Using the Vaccine Adverse Event Reporting System, the number and demographic characteristics of cases of thrombosis with thrombocytopenia syndrome occurring after receipt of COVID-19 vaccines in the United States were determined.
Visual Abstract. Thrombosis With Thrombocytopenia Syndrome After COVID-19 Vaccination.
Using the Vaccine Adverse Event Reporting System, the number and demographic characteristics of cases of thrombosis with thrombocytopenia syndrome occurring after receipt of COVID-19 vaccines in the United States were determined.
Abstract
Background:
Thrombosis with thrombocytopenia syndrome (TTS) is a potentially life-threatening condition associated with adenoviral-vectored COVID-19 vaccination. It presents similarly to spontaneous heparin-induced thrombocytopenia. Twelve cases of cerebral venous sinus thrombosis after vaccination with the Ad26.COV2.S COVID-19 vaccine (Janssen/Johnson & Johnson) have previously been described.
Objective:
To describe surveillance data and reporting rates of all reported TTS cases after COVID-19 vaccination in the United States.
Design:
Case series.
Setting:
United States.
Patients:
Case patients receiving a COVID-19 vaccine from 14 December 2020 through 31 August 2021 with thrombocytopenia and thrombosis (excluding isolated ischemic stroke or myocardial infarction) reported to the Vaccine Adverse Event Reporting System. If thrombosis was only in an extremity vein or pulmonary embolism, a positive enzyme-linked immunosorbent assay for antiplatelet factor 4 antibodies or functional heparin-induced thrombocytopenia platelet test result was required.
Measurements:
Reporting rates (cases per million vaccine doses) and descriptive epidemiology.
Results:
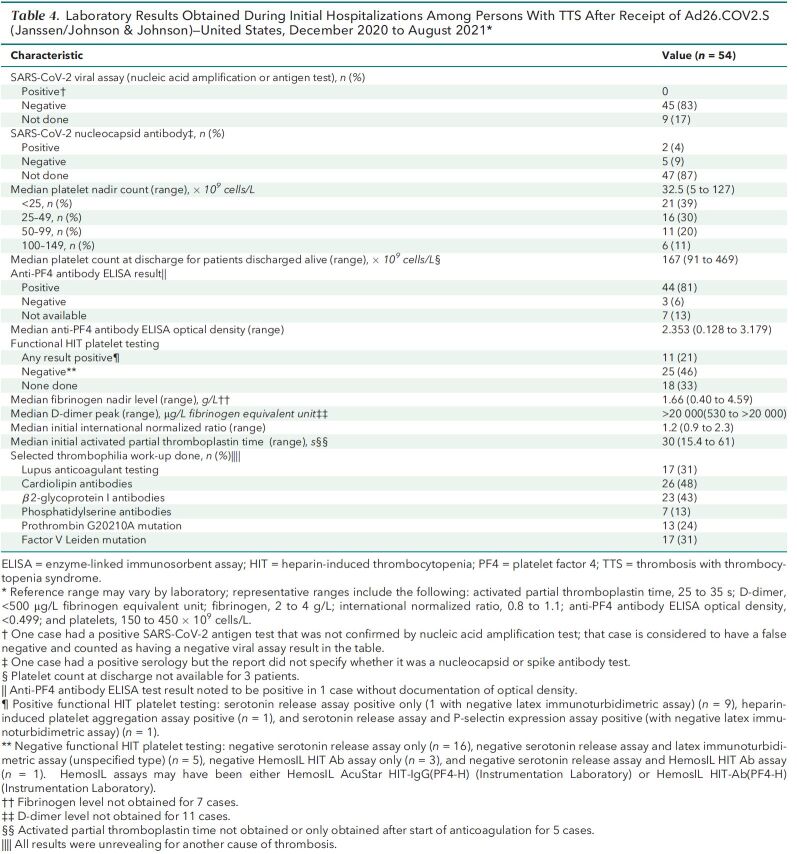
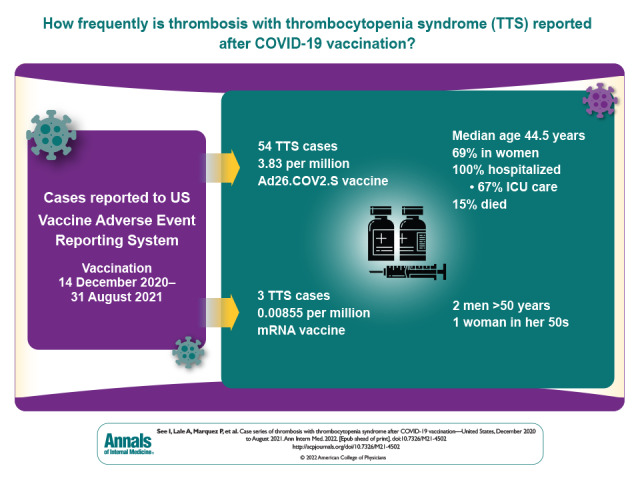
A total of 57 TTS cases were confirmed after vaccination with Ad26.COV2.S (n = 54) or a messenger RNA (mRNA)–based COVID-19 vaccine (n = 3). Reporting rates for TTS were 3.83 per million vaccine doses (Ad26.COV2.S) and 0.00855 per million vaccine doses (mRNA-based COVID-19 vaccines). The median age of patients with TTS after Ad26.COV2.S vaccination was 44.5 years (range, 18 to 70 years), and 69% of patients were women. Of the TTS cases after mRNA-based COVID-19 vaccination, 2 occurred in men older than 50 years and 1 in a woman aged 50 to 59 years. All cases after Ad26.COV2.S vaccination involved hospitalization, including 36 (67%) with intensive care unit admission. Outcomes of hospitalizations after Ad26.COV2.S vaccination included death (15%), discharge to postacute care (17%), and discharge home (68%).
Limitations:
Underreporting and incomplete case follow-up.
Conclusion:
Thrombosis with thrombocytopenia syndrome is a rare but serious adverse event associated with Ad26.COV2.S vaccination. The different demographic characteristics of the 3 cases reported after mRNA-based COVID-19 vaccines and the much lower reporting rate suggest that these cases represent a background rate.
Primary Funding Source:
Centers for Disease Control and Prevention.
Thrombosis with thrombocytopenia syndrome (TTS) is a rare condition recently described among persons who received the adenoviral-vectored COVID-19 vaccines Ad26.COV2.S (Janssen/Johnson & Johnson) and ChAdOx1 nCoV-19 (Oxford/AstraZeneca) (1–5). The term TTS is used by the Brighton Collaboration, an organization that develops consensus definitions for adverse events after immunization, and by various governmental agencies (6–8). Many clinical experts use the term vaccine-induced immune thrombotic thrombocytopenia (VITT) to refer to this syndrome (8). Initial recognition of the condition precipitated a pause in the administration of Ad26.COV2.S in the United States and modifications of ChAdOx1 nCoV-19 vaccine policy in other countries (9, 10). Currently available evidence supports a causal relationship between TTS and Ad26.COV2.S vaccination (11).
Reported rates of TTS after ChAdOx1 nCoV-19, which is not approved or authorized for use in the United States, range from 13 to 39 cases per million vaccine doses administered, depending on the publication (2, 12–14). The first reports of TTS after ChAdOx1 nCoV-19 vaccination described a high case-fatality rate and features shared with spontaneous heparin-induced thrombocytopenia (HIT) (1–3), including thrombocytopenia, thrombosis, and the presence of antibodies to platelet factor 4 (PF4) (1–3, 15) in the absence of prior exposure to heparin. These reports described positive results for antibodies to PF4 measured by enzyme-linked immunosorbent assay (ELISA) and, in many cases, positive results for various functional HIT platelet assays, which evaluate the ability of anti-PF4 antibodies to activate platelets and cause thrombosis (1–3, 16). Preliminary hypotheses on the pathogenesis of TTS generally posit mechanisms related to the adenovirus vector platform, including triggering of HIT-like antibodies from adenoviral DNA, cell line–derived factors, or splice variants of the SARS-CoV-2 spike protein caused by the adenoviral vector platform (1, 17, 18).
Thrombosis with thrombocytopenia syndrome after receipt of the ChAdOx1 nCoV-19 vaccine has been described in detail (19). In the United States, the first 12 cases of TTS after vaccination with Ad26.COV2.S involving cerebral venous sinus thrombosis were described in a preliminary report (5); additional case reports have been published, including 1 case of TTS after messenger RNA (mRNA) COVID-19 vaccination (20).
This report is the first comprehensive review of TTS after COVID-19 vaccination in the United States. We describe the epidemiology and clinical characteristics of the case reports to inform clinicians and public health officials.
Methods
Data Sources
The Vaccine Adverse Event Reporting System (VAERS) is a passive surveillance (spontaneous reporting) system for adverse events after immunization that is jointly administered by the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (21). The system accepts all reports of possible adverse events after vaccination from patients, clinicians, vaccine manufacturers, and the public, regardless of clinical severity of the event or its causal association with vaccination. The VAERS database was searched for potential reports of TTS received during 14 December 2020 through 30 September 2021 in the following manner. First, on 9 April 2021, a retrospective search was done for reports received during 14 December 2020 through 8 April 2021. Then, prospective searches were done beginning on 9 April 2021. All data for this analysis were retrieved by 15 October 2021. Reports were screened for Medical Dictionary for Regulatory Activities standardized codes (preferred terms [PTs]) (22) assigned to reports by professional medical coders as well as language in the symptom or laboratory text fields of the report that described symptoms, laboratory values, and medical conditions that could indicate TTS (see the Methods section in the Appendix). For inclusion in this analysis, patients needed to have received COVID-19 vaccination by 31 August 2021.
Vaccine administration data were reported by public health jurisdictions to the CDC (Methods section in the Appendix) (23).
Case Definitions
We used the following case definitions for TTS. A tier 1 case had thrombosis in an unusual location for a thrombus (that is, cerebral vein, visceral artery or vein, extremity artery, or central artery or vein) and new-onset thrombocytopenia (that is, platelet count <150 × 109 cells/L) occurring any time after receipt of a COVID-19 vaccine. Tier 2 cases had new-onset thrombocytopenia, thrombosis in an extremity vein or pulmonary artery in the absence of thrombosis at a tier 1 location, and a positive anti-PF4 antibody ELISA test result or functional HIT platelet test result occurring any time after receipt of a COVID-19 vaccine. Given the unusual clinical presentation of the tier 1 cases, additional laboratory abnormalities were not required, whereas the inclusion of positive HIT testing results for cases with only common thromboses (tier 2) was intended to limit the number of potential false-positive cases.
Confirmation of case status required review of patient data from medical records and, in some cases, also discussion with providers. All cases were also reviewed with clinicians from CDC's Clinical Immunization Safety Assessment (CISA) Project (Methods section in the Appendix) (24), including specialists in infectious diseases, neurology, and hematology. If, after review and consideration of the differential diagnoses, the clinical syndrome was not considered consistent with TTS, or if a more likely cause of the patient's signs and symptoms was identified, that report was removed from the case series.
Statistical Analysis
Reporting rates for TTS were calculated as confirmed cases per million doses of Ad26.COV2.S vaccine administered and stratified by sex and age group. Overall reporting rates were calculated as confirmed cases per million doses of mRNA COVID-19 vaccine (that is, Moderna and Pfizer–BioNTech) administered. Booster dose administration (that is, a second dose of Ad26.COV2.S or a third dose of an mRNA COVID-19 vaccine) was excluded from reporting rate calculations.
Data were collected and managed using Research Electronic Data Capture tools hosted at CDC. Research Electronic Data Capture is a secure, web-based software platform designed to support data entry and management (25, 26). Descriptive analysis of demographic and clinical data associated with TTS cases was done. Demographic characteristics, risk factors for thrombosis, platelet nadir, and timing of clinical presentation were compared between tier 1 and tier 2 cases using chi-square or Fisher exact test, as appropriate, for categorical variables and Wilcoxon rank-sum test for continuous variables. To permit comparisons with other reports about TTS, the Results section of the Appendix describes whether cases met “definite VITT” or “probable VITT” criteria established by the U.K. Expert Haematology Panel (19). Venous thromboembolism risk factors were assessed according to published literature (27, 28). Data analyses were done with SAS, version 9.4 (SAS Institute).
This activity was reviewed by the CDC Human Subjects Office, determined to be a nonresearch public health surveillance project, and conducted consistent with applicable federal law and policy (see, for example, 45 C.F.R. part 46.102(I)(2), 21 C.F.R. part 56, 42 U.S.C. §241(d), 5 U.S.C. §552a, and 44 U.S.C. §3501 et seq.).
Role of the Funding Source
This activity was funded by the CDC. The CDC and non-CDC coauthors conducted the investigations; performed collection, management, analysis, and interpretation of the data; were involved in preparation, review, and approval of the manuscript; and made the decision to submit the manuscript for publication.
Results
Reporting Rates for TTS
Of 579 301 total reports to VAERS after COVID-19 vaccination during the analytic period, 1122 were identified as potential cases on the basis of initial screening of VAERS reports. Of these, for 2, receipt of complete medical records are still pending (as of 19 December 2021), precluding a final determination of whether the reports satisfied the TTS case definition; for 11, insufficient information was contained in the report to make a determination (for example, the report included information about thrombosis only and no provider listed to obtain further information); and, for 976, the case definition criteria after review was not met (for example, either thrombosis or thrombocytopenia was not present). Of the 133 remaining reports that were confirmed to involve thrombosis and thrombocytopenia after COVID-19 vaccination, 76 were excluded because they were duplicate reports (n = 30), active COVID-19 infection was documented at the time of TTS diagnosis (n = 5), thrombocytopenia was not new-onset (n = 2), TTS symptoms preceded COVID-19 vaccination (n = 3), thrombocytopenia was most likely spurious (n = 1), or thrombosis was more likely due to another cause (n = 35). After these exclusions, 57 reports were determined to meet the CDC case definition for TTS: 54 cases after Ad26.COV2.S vaccination and 3 after mRNA COVID-19 vaccination (Figure).
Figure. Reports to VAERS confirmed to be TTS—United States, December 2020 to August 2021.
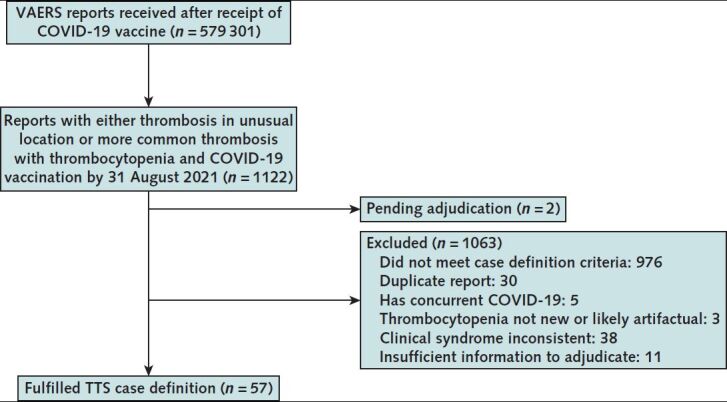
TTS = thrombosis with thrombocytopenia syndrome; VAERS = Vaccine Adverse Event Reporting System.
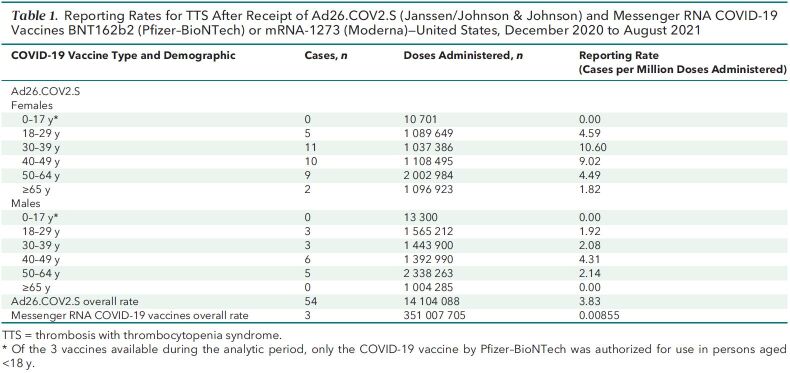
Given administration of 14.1 million doses of Ad26.COV2.S and 351 million doses of mRNA COVID-19 vaccines, the overall TTS reporting rate (per million vaccine doses administered) was 3.83 after Ad26.COV2.S and 0.00855 after mRNA COVID-19 vaccination. Reporting rates were highest after receipt of the Ad26.COV2.S vaccine among women aged 30 to 39 years (10.6 per million doses) and 40 to 49 years (9.02 per million doses) (Table 1).
Table 1.
Reporting Rates for TTS After Receipt of Ad26.COV2.S (Janssen/Johnson & Johnson) and Messenger RNA COVID-19 Vaccines BNT162b2 (Pfizer–BioNTech) or mRNA-1273 (Moderna)—United States, December 2020 to August 2021
Demographic Characteristics, Locations of Thrombosis, Treatments, and Outcomes of TTS Cases After Ad26.COV2.S Vaccination
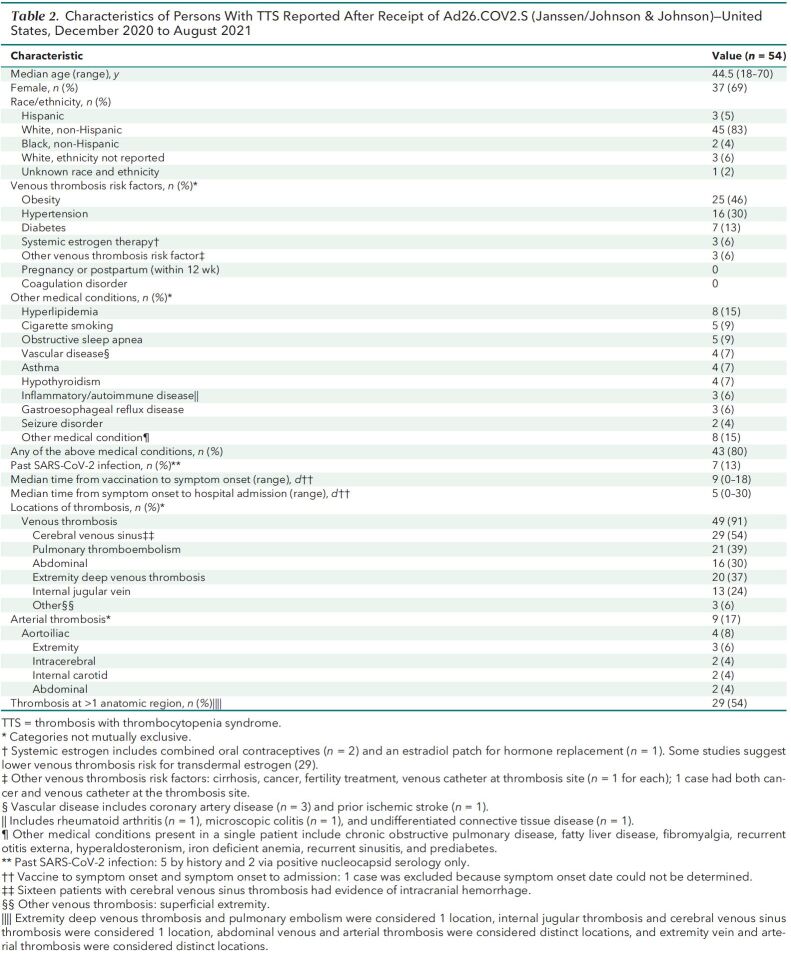
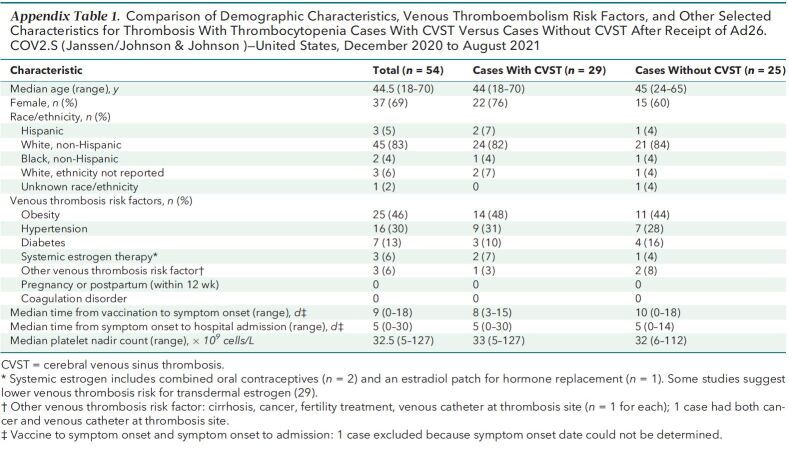
The 54 TTS cases reported after Ad26.COV2.S vaccination occurred in patients with median age of 44.5 years (range, 18 to 70 years [interquartile range, 36 to 52 years]); 37 (69%) were women, and 45 (83%) were non-Hispanic White (Table 2) . Forty-six cases were tier 1 (85%), and 8 (15%) were tier 2. Thirty-nine of the cases (72%) were in patients vaccinated before the U.S. pause in administration of Ad26.COV2.S and the accompanying health alert on 13 April 2021. Either a venous thrombosis or thromboembolism was found in 53 of 54 cases (98%). The most common anatomical region for thrombosis was the cerebral venous sinuses (n = 29 [54%]). Nine TTS cases (17%) had arterial thrombosis (including 8 with an accompanying venous thrombosis or thromboembolism). Thrombosis was present in more than 1 anatomical region for 29 cases (54%). A recognized risk factor for venous thromboembolism (24, 25) was present in 35 (65%) cases, most commonly obesity (n = 25 [46%]). No TTS cases occurred in women who were pregnant or within 12 weeks of childbirth or in patients with known thrombophilia. Seven cases (13%) occurred in patients with prior SARS-CoV-2 infection. Demographic characteristics, presence of risk factors for venous thrombosis, and timing of illness did not differ substantially between cases with cerebral venous sinus thrombosis and other cases (Appendix Table 1).
Table 2.
Characteristics of Persons With TTS Reported After Receipt of Ad26.COV2.S (Janssen/Johnson & Johnson)—United States, December 2020 to August 2021
Appendix Table 1.
Comparison of Demographic Characteristics, Venous Thromboembolism Risk Factors, and Other Selected Characteristics for Thrombosis With Thrombocytopenia Cases With CVST Versus Cases Without CVST After Receipt of Ad26.COV2.S (Janssen/Johnson & Johnson)—United States, December 2020 to August 2021
Heparin was administered to manage thrombosis in 11 of 15 cases (73%) presenting before the pause in use of Ad26.COV2.S and 11 of 39 (28%) cases presenting after. Other treatments used to manage TTS were nonheparin anticoagulants (n = 48 [89%]), intravenous immunoglobulin (n = 36 [67%]), corticosteroids (n = 24 [44%]), platelet transfusion (n = 22 [41%]), and plasmapheresis (n = 4 [7%]) (Table 3). A surgical or radiologic procedure was done in 23 (43%) cases to manage thrombosis or suspected complication, of which the most common was thrombectomy or catheter-directed thrombolysis (n = 16 [30%]).
Table 3.
In-Hospital Treatment and Outcomes After Hospitalization for TTS Cases After Receipt of Ad26.COV2.S (Janssen/Johnson & Johnson)—United States, December 2020 to August 2021
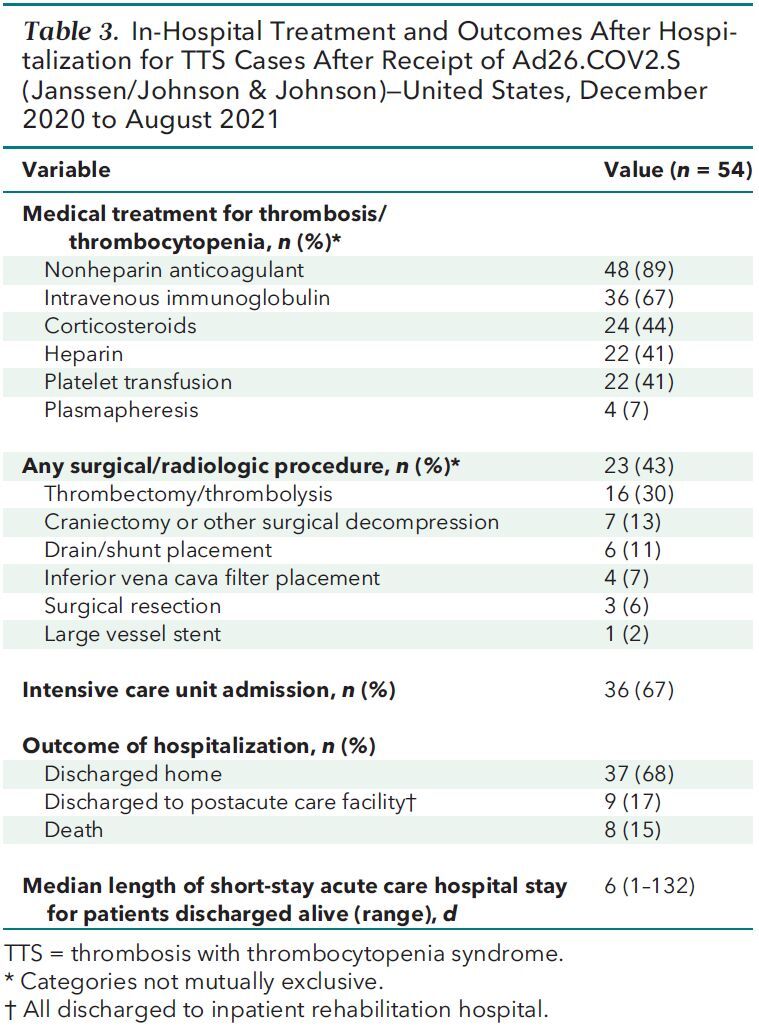
All patients were hospitalized; 36 (67%) were admitted to an intensive care unit (ICU). Outcomes of the initial hospitalization were discharge to home (n = 37 [68%]), discharge to postacute care facility (n = 9 [17%]), and death (n = 8 [15%]). For patients discharged from the hospital alive, median hospital length of stay was 6 days (range, 1 to 132 days) (Appendix Figure 1).
Appendix Figure 1. Distribution of hospital length of stay (in days) for thrombosis with thrombocytopenia syndrome cases after receipt of Ad26.COV2.S (Janssen/Johnson & Johnson) for patients discharged alive (n = 46)—United States, December 2020 to August 2021.
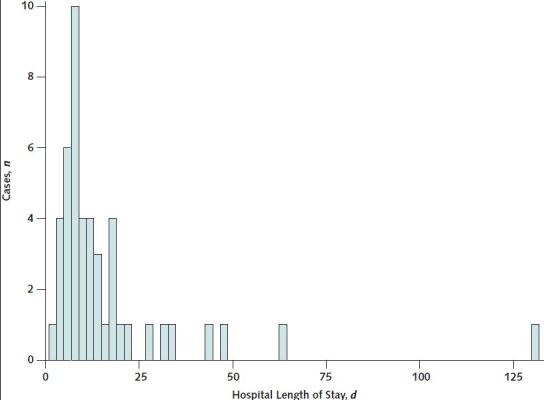
Each bar represents a 2-d range for hospital length of stay.
All 8 deaths occurred in non-Hispanic White patients. Six were diagnosed with cerebral venous sinus thrombosis, including 5 with superior sagittal sinus thrombosis; 2 of those with superior sagittal sinus thrombosis also had thrombosis of another cerebral venous sinus. Two decedents did not have neuroimaging results reporting on the cerebral venous sinuses, and therefore did not have a diagnosis of cerebral venous sinus thrombosis. All had cerebral hemorrhage and evidence of mass effect on initial neuroimaging and died within 2 days of presentation. Six decedents were women; ages ranged from 29 to 62 years (median, 45 years). Six of the decedents had underlying medical conditions (6 with a risk factor for venous thromboembolism [obesity, n = 6; hypertension, n = 3; diabetes, n = 2]; 1 each with asthma, hyperlipidemia, hypothyroidism, iron deficiency anemia, obstructive sleep apnea, and seizure disorder). None had documentation of prior SARS-CoV-2 infection. None received heparin. Median platelet count nadir among decedents was 23.5 × 109 cells/L (range, 9 to 44 × 109 cells/L). Anti-PF4 antibody ELISA testing was done for 4 decedents; all test results were positive, with optical density values greater than 2.0.
Laboratory Findings and Clinical Presentation of TTS Cases After Ad26.COV2.S Vaccination
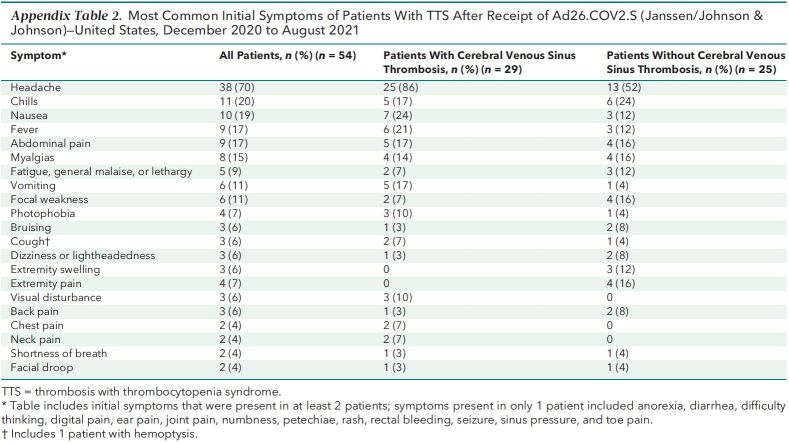
Symptoms began a median of 9 days after vaccination (range, 0 to 18 days) (Table 2; Appendix Figure 2). The most common initial symptom reported among cases was headache (n = 38 [70%]); headache was an initial symptom for 25 of 29 (86%) cases with cerebral venous sinus thrombosis and for 13 of 25 (52%) cases without cerebral venous sinus thrombosis (Appendix Table 2). Twenty (37%) patients had at least 1 prior health care encounter for evaluation of symptoms, with discharge home before a hospital admission and eventual TTS diagnosis; of these, 9 were documented to have thrombocytopenia during the initial encounter (that is, before hospital admission). In 7 cases, radiologic evaluation on initial presentation was negative for thrombosis, including 5 (of whom 1 later died) with initial radiologic examination negative at a site where thrombosis was later diagnosed.
Appendix Figure 2. Distribution of days from vaccination to symptom onset for thrombosis with thrombocytopenia syndrome after receipt of Ad26.COV2.S (Janssen/Johnson & Johnson) (n = 53)—United States, December 2020 to August 2021.
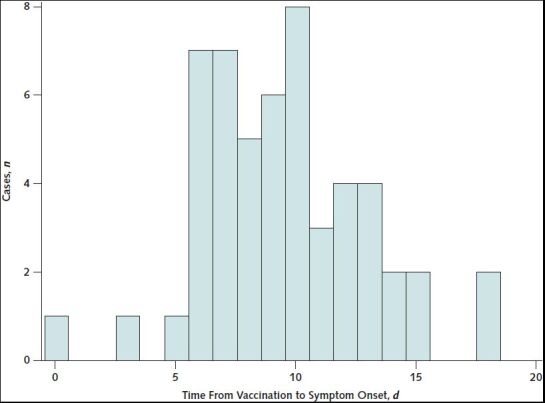
Excludes 1 patient whose symptom onset date could not be determined.
Appendix Table 2.
Most Common Initial Symptoms of Patients With TTS After Receipt of Ad26.COV2.S (Janssen/Johnson & Johnson)—United States, December 2020 to August 2021
Thrombocytopenia was present during TTS hospitalization in all except 1 case in which thrombocytopenia was found only at the first emergency department visit after Ad26.COV2.S vaccination, before evaluation for thrombosis was done. The platelet count had normalized by the time the patient was hospitalized 6 days later, when pulmonary embolism was diagnosed and a positive anti-PF4 antibody ELISA test result was obtained.
The median platelet count nadir was 32.5 × 109 cells/L (range, 5 to 127 × 109 cells/L) (Table 4; Appendix Figure 3). Platelet nadirs differed by clinical characteristics and course of disease. Of the patients with hospital length of stay greater than 20 days (n = 7), 6 had platelet count nadirs less than 25 × 109 cells/L (Appendix Figure 4). All patients whose hospital admission date was more than 12 days after symptom onset (n = 6) had platelet nadirs greater than 75 × 109 cells/L (Appendix Figure 5). Median platelet nadir for cases admitted to the ICU was 30.5 × 109 cells/L (range, 5 to 127 × 109 cells/L) versus 44 × 109 cells/L for those not admitted to the ICU (range, 8 to 112 × 109 cells/L). For 43 patients discharged from the hospital alive, with discharge platelet counts available, median platelet count at discharge was 167 × 109 cells/L (range, 91 to 469 × 109 cells/L) (Table 4). Of these patients, 17 (40%) had thrombocytopenia at discharge.
Table 4.
Laboratory Results Obtained During Initial Hospitalizations Among Persons With TTS After Receipt of Ad26.COV2.S (Janssen/Johnson & Johnson)—United States, December 2020 to August 2021*
Appendix Figure 3. Distribution of platelet nadir counts for thrombosis with thrombocytopenia syndrome cases after receipt of Ad26.COV2.S (Janssen/Johnson & Johnson) (n = 54)—United States, December 2020 to August 2021.
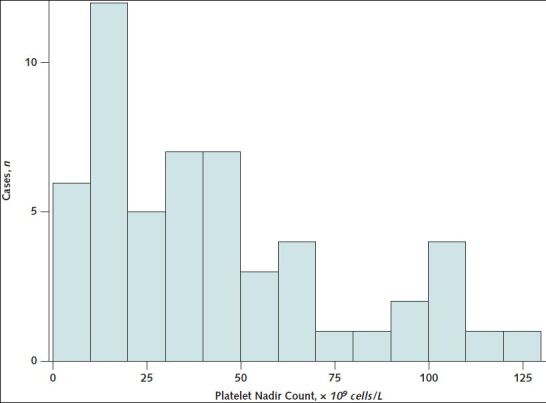
Each bar corresponds to a 10 × 109 cells/L range of platelet counts.
Appendix Figure 4. Scatter plot of platelet nadir count versus hospital length of stay (in days) for thrombosis with thrombocytopenia syndrome cases after receipt of Ad26.COV2.S (Janssen/Johnson & Johnson) (n = 46)—United States, December 2020 to August 2021.
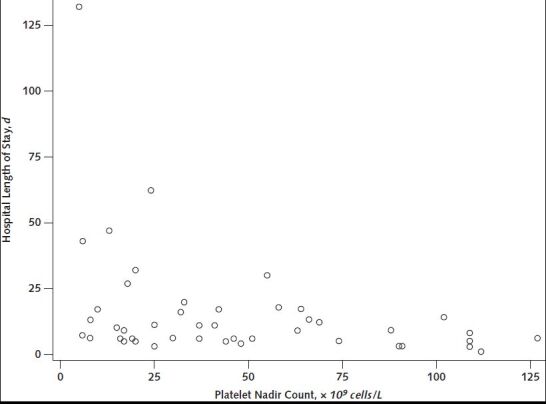
Limited to patients who were discharged from the hospital alive.
Appendix Figure 5. Scatter plot of platelet nadir count versus time (in days) from symptom onset to hospital admission for thrombosis with thrombocytopenia syndrome cases after receipt of Ad26.COV2.S (Janssen/Johnson & Johnson) (n = 53)—United States, December 2020 to August 2021.
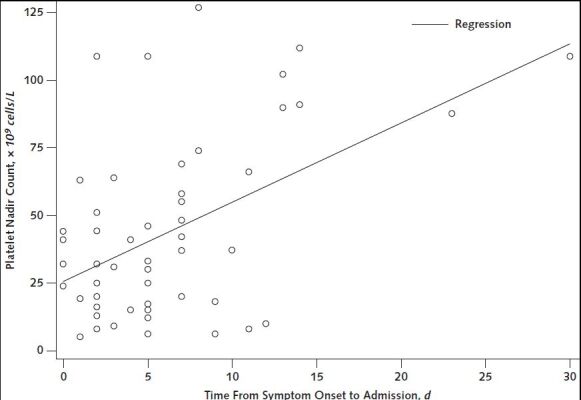
Excludes 1 patient whose symptom onset date could not be determined. R2 value = 0.23 (P < 0.001).
Anti-PF4 antibody ELISA test results were positive in 44 (81%), negative in 3 (6%), and not done in 7 (13%) cases (Table 4). Functional HIT platelet testing results were available for 36 and were positive in 11. Of the 3 TTS cases after Ad26.COV2.S vaccination with negative anti-PF4 antibody ELISA test results, 2 occurred in male patients, and 2 were Hispanic or of non-White race. All had underlying medical conditions, including hyperlipidemia (n = 2) and prior vascular disease (n = 2; history of myocardial infarction or ischemic stroke). All had platelet nadir counts greater than 100 × 109 cells/L. Two had cerebral venous sinus thrombosis, and 1 had abdominal vein thrombosis.
A thrombophilia evaluation (including testing for 1 or more of the following: lupus anticoagulant; cardiolipin, β2-glycoprotein I, phosphatidylserine antibodies, prothrombin G20210A mutation, and factor V Leiden mutation) was reported for 34 cases (63%); results for these 34 did not suggest an alternative cause for thrombosis (Table 4). For the 20 cases that did not have any of the tests listed earlier done for thrombophilia evaluation, anti-PF4 antibody ELISA testing was done in 18 and was positive in 16.
The Results section in the Appendix describes whether U.S. TTS cases after Ad26.COV2.S vaccination satisfied the U.K. Expert Haematology Panel VITT case definition.
Description of TTS Cases After mRNA COVID-19 Vaccination
Three TTS cases were identified after mRNA COVID-19 vaccination, 2 after dose 2 of the Moderna vaccine and 1 after the first dose of the Moderna vaccine. Two occurred in men older than 50 years who were either non-White or Hispanic and had cardiovascular risk factors (hyperlipidemia and hypertension in both; diabetes in 1); the other was in a woman aged 50 to 59 years with hyperlipidemia. Both male patients had cerebral venous sinus thrombosis, and 1 also had deep venous thrombosis as well as pulmonary emboli. The female patient had pulmonary embolism, superior mesenteric artery thrombosis, lower extremity deep venous thrombosis, and an aortic mural thrombus in addition to renal and splenic infarctions. In these 3 patients, symptom onset occurred 2, 9, and 11 days after vaccination. Platelet nadirs for the 3 cases were less than 25 × 109 cells/L, between 25 and 49 × 109 cells/L, and between 50 and 99 × 109 cells/L. D-dimer values were greater than 10 000 μg/L fibrinogen equivalent unit for all 3. Initial anti-PF4 antibody ELISA test results were positive, with optical density values greater than 2.0 for the 2 male patients and greater than 1.0 for the female patient, although subsequent anti-PF4 antibody ELISA test results were indeterminate. Of the male patients, 1 had a positive serotonin release assay, and the other did not have a functional HIT platelet test done. The female patient had a serotonin release assay with indeterminate results. All 3 had negative cardiolipin and β2-glycoprotein 1 antibody test results. One patient received intensive care and died during hospitalization, 1 was discharged home, and 1 was discharged to a postacute care facility.
Additional Potential and Confirmed TTS Cases
As of 16 December 2021, three TTS cases with Ad26.COV2.S vaccination after 31 August 2021 have been confirmed. Of these, 1 resulted in death. The death was in a woman aged younger than 30 years with obesity who developed superior sagittal sinus thrombosis and multiple cerebral hemorrhages and died within 24 hours of admission. The platelet count nadir for the decedent was between 50 and 99 × 109 cells/L, and no anti-PF4 antibody ELISA or D-dimer testing was done.
In addition, 2 deaths with cerebral hemorrhage and thrombocytopenia have been identified after Ad26.COV2.S vaccination for reports that were not confirmed as TTS. One was in a woman aged 50 to 59 years and the other in a man aged 40 to 49 years. Both had symptom onset within 7 to 14 days after receipt of the Ad26.COV2.S vaccine and died within 2 to 3 days of hospital admission with large intraparenchymal hemorrhage accompanied by subarachnoid or subdural hemorrhage in the absence of known trauma. For both deaths, arterial imaging ruled out the presence of intracranial aneurysm, arteriovenous fistula, or malformation, but neither had venous imaging or another imaging report that ruled out the presence of underlying cerebral venous sinus thrombosis. Venous thrombosis was considered clinically to be the likely cause of hemorrhage for 1 patient during surgery to address hemorrhage; for the other, the D-dimer level was above the upper limit of reporting at the time of initial presentation. Platelet count nadirs were less than 10 × 109 cells/L for 1 patient and between 50 and 99 × 109 cells/L for the other. Both of the decedents were vaccinated before the U.S. pause in Ad26.COV2.S vaccination.
Discussion
This case series provides further evidence that TTS is a rare but serious condition causally associated with receipt of the Ad26.COV2.S vaccine. It is characterized predominantly by cerebral venous sinus thrombosis or venous thromboembolism with thrombocytopenia, although arterial thrombosis occurs in a minority of cases. Continued vigilance for TTS in patients with a history of Ad26.COV2.S vaccination, thrombocytopenia, and compatible symptoms is needed to ensure proper evaluation (for example, anti-PF4 antibody ELISA testing) and treatment (for example, use of nonheparin anticoagulation and intravenous immunoglobulin) of these patients (30). In addition, this case series provides information to contextualize 3 reported TTS cases after mRNA COVID-19 vaccination. Two of these cases were in men with cardiovascular risk factors, and the third patient technically had a positive anti-PF4 antibody ELISA test result but indeterminate serotonin release assay testing, which was not seen in any of the TTS cases after Ad26.COV2.S vaccination. The different demographic characteristics for 2 of the cases, difference in HIT testing result for the third, and the much lower reporting rate for TTS after mRNA-based COVID-19 vaccines suggest that these 3 cases likely represent background cases or a different pathogenesis than the cases associated with Ad26.COV2.S vaccination.
This case series highlights important clinical characteristics of TTS after Ad26.COV2.S vaccination. For example, although arterial thrombosis does occur and there are case reports of TTS involving only arterial thrombosis (31, 32), TTS after Ad26.COV2.S vaccination is largely a syndrome of venous thrombosis or thromboembolism. The frequency of ICU admission, need for procedures to address thrombosis or complications, long length of hospital stay, and fatalities confirm that TTS occurring after Ad26.COV2.S vaccination is a serious condition. The proportion of TTS cases with death presented in this U.S. series (15% for cases after Ad26.COV2.S vaccination) is lower than the 22% described for TTS after ChAdOx1 nCoV-19 vaccination described from the United Kingdom (19). We found higher rates of TTS among female persons after Ad26.COV2.S vaccination, whereas no male or female predominance was seen in a large U.K. series of TTS after ChAdOx1 nCoV-19 vaccination (19). Consistent with this U.K. series is our finding that among TTS cases after Ad26.COV2.S vaccination, the platelet count nadir may be a marker of severity or how quickly symptoms progress. Although many of the U.S. TTS cases after Ad26.COV2.S vaccination occurred in patients with a recognized venous thromboembolism risk factor (for example, obesity), because of the lack of a comparison population, the actual risk factors for TTS after Ad26.COV2.S vaccination are not known.
The diagnosis of TTS can be challenging. A negative initial radiologic examination in our cases did not preclude later development of thrombosis in the same location. Repeated imaging may be needed for patients who present more than once with persistent or progressive signs and symptoms of TTS. Headache was the most common initial symptom not only for patients with cerebral venous sinus thrombosis but also extracranial thrombosis, with onset usually beginning several days after vaccination. In patients with prolonged or persistent headache and thrombocytopenia after Ad26.COV2.S vaccination, a search for extracranial sites of thrombosis should be considered. In addition, the American Society of Hematology recommends that anticoagulation and intravenous immunoglobulin be strongly considered for patients with thrombocytopenia, high D-dimer level, and severe headache within a compatible timeframe after Ad26.COV2.S vaccination even in the absence of diagnosed thrombosis pending PF4 ELISA testing (30), which follows similar recommendations from investigators for ChAdOx1 nCoV-19 vaccination (33).
All deaths associated with confirmed TTS cases after Ad26.COV2.S vaccination (that is, 8 with vaccination by 31 August 2021 and 1 with vaccination after 31 August 2021) occurred in patients with cerebral hemorrhage; we also noted 2 additional deaths involving cerebral hemorrhage after Ad26.COV2.S vaccination but without documented thrombosis where the clinical picture was otherwise compatible with TTS. It may be important to have a high suspicion for cerebral venous sinus thrombosis or other thromboses in patients presenting with cerebral hemorrhage and thrombocytopenia within 30 days of vaccination with Ad26.COV2.S.
The TTS reporting rate from U.S. VAERS data after Ad26.COV2.S vaccination (3.83 cases per million doses) is lower than rates (13 to 39 cases per million doses) reported for TTS after ChAdOx1 nCoV-19 vaccination in other countries (2, 12–14). Although information about TTS was disseminated by the U.S. media (34), differences in reporting rates could be associated with relative underreporting or underascertainment in the United States, true differences in TTS risk after Ad26.COV2.S versus ChAdOx1 nCoV-19 vaccination, differences in the populations of the countries vaccinated, or other factors.
A recent report estimated that the background incidence of cerebral venous sinus thrombosis with thrombocytopenia during the pre–COVID-19 era (2018 to 2019) (Payne A. Personal communication.) was 0.16 to 0.22 cases per 100 000 persons per year. On the basis of these data, the expected 2-week incidence per million persons is 0.062; the reporting rate for TTS after mRNA COVID-19 vaccination (0.00855) found here falls below that estimate of background incidence before COVID-19 vaccines were available. The small number of TTS cases reported after mRNA COVID-19 vaccination were among patients with different demographic characteristics (older, non-White men) and medical histories (for example, presence of cardiovascular risk factors) than most cases reported after Ad26.COV2.S vaccination. These findings suggest that the TTS cases after mRNA COVID-19 vaccination represent a background rate of spontaneous HIT or TTS associated with a different risk factor than cases associated with Ad26.COV2.S vaccination.
On the basis of positive results from functional HIT platelet assays among early case reports, the pathophysiology of TTS after ChAdOx1 nCoV-19 vaccination is believed to be similar to spontaneous HIT, in which anti-PF4 antibodies activate platelets and cause thrombosis in the absence of prior heparin exposure (1–3). The binding site for anti-PF4 antibodies in patients with TTS reported after ChAdOx1 nCoV-19 vaccination is contained within the site where heparin binds to PF4 (35). However, as was described with the initial 12 cases of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, most TTS cases reported after Ad26.COV2.S vaccination in which a serotonin release assay (the most common functional HIT platelet assay performed) was done had negative results. Several groups have described the need to add PF4 to the serotonin release assay to identify patients with TTS after ChAdOx1 nCoV-19 vaccination (36, 37), and the same modifications in testing procedure may be needed for confirmation of TTS after Ad26.COV2.S vaccination.
Compared with the U.K. Expert Haematology Panel case definitions for definite VITT or probable VITT (19), the TTS case definition used here excludes cases without thrombocytopenia and can include cases with negative anti-PF4 antibody ELISA results, symptom onset fewer than 5 days after vaccination, and normal D-dimer results. More than 70% of cases occurred in persons vaccinated before the pause in Ad26.COV2.S use in the United States, with the accompanying health advisory announcement. Therefore, many cases occurred before there was widespread recognition of the testing recommended for evaluation of TTS (30). In our application of an epidemiologic analysis seeking to quantify the occurrence of a disease, we chose to use a case definition with greater sensitivity (Results section of the Appendix ). The tight clustering of symptom onset within 6 to 18 days after Ad26.COV2.S vaccination provides some additional epidemiologic evidence that we may indeed be largely capturing cases linked to vaccination as intended. In contrast, half of our deaths did not have anti-PF4 antibody ELISA testing, and a definition requiring positive anti-PF4 antibody ELISA results may result in false low estimates of death rates. Although the presence of anti-PF4 antibodies is considered a key aspect of the pathogenesis of TTS after adenoviral-vectored COVID-19 vaccines (38), we did note 3 cases with negative anti-PF4 antibody ELISA results. Although it has been reported that anti-PF4 antibody ELISA results can be falsely negative in a minority of instances (39), the differences in characteristics for these cases suggest that they may be associated with another pathophysiologic mechanism.
Moving forward, additional modifications to the case definition used here may be needed. For example, as has been suggested by others (30, 33), TTS may present with a positive anti-PF4 antibody ELISA test result and compatible symptoms but without thrombosis and/or thrombocytopenia. In addition, requiring cases after mRNA COVID-19 vaccination (or other nonadenoviral-vector COVID-19 vaccines in the future) to have positive anti-PF4 antibody ELISA results may improve specificity. Because evidence to date is insufficient to conclude that TTS is epidemiologically linked to receipt of mRNA COVID-19 vaccines, this requirement may limit false positives for the rare events that meet the TTS case definition after mRNA COVID-19 vaccination. Note that our case definition for TTS was designed for national surveillance and not for clinical purposes; clinical guidance from the American Society of Hematology recommends anti-PF4 antibody ELISA testing when TTS is suspected, regardless of the location of thrombosis (30).
This report is subject to limitations. The Vaccine Adverse Event Reporting System is a passive surveillance system, so the TTS reporting rates described likely underestimate true rates of occurrence. In addition, lack of anti-PF4 antibody ELISA testing in the clinical setting could lead to both underreporting and underrepresentation of tier 2 cases compared with tier 1 TTS cases. Not all cases had evaluation for alternative causes, such as antiphospholipid antibody syndrome (38), so it is possible that some cases included as TTS could have another underlying cause.
A strength of this analysis is that because the COVID-19 immunization program was federally coordinated, all U.S. public health jurisdictions reported the number of COVID-19 vaccines that were administered, allowing a better estimate of reporting rates than usually possible when using VAERS data. In addition, although some laboratory testing (for example, anti-PF4 antibody ELISA) was not done in every patient, another strength is the adjudication by a panel of expert academic clinicians in various specialty fields to ensure that the cases' clinical syndromes were consistent with TTS and not likely due to another cause.
We estimate reporting rates of TTS after COVID-19 vaccination and describe the epidemiology of this syndrome through a systematic national surveillance effort. The epidemiology and hypothesized pathogenesis of TTS support a causal association with receipt of the Ad26.COV2.S vaccine. However, the much lower reported rates and difference in demographic characteristics for TTS cases after mRNA-based COVID-19 vaccines suggest that reports occurring after mRNA-based vaccination likely represent a background rate of cases of spontaneous HIT. Continued vigilance for TTS cases after Ad26.COV2.S vaccination is needed; the reporting rates and epidemiologic data about TTS after Ad26.COV2.S vaccination will be useful in formulating benefit–risk assessments for COVID-19 immunization programs in the United States and other countries (40, 41).
Appendix: Additional Methods and Results
Methods
Review of VAERS
Potential reports of TTS were identified using combinations of searches among VAERS reports in which each search used a group of selected PTs or text strings.
Search 1 searched for the words “thrombocytopenia” or “low platelets” in the text of the symptom or laboratory test fields or for 1 or more of the following PTs: autoimmune heparin-induced thrombocytopenia, heparin-induced thrombocytopenia, immune thrombocytopenia, non-immune heparin associated thrombocytopenia, spontaneous heparin-induced thrombocytopenia syndrome, thrombocytopenia, or thrombocytopenic purpura.
Search 2 searched for 1 or more of the following PTs: acute myocardial infarction, basal ganglia stroke, brain stem stroke, hemorrhagic stroke, hemorrhagic transformation stroke, ischemic stroke, lacunar stroke, myocardial infarction, perinatal stroke, silent myocardial infarction, spinal stroke, thrombotic stroke, or vertebrobasilar stroke.
Search 3 searched for 1 or more of the following PTs: axillary vein thrombosis, deep vein thrombosis, or pulmonary embolism.
Search 4 searched for 1 or more of the following PTs: aortic embolus, aortic thrombosis, aseptic cavernous sinus thrombosis, brachiocephalic vein thrombosis, brain stem embolism, brain stem thrombosis, carotid arterial embolus, carotid artery thrombosis, cavernous sinus thrombosis, cerebral artery thrombosis, cerebral venous sinus thrombosis, cerebral venous thrombosis, femoral artery embolism, hepatic artery embolism, hepatic artery thrombosis, hepatic vein embolism, hepatic vein thrombosis, iliac artery embolism, jugular vein embolism, jugular vein thrombosis, mesenteric artery embolism, mesenteric artery thrombosis, mesenteric vein thrombosis, obstetrical pulmonary embolism, portal vein embolism, portal vein thrombosis, portosplenomesenteric venous thrombosis, pulmonary artery thrombosis, pulmonary thrombosis, pulmonary venous thrombosis, renal artery thrombosis, renal embolism, renal vein embolism, renal vein thrombosis, splenic artery thrombosis, splenic embolism, splenic thrombosis, splenic vein thrombosis, spontaneous heparin-induced thrombocytopenia syndrome, subclavian artery embolism, subclavian vein thrombosis, superior sagittal sinus thrombosis, thrombosis mesenteric vessel, transverse sinus thrombosis, truncus celiacus thrombosis, vena cava embolism, vena cava thrombosis, or visceral venous thrombosis.
Search 5 searched for 1 or more of the following PTs: autoimmune heparin-induced thrombocytopenia, heparin-induced thrombocytopenia, heparin-induced thrombocytopenia test positive, idiopathic thrombocytopenic purpura, immune thrombocytopenia, immune thrombocytopenic purpura, non-immune heparin associated thrombocytopenia, platelet count decreased, severe fever with thrombocytopenia syndrome, spontaneous heparin-induced thrombocytopenia syndrome, thrombocytopenia, thrombocytopenic purpura, or thrombotic thrombocytopenic purpura.
Search 6 searched for an extensive list of PTs describing various additional conditions or procedures that could indicate thrombosis (available on request).
Potential reports of TTS were identified as reports containing PTs and/or text for both search 1 and search 2, both search 1 and search 3, both search 1 and search 4, or both search 5 and search 6. Reports identified by these preliminary searches were then reviewed by trained clinical abstractors. Reports with COVID-19 vaccine receipt date through 31 August 2021 were included in this review. Some cases were first identified through CDC's CISA Project (24) when health care providers requested clinical consultation about a patient and then subsequently a VAERS report was made.
For reports that, on initial review, seemed consistent with this case definition, an abstractor attempted to reach the best contact listed on the VAERS report form (typically the medical care provider for the reported patient), and medical records for the reported case were requested. Confirmation of case status was obtained through medical record review of the initial hospital course and/or discussions with treating clinicians. Clinical data about potential cases were reviewed with physician investigators from CDC's CISA Project (24). Specifically, we excluded cases with any of the following characteristics: active COVID-19 infection at the time of TTS diagnosis, preexisting thrombocytopenia, TTS symptoms preceding COVID-19 vaccination, thrombocytopenia that was most likely spurious artifactual, or thrombosis that was more likely due to other causes.
Vaccination Administration Data
Vaccine administration data were obtained for doses 1 and 2 of the Pfizer–BioNTech COVID-19 vaccine and Moderna COVID-19 vaccine and dose 1 of the Ad26.COV2.S vaccine from 14 December through 31 August 2021, as reported to the CDC from state health departments and other U.S. public health jurisdictions (for example, U.S. territories) (23). Dose administered data from Texas were available stratified by age group, with 1 age group representing all persons aged 25 to 39 years. Doses administered from Texas for persons aged 25 to 29 years were estimated by (doses administered among persons aged 25 to 39 years in Texas) × (doses administered among persons aged 25 to 29 years outside Texas) ÷ (doses administered among persons aged 25 to 39 years outside Texas). Doses administered from Texas for persons 30 to 39 years were estimated in a similar fashion. For other jurisdictions (that is, other than Texas), doses with unknown sex or age (<1% of total) were excluded from calculation of reporting rates. For Texas, doses with unknown sex (0.5% of total) were excluded from calculation of reporting rates (no cases with unknown age were reported).
Data Analysis
D-dimer values reported in D-dimer units were converted to fibrinogen equivalent units by multiplying by 2 (42). Potential correlation between platelet nadir values and outcomes for TTS cases after Ad26.COV2.S vaccination was assessed through scatter plots; linear (Pearson) correlation between platelet nadir values and time from symptom onset to admission was assessed. A public announcement in the United States of the existence of TTS cases after Ad26.COV2.S vaccination occurred on 13 April 2021 (10); a pause in Ad26.COV2.S administration began, and preliminary treatment guidance was also provided on the same date, including a recommendation to avoid use of heparin in patients with TTS. Treatment of patients admitted to the hospital before the pause in use of the Ad26.COV2.S vaccine was compared with treatment of patients admitted after the pause (10). Anti-PF4 antibody ELISA results and functional HIT platelet assay findings (for example, serotonin release assay, latex immunoturbidimetric assay, and P-selectin expression assay) were summarized (16).
Results
Comparison With U.K. Expert Haematology Panel Case Definition
Of the 54 TTS cases reported after Ad26.COV2.S vaccination, 32 (59%) were definite VITT, 16 (30%) were probable VITT, and 3 (6%) were “possible VITT,” using the U.K. Expert Haematology Panel case definition (19). Three cases did not fall into any category using the U.K. case definition. These 3 cases all had cerebral venous sinus thrombosis, thrombocytopenia, and D-dimer values less than 2000 µg/L fibrinogen equivalent unit. One had a positive anti-PF4 antibody ELISA test result, 1 did not have anti-PF4 antibody ELISA testing done, and 1 had a negative anti-PF4 antibody ELISA test result.
Footnotes
This article was published at Annals.org on 18 January 2022.
References
- 1. Greinacher A , Thiele T , Warkentin TE , et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092-2101. [PMID: ] doi: 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schultz NH , Sørvoll IH , Michelsen AE , et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. [PMID: ] doi: 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scully M , Singh D , Lown R , et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202-2211. [PMID: ] doi: 10.1056/NEJMoa2105385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shay DK , Gee J , Su JR , et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine - United States, March-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:680-684. [PMID: ] doi: 10.15585/mmwr.mm7018e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. See I , Su JR , Lale A , et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. [PMID: ] doi: 10.1001/jama.2021.7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brighton Collaboration. Interim case definition of thrombosis with thrombocytopenia syndrome (TTS). Accessed at https://brightoncollaboration.us/thrombosis-with-thrombocytopenia-syndrome-interim-case-definition/ on 20 December 2021.
- 7. Brighton Collaboration. About: Brighton Collaboration is a community aiming to promote and improve vaccine safety. Accessed at https://brightoncollaboration.us/about/ on 20 December 2021.
- 8. Favaloro EJ , Pasalic L , Lippi G . Review and evolution of guidelines for diagnosis of COVID-19 vaccine induced thrombotic thrombocytopenia (VITT). Clin Chem Lab Med. 2022;60:7-17. [PMID: ] doi: 10.1515/cclm-2021-1039 [DOI] [PubMed] [Google Scholar]
- 9. Solsvik T, Adomaitis N. Norway drops AstraZeneca vaccine, J&J remains on hold. Reuters. 12 May 2021. Accessed at www.reuters.com/business/healthcare-pharmaceuticals/norway-will-not-use-astrazeneca-covid-19-vaccine-says-daily-vg-2021-05-12/ on 3 September 2021.
- 10. MacNeil JR , Su JR , Broder KR , et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients - United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. [PMID: ] doi: 10.15585/mmwr.mm7017e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. U.S. Food and Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Accessed at www.fda.gov/media/146304/download on 11 January 2021.
- 12. Public Health Agency of Canada. Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI): recommendations on the use of COVID-19 vaccines. Accessed at www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/recommendations-use-covid-19-vaccines-en.pdf?hq_e=el&hq_m=2184507&hq_l=1&hq_v=911ba6e5ee on 2 September 2021.
- 13. Australian Government, Department of Health, Therapeutic Goods Administration. COVID-19 vaccine weekly safety report—02-09-2021. Accessed at www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-02-09-2021 on 2 September 2021.
- 14. United Kingdom Medicines & Healthcare products Regulatory Agency. Research and analysis: coronavirus vaccine—weekly summary of Yellow Card reporting. Accessed at www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting#yellow-card-reports on 2 September 2021.
- 15. Warkentin TE , Basciano PA , Knopman J , et al. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood. 2014;123:3651-4. [PMID: ] doi: 10.1182/blood-2014-01-549741 [DOI] [PubMed] [Google Scholar]
- 16. Sahu KK , Jindal V , Anderson J , et al. Current perspectives on diagnostic assays and anti-PF4 antibodies for the diagnosis of heparin-induced thrombocytopenia. J Blood Med. 2020;11:267-277. [PMID: ] doi: 10.2147/JBM.S232648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greinacher A, Selleng K, Wesche J, et al. Towards understanding ChAdOx1 nCoV-19 vaccine-induced thrombotic thrombocytopenia (VITT). Research Square. Preprint posted online 20 April 2021. doi:10.21203/rs.3.rs-440461/v1
- 18. Kowarz E, Krutzke L, Reis J, et al. “Vaccine-induced Covid-19 mimicry” syndrome: splice reactions within the SARS-CoV-2 spike open reading frame result in spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. Research Square. Preprint posted online 26 May 2021. doi:10.21203/rs.3.rs-558954/v1
- 19. Pavord S , Scully M , Hunt BJ , et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680-1689. [PMID: ] doi: 10.1056/NEJMoa2109908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sangli S , Virani A , Cheronis N , et al. Thrombosis with thrombocytopenia after the messenger RNA-1273 vaccine [Letter]. Ann Intern Med. 2021;174:1480-1482. [PMID: ] doi: 10.7326/L21-0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimabukuro TT , Nguyen M , Martin D , et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33:4398-405. [PMID: ] doi: 10.1016/j.vaccine.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Medical Dictionary for Regulatory Activities. Welcome to MedDRA. Accessed at www.meddra.org/ on 3 September 2021.
- 23. Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Accessed at https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total on 7 September 2021.
- 24. Centers for Disease Control and Prevention. Clinical Immunization Safety Assessment (CISA) Project. Accessed at www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html on 7 September 2021.
- 25. Harris PA , Taylor R , Thielke R , et al. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-81. [PMID: ] doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris PA , Taylor R , Minor BL , et al; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [PMID: ] doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lijfering WM , Rosendaal FR , Cannegieter SC . Risk factors for venous thrombosis—current understanding from an epidemiological point of view. Br J Haematol. 2010;149:824-33. [PMID: ] doi: 10.1111/j.1365-2141.2010.08206.x [DOI] [PubMed] [Google Scholar]
- 28. Crous-Bou M , Harrington LB , Kabrhel C . Environmental and genetic risk factors associated with venous thromboembolism. Semin Thromb Hemost. 2016;42:808-820. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinkerton JV , James AH . Management of menopausal symptoms for women who are at high risk of thrombosis. Clin Obstet Gynecol. 2018;61:260-268. [PMID: ] doi: 10.1097/GRF.0000000000000358 [DOI] [PubMed] [Google Scholar]
- 30. American Society of Hematology. Thrombosis with thrombocytopenia syndrome (also termed vaccine-induced thrombotic thrombocytopenia). Accessed at www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia on 8 September 2021.
- 31. Walter U , Fuchs M , Grossmann A , et al. Adenovirus-vectored COVID-19 vaccine-induced immune thrombosis of carotid artery: a case report. Neurology. 2021. [PMID: ] doi: 10.1212/WNL.0000000000012576 [DOI] [PubMed] [Google Scholar]
- 32. Tiede A , Sachs UJ , Czwalinna A , et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood. 2021;138:350-353. [PMID: ] doi: 10.1182/blood.2021011958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salih F , Schönborn L , Kohler S , et al. Vaccine-induced thrombocytopenia with severe headache [Letter]. N Engl J Med. 2021;385:2103-2105. [PMID: ] doi: 10.1056/NEJMc2112974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller Z, Neergaard L, Perrone M. US recommends ‘pause' for J&J shots in blow to vaccine drive. Associated Press. 13 April 2021. Accessed at https://apnews.com/article/us-pause-j-and-j-vaccine-blood-clot-reports-2dde2aacf486bab59844ef907a28cbce on 14 September 2021.
- 35. Huynh A , Kelton JG , Arnold DM , et al. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021;596:565-569. [PMID: ] doi: 10.1038/s41586-021-03744-4 [DOI] [PubMed] [Google Scholar]
- 36. Vayne C , Rollin J , Gruel Y , et al. PF4 immunoassays in vaccine-induced thrombotic thrombocytopenia [Letter]. N Engl J Med. 2021;385:376-378. [PMID: ] doi: 10.1056/NEJMc2106383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bourguignon A , Arnold DM , Warkentin TE , et al. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;385:720-728. [PMID: ] doi: 10.1056/NEJMoa2107051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klok FA , Pai M , Huisman MV , et al. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022;9:e73-e80. [PMID: ] doi: 10.1016/S2352-3026(21)00306-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Platton S , Bartlett A , MacCallum P , et al. Evaluation of laboratory assays for anti-platelet factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J Thromb Haemost. 2021;19:2007-2013. [PMID: ] doi: 10.1111/jth.15362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosenblum HG , Hadler SC , Moulia D , et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): update from the Advisory Committee on Immunization Practices - United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1094-1099. [PMID: ] doi: 10.15585/mmwr.mm7032e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Custers J , Kim D , Leyssen M , et al; Brighton Collaboration Viral Vector Vaccines Safety Working Group (V3SWG). Vaccines based on replication incompetent Ad26 viral vectors: standardized template with key considerations for a risk/benefit assessment. Vaccine. 2021;39:3081-3101. [PMID: ] doi: 10.1016/j.vaccine.2020.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weitz JI , Fredenburgh JC , Eikelboom JW . A test in context: D-dimer. J Am Coll Cardiol. 2017;70:2411-2420. [PMID: ] doi: 10.1016/j.jacc.2017.09.024 [DOI] [PubMed] [Google Scholar]