Abstract
Background:
There is increasing recognition of the importance of early incorporation of palliative care services in the care of patients with advanced cancers. Hospice-based palliative care remains underutilized for black patients with cancer, and there is limited literature on racial disparities in use of non-hospice-based palliative care services for patients with cancer.
Objective:
The primary objective of this study is to describe racial differences in the use of inpatient palliative care consultations (IPCC) for patients with advanced cancer who are admitted to a hospital in the United States.
Design:
This retrospective cohort study analyzed 204 175 hospital admissions of patients with advanced cancers between 2012 and 2014. The cohort was identified through the National Inpatient Dataset. International Classification of Disease, Ninth Revision codes were used to identify receipt of a palliative care consultation.
Results:
Of this, 57.7% of those who died received IPCC compared to 10.5% who were discharged alive. In multivariable logistic regression models, black patients discharged from the hospital, were significantly less likely to receive a palliative care consult compared to white patients (odds ratio [OR] black: 0.69, 95% CI: 0.62–0.76).
Conclusions:
Death during hospitalization was a significant modifier of the relationship between race and receipt of palliative care consultation. There are significant racial disparities in the utilization of IPCC for patients with advanced cancer.
Keywords: palliative care, disparities, inpatient, National Inpatient Sample, black
Introduction
In 2018, over 600 000 people died from cancer in the United States with higher mortality rates for black patients.1,2 Indeed, there are disparities in health care and outcomes for black patients throughout the continuum of oncologic care.3 Black women with breast cancer and black men with lung or prostate cancer have higher death rates than other racial groups.2,4 Furthermore, black patients are more likely to have untreated pain, more likely to have care discordant with their preferences, and less likely to have advance directives or enroll in hospice at end of life (EOL).5–9 Black patients and caregivers are also less satisfied with physician communication and overall quality of health at EOL.10,11
Palliative care improves quality of life, decreases depressive symptoms, and increases survival when incorporated early for patients with advanced malignancies.12–14 Based on the extensive body of literature describing the benefits of palliative care, several professional societies advocate for the early incorporation of palliative care for patients with cancer.15,16 Despite the strong association of palliative care with improved outcomes at EOL, there is limited literature about racial differences in the utilization of non-hospice-based palliative care consultations for patients with cancer.9 Existing studies focus on the use of hospice-based palliative care services, which has been shown to be underutilized among black adults.17–20 Furthermore, studies evaluating the use of non-hospice-based palliative care are often limited to a single center, a single type of malignancy, or do not specifically aim to investigate racial disparities in health care.21–26
Black patients have higher rates of emergency department visits, hospitalizations, and intensive care unit care at EOL compared to white patients.6,27,28 Inpatient palliative care consultations (IPCC) offer a unique opportunity to introduce black patients and their families to non-hospice-based palliative care services as blacks are more likely to be treated at the EOL in an inpatient setting.29 Given the gaps in the literature regarding disparities in use of non-hospice-based palliative care and the potential benefits of using IPCC for black patients, we seek to examine racial disparities in IPCC for patients with advanced cancer using a national population–based hospital database.
Methods
Data Sources and Patient Population
This study was performed using data from the Nationwide Inpatient Sample (NIS) from 2012 to 2014. The NIS is an all payer database that collects data from approximately 20% of all inpatient discharges from community hospitals across the United States. It is collected and maintained by the Agency for Healthcare Research and Quality (AHRQ). For each patient record, the NIS collects demographic data, hospital-level characteristics, and up to 25 diagnostic and procedure codes, coded using International Classification of Disease, Ninth Revision, Clinical Modification codes (ICD-9-CM).30
Patient records with a primary or secondary diagnosis of advanced solid organ malignancies as defined by relevant ICD-9-CM codes (Supplemental Table 1) were included in the study. Patients younger than 18 years and with a length of stay (LOS) less than one day were excluded from our final study population. Patient comorbidity was categorized according to their Charlson Comorbidity Index score (CCI), into 1 of 3 groups; CCI = 2, CCI = 3–6 and CCI >6.31 Receipt of major surgery during the inpatient admission was determined using criteria outlined by the AHRQ.32
Study Outcomes
The primary outcome of the study was the receipt of a palliative care consultation during the inpatient admission determined using the ICD-9-CM diagnosis code “v66.7.”33 This code has been used in previous studies to identify inpatient palliative care consultation in NIS data.34,35 Other secondary outcomes of the study included inpatient LOS and inpatient mortality.
Study Exposure
The primary exposure of this study was race. Patient race was classified as white, black, Hispanic, Asian, or Pacific Islander, and all other races were grouped as “Other.” Even though our primary objective is to examine black–white differences in palliative care, we have included data on races other than white and black to allow for hypothesis-generating ideas pertaining to other racial and ethnic minority groups.
Study Confounders
Directed acyclic graphs were used to visualize relationship between race and IPCC. Factors that impact race or IPCC but do not lie on pathway wherein race affects IPCC were included in final model as confounders. These include demographic characteristics including patient age and sex, and markers of socioeconomic status such as insurance status and median household income for the patient’s zip code. Charlson Comorbidity Index score, receipt of a major operation during the inpatient admission, receipt of chemotherapy or radiation while inpatient, code status, gastrostomy tube placement, receipt of parenteral nutrition, inpatient hemodialysis, the development of postoperative complications and hospital LOS all represent illness severity. Finally, hospital-level characteristics including hospital region, hospital bed size, and hospital teaching status can all affect availability of palliative care consults.
Given our primary exposure and covariates of interest, we excluded hospital events where patient age, sex, race, insurance status, or patient outcome at discharge (dead or alive) were missing.
Statistical Analysis
Categorical data were compared between patient groups using Pearson χ2 test, while continuous data were compared using the Kruskal-Wallis test. Multivariable logistic regression analyses were performed to assess the association between patient race and the receipt of palliative care services during the inpatient admission. Additional stratified analyses were also performed to identify factors associated with use of palliative care services within each patient group (by race and inpatient mortality). All multivariable models in the study adjusted for the following confounders of the relationship between race and IPCC described above: Collinearity of variables was examined using variance inflation factor analysis, and model fit was evaluated using the Akaike Information Criterion. Results of the multivariable analysis were presented as odds ratios (ORs) with corresponding 95% CIs. All analyses were performed using Stata version 14.0, and a P value of <.05 was used to define statistical significance.36 The study was approved by the institutional review board of Johns Hopkins University.
Results
Baseline Patient and Hospital Characteristics
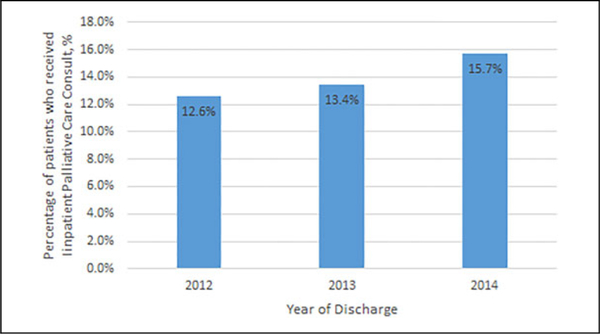
A total of 204 175 hospital admission records met inclusion criteria. Of these the majority were white 146 306 (71.7%), 28 286 (13.8%) were black, 16 240 (7.9%) were Hispanic, and 6444 (3.2%) were Asian or Pacific Islanders. Median age was 65 years (interquartile range: 55–74 years) and 53% were females (Table 1). Eighty-six percent of all hospital admission records had an associated code status of full code, while 80% had aggressive inpatient care such as inpatient chemotherapy or radiation therapy, hemodialysis, mechanical ventilation, gastrostomy tube placement, or administration of parenteral nutrition. Approximately 14% (28 367) of included hospital admissions for patients with advanced solid organ malignancies had an associated palliative care consultation. The proportion of IPCC increased with time (Figure 1).
Table 1.
Baseline Characteristics by Race and Ethnicity.
| Characteristic | White n(%) |
Black n (%) |
Hispanic n (%) |
Asian/Pacific Islander n (%) |
Other n(%) |
P value | Total N (%) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age, years, median (IQR) | 66 (57–75) | 62 (54–71) | 61 (50–71) | 63 (53–72) | 62 (53–72) | <.001 | 65 (55–74) |
| Sex | <.001 | ||||||
| Male | 69 907 (47.8%) | 12 400 (43.8%)) | 7557 (46.5%) | 2952 (45.8%) | 3235 (46.9%) | 96 051 (47.0%) | |
| Female | 76 399 (52.2%) | 15 886 (56.2%) | 8683 (53.5%) | 3492 (54.2%) | 3664 (53.1%) | 108 124 (53.0%) | |
| Insurance | <.001 | ||||||
| Private | 48 530 (33.2%) | 7409 (26.2%) | 3984 (24.5%) | 2320 (36.0%) | 2217 (32.1%) | 64 460 (31.6%) | |
| Medicare | 77 839 (53.2%) | 12 742 (45.0%) | 6253 (38.5%) | 2518 (39.1%) | 2784 (40.4%) | 102 136 (50.0%) | |
| Medicaid | 11 890 (8.1%) | 5902 (20.9%) | 4073 (25.1%) | 1174 (18.2%) | 1143 (16.6%) | 24 182 (11.8%) | |
| Other | 8047 (5.5%) | 2233 (7.9%) | 1930 (11.9%) | 432 (6.7%) | 755 (10.9%) | 13 397 (6.6%) | |
| Income quartile | <.001 | ||||||
| Q1 | 31 817 (22.2%) | 13 497 (49.1%) | 5845 (37.1%) | 792 (12.6%) | 1739 (26.49%) | 53 690 (26.9%) | |
| Q2 | 37 482 (26.1%) | 5907 (21.5%) | 3802 (24.1%) | 1079 (17.1%) | 1411 (21.49%) | 49 681 (24.9%) | |
| Q3 | 36 441 (25.4%) | 4722 (17.2%) | 3723 (23.6%) | 1692 (26.8%) | 1525 (23.23%) | 48 103 (24.1%) | |
| Q4 | 37 746 (26.3%) | 3339 (12.2%) | 2385 (15.1%) | 2748 (43.5%) | 1890 (28.79%) | 48 108 (24.1%) | |
| Charlson Comorbidity | 7(6–7) | 7 (6–8) | 6 (6–7) | 6 (6–7) | 6 (6–7) | <.001 | 7 (6–7) |
| Index score, median (IQR) | |||||||
| Inpatient surgical procedure | <.001 | ||||||
| No | 72 623 (49.6%) | 16 989 (60.1%) | 8900 (54.8%) | 3353 (52.0%) | 3441 (49.88%) | 105 306 (51.6%) | |
| Yes | 73 683 (50.4%) | 11 297 (39.9%) | 7340 (45.2%) | 3091 (48.0%) | 3458 (50.12%) | 98 869 (48.4%) | |
| DNR status | <.001 | ||||||
| Full code | 125 279 (85.6%) | 24 516 (86.7%) | 14 100 (86.8%) | 5444 (84.5%) | 5922 (85.84%) | 175 261 (85.8%) | |
| DNR | 21 027 (14.4%) | 3770 (13.3%) | 2140 (13.2%) | 1000 (15.5%) | 977 (14.16%) | 28 914 (14.2%) | |
| Hospital location | <.001 | ||||||
| South | 54 088 (37.0%) | 13 944 (49.3%) | 6429 (39.6%) | 993 (15.1%) | 2382 (34.53%) | 77 836 (38.1%) | |
| Midwest | 31 935 (21.8%) | 5896 (20.8%) | 1162 (7.2%) | 505 (7.8%) | 914 (13.25%) | 40 412 (19.8%) | |
| West | 24 614 (16.8%) | 2316 (8.2%) | 5922 (36.5%) | 3659 (56.8%) | 1226 (17.77%) | 37 737 (18.5%) | |
| Northeast | 35 669 (24.4%) | 6130 (21.7%) | 2727 (16.2%) | 1287 (20.0%) | 2377 (34.45%) | 48 190 (23.6%) | |
| Hospital bed size | <.001 | ||||||
| Small | 17 149 (11.7%) | 3259 (11.5%) | 1726 (10.6%) | 742 (11.5%) | 680 (9.9%) | 23 556 (11.5%) | |
| Medium | 33 172 (22.7%) | 6913 (24.4%) | 3873 (23.9%) | 1295 (20.1%) | 1357 (19.7%) | 46 610 (22.8%) | |
| Large | 95 985 (65.6%) | 18 114 (64.0%) | 10 641 (65.5%) | 4407 (68.4%) | 4862 (70.5%) | 134 009 (65.6% | |
| Hospital teaching status | <.001 | ||||||
| Rural | 9983 (6.8% | 786 (2.8% | 271 (1.7% | 137 (2.1% | 248 (3.6% | 11 425 (5.6% | |
| Urban nonteaching | 40 207 (27.5% | 5835 (20.6% | 4781 (29.4% | 1796 (27.9% | 1536 (22.3% | 54 155 (26.5% | |
| Urban teaching | 96 116 (65.7% | 21 665 (76.6% | 11 188 (68.9% | 4511 (70.00% | 5115 (74.1% | 138 595 (67.9%) | |
| Aggressive care (any) | <.001 | ||||||
| No | 118 021 (80.7%) | 21 814 (77.1%) | 12 909 (79.5%) | 5125 (79.5%) | 5319 (77.1%) | 163 188 (79.9%) | |
| Yes | 28 285 (19.3%) | 6472 (22.9%) | 3331 (20.5%) | 1319 (20.5%) | 1580 (22.9%) | 40 987 (20.1%) | |
| Inpatient radiation therapy | <.001 | ||||||
| No | 139 333 (95.2%) | 26 512 (93.7%) | 15 521 (95.6%) | 6166 (95.7%) | 6562 (95.1%) | 194 094 (95.1%) | |
| Yes | 6973 (4.8%) | 1774 (6.3%) | 719 (4.4%) | 278 (4.3%) | 337 (4.9%) | 10 081 (4.9%) | |
| Inpatient chemotherapy | <.001 | ||||||
| No | 136 304 (93.2%) | 26 161 (92.5%) | 14 898 (91.7%) | 5935 (92.1%) | 6240 (90.5%) | 189 538 (92.8%) | |
| Yes | 10 002 (6.8%) | 2125 (7.5%) | 1342 (8.3%) | 509 (7.9%) | 659 (9.5%) | 14 637 (7.2%) | |
| Mechanical ventilation | <.001 | ||||||
| No | 141 554 (96.8%) | 27 097 (95.8%) | 15 686 (96.6%) | 6211 (96.4%) | 6634 (96.2%) | 197 182 (96.6%) | |
| Yes | 4752 (3.2%) | 1189 (4.2%) | 554 (3.4%) | 233 (3.6%) | 265 (3.8%) | 6993 (3.4%) | |
| Gastrostomy tube | <.001 | ||||||
| No | 142 765 (97.6%) | 27 547 (97.4%) | 15 862 (97.7%) | 6292 (97.6%) | 6698 (97.1%) | 199 164 (97.5%) | |
| Yes | 3541 (2.4%) | 739 (2.6%) | 378 (2.3%) | 152 (2.4%) | 201 (2.9%) | 5011 (2.5%) | |
| Parenteral nutrition | <.001 | ||||||
| No | 139 096 (95.1%) | 26 834 (94.9%) | 15 480 (95.3%) | 6058 (94.0%) | 6464 (93.7%) | 193932 (95.0%) | |
| Yes | 7210 (4.9%) | 1452 (5.1%) | 760 (4.7%) | 386 (6.0%) | 435 (6.3%) | 10 243 (5.0%) | |
| Hemodialysis | <.001 | ||||||
| No | 145 271 (99.3%) | 27 733 (98.0%) | 16 056 (98.9%) | 6391 (99.2%) | 6842 (99.7%) | 202 293 (99.1%) | |
| Yes | 1035 (0.7%) | 533 (2.0%) | 184 (1.1%) | 53 (0.8%) | 57 (0.8%) | 1882 (0.9%) | |
| Palliative care consultation | <.001 | ||||||
| No | 126 392 (86.4%) | 24 080 (85.1%) | 13 944 (85.9%) | 5456 (84.7%) | 5936 (86.0%) | 175 808 (86.1%) | |
| Yes | 19 914 (13.6%) | 4206 (14.9%) | 2296 (14.1%) | 988 (15.3%) | 963 (14.0%) | 28 367 (13.9%) | |
Abbreviations: IQR, interquartile range; DNR, Do Not Resuscitate.
Figure 1.
Proportion of patients who received inpatient palliative care consult by year of inpatient admission.
Factors Associated With Use of Palliative Care
In univariable analyses, black and Asian or Pacific Islander race (OR black: 1.11; 95% CI: 1.07–1.15; OR Asian/Pacific Islander: 1.15; 95% CI: 1.07–1.23, Table 2), increasing age (OR: 1.27; 95% CI: 1.24–1.31), insurance status other than private insurance, and comorbidity score (OR: 1.14; 95% CI: 1.13–1.15) were associated with significant increased odds of IPCC. Do-Not-Resuscitate status was associated with 12 times increased odds of receiving IPCC (OR: 12.1; 95% CI: 117–12.4), and receipt of aggressive care was also associated with increased odds of receiving IPCC (OR: 1.24; 95% CI: 1.21–1.18). In terms of hospital characteristics, only hospital bed size was associated with IPCC, with medium and large hospitals having increased odds of IPCC compared to small hospitals (OR medium: 1.16; 95% CI: 1.11–1.21; OR large: 1.10; 95% CI: 1.10–1.156).
Table 2.
Univariable and Multivariable Logistic Regression Analyses of Factors Associated With Receipt of Inpatient Palliative Care.
| Univariable logistic regression |
Multivariable logistic regression |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | ||
|
| ||||||||
| Patient race | ||||||||
| White | Reference | – | – | – | Reference | – | – | – |
| Black | 1.11 | 1.069 | 1.149 | <.001 | 1.04 | 0.998 | 1.083 | .065 |
| Hispanic | 1.05 | 0.997 | 1.095 | .064 | 1.02 | 0.967 | 1.076 | .466 |
| Asian/Pacific Islander | 1.15 | 1.072 | 1.232 | <.001 | 1.08 | 0.998 | 1.170 | .056 |
| Other | 1.03 | 0.960 | 1.104 | .411 | 1.00 | 0.924 | 1.083 | .994 |
| Age, years | 1.27 | 1.241 | 1.305 | <.001 | 1.01 | 1.006 | 1.009 | <.001 |
| Sex | ||||||||
| Male | Reference | – | – | – | Reference | – | – | – |
| Female | 1.04 | 0.979 | 1.030 | .741 | 1.03 | 1.002 | 1.061 | .038 |
| Insurance | ||||||||
| Private | Reference | – | – | – | Reference | – | – | – |
| Medicare | 1.25 | 1.215 | 1.289 | <.001 | 0.85 | 0.814 | 0.882 | <.001 |
| Medicaid | 1.33 | 1.278 | 1.391 | <.001 | 1.17 | 1.110 | 1.223 | <.001 |
| Other | 1.51 | 1.432 | 1.569 | <.001 | 1.32 | 1.243 | 1.396 | <.001 |
| Income quartile | ||||||||
| Q1 | Reference | – | – | – | – | – | – | – |
| Q2 | 0.98 | 0.943 | 1.013 | .209 | – | – | – | – |
| Q3 | 1.02 | 0.981 | 1.053 | .374 | – | – | – | – |
| Q4 | 1.02 | 0.983 | 1.056 | .300 | – | – | – | – |
| Charlson Comorbidity Index score | 1.14 | 1.129 | 1.149 | <.001 | 1.04 | 1.033 | 1.056 | <.001 |
| Inpatient surgical procedure | ||||||||
| No | Reference | – | – | – | Reference | – | – | – |
| Yes | 0.17 | 0.169 | 0.181 | <.001 | 0.28 | 0.269 | 0.288 | <.001 |
| DNR status | ||||||||
| Full code | Reference | – | – | – | Reference | – | – | – |
| DNR | 12.08 | 11.738 | 12.437 | <.001 | 8.60 | 8.345 | 8.866 | <.001 |
| Hospital location | ||||||||
| South | Reference | – | – | – | – | – | – | – |
| Midwest | 0.98 | 0.944 | 1.012 | .201 | – | – | – | – |
| West | 1.10 | 1.057 | 1.134 | <.001 | – | – | – | – |
| Northeast | 1.03 | 1.001 | 1.069 | .046 | – | – | – | – |
| Hospital bed size | ||||||||
| Small | Reference | – | – | – | Reference | – | – | – |
| Medium | 1.16 | 1.106 | 1.213 | <.001 | 1.15 | 1.094 | 1.214 | <.001 |
| Large | 1.10 | 1.060 | 1.151 | <.001 | 1.18 | 1.128 | 1.237 | <.001 |
| Hospital teaching status | ||||||||
| Rural | Reference | – | – | – | – | – | – | – |
| Urban nonteaching | 0.91 | 0.859 | 0.964 | .001 | – | – | – | – |
| Urban teaching | 0.93 | 0.884 | 0.985 | .012 | – | – | – | – |
| Aggressive care (any) | ||||||||
| No | Reference | – | – | – | Reference | – | – | – |
| Yes | 1.24 | 1.205 | 1.279 | <.001 | 1.22 | 1.180 | 1.263 | <.001 |
| Inpatient radiation therapy | ||||||||
| No | Reference | – | – | – | – | – | – | – |
| Yes | 1.39 | 1.324 | 1.467 | <.001 | – | – | – | – |
| Inpatient chemotherapy | ||||||||
| No | Reference | – | – | – | – | – | – | – |
| Yes | 0.84 | 0.802 | 0.889 | <.001 | – | – | – | – |
| Mechanical ventilation | ||||||||
| No | Reference | – | – | – | – | – | – | – |
| Yes | 1.73 | 1.630 | 1.832 | <.001 | – | – | – | – |
| Gastrostomy tube | ||||||||
| No | Reference | – | – | – | – | – | – | – |
| Yes | 1.37 | 1.270 | 1.470 | <.001 | – | – | – | – |
| Parenteral nutrition | ||||||||
| No | Reference | – | – | – | – | – | – | – |
| Yes | 1.27 | 1.199 | 1.334 | <.001 | – | – | – | – |
| Hemodialysis | ||||||||
| No | Reference | – | – | – | – | – | – | – |
| Yes | 1.53 | 1.368 | 1.719 | <.001 | – | – | – | – |
Abbreviation: DNR, Do Not Resuscitate.
In multivariable analyses, all of the significant factors from univariable analyses remained statistically significant with the exception of race. Once adjusted for age, gender, insurance status, comorbidity score, code status, hospital bed size, and receipt of aggressive care, there were no longer any statistically significant associations between race and receipt of IPCC (Table 2).
Palliative Care Utilization by Patient Race/Ethnicity
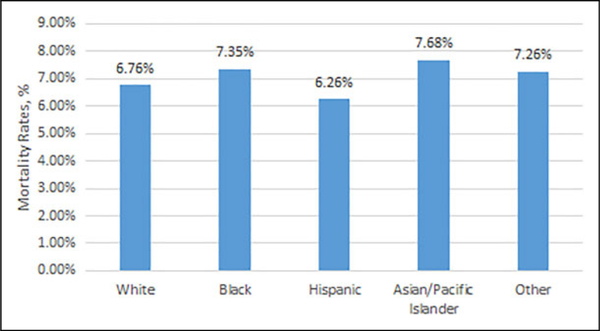
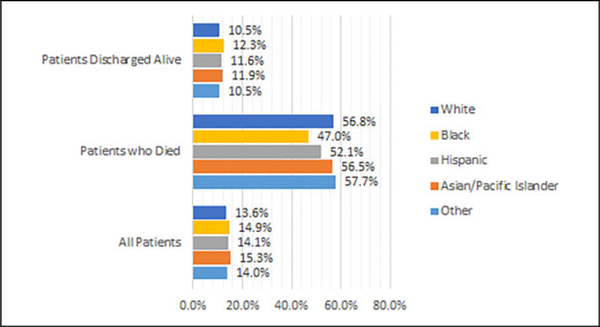
The highest utilization of IPCC by race or ethnicity was for Asian or Pacific Islanders (15.3%), followed by black (14.9%), Hispanic (14.1%), other (14.0%), and white (13.6%) patients. Rates of in-hospital mortality varied from 6.3% for Hispanic patients to 7.7% for Asian or Pacific Islander patients (Figure 2). When stratified by death during hospitalization event, 57.7% of those who died received IPCC compared to 10.5% who were discharged alive. This difference persisted when further broken down by race. 56.8% of whites and 47.0% of blacks who died received IPCC versus 10.5% of whites and 12.3% of blacks those who were discharged alive (Figure 3).
Figure 2.
Mortality rates among all patients by race/ethnicity.
Figure 3.
Vital status at discharge by race/ethnicity.
Due to the strong effect of death on our outcome of interest, the multivariable analysis was repeated stratifying by death status. Adjusting for the same covariates as our original multivariable analysis, we see statistically significant increase in odds of IPCC for black and Hispanic patients compared to white (OR black: 1.14, 95% CI: 1.09–1.19; OR Hispanic: 1.11, 95% CI: 1.05–1.18). However, for those discharged alive, we see significant reductions in odds of IPCC for blacks and Hispanics compared to whites (OR black: 0.69, 95% CI: 0.62–0.76; OR Hispanic: 0.75, 95% CI: 0.65–0.87, Table 3.
Table 3.
Association of Race/Ethnicity and Receipt of Inpatient Palliative Care Stratified by Vital Status Results of a Multivariable Logistic Regression.a
| All patients |
Patients who died |
Patients discharged alive |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
|
| |||||||||
| White | Reference | – | – | Reference | – | – | Reference | – | – |
| Black | 1.04 | 0.998–1.083 | .065 | 1.14 | 1.087–1.190 | <.001 | 0.69 | 0.622–0.764 | <.001 |
| Hispanic | 1.02 | 0.967–1.076 | .466 | 1.11 | 1.049–1.179 | <.001 | 0.75 | 0.655–0.867 | <.001 |
| Asian/Pacific Islander | 1.08 | 0.998–1.170 | .056 | 1.11 | 1.014–1.214 | .023 | 0.92 | 0.755–1.114 | .384 |
| Other | 1.00 | 0.924–1.083 | .994 | 1.01 | 0.923–1.106 | .824 | 0.96 | 0.789–1.162 | .660 |
This model adjusted for age, gender, race/ethnicity, insurance status, comorbidities, code status, hospital bed size, and receipt of aggressive care.
Discussion
Although much of the existing literature focuses on disparities in the use of hospice, there are limited studies examining racial differences in the utilization of non-hospice-based palliative care services.9 This study analyzed racial disparities in the use of palliative care for a nationally representative sample of patients with advanced cancer admitted to an inpatient unit. Black patients with cancer have been shown to have higher burden of symptoms at EOL and would therefore greatly benefit from palliative care interventions prior to initiation of hospice care.5–7,9,11,37 However, racial differences were found in the use of IPCC for black patients with advanced cancers discharged alive versus white patients of the same condition.
Among patients with advanced cancers hospitalized in the United States, blacks were more likely than whites to receive IPCC if they died in the hospital. However, among patients who were discharged from hospital alive, this association reversed and blacks were less likely to have received IPCC than whites. This association remained true after adjusting for age, insurance status, and comorbidities. Among our entire cohort, a significantly higher proportion of IPCC were done for patients who died compared to those who were discharged alive. These findings suggest that death or perceived time to death is a significant mediator of receipt of IPCC for black patients.
Perceived time to death or death acting as a mediator on the relationship between race and receipt of IPCC may contribute to the mixed findings of the association between race and IPCC in the literature.21–26 Although several studies have found higher odds of IPCC for hospitalized black patients with cancer, a few have found no difference or higher odds of IPCC for black patients compared to white patients.21–26 However, only one of these studies investigated death as a mediator of receipt of IPCC.25 The absence of measurement of the potential mediator of death could explain the differences among these studies as highlighted by our findings of a transposed relationship between black race and IPCC when stratified by death. Furthermore, these studies included a single tumor type or were done at a single institution; factors that may lead to differences in racial distribution of people who died. Dissimilarities in study populations based on inclusion and exclusion criteria and temporal trends in IPCC may also explain the heterogeneity of study results.
Physicians who work in the inpatient setting are more likely to have EOL discussions when they believe prognosis to be grim and death imminent.38,39 It has also been demonstrated that physicians who care for patients in a hospital setting are often unfamiliar with advance directives, lack knowledge of criteria for hospice or services available through hospice, and have discomfort in discussing futility of treatment with patients and their caregivers.40 All of these factors may increase likelihood of using a palliative care consultation to overcome some of these barriers when death is believed to be close. This is supported by our findings of higher rates of IPCC among those who died compared to those discharged from hospital alive. Furthermore, critically ill nonwhite patients have been shown to have higher rates of discord with clinical providers and family members.8 In this setting, IPCC may be used to facilitate EOL discussions which are perceived to be necessary but difficult. These factors may contribute to our finding of 14% higher odds of IPCC for black patients who died during admission compared to white patients.
The lower odds of IPCC utilization among black patients who were discharged alive reveal a critical disparity in the utilization of an effective medical tool which can improve health outcomes for black patients. In fact, these patients who are discharged alive are arguably the ones that would benefit the most from non-hospice-based palliative care; as they are going home to live with the sequelae of their cancer. Black patients potentially have more to gain from palliative care given their higher symptom burden at EOL.9,11,37 Yet, this evidence-based practice is not being delivered to this vulnerable population at a rate equivalent to their white counterparts. Given our adjustments for medical and socioeconomic factors, which confound the relationship between race and IPCC, these results demonstrate that race and ethnicity play a role in the decision to provide IPCC. Further study is needed to understand the role of patient and family attitudes and choices in the rates of IPCC seen in this study.
Limitations
This study includes a large sample size and covers a variety of hospital settings across the United States, increasing the generalizability of our findings. However, there are some limitations. This is an administrative database which may have coding errors and lacks clinical details such as reason for palliative care consults and when consults were refused by patients. The ICD-9 code “v66.7” used to identify palliative care consults may also be used to code other medical care such as initiation of EOL care without a formal palliative care consultations. This would result in an overestimation of actual inpatient palliative care consults. However, validation studies have found high specificity of this code for capturing IPCC, especially among patients with advanced cancer.33 We are also unable to ascertain whether palliative care consults were arranged for the outpatient setting postdischarge or whether patients had established care with palliative care team prior to admission. Using the NIS database, we are unable to distinguish between new admissions and readmissions. This may have led to an over- or underestimation of the prevalence of palliative care consultations in our study population depending on the racial distribution of readmissions in this sample.
Conclusions
To the best of our knowledge, this is the first study to use a nationally representative sample to address the question of whether or not disparities in utilization of palliative care consults persist for racial minorities in the inpatient setting. Although IPCC is more likely to be used for black patients who die, the disparity is reversed for black patients with advanced cancer who leave the hospital alive. As palliative care consult must be placed prior to death, this finding suggests that in the clinical setting, perceived risk of death mediates the relationship between race and use of palliative care consultation. Secondly, there remain significant disparities in utilization of palliative care for black patients most likely to benefit—those who were discharged from hospital alive. IPCC could be used to help address disparities in quality of care at EOL such as uncontrolled pain and foster a bridge for ongoing palliative care as an outpatient. Inpatient providers who care for patients with cancer should be aware of the benefits of IPCC, especially for racial minority patients. Further research is warranted to understand the underlying factors contributing the racial disparities in IPCC utilization and to develop interventions to improve utilization of IPCC among patients with advanced cancers.9
Supplementary Material
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Agency for Healthcare Research and Quality [1K08HS024736-01] and the Ruth L. Kirschtein National Research Service Award [5T32HL139426-2].
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016. National Cancer Institute; 2019. [Google Scholar]
- 2.O’Keefe EB, Meltzer JP, Bethea TN. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front Public Health 2015;3(4):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004; 54(2):78–93. [DOI] [PubMed] [Google Scholar]
- 4.Hill DA, Prossnitz ER, Royce M, Nibbe A. Temporal trends in breast cancer survival by race and ethnicity: a population-based cohort study. PLoS One. 2019;14(10):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamal AH, Bull J, Wolf SP, Portman D, Strand J, Johnson KS. Unmet needs of African Americans and whites at the time of palliative care consultation. Am J Hosp Palliat Care. 2017; 34(5):461–465. [DOI] [PubMed] [Google Scholar]
- 6.Mack JW, Paulk ME, Viswanath K, Prigerson HG: Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med. 2010;170(17):1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucher NA, Raghavan M, Smith A, et al. Palliative care in the African American community #204. J Palliat Med. 2016;19(2): 228–230. [DOI] [PubMed] [Google Scholar]
- 8.Muni S, Engelberg RA, Treece PD, et al. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139(5):1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson KS. Racial and ethnic disparities in palliative care. J Palliat Med. 2013;16(11):1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mc Cleskey SG, Cain CL. Improving end-of-life care for diverse populations: communication, competency, and system supports. Am J Hosp Palliat Care. 2019;36(6):453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch LC, Teno JM, Mor V. End-of-life care in black and white: race matters for medical care of dying patients and their families. J Am Geriatr Soc. 2005;53(7):1145–1153. [DOI] [PubMed] [Google Scholar]
- 12.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 13.Bakitas MA, Tosteson TD, Li Z, et al. : Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015; 33(13):1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temel JS, Greer JA, El-Jawahri A, et al. : Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice guideline update. J Clin Oncol. 2017;35(1): 96–112. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. Palliative care (version 2.2019). Updated February 8, 2019. Accessed January 16, 2020. https://www.nccn.org/professionals/physician_gls/pdf/palliative.pdf.
- 17.Lepore MJ, Miller SC, Gozalo P. Hospice use among urban black and white U.S. nursing home decedents in 2006. Gerontologist. 2011;51(2):251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramey SJ, Chin SH. Disparity in hospice utilization by African American patients with cancer. Am J Hosp Palliat Care. 2012; 29(5):346–354. [DOI] [PubMed] [Google Scholar]
- 19.Haines KL, Jung HS, Zens T, Turner S, Warner-Hillard C, Agarwal S. Barriers to hospice care in trauma patients: the disparities in end-of-life care. Am J Hosp Palliat Care. 2018;35(8):1081–1084. [DOI] [PubMed] [Google Scholar]
- 20.Payne R. Racially associated disparities in hospice and palliative care access: acknowledging the facts while addressing the opportunities to improve. J Palliat Med. 2016;19(2):131–133. [DOI] [PubMed] [Google Scholar]
- 21.Cervantez SR, Tenner LL, Schmidt S, et al. Symptom burden and palliative referral disparities in an ambulatory south Texas cancer center. Front Oncol. 2018;8:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blue B, Vegunta R, Rodin M, et al. Impact of an inpatient palliative care consultation in terminally ill cancer patients. Cureus. 2018;10(7):e3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chuang E, Hope AA, Allyn K, et al. Gaps in provision of primary and specialty palliative care in the acute care setting by race and ethnicity. J Pain Symptom Manage. 2017;54(5): 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma RK, Cameron KA, Chmiel JS, et al. Racial/ethnic differences in inpatient palliative care consultation for patients with advanced cancer. J Clin Oncol. 2015;33(32): 3802–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubens M, Ramamoorthy V, Saxena A, et al. Palliative care consultation trends among hospitalized patients with advanced cancer in the United States, 2005 to 2014. Am J Hosp Palliat Care. 2019; 36(4):294–301. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld EB, Chan JK, Gardner AB, et al. Disparities associated with inpatient palliative care utilization by patients with metastatic gynecologic cancers: a study of 3337 women. Am J Hosp Palliat Care. 2018;35(4):697–703. [DOI] [PubMed] [Google Scholar]
- 27.Burgio KL, Williams BR, Dionne-Odom JN, et al. Racial differences in processes of care at end of life in VA medical centers: Planned secondary analysis of data from the BEACON trial. J Palliat Med. 2016;19(2):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith AK, Earle CC, Mc Carthy EP. Racial and ethnic differences in end-of-life care in fee-for-service Medicare beneficiaries with advanced cancer. J Am Geriatr Soc. 2009;57(1): 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanchate A, Kronman AC, Young-Xu Y, et al. Racial and ethnic differences in end-of-life costs: why do minorities cost more than whites? Arch Intern Med. 2009;169(5):493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Introduction to the HCUP National Inpatient Sample (NIS). Updated July 2018. Accessed January 16, 2020. https://www.hcup-us.ahrq.gov/db/nation/nis/NIS%20Introduction%202016.pdf.
- 31.Charlson ME, Pompei P, Ales KL, Mac Kenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(1):373–383. [DOI] [PubMed] [Google Scholar]
- 32.Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project, (HCUP). NIS description of data elements. Updated September 17, 2008. Accessed January 16, 2020. https://www.hcup-us.ahrq.gov/db/vars/pclassn/nisnote.jsp.
- 33.Hua M, Li G, Clancy C, et al. Validation of the V66.7 code for palliative care consultation in a single academic medical center. J Palliat Med. 2017;20(4):372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulvey CL, Smith TJ, Gourin CG. Use of inpatient palliative care services in patients with metastatic incurable head and neck cancer. Head Neck. 2016;38(3):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gani F, Enumah ZO, Conca-Cheng AM, et al. Palliative care utilization among patients admitted for gastrointestinal and thoracic cancers. J Palliat Med. 2018;21(4):428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.StataCorp. Stata Statistical Software: Release 14. StataCorp LP; 2015. [Google Scholar]
- 37.Sharma RK, Freedman VA, Mor V, et al. Association of racial differences with end-of-life care quality in the United States. JAMA Intern Med. 2017;177(12):1858–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schenker Y, Tiver G, Hong S, White D. Association between physicians’ beliefs and the option of comfort care for critically ill patients. Intensive Care Med. 2012;38(10):1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White DB, Braddock CH, Bereknyei S, Curtis JR. Toward shared decision making at the end of life in intensive care units: opportunities for improvement. Arch Intern Med. 2007;167(5): 461–467. [DOI] [PubMed] [Google Scholar]
- 40.Visser M, Deliens L, Houttekier D. Physician-related barriers to communication and patient- and family-centred decision-making towards the end of life in intensive care: a systematic review. Crit Care. 2014;18(6):604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.