insertion sites, all in the same orientation, have been described as being within a 267-bp intergenic region, ipl (15).
Two other reports of IS6110 insertions in the direct repeat region of the M. tuberculosis genome preventing efficient amplification of direct repeat regions and thus loss of spacers on spoligotyping have been reported. One report describes an outbreak of multidrug-resistant Mycobacterium bovis in which a change in the IS6110 insertion site resulted in a deletion of spacer 30 (or 40 in the new numbering scheme) (36). IS6110 was inserted into spacer 30, thus preventing amplification. The second report explains differences between two common spoligotypes found in the IS6110 Harlem family of strains. These strains differ only by detection or nondetection of spacer 31. Genomic sequencing showed that the spacer DNA was intact but an IS6110 inserted 8 bp proximal to spacer 31 in the direct repeat, thus preventing amplification of spacer 31 (17).
In this report, the apparent loss of spacer 9 on the spoligotype resulted from a similar asymmetric insertion of IS6110 into direct repeat 8. As previously mentioned, the most common hot spot for IS6110 insertion is within direct repeat 24. This usually does not result in the loss of spacer 25 on the spoligotype because IS6110 is usually found in the center of the direct repeat and the primers DRa and DRb were designed to hybridize to the 18 bp on either side of the insertion. Thus, no loss of spacer 24 or 25 was seen by spoligotyping. In patient no. 2's strain, however, IS6110 inserted 5 bp from the left end of the direct repeat that included the 3 bp duplicated from the IS6110 insertion. The limited sequence did not allow the primer to adequately bind, and therefore, no PCR product was seen that corresponded to spacer 9 on spoligotyping. Without the IS6110 primer INS1, the results of PCR amplification of this region would need to be 1.3 kb, which is much longer than the other PCR products in spoligotyping and results in minimal amplification. Two recent studies found analogous results with an M. bovis strain which is missing spacer 30 (36) and members of an M. tuberculosis family which differ by spacer 31 (17). In both instances, the missing spacer could be amplified using IS6110-specific primers instead of primers targeted to the direct repeat.
This is the first known report of M. tuberculosis contamination from a bronchoscope within our state. Since 1994, approximately 90% of the M. tuberculosis cultures from patients within our state with tuberculosis disease have been monitored by IS6110 fingerprinting. From the 2107 IS6110 fingerprints, we have identified several major tuberculosis outbreaks and a high incidence of clustering (48%). Five percent of the positive cultures resulted from laboratory contamination (14). In this incident of contamination from a bronchoscope, because the results of two M. tuberculosis typing methods were changed by a single transposition event, it was more difficult to confirm the relatedness of the two strains. The confirmation of this bronchoscope contamination illustrates the importance of linking molecular epidemiology to traditional epidemiology. Using molecular techniques alone, we would have concluded that these two strains were unrelated. However, with the strong traditional epidemiologic link, we probed deeper and found that a single transposition event was responsible for the differences found with both typing methods, thus suggesting that the strains were closely related.
Conclusion.
We report a case of M. tuberculosis contamination from a bronchoscope that was difficult to detect by molecular typing because a single transposition event changed both the IS6110 and the spoligotyping patterns. By sequencing of the M. tuberculosis DNA, it was revealed that an IS6110 sequence inserted asymmetrically within spacer 8 caused both an extra band in the IS6110 fingerprint and loss of spacer 9. Both traditional epidemiology and molecular investigation were needed to confirm contamination.
ACKNOWLEDGMENTS
This work was supported by Tuberculosis Program Medical Consultant Contract no. C00118096 and CDC TB Cooperative Agreement–Region IV Contract no. C00118092.
REFERENCES
- 1.Agerton T, Valway S, Gore B, Pozsik C, Plikaytis B, Woodley C, Onorato I. Transmission of a highly drug-resistant strain (strain W1) of Mycobacterium tuberculosis. Community outbreak and nosocomial transmission via a contaminated bronchoscope. JAMA. 1997;278:1073–1077. [PubMed] [Google Scholar]
- 2.Akamatsu T, Tabata K, Hironaga M, Uyeda M. Evaluation of the efficacy of a 3.2% glutaraldehyde product for disinfection of fibreoptic endoscopes with an automatic machine. J Hosp Infect. 1997;35:47–57. doi: 10.1016/s0195-6701(97)90167-5. [DOI] [PubMed] [Google Scholar]
- 3.Aranaz A, Liébana E, Mateos A, Dominguez L, Vidal D, Domingo M, Gonzolez O, Rodriguez-Ferri E F, Bunschoten A E, Van Embden J D, Cousins D. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. J Clin Microbiol. 1996;34:2734–2740. doi: 10.1128/jcm.34.11.2734-2740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer J, Thomsen V, Poulsen S, Andersen A. False-positive results from cultures of Mycobacterium tuberculosis due to laboratory cross-contamination confirmed by restriction fragment length polymorphism. J Clin Microbiol. 1997;35:988–991. doi: 10.1128/jcm.35.4.988-991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beggs M L, Cave M D, Marlave C, Cloney L, Duck P, Eisenach K D. Characterization of Mycobacterium tuberculosis complex direct repeat sequence for use in cycling probe reaction. J Clin Microbiol. 1996;34:2985–2989. doi: 10.1128/jcm.34.12.2985-2989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braden C R, Templeton G L, Stead W W, Bates J H, Cave M D, Valway S E. Retrospective detection of laboratory cross-contamination of Mycobacterium tuberculosis cultures with use of DNA fingerprint analysis. Clin Infect Dis. 1997;24:35–40. doi: 10.1093/clinids/24.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Bryce E A, Walker M, Bevan C, Smith J A. Contamination of bronchoscopes with Mycobacterium tuberculosis. Can J Infect Control. 1993;8:35–36. [PubMed] [Google Scholar]
- 8.Burman W J, Stone B L, Reves R R, Wilson M L, Yang Z, El-Hajj H, Bates J H, Cave M D. The incidence of false-positive cultures for Mycobacterium tuberculosis. Am J Respir Crit Care Med. 1997;155:321–326. doi: 10.1164/ajrccm.155.1.9001331. [DOI] [PubMed] [Google Scholar]
- 9.Cave M D, Eisenach K D, McDermott P F, Bates J H, Crawford J T. IS6110: conservation of sequence in the Mycobacterium tuberculosis complex and its utilization in DNA fingerprinting. Mol Cell Probes. 1991;5:73–80. doi: 10.1016/0890-8508(91)90040-q. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Multiple misdiagnoses of tuberculosis resulting from laboratory error—Wisconsin, 1996. Morb Mortal Wkly Rep. 1997;46:797–801. [PubMed] [Google Scholar]
- 11.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry C E, 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 12.Cox H M, Cheung K, Fletcher A J, Whitby R M, Hickman P E. Glutaraldehyde concentration in the sterilization of endoscopes. Pathology. 1995;27:362–364. doi: 10.1080/00313029500169313. [DOI] [PubMed] [Google Scholar]
- 13.de C. Ramos M, Soini H, Roscanni G C, Jaques M, Villares M C, Musser J M. Extensive cross-contamination of specimens with Mycobacterium tuberculosis in a reference laboratory. J Clin Microbiol. 1999;37:916–919. doi: 10.1128/jcm.37.4.916-919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunlap N E, Harris R H, Benjamin W H J, Harden J W, Hafner D. Laboratory contamination of Mycobacterium tuberculosis cultures. Am J Respir Crit Care Med. 1995;152:1702–1704. doi: 10.1164/ajrccm.152.5.7582316. [DOI] [PubMed] [Google Scholar]
- 15.Fang Z, Forbes K J. A Mycobacterium tuberculosis IS6110 preferential locus (ipl) for insertion into the genome. J Clin Microbiol. 1997;35:479–481. doi: 10.1128/jcm.35.2.479-481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Z, Morrison N, Watt B, Doig C, Forbes K J. IS6110 transposition and evolutionary scenario of the direct repeat locus in a group of closely related Mycobacterium tuberculosis strains. J Bacteriol. 1998;180:2102–2109. doi: 10.1128/jb.180.8.2102-2109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filliol I, Sola C, Rastogi N. Detection of a previously unamplified spacer within the DR locus of Mycobacterium tuberculosis: epidemiological implications. J Clin Microbiol. 2000;38:1231–1234. doi: 10.1128/jcm.38.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fomukong N, Beggs M, el Hajj H, Templeton G, Eisenach K, Cave M D. Differences in the prevalence of IS6110 insertion sites in Mycobacterium tuberculosis strains: low and high copy number of IS6110. Tuber Lung Dis. 1997;78:109–116. doi: 10.1016/s0962-8479(98)80003-8. [DOI] [PubMed] [Google Scholar]
- 19.Frothingham R, Meeker-O'Connell W A. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology. 1998;144:1189–1196. doi: 10.1099/00221287-144-5-1189. [DOI] [PubMed] [Google Scholar]
- 20.Groenen P M, Bunschoten A E, van Soolingen D, van Embden J D. Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis; application for strain differentiation by a novel typing method. Mol Microbiol. 1993;10:1057–1065. doi: 10.1111/j.1365-2958.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 21.Hermans P W M, van Soolingen D, Bik E M, de Haas P E W, Dale J W, van Embden J D A. Insertion element IS987 from Mycobacterium bovis BCG is located in a hot-spot integration region for insertion elements in Mycobacterium tuberculosis complex strains. Infect Immun. 1991;59:2695–2705. doi: 10.1128/iai.59.8.2695-2705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izbicki G, Mosimann W, Bolliger C T, Frei R, Perruchoud A P. Endoscopic contamination with Mycobacterium tuberculosis. Schweiz Med Wochenschr. 1997;127:182–186. [PubMed] [Google Scholar]
- 23.Kimerling M, Benjamin W, Lok K, Curtis G, Dunlap N. Restriction fragment length polymorphism screening of Mycobacterium tuberculosis isolates: population surveillance for targeting disease transmission in a community. Int J Tuberc Lung Dis. 1998;2:655–662. [PubMed] [Google Scholar]
- 24.Kremer K, van Soolingen D, Frothingham R, Haas W H, Hermans P W, Martin C, Palittapongarnpim P, Plikaytis B B, Riley L W, Yakrus M A, Musser J M, van Embden J D. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol. 1999;37:2607–2618. doi: 10.1128/jcm.37.8.2607-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurepina N E, Sreevatsan S, Plikaytis B B, Bifani P J, Connell N D, Donnelly R J, van Sooligen D, Musser J M, Kreiswirth B N. Characterization of the phylogenetic distribution and chromosomal insertion sites of five IS6110 elements in Mycobacterium tuberculosis: non-random integration in the dnaA-dnaN region. Tuber Lung Dis. 1998;79:31–42. doi: 10.1054/tuld.1998.0003. [DOI] [PubMed] [Google Scholar]
- 26.MacGregor R R, Clark L W, Bass F. The significance of isolating low numbers of Mycobacterium tuberculosis in culture of sputum specimens. Chest. 1975;68:518–523. doi: 10.1378/chest.68.4.518. [DOI] [PubMed] [Google Scholar]
- 27.Mazurek G H, Cave M D, Eisenach K D, Wallace R J, Jr, Bates J H, Crawford J T. Chromosomal DNA fingerprint patterns produced with IS6110 as strain-specific markers for epidemiologic study of tuberculosis. J Clin Microbiol. 1991;29:2030–2033. doi: 10.1128/jcm.29.9.2030-2033.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbithi J N, Springthorpe V S, Sattar S A, Pacquette M. Bactericidal, virucidal, and mycobactericidal activities of reused alkaline glutaraldehyde in an endoscopy unit. J Clin Microbiol. 1993;31:2988–2995. doi: 10.1128/jcm.31.11.2988-2995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michele T M, Cronin W A, Graham N M, Dwyer D M, Pope D S, Harrington S, Chaisson R E, Bishai W R. Transmission of Mycobacterium tuberculosis by a fiberoptic bronchoscope. Identification by DNA fingerprinting. JAMA. 1997;278:1093–1095. [PubMed] [Google Scholar]
- 30.Nelson K E, Larson P A, Schraufnagel D E, Jackson J. Transmission of tuberculosis by flexible fiberbronchoscopes. Am Rev Respir Dis. 1983;127:97–100. doi: 10.1164/arrd.1983.127.1.97. [DOI] [PubMed] [Google Scholar]
- 31.Otal I, Martin C, Vincent-Levy-Frebault V, Thierry D, Gicquel B. Restriction fragment length polymorphism analysis using IS6110 as an epidemiological marker in tuberculosis. J Clin Microbiol. 1991;29:1252–1254. doi: 10.1128/jcm.29.6.1252-1254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prigogine T, Glupczynski Y, Van Molle P, Schmerber J. Mycobacterial cross-contamination of bronchoscopy specimens. J Hosp Infect. 1988;11:93–95. doi: 10.1016/0195-6701(88)90047-3. [DOI] [PubMed] [Google Scholar]
- 33.Reeves D S, Brown N M. Mycobacterial contamination of fibreoptic bronchoscopes. J Hosp Infect. 1995;30(Suppl.):531–536. doi: 10.1016/0195-6701(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 34.Uttley A H, Honeywell K M, Fitch L E, Yates M D, Collins C H, Simpson R A. Cross contamination of bronchial washings. BMJ. 1990;301:1274. doi: 10.1136/bmj.301.6763.1274-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Embden J D, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Embden J D A, van Gorkom T, Kremer K, Jansen R, van Der Zeijst B A M, Schouls L M. Genetic variation and evolutionary origin of the direct repeat locus of Mycobacterium tuberculosis complex bacteria. J Bacteriol. 2000;182:2393–2401. doi: 10.1128/jb.182.9.2393-2401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenzel R P, Edmond M B. Tuberculosis infection after bronchoscopy. JAMA. 1997;278:1111. [PubMed] [Google Scholar]
- 38.Wheeler P W, Lancaster D, Kaiser A B. Bronchopulmonary cross-colonization and infection related to mycobacterial contamination of suction valves of bronchoscopes. J Infect Dis. 1989;159:954–958. doi: 10.1093/infdis/159.5.954. [DOI] [PubMed] [Google Scholar]
- 39.Yeh R, Ponce de Leon A, Agasino C, Hahn J, Daley C, Hopewell P, Small P. Stability of Mycobacterium tuberculosis DNA genotypes. J Infect Dis. 1998;177:1107–1111. doi: 10.1086/517406. [DOI] [PubMed] [Google Scholar]