Abstract
Metastatic spread of cancer is an unfortunate consequence of disease progression, aggressive cancer subtypes, and/or late diagnosis. Brain metastases are particularly devastating, difficult to treat, and confer a poor prognosis. While the precise incidence of brain metastases in the United States remains hard to estimate, it is likely to increase as extracranial therapies continue to become more efficacious in treating cancer. Thus, it is necessary to identify and develop novel therapeutic approaches to treat metastasis at this site. To this end, intracranial injection of cancer cells has become a well-established method in which to model brain metastasis. Previously, the inability to directly measure tumor growth has been a technical hindrance to this model; however, increasing availability and quality of small animal imaging modalities, such as magnetic resonance imaging (MRI), are vastly improving the ability to monitor tumor growth over time and infer changes within the brain during the experimental period. Herein, intracranial injection of murine mammary tumor cells into immunocompetent mice followed by MRI is demonstrated. The presented injection approach utilizes isoflurane anesthesia and a stereotactic setup with a digitally controlled, automated drill and needle injection to enhance precision, and reduce technical error. MRI is measured over time using a 9.4 Tesla instrument in The Ohio State University James Comprehensive Cancer Center Small Animal Imaging Shared Resource. Tumor volume measurements are demonstrated at each time point through use of ImageJ. Overall, this intracranial injection approach allows for precise injection, day-to-day monitoring, and accurate tumor volume measurements, which combined greatly enhance the utility of this model system to test novel hypotheses on the drivers of brain metastases.
Keywords: Cancer Research, Issue 160, Intracranial injection, cancer, brain metastasis, magnetic resonance imaging, image analysis, stereotactic
Introduction
Brain metastases are 10 times more common than adult primary central nervous system tumors1, and have been reported in almost every solid tumor type with lung cancer, breast cancer, and melanoma exhibiting the highest incidence2. Regardless of the primary tumor site, the development of brain metastasis leads to a poor prognosis often associated with cognitive decline, persistent headaches, seizures, behavioral and/or personality changes1,3,4,5. In terms of breast cancer, there have been many advances in the prevention and treatment of the disease. However, 30% of women diagnosed with breast cancer will go on to develop metastases, and of those with stage IV disease, approximately 7% (SEER 2010-2013) have brain metastasis6,7. Current treatment options for brain metastasis involve surgical resection, stereotactic radiosurgery and/or whole brain radiotherapy. Yet, even with this aggressive therapy, the median survival for these patients is a short 8-11 months7,8,9. These grim statistics strongly support the need for the identification and implementation of novel, effective therapeutic strategies. Thus, as with all cancers that metastasize to the brain, it is essential to properly model breast cancer associated brain metastasis (BCBM) in the laboratory to ensure significant advancements in the field.
To date, researchers have utilized a variety of methodologies to study mechanisms of metastasis to the brain, each with distinct advantages and limitations10,11. Experimental metastasis methods such as tail vein and intracardiac injection spread tumor cells throughout the body and can result in immense tumor burden at other metastatic sites depending on the cells injected. These results are then confounding if specifically studying metastasis to the brain. The intracarotid artery injection method is advantageous as it specifically targets brain-seeding of tumor cells but is limited as it can be technically difficult to perform. Orthotopic primary tumor resection is often considered the most clinically relevant model of metastasis as it recapitulates the entire metastatic cascade. Yet, this approach involves prolonged wait periods for spontaneous metastasis to occur with dramatically lower rates of brain metastasis compared to the other metastatic sites such as the lymph node, the lung and the liver. Often, animals must be removed from studies due to tumor burden at these other metastatic sites prior to the development of brain metastasis. Other methods involving brain tropic cell lines are effective at metastasizing to the brain; however, these models are limited in that they take time to develop and often lose their tropism with propagation. Given these limitations, researchers have routinely used the intracranial injection method to model cancer metastasis to the brain11,12,13,14 with varying methodologies15,16,17,18,19. It is acknowledged that this approach similarly has limitations, most importantly in that it does not allow for investigation of early metastatic steps including intravasation out of the primary tumor, penetrance through the blood brain barrier, and establishment within the brain. However, it does allow for researchers to test (1) what tumor derived factors mediate growth within the brain (e.g., genetic manipulation of an oncogenic factor in tumor cells), (2) how changes in the metastatic microenvironment alter cancer growth at this site (e.g., comparison between transgenic mice with altered stromal components) and (3) effectiveness of novel therapeutic strategies on growth of established lesions.
Given the potential utility of the intracranial injection model, it is absolutely necessary to reduce technical error during injection and to precisely monitor tumor growth over time. The method described herein involves continuous dosing of inhaled gas anesthesia, and direct implantation of tumor cells into the brain parenchyma using a stereotactic drill and injection stand. Administering gas anesthetic allows for fine tuning the depth and length of anesthesia as well as ensuring a quick and smooth recovery. A digitally controlled, automated drill and needle injection system enhances injection-site precision and reduces technical error often incurred by drilling and free-hand injection methods. The use of magnetic resonance imaging (MRI) further increases precision in monitoring tumor growth, tumor volume, tissue response, tumor necrosis, and response to treatment. MRI is the imaging modality of choice for soft tissues20,21. This imaging technique does not use ionizing radiation and is preferred over Computed Tomography (CT), especially for multiple imaging sessions during the course of a study. MRI has much greater range of available soft tissue contrast then CT or ultrasound imaging (USG) and presents anatomy in greater detail. It is more sensitive and specific for abnormalities within the brain itself. MRI can be performed in any imaging plane without having to physically move the subject as is the case in 2D USG or 2D optical imaging. It is important to mention that the skull does not attenuate the MRI signal as in other imaging modalities. MRI allows the evaluation of structures that may be obscured by artifacts from bone in CT or USG. An additional advantage is that there are many contrast agents available for MRI, which enhances the lesion detection limit, with relatively low toxicity or side effects. Importantly, MRI allows monitoring in real-time unlike histological evaluation at the time of necropsy, which is limited in deciphering tumor volume. Other imaging modalities, such as bioluminescent imaging, are indeed effective for early tumor detection and monitoring over time; however, this method requires genetic manipulation (e.g., luciferase/GFP tagging) of cell lines and does not allow for volumetric measurements. MRI is further advantageous as it mirrors patient monitoring and downstream volumetric analysis of the MR images is known to be strongly correlated to histologic tumor size at necropsy22. Serial monitoring with MRI screening also increases the clinical monitoring of neurologic impairments, should they arise.
Overall, the presented method of stereotactic intracranial tumor injection followed by serial MRI enables us to produce reliable, predictable, and measurable results to study mechanisms of brain metastasis in cancer.
Protocol
All methods described herein have been approved by the Institutional Animal Care and Use Committee (IACUC) at The Ohio State University (P.I. Gina Sizemore; Protocol #2007A0120). All rodent survival surgery IACUC policies are followed, including use of sterile techniques, supplies, instruments, as well as fur removal and sterile preparation of the incision site.
1. Intracranial injection of breast cancer cells
NOTE: The method described herein utilized the DB7 murine mammary tumor cell line derived from a primary MMTV-PyMT tumor23. Previous studies have established intracranial injection of DB7 cells as a model of BCBM with histology that mimics that of the human disease12. Importantly, immune-competent FVB/N mice are used for this model as DB7 cells were derived from this mouse strain. As this is a breast cancer model, adult female mice are used for these studies.
- Prepare cells.
- In a sterile hood, aspirate media from cell culture plates using standard techniques.
- Wash cells with 1x DPBS and trypsinize using manufacturer’s protocol.
- Add an appropriate volume of FBS-containing media to stop the trypsin reaction and determine the concentration of cells using a hemocytometer or preferred method.
- Pellet cells at 300 x g for 4 min at 4 °C.
- Aspirate media, wash with 1x DPBS, re-spin at 300 x g for 4 min at 4 °C.
-
Resuspend cells in 1x DPBS to an appropriate concentration, approximately 50,000 cells per injectable volume of 2 μL
- Place resuspended cells on ice until injected to maintain viability.
- Prepare mice for surgery.
-
For mice with fur: remove fur from the cranium, either by depilatory cream/lotion or by shaving. Do this within 24-48 h prior to surgery as performing this step too close to surgery can interfere with skin quality and suture strength.NOTE: Female FVB/N mice weighing approximately 25 g were used due to the study of metastatic breast cancer, a predominantly female disease.
-
Administer analgesics as determined by the IACUC and attending veterinarian: a subcutaneous injection of Buprenorphine SR-LAB (0.05-0.1 mg/kg dose, Buprenorphine stock: 0.5 mg/mL for a dosage of 0.0025-0.088 mL) at least 24 h prior to surgery to provide up to 72 h analgesia, which may be repeated 48-72 h after the first dose, if needed. Also administer NSAIDs (20% ibuprofen in drinking water e.g., 1 mg/5 mL) at least 24-48 h before surgery and continue for 2-7 days after surgery to provide a minimum 72 h postoperative analgesia.NOTE: At The Ohio State University, Buprenorphine SR-LAB is administered by the University Lab Animal Resources Veterinary staff as it is a controlled substance.
-
- Prepare stereotactic units for surgery.
-
Turn on all anesthesia machines, digital Vernier scales, and digital injectors.NOTE: All surgical tools should be adequately cleaned and sterilized prior to surgery.
- Utilize anesthetic machines with an induction chamber attachment in a biological safety cabinet (Figure 1A).
-
Set anesthesia machines to manufacturer recommended nose cone flow rate based on the mouse weight (e.g., 25 g animal weight: nose cone flow rate 34 mL/min).NOTE: The head holder included in this stereotactic set up is recommended only for adult mice. Ensure that the manufacturer recommendations included with the stereotactic set up are followed.
-
Ensure that the appropriate anesthetic (e.g., isoflurane) prefilled in the syringe is attached to the anesthesia machine (Figure 1B).NOTE: Over-priming the syringe can cause too much anesthesia to be delivered to the mice during surgery and result in an anesthetic overdose.
- Prepare the drills by twisting the stage lock, inserting a drill bit adaptor and a 1 mm drill bit into each drill and lock the drill by manually tightening the bit-lock.
- Attach drills onto the stereotactic frames (Figure 1C).
- Clean Hamilton syringes with 5 alternating washes of 1x DPBS, then 70% ethanol, then once again in 1x DPBS. Place aside until animal is prepped for injection.
- Set digital injector to deliver at a rate of 0.4 μL/min and a target of 2 μL. This rate and volume allow for slow introduction of tumor cells into the brain, which reduces pressure and associated damage.
-
- Place mice on stereotactic frames.
- Anesthetize mice (e.g., isoflurane) using the aforementioned induction chamber.
-
Maintain mice throughout the procedure at a deep anesthetic plane. The % anesthesia administered by the machine depends on a number of factors including: flow rate, degree of stimulation, and body temperature. Monitor the mice frequently throughout the procedure for depth of anesthesia by evaluating for rhythmic respirations (animal is not gasping); lack of palpebral reflex (fluttering of the eyelids when stimulated with a cotton tip applicator); and lack of toe pinch (withdrawal of limb upon the noxious stimulus of pinching the toes).NOTE: Different strains of mice will have a different response to anesthesia.
-
After mice are at an appropriate, deep anesthetic plane, transfer the mice to the stereotactic unit. Use a heating pad while the mouse is on the stereotactic machine to maintain body temperature and an appropriate anesthetic plane.NOTE: To achieve low profile we use air-activated hand warmers placed within an inverted pipette tip box (mouse elevating box in Figure 1D).
- Open the mouse’s mouth and place teeth in the trough of the mouth bar located on the nose piece on the stereotactic frame (Figure 2A). Slide the nose cone over the mouse’s nose/mouth (Figure 2B).
- Place mice with their heads level to the mouth bar. Gently open the mouth with the blunt end of a cotton tip applicator and slide into place. Ensure the nose cone is fully over the mouse’s nose or anesthesia will not be delivered properly. The nose cone will not engage with the nose if the teeth are not seated within this groove (Inset Figure 2A).
- Using a sterile cotton swab, place eye lubricant on each of the mouse’s eyes. Application of eye lubricant mitigates drying of the cornea and reduces the chance of corneal damage and postoperative complications related to corneal trauma.
-
Stabilize the mouse’s skull by depressing the left ear bar up against the medial canthus of the left ear, locking it in place using the screw on the stereotactic frame. Then slide the right ear bar against the medial canthus of the right ear and screw tight on the stereotactic frame (Figure 2B).NOTE: Make sure the mouse’s head is level and straight when placing ear bars. If the head is crooked or angled, the injection will be in the incorrect place in the brain. The ear bars should be tightened ONLY until the skull is immobilized upon stimulation with moderate manual probing.
- Make a calvarial window.
- Prepare and clean the scalp with 3x alternating passes each of a betadine solution and 70% ethanol. Due to the close proximity of the surgical site to the eyes, use the betadine solution over the surgical scrub.
- Using a sterile scalpel, make a 3 mm incision through the skin along the central median aspect of the cranium (following the sagittal suture line). Bleeding at the incision should be minimal. Should it occur, apply consistent, firm pressure at the site of bleeding with a sterile cotton tip applicator for >30 seconds.
- Identify and orient the drill perpendicular to the bregma (Figure 2C), making sure to reset the digital Vernier scale to zero.
- Move the drill 2 mm lateral to the sagittal suture and 1 mm anterior to the coronal suture (Figure 2C). For reproducibility, ensure the location to the left or right of the sagittal suture line remains consistent for all animals.
- Turn the drill onto its highest speed. Ensure skin is moved away from drill to avoid tissue damage caused by the rotating bit and carefully initiate the drill onto the skull. Drill a hole roughly 0.5 mm deep through the calvaria, resulting in the calvarial window. Be careful not to lower the drill too far or it will drill into the brain. Dropping sterile saline at the drill site while the drill is in motion can offset any heat generated by the machine that may cause thermal damage to the surrounding tissue.
- Once the calvarial window is made, carefully raise the drill and remove it from the stereotactic frame.
- Clean the drill bits using 70% ethanol and set aside if being used again. If not, remove drill bits and submerge in 70% ethanol.
- Injecting cancer cells into the brain
- Attach the automatic injector unit to the stereotactic apparatus (Figure 1D).
- Pull up >2 μL of cells thoroughly resuspended in 1x DPBS in a clean Hamilton syringe. Be sure to resuspend cells immediately prior to filling the syringe to reduce clump formation and ensure a homogenous cell slurry. It is ideal to pull up 6-8μL of cell volume to ensure there are no air pockets/bubbles.
- Place the Hamilton syringe onto the injector apparatus, and prime the needle for injection by dispensing a small amount of volume onto a disposable sterile drape to ensure the injector is working properly. Wipe the syringe with 70% ethanol with a cotton tip applicator (Figure 1D, inset). This removes tumor cells from the outer barrel of the needle shaft reducing the risk of tumor cell seeding along the injection tract.
- Align the needle tip to the center of the calvarial window, nearly touching the exposed cerebrum.
- Reset the digital Vernier scale to zero.
- Slowly insert the needle to a depth of 3 mm into the brain and allow the needle to remain in the brain for at least 60 seconds before proceeding. This time frame allows the brain parenchyma to conform around the needle, which reduces back pressure and potential expulsion of tumor cells along the needle tract.
- Select Run on the injector screen to begin the delivery of cells to the injection site. It will take approximately 5 min to inject this volume. The prolonged time for this step is to reduce secondary damage caused by injection force on the brain parenchyma.
- Once the injection protocol is finished, allow the needle to rest in the brain for at least 3 min, again allowing the brain parenchyma to acclimate to the injection.
- After at least 3 min, raise the needle from the brain at a rate of 0.75 mm/min. Do this at an extremely slow and consistent manner to reduce back pressure and tumor tracking up the needle tract.
- Once the needle has exited the brain, carefully remove the Hamilton syringe from injector and clean as described in step 1.2.8.
- Apply warm bone wax to the calvarial window using a sterile cotton swab. The bone wax acts as a physical barrier to keep the tumor within the skull.
- Close the incision (e.g., 5-0 PDS dissolvable sutures in a simple interrupted pattern OR suture clips, whichever is most comfortable for the surgeon).
- Stop the administration of anesthesia and remove the mouse from the apparatus by unlocking and sliding out the ear bars, sliding the nose cone off the mouse, and disengaging the teeth from the mouth bar.
- Place the mouse in a clean cage that is on a warmer set to 37 °C for recovery. Monitor mice during recovery, which typically occurs 10-15 minutes after anesthesia has been discontinued.
- After the mouse is recovered, monitor for early removal criteria as determined by the host institution’s IACUC.
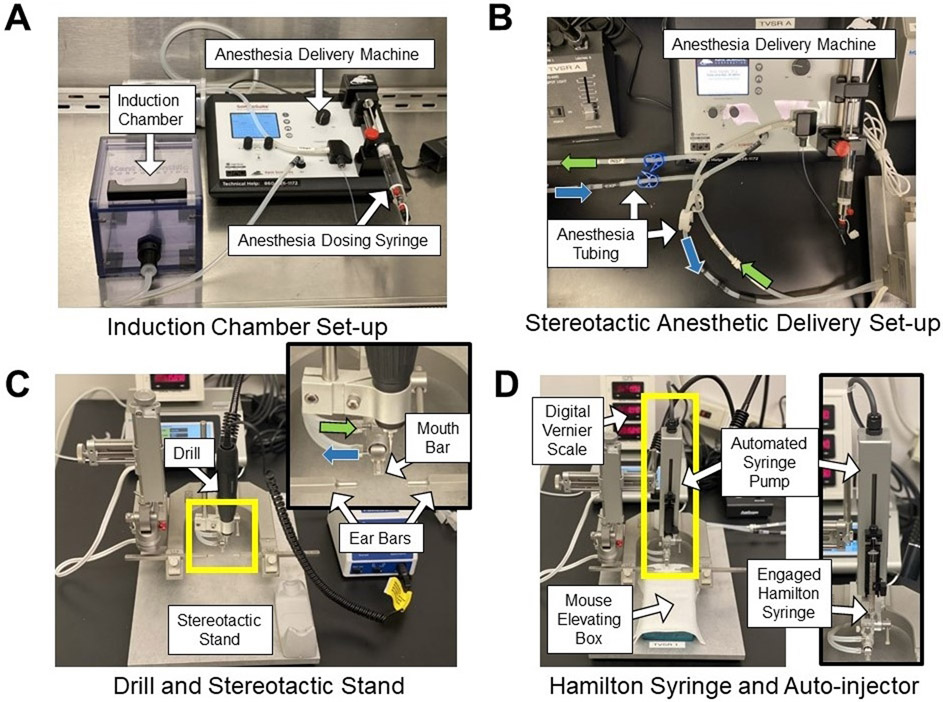
Figure 1: Stereotactic and anesthesia systems.
(A) Anesthesia induction chamber setup within a biological safety cabinet. (B) Stereotactic anesthetic delivery setup highlighting anesthesia tubing from the anesthetic machine to the nose cone on the stereotactic apparatus (see inset in (C)). Green arrows indicate delivered anesthetic gas tubing, and blue arrows indicate scavenged gas tubing. (C) Stereotactic stand with drill attachment. Inset shows anesthetic tubing (green and blue arrows), mouth bar, and ear bars. (D) Stereotactic stand with automated syringe pump and mouse elevating box. The box is an inverted pipette tip box containing hand warmers used to elevate the mouse to the appropriate height and maintain body temperature. Inset shows the orientation of the Hamilton syringe in the automated injection apparatus.
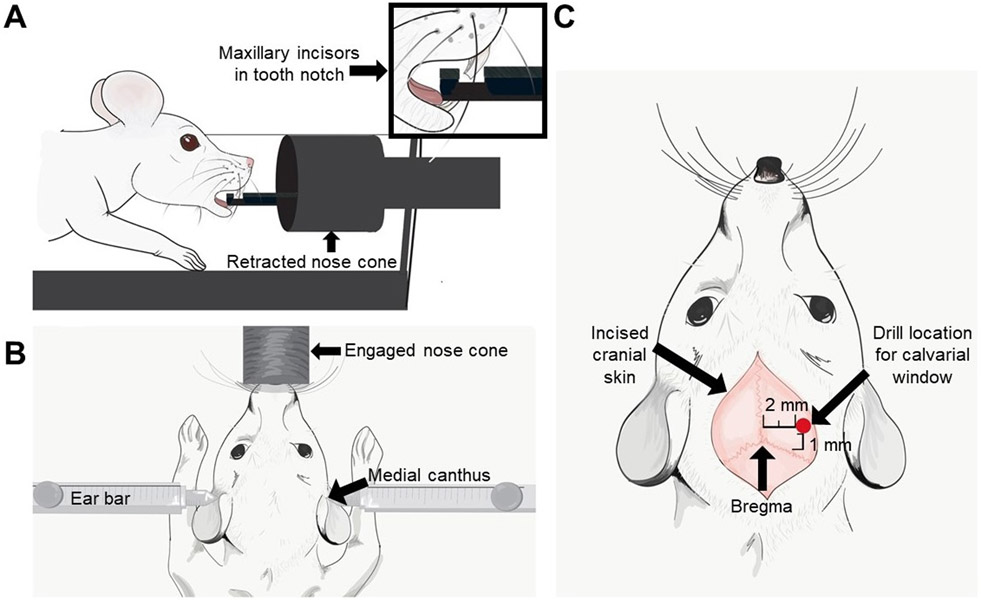
Figure 2: Pictorial representation of the tooth placement on a stereotactic device, location of ear bars, and calvarial window relative to Bregma.
(A) The pictorial of the maxillary incisors in the tooth notch on the nose cone. (B) The location of the left and right ear bars within the medial canthus of the respective ears. (C) An arrow indicates the bregma and a red dot indicates the location where the calvarial window should be made (2 mm lateral to the sagittal suture and 1 mm anterior to the coronal suture).
2. Magnetic resonance imaging
Administer Gadolinium-based contrast agent (100 μL/20 g body weight mouse of 0.1 M MultiHance) to mice by standard intraperitoneal injection24 10-20 minutes prior to imaging. Then anesthetize using an induction chamber with an inhaled anesthetic (e.g., isoflurane) mixed with 95% O2 and 5% CO2 (i.e., supplied room air gas).
Place mouse on a heated holder to maintain body temperature. Secure the head with ear prongs and a bite bar, and place holder in the 9.4 T magnet equipped with a mouse brain surface coil. Maintain anesthesia during imaging time, a study typically takes about 20 min per mouse. Monitor respiratory rate and heart rate (~70 bpm) throughout the procedure using a pneumatic pillow and small animal monitoring system.
Obtain a localizer image, and then image the mouse brain using a T2-weighted RARE sequence (TR = 3500-4228 ms, TE=12 ms, RARE Factor = 8, FOV=2.0 x 2.0 cm, matrix size = 256 x 256, 1 mm or 0.5 mm slice thickness, NA=2-4, 15-30 contiguous slices) and post Gadolinium-based contrast T1 -weighted RARE sequence (TR = 1200 ms, TE=7.5 ms, RARE Factor = 4, FOV=2.0 x 2.0 cm, matrix size = 256 x 256, 1 mm or 0.5 mm slice thickness, NA=2-4, 15-30 contiguous slices).
Post-imaging, place mouse in a cage on a warmer set to 37 °C for recovery.
3. Volumetric tumor measurements
- Obtaining total tumor volume
-
Open MRI DICOM data file in ImageJ, an image processing software available as a free download through the National Institutes of Health (https://imagej.nih.gov/ij/)25.NOTE: ImageJ allows viewing of DICOM files, which is required to utilize the embedded pixel dimensions for tumor volume calculations (see Image, Properties where “unit of length” can be set as desired (e.g., mm)).
- Use the Freehand Selections tool to draw an outline around the tumor. Perform these selections in a dark room to enhance visibility. Do not adjust brightness/contrast to maintain consistency between sessions.
- Under the Analyze tab, select Measure to obtain the area of the selected region. If unit of millimeters was chosen in step 3.1.1., area will be given in cubic millimeters. If desired, embed the Freehand Selection by selecting ctrl+D. Change the output color by going to Edit ∣ Options ∣ Color. Convert the image to an RGB image (Image ∣ Type ∣ RGB color) prior to saving as a .tif file.
- Proceed with measuring all tumor-containing slices for an individual mouse and copy values into an appropriate data analysis software program (e.g., Microsoft Excel or GraphPad Prism).
- Sum the areas from each slice to get total tumor volume. Be sure to correct the area based on slice thickness (area/thickness).
- Complete steps 3.1.1.-3.1.5. until all mice have a total tumor volume. Given the somewhat subjective nature of outlining the tumors, it is ideal if the same methodology is repeated multiple times and averaged to reduce technical error.
- To set scale bars, open DICOM data file and go to Analyze ∣ Tools ∣ Scale Bar. Since the dimensions are already embedded in the DICOM file, just pick the desired length/width. Covert to an RGB image (Image ∣ Type ∣ RGB color) prior to saving as a .tif file.
-
Representative Results
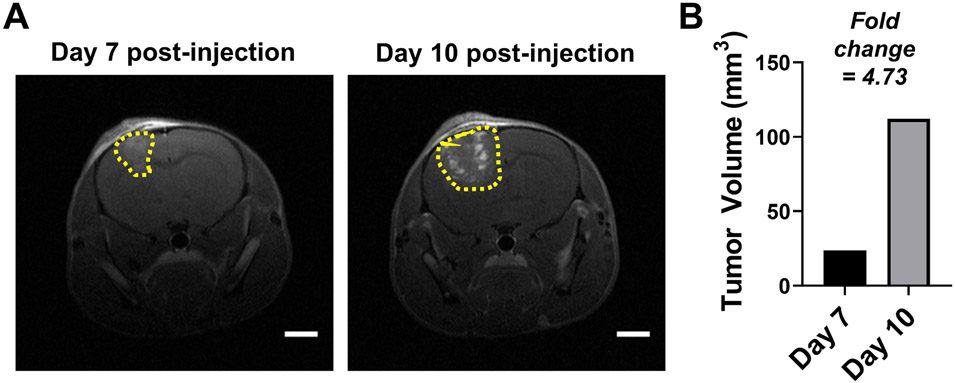
Figure 3 overviews the tumor volume quantification for a single mouse at two time points (day 7 and day 10) post-injection of murine mammary tumor cells. For this experiment, 50,000 DB7 cells were injected, and the animal’s brain was evaluated by MRI. For each scan, 30 slices (0.5 mm thickness) were captured. Evaluation of the 30 slices per scan revealed that at day 7 post-injection, 5 slices exhibited tumor burden (Figure 3A) and at day 10 post-injection, 9 slices exhibited tumor burden (Figure 3B). Each image was evaluated for tumor area as described and the area for each frame is depicted in Figure 3C. The total tumor volume is determined and adjusted for slice thickness. Figure 4 depicts the representative data in publication format including representative images (Figure 4A) and a histogram of tumor volume over time (Figure 4B).
Figure 3: Example of tumor volume quantification for a single mouse over two time points post-injection.
Images of the slices containing tumor at (A) day 7 and (B) day 10 post-injection. Yellow denotes tumor area quantified in ImageJ. (C) Slice area and total volumetric quantification as determined in ImageJ (*=correction for slice thickness (0.5 mm)).
Figure 4: Representative tumor volume quantification in publication format.
(A) Representative images with scale bars (=2 mm). (B) Histogram depicting tumor volume (mm3) for the two time points. Fold change is noted.
Discussion
The utilization of intracranial injection followed by serial monitoring with MRI provides the unique ability to visualize tumor growth with tumor volume accuracy over time. The application of digital imaging analysis allows for interpretation of brain lesions for tumor volume, hemorrhage, necrosis, and response to treatment.
As with any procedure, there are key steps that must be followed for success. First, careful setup of the stereotactic devices is imperative to the success of this technique. Due to the small size of the murine cranium, slight incongruencies can result in dramatically different effects on tumor growth rate and take in experimental animals. As such, proper training on the use of these instruments is necessary. Second, a heating source throughout the procedure ensures the anesthetic plane does not drop too low. Low body temperatures place the animals at risk of suddenly dying under anesthesia due to decreased drug metabolism and inadvertent overdose of anesthesia and/or can prolong recovery times. The prefabricated heating pads can be bulky and difficult to maneuver around on the stereotactic machine, but small, air-activated hand warmers purchased through any commercial vendor maintain temperature and are small enough to be placed within the inverted pipette tip box used to elevate the mice to the appropriate level for the stereotactic setup. Lastly, quantification is straightforward, but often determining what is tumor versus what is not tumor can be challenging. It is recommended that investigators consult with imaging staff to ensure accurate assessment. It is also helpful to repeat tumor volume measurements multiple times to reduce error. As well, each study should be analyzed by the same person for all images.
The presented protocol can be modified depending on user preferences. First, the use of injectable anesthesia (e.g., ketamine/xylazine) is common and can certainly be utilized in lieu of an inhaled anesthesia (e.g., isoflurane) depending on investigator preference. However, it is important to consider the advantages of an inhaled anesthesia: (1) not a controlled substance, (2) level of anesthesia can be adjusted over time (compared to an upfront dose of ketamine determined by animal weight), and (3) recovery is relatively quick compared to ketamine/xylazine. Second, the use of the automatic drill provides a high level of accuracy but adds time to the procedure given the time needed to set up and tear down the unit. It is certainly reasonable to use a free handing technique if desired.
This protocol utilizes both a stereotactic setup as well as the use of MRI, both are associated with increased cost. Alternative methods for intracranial injection have been described previously15,16,17,18,19. Some of these methods also employ the use of bioluminescent imaging to monitor tumor growth throughout the study if preferred.
As mentioned above, it is important to determine proper cell number for injection depending on the model system being studied. Injection of murine cells into an immunocompetent host tends to require fewer cells than injection of human cells into an immunodeficient host. Post-injection tumor burden monitoring by MRI helps determine whether the number of cells injected is appropriate as it is possible to see when tumors reach a certain volume and at what point mice start reaching early removal criteria.
The utility and broad application of MRI for noninvasive monitoring has been used by others in small animal research studies26. While MRI provides several advantages as already discussed, there are indeed limitations worth mentioning. First, use of the machine is dependent on core service personnel and machine availability. Second, use can be costly. If these are concerns, the use of bioluminescent imaging is a valid alternative for tumor monitoring17,19. Third, contrast between tumor and the surrounding tissue (i.e., brain) can vary among different cell lines; however, this methodology offers the greatest chance of identifying tumor in vivo in the absence of cell labeling. Lastly, MRI is limited in its resolution, which can skew tumor volume data to where it seems as if there is exponential growth when in fact the growth is linear. There is also a maximum tumor volume that can be obtained through MR imaging, but this is less of a concern because it is unlikely a mouse could survive a tumor at the upper end. The tumor detection limit depends on the type and location of the tumor in the brain, whether there is blood brain barrier leakage, and whether the tumor is well circumscribed or is infiltrating into the surrounding tissue. It is also dependent upon whether it is a contrast enhancing tumor and what type of the MR imaging protocol is used. In our experience, with an in-plane voxel size of 78x78 μm and a 0.5 mm slice thickness, we can observe a 0.5 mm diameter tumor routinely with a minimum limit around 0.3 mm.
Regarding intracranial injection, there are several limitations to consider. First, intracranial injections do not mirror the metastatic cascade in that they completely bypass the development of metastatic properties within the primary tumor, intravasation into the bloodstream, and penetration through the blood brain barrier11. Second, by directly injecting into the brain this method induces inflammation, which may confound findings associated with neuroinflammation. Lastly, direct injection at this site can result in rapid growth over a short period of time. It is crucial to monitor mice for neurological symptoms including hind limb paralysis, lethargy, and ataxia.
All considered, injection directly into the brain allows for monitoring of tumor take rate, which can inform the researcher about growth specifically within this metastatic site as well as interaction with the brain metastatic microenvironment. Furthermore, monitoring of tumor burden over time allows the investigator to directly compare altered tumor phenotypes, altered microenvironment phenotypes and response to experimental therapeutic strategies. Given the relatively low incidence of both spontaneous and experimental brain metastasis in mouse models, the intracranial technique is indeed a valuable tool.
Cancer metastasis to the brain is a catastrophic diagnosis with poor response to current treatment strategies1,11,27. While breast cancer is the predominant cause of brain metastasis in women and the focus herein, lung cancer is the most common cause of brain metastasis in all cancer patients2. Further, brain metastases have been reported in patients diagnosed with an array of solid tumor types, and it is expected that incidence will continue to increase as therapies continue to improve to treat extracranial disease. Thus, while the focus of this proposal is on BCBM, intracranial cancer injection and MRI analysis is applicable to studying brain metastases of other solid tumor types as well as primary brain tumors. Utilizing the intracranial injection model of brain metastasis enables researchers to recapitulate disease in large cohorts of animals to test a variety of research questions in hopes to better patient care. By coupling this model with high resolution digital images obtained by MRI, it is possible to monitor tumor volume at multiple time points as well as tumor response to therapy.
Acknowledgments
Representative data was funded through the National Cancer Institute (K22CA218472 to G.M.S.). Intracranial injections are performed in The Ohio State University Comprehensive Cancer Center Target Validation Shared Resource (Director – Dr. Reena Shakya) and MRI is completed in The Ohio State University Comprehensive Cancer Center Small Animal Imaging Shared Resource (Director – Dr. Kimerly Powell). Both shared resources are funded through the OSUCCC, the OSUCCC Cancer Center Support Grant from the National Cancer Institute (P30 CA016058), partnerships with The Ohio State University colleges and departments, and established chargeback systems.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/61272.
Disclosures
The authors do not have any disclosures.
References
- 1.Lin X, DeAngelis LM Treatment of Brain Metastases. Journal of Clinical Oncology. 33 (30), 3475–3484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Wright CH, Barnholtz-Sloan JS Brain metastases: epidemiology. Handbook of Clinical Neurology. 149, 27–42 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Eichler AF et al. The biology of brain metastases-translation to new therapies. Nature Reviews Clinical Oncology. 8 (6), 344–356 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steeg PS, Camphausen KA, Smith QR Brain metastases as preventive and therapeutic targets. Nature Reviews Cancer. 11 (5), 352–363 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valiente M et al. The Evolving Landscape of Brain Metastasis. Trends in Cancer. 4 (3), 176–196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H et al. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: a SEER based study. Oncotarget. 8 (16), 26368–26379 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R et al. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer. 19 (1), 1091 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong Y, Liu YR, Ji P, Hu X, Shao ZM Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Scientific Reports. 7, 45411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YJ, Kim JS, Kim IA Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. Journal of Cancer Researchearch and Clinical Oncology. 144 (9), 1803–1816 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Cuadrado L, Tracey N, Ma R, Qian B, Brunton VG Mouse models of metastasis: progress and prospects. Disease Models & Mechanisms. 10 (9), 1061–1074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodack DP, Askoxylakis V, Ferraro GB, Fukumura D, Jain RK Emerging strategies for treating brain metastases from breast cancer. Cancer Cell. 27 (2), 163–175 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meisen WH et al. Changes in BAI1 and nestin expression are prognostic indicators for survival and metastases in breast cancer and provide opportunities for dual targeted therapies. Molecular Cancer Therapeutics. 14 (1), 307–314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell L et al. PTEN expression by an oncolytic herpesvirus directs T-cell mediated tumor clearance. Nature Communications. 9 (1), 5006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thies KA et al. Stromal platelet-derived growth factor receptor-beta signaling promotes breast cancer metastasis in the brain. Cancer Research. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramp TR, Camphausen K Combination radiotherapy in an orthotopic mouse brain tumor model. Journal of Visualized Experiments. (61), e3397 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce AM, Keating AK Creating anatomically accurate and reproducible intracranial xenografts of human brain tumors. Journal of Visualized Experiments. (91), 52017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdelwahab MG, Sankar T, Preul MC, Scheck AC Intracranial implantation with subsequent 3D in vivo bioluminescent imaging of murine gliomas. Journal of Visualized Experiments. (57), e3403 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donoghue JF, Bogler O, Johns TG A simple guide screw method for intracranial xenograft studies in mice. Journal of Visualized Experiments. (55) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozawa T, James CD Establishing intracranial brain tumor xenografts with subsequent analysis of tumor growth and response to therapy using bioluminescence imaging. Journal of Visualized Experiments. (41) (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink JR, Muzi M, Peck M, Krohn KA Multimodality Brain Tumor Imaging: MR Imaging, PET, and PET/MR Imaging. Journal of Nuclear Medicine. 56 (10), 1554–1561 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borges AR, Lopez-Larrubia P, Marques JB, Cerdan SG MR imaging features of high-grade gliomas in murine models: how they compare with human disease, reflect tumor biology, and play a role in preclinical trials. American Journal of Neuroradiology. 33 (1), 24–36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhu SS, Broaddus WC, Oveissi C, Berr SS, Gillies GT Determination of intracranial tumor volumes in a rodent brain using magnetic resonance imaging, Evans blue, and histology: a comparative study. IEEE Transactions on Biomedical Engineering. 47 (2), 259–265 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Borowsky AD et al. Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clinical & Experimental Metastasis. 22 (1), 47–59 (2005). [DOI] [PubMed] [Google Scholar]
- 24.JoVE Science Education Database. Lab Animal Research. Compound Administration I. Journal of Visualized Experiments. Cambridge, MA: (2020). [Google Scholar]
- 25.Abramoff MD, Magelhaes PJ, Ram SJ Image Processing with ImageJ. Biophotonics International. 11, 36–42 (2004). [Google Scholar]
- 26.Lee D, Marcinek D Noninvasive in vivo small animal MRI and MRS: basic experimental procedures. Journal of Visualized Experiments. (32) (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah N et al. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacological Research. 132, 47–68 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]