ABSTRACT
Background
The World Health Organization declared COVID-19 a pandemic in March 2020. The first vaccine became available in December, with practically no post-marketing data.
Methods
An analytical cross-sectional survey-based study was conducted in a third-level hospital in Spain between March and April 2021 to describe the difference in adverse events with the BNT162b2 and mRNA-1273 COVID-19 vaccines. The participants were hospital workers who completed a survey voluntarily at least 14 days after the last vaccine. The STROBE checklist was followed.
Results
One thousand two hundred and forty-nine respondents completed the survey; 48% (599) received mRNA-1273 and 52% (650) BNT162b2. Fourteen thousand four hundred and two adverse reactions were recorded, 6896 local (3939 with mRNA-1273 and 2957 with BNT162b2 (6.6 vs 4.4 reactions per patient)) and 7506 systemic (4460 with mRNA-1273 and 3046 with BNT162b2 (7.4 vs 4.7 per patient)). Local reactions were more frequent after the first dose, while systemic reactions were higher after the second, for both vaccines and in a higher percentage with mRNA-1273 compared to BNT162b2 (p-value<0.05).
Conclusions
Licensed mRNA vaccines were highly safe when administered under post-marketing conditions among working-age adults. The main adverse events were mild, although they occurred in most patients, especially after the mRNA-1273 vaccine.
KEYWORDS: COVID19, vaccine, adverse events, BNT162b2, mRNA-1273
1. Introduction
Since December 2019, the world has been facing a new disease known as COVID-19, caused by the coronavirus type 2 (SARS-CoV-2), responsible for the severe acute respiratory syndrome [1]. On 11 March 2020 the World Health Organization officially declared a pandemic situation for COVID-19 [2].
Symptoms of SARS-CoV-2 infection vary widely, ranging from asymptomatic disease to pneumonia and life-threatening complications, including acute respiratory distress syndrome, multi-organ failure and ultimately death [3–5].
Since the detection of the first outbreak in China on 31 December 2019, there have been more than 183,198,019 confirmed cases of COVID-19 and 3,971,687 deaths [6].
Preventive measures such as hand washing, use of masks and social distancing have been found to be effective in helping to curb the epidemic [7,8]. Strong promotion of these proven measures by governments or public health institutions may have been a key ingredient in tackling the pandemic. Despite this, the number of COVID-19 diagnoses has continued to grow [9].
In the absence of a specific and fully effective antiviral drug against SARS-CoV-2, a SARS-CoV-2 vaccine was a high priority target [10]. Therefore, the European Medicines Agency (EMA) recommended conditional approval for BNT162b2 mRNA COVID-19 vaccine, developed by Pfizer-BioNTech in late December 2020 [11] and for mRNA-1273 COVID-19 vaccine developed by the US company Moderna in early January 2021 [12].
The safety data in the datasheets of the two vaccines are based on the results of the pivotal trials that led to their licensing and state that the type of adverse reaction expected is moderate-mild, generally self-limiting within the first 7 days post administration of each of the doses and have similar prevalence values in between the two presentations. In addition, they confirm that the reactogenicity and safety profile in subjects who received these vaccines and were seropositive for SARS-CoV-2 at baseline was comparable to those of seronegative subjects [13,14]. However, there are already publications with small population samples suggesting that the incidence of adverse events is higher in the first dose in seropositive subjects [15,16]. It has also been observed that the presence of adverse effects is higher in the mRNA-1273 vaccine compared to the BNT162b2 vaccine and that the clinical symptoms of this first vaccine are mild-moderate after the administration of the first dose and moderate-severe with the administration of the second dose [17,18].
The objectives of this study were to describe the incidence of adverse events with the BNT162b2 and mRNA-1273 vaccines used among our hospital workers from a third level hospital in Spain and to demonstrate whether or not there are differences in the safety profiles between the two vaccines, as well as between the first and second dose, and also between subjects who had previously tested positive or negative for SARS-CoV-2 coronavirus infection.
2. Methods
2.1. Design
After obtaining the approval of the hospital’s human research ethics committee for this study, an analytical cross-sectional survey-based study was conducted by circulating an online questionnaire through an internet-based survey platform called Limesurvey® that gathered anonymous responses from healthcare workers of a third level hospital in Spain. The safety aspects collected in the survey were graded according to the US Food and Drugs Administration (FDA) guidelines ‘Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials’ [19,20] although some variables not included in the guidelines were also added (see Tables 1 and 2).
Table 1.
Local reactions reported within 7 days after injection of mRNA-1273 or BNT162b2 vaccines
| Local adverse reactions in the first seven days after each injection | |||||
|---|---|---|---|---|---|
| LOCAL REACTION | NO | MILD (Grade 1) | MODERATE (Grade 2) | SEVERE (Grade 3) | EMERGENCY (Potentially life threatening – Grade 4) |
| Redness at the injection site | NO | 2,5–5 cm | 5,1 cm −10 cm | > 10 cm | Necrosis or exfoliative dermatitis |
| Swelling at the injection site | NO | 2,5–5 cm or does not interfere with activity | 5,1 cm −10 cm or interferes with activity | > 10 cm and prevents daily activity | Necrosis |
| Pain at the injection site | NO | No interference with activity | Interferes with activity or repeated use of non-narcoticpain reliever >24 hours | Any use of narcotic pain reliever or prevents daily activity | Emergency room visit or hospitalization |
| Itching at the injection site* | NO | Yes, punctual but did not require antihistamines | Yes, more than one day but did not require antihistamines | Yes, more than one day and required antihistamines | Emergency room visit or hospitalization |
| Tenderness at the injection site/ Arm movility | NO | Mild discomfort to touch | Discomfort with movement | Significant discomfort at rest | Emergency room visit or hospitalization |
| Focusing on arm mobility, did you have problems moving the arm where the vaccine was administered?* | NO | Yes, for less than 3 days | Yes, for more than 3 days but resolved within a week of injection | Yes, for more than a week | Emergency room visit or hospitalization |
Table 2.
Systemic reactions reported within 7 days after injection of mRNA-1273 or BNT162b2 vaccines
| Systemic adverse reactions in the first seven days after each injection | |||||
|---|---|---|---|---|---|
| SYSTEMIC REACTION | NO | MILD (Grade 1) | MODERATE (Grade 2) | SEVERE (Grade 3) | EMERGENCY (Potentially life threatening - Grade 4) |
| Fever | NO | 38,0 - 38,4ºC | 38,5 - 38,9ºC | 39,0 - 40ºC | More than 40ºC |
| Nausea/vomiting | NO | No interference with activity or 1-2 episodes/24 hours | Some interference with activity or >2 episodes/24 hours | Prevents daily activity, requires intravenous hydration | Emergency room visit or hospitalisation |
| Diarrhea | NO | Yes, 2 to 3 times a day | Yes, 4 to 5 times a day | Yes, more than 6 times a day or requires intravenous hydration | Emergency room visit or hospitalisation |
| Joint pain* | NO | No interference with activity | Interferes with daily activity | Any use of narcotic pain reliever or prevents daily activity | Emergency room visit or hospitalisation |
| Muscle pain | NO | No interference with activity | Interferes with daily activity | Any use of narcotic pain reliever or prevents daily activity | Emergency room visit or hospitalisation |
| Fatigue | NO | No interference with activity | Interferes with daily activity | Significant; prevents daily activity | Emergency room visit or hospitalisation |
| Chills* | NO | No interference with activity | Interferes with daily activity | Significant; prevents daily activity | Emergency room visit or hospitalisation |
| Headache | NO | No interference with activity | Interferes with daily activity | Significant; prevents daily activity | Emergency room visit or hospitalisation |
| Lymphadenopathy* | NO | No interference with activity | Interferes with daily activity | Significant; prevents daily activity | Emergency room visit or hospitalisation |
| Insomnia* | NO | No interference with activity | Interferes with daily activity | Significant; prevents daily activity | Emergency room visit or hospitalisation |
| Leg pain* | NO | No interference with activity | Interferes with daily activity | Significant; prevents daily activity | Emergency room visit or hospitalisation |
| General discomfort* | NO | No interference with activity | Interferes with daily activity | Significant; prevents daily activity | Emergency room visit or hospitalisation |
| Anaphylactic reaction (allergic reaction to the components of the vaccine)* | NO | No interference with activity | Interferes with daily activity | Significant; prevents daily activity | Emergency room visit or hospitalisation |
| Did you require the use of painkillers or antipyretics in the first week after this dose?* | NO | Yes, only 1 day | Yes, 2-3 days | Yes, more than 4 days | Emergency room visit or hospitalisation |
*Out of FDA toxicity guidance.
The hospital’s management team e-mail address was used as the dissemination channel for a down-stream emission of the survey to lower categories, which could be completed via computer or any mobile device with a web browser. No personal data was collected.
Informed consent was obtained at the beginning of the survey. It was completed voluntarily by each participant, at least 2 weeks after the second dose of the vaccine had been administered (or first dose if they had received a single dose).
A sample size of 1160 patients was estimated. It was considered an appearance of headache after the second dose ratio of 49% and 58% (BNT162b2 and mRNA-1273 COVID-19 vaccines respectively) after the second dose as the baseline systemic adverse event. A two-sided test was established with an alpha cutoff of 5%, a beta of 20% and an expected loss ratio of 15%.
This study was approved by the local Clinical Research Ethics Committee of University Hospital Virgen Macarena (Seville-Spain) and was conducted under the tenets of the Declaration of Helsinki. The study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline.
2.2. Inclusion criteria
All healthcare and non-healthcare workers of all ages at the Hospital, as well as workers from the healthcare centers linked the hospital and external companies that provide services directly to the center, who have received one or two doses of any of the Pfizer-Biontech or Moderna vaccines against COVID-19 disease in the months from December 2020 to April 2021 and whose vaccination has been managed by the hospital itself and were selected to receive the survey.
2.3. Exclusion criteria
Subjects who have been vaccinated in the same period, but who are not employed at the hospital or at the health centers or companies dependent on the hospital. Workers who have been vaccinated with additional business services other than those considered as the object of this study.
2.4. Duration of the study
The online survey was open to collect responses between 1st of March and 30th of April 2021.
2.5. Statistical analysis
Descriptive statistics were performed on the study variables. Absolute frequencies and percentages were used for qualitative variables. Quantitative variables were summarized by median and range (minimum and maximum) according to their asymmetry. The distributions were tested for normality using the Kolmogorov-Smirnov and Shapiro-Wilk tests.
Comparison of the study groups was performed using the χ2 test and Fischer’s exact test when necessary, for qualitative variables.
Non-parametric ‘marginal homogeneity’ tests were used to compare before and after reactions, as the variable to be analyzed was in ordinal form (non-mild-moderate-severe-severe-emergence).
Data analysis was performed with IBM SPSS v.20 statistical software.
3. Results
1765 workers responded to the survey; 70,8% (1249) with complete responses and 29,2% (516) with incomplete responses being excluded from the study. Of the 1249 respondents who completed the survey, 52% (650) received the BNT162b2 vaccine and the remaining received the mRNA-1273 vaccine (599) (the study disposition is set in Supplementary Figure 1).
Table 3 summarizes the demographic characteristics of the volunteers surveyed for each type of vaccine received. 79.1% were female and the rest were male. 13% were in the age group 18–30 years, 59.7% were between 31–55 years and 27.3% were over 55 years. The median age of the respondents was 47 years (19–67). 88.3% were healthcare professionals. The majority of respondents had a high level of education with a university degree. 36.4% confirmed that they had one or more allergies (mRNA-1273: 38.7%; BNT162b2: 34.3%) and 37.9% had some co-morbidity (mRNA-1273: 41.4%; BNT162b2: 34.6%). Among the most prevalent allergies were: pollen allergy (19.4%), dust mite allergy (11.8%), drug allergy (10.8%) and food allergy (7%). Among the comorbidities reported we highlight: hypertension (7.9%), cardiovascular diseases (7.2%), autoimmune diseases (6.8%), other rheumatic diseases (5.5%), thyroid disease (5,1%) and diabetes (4%).
Table 3.
Demographic characteristics of the participants
| mRNA-1273 | BNT162b2 | Total | p | |
|---|---|---|---|---|
| Sex – nº (%) | ||||
| Male | 156 (26) | 180 (27,7) | 336 (26,9) | 0,511 |
| Female | 443 (74) | 470 (72,3) | 913 (73,1) | |
| Age group – nº (%) | ||||
| 18–30 | 74 (12,4) | 88 (13,5) | 162 (13) | < 0,0005 |
| 31–55 | 320 (53,4) | 426 (65,5) | 746 (59,7) | |
| >55 | 205 (34,2) | 136 (20,9) | 341 (27,3) | |
| Age at vaccination – years | ||||
| Median | 50 | 45 | 47 | < 0,0005 |
| Range | 19–67 | 20–66 | 19–67 | |
| Company which belong to – nº (%) | ||||
| Public health service | 549 (91,7) | 600 (92,3) | 1149 (92) | 0,031 |
| University (internship students) | 8 (1,3) | 1 (0,2) | 9 (0,7) | |
| Other | 42 (7) | 49 (7,5) | 91 (7,3) | |
| Academic Degree – nº (%) | ||||
| Lower secondary education | 18 (3) | 22 (3,4) | 40 (3,2) | 0,009 |
| Upper secondary education | 20 (3,3) | 5 (0,8) | 25 (2) | |
| Vocational training | 138 (23) | 118 (18,2) | 256 (20,5) | |
| University degree or equivalent | 370 (61,8) | 450 (69,2) | 820 (65,7) | |
| Medical residents | 53 (8,9) | 55 (8,5) | 108 (8,6) | |
| Presence of any type of allergy – nº (%) | ||||
| Yes | 232 (38,7) | 223 (34,3) | 455 (36,4) | 0,105 |
| No | 367 (61,3) | 427 (65,7) | 794 (63,6) | |
| Presence of any type of comorbility – nº (%) | ||||
| Yes | 248 (41,4) | 225 (34,6) | 473 (37,9) | 0,013 |
| No | 351 (58,6) | 425 (65,4) | 776 (62,1) | |
| Symptoms and positivity to COVID-19 – nº (%) | ||||
| Previously positive to COVID-19 | 54 (9) | 64 (9,8) | 118 (9,4) | 0,442 |
| Never positive to COVID-19 | 545 (91) | 586 (90,2) | 1131 (90,6) | |
47.3% stated that they were taking medication on a regular basis. 90.6% confirmed never having been COVID-19 positive, 7.6% confirmed having been COVID-19 positive with symptoms and only 1.8% confirmed having been COVID-19 positive asymptomatically.
Eleven (1.7%) of the respondents who received the BNT162b2 vaccine and 4 (0.67%) of those who received mRNA-1273 were administered a single dose.
3.1. Local adverse events
The presence of local reactions showed significant differences when compared by vaccine with a p-value of 0.003; where the proportion of patients with these reactions was 98.3% for mRNA-1273 vs. 95.4% for BNT162b2.
In total, 6896 local reactions were recorded: 3939 among the 599 patients in the mRNA-1273 group (6.6 reactions per patient) and 2957 among the 650 patients in the BNT162b2 group (4.5 reactions per patient).
In the mRNA-1273 group 2495 (63.3%) were mild, 1056 (26.8%) moderate, 381 (9.6%) severe and 7 (0.2%) emergency reactions of which 2 (0,06%) were injection site pain, 2 (0,06%) were injection site itching and 3 (0,09%) were arm mobility. In the BNT162b2 group, 2222 (75.1%) were mild, 577 (19.5%) moderate, 155 (5.3%) severe and 3 (0.1%) emergency reactions of which 1 (0,03%) were injection site swelling, 1 (0,03%) were injection site itching and 1 (0,03%) were arm mobility.
In both the mRNA-1273 and BNT162b2 groups, the most frequent localized adverse reactions after the first and second dose were as follows: pain at the injection site (mRNA-1273: 90.7% vs 84.1%; BNT162b2: 84.6% vs 76.1%), injection site tenderness (mRNA-1273: 72.5% vs 68.1%; BNT162b2: 60.9% vs 55.1%) and arm mobilization problems (mRNA-1273: 62.4% vs 52.9%; BNT162b2: 45.8% vs 39.7%) as can be seen in Figure 1.
Figure 1.
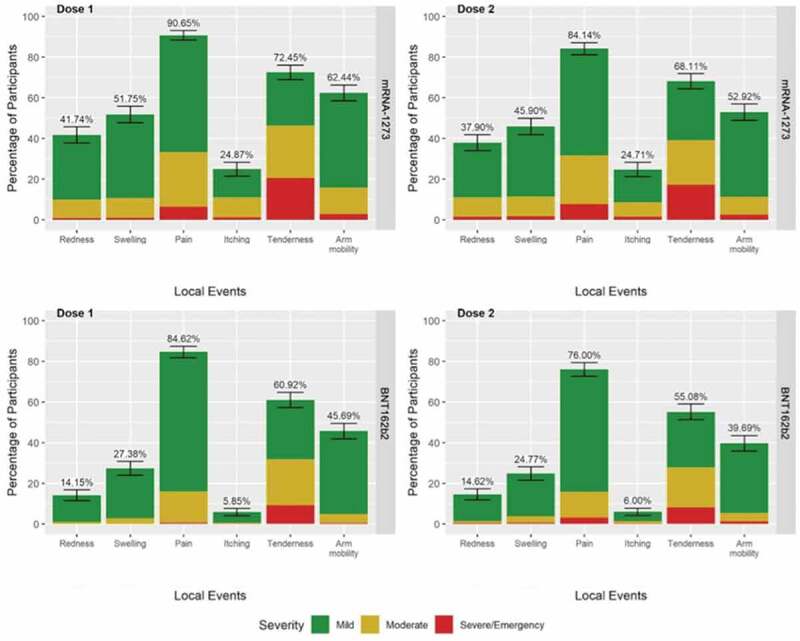
Local reactions reported within 7 days after injection of mRNA-1273 or BNT162b2 vaccines.
Local reactions occurred in a higher percentage of individuals after administration of the first dose compared to the second dose for both vaccines, and in a higher percentage in the mRNA-1273 group compared to the BNT162b2 group, the difference being statistically significant.
3.2. Systemic adverse reactions
The presence of systemic reactions showed significant differences when compared by vaccine with p-value<0.05; where the proportion of patients with these reactions was 96.3% for mRNA-1273 vs 81.5% for BNT162b2.
In total, 7506 systemic reactions were recorded: 4460 among the 599 patients in the mRNA-1273 group (7.4 per patient) and 3046 among the 650 patients in the BNT162b2 group (4.7 per patient).
In the mRNA-1273 group 2167 (48.6%) were mild, 1351 (30.3%) moderate, 918 (20.6%) severe and 24 (0.5%) emergency reactions of which 3 (0,06%) were fever, 2 (0,04%) were nausea/vomiting, 3 (0,06%) were diarrhea, 4 (0,08%) were joint pain, 1 (0,02%) were muscle pain, 1 (0,02%) were fatigue, 1 (0,02%) were chills, 2 (0,04%) were headache, 2 (0,04%) were lymphadenopathy, 1 (0,02%) were leg pain and 4 (0,08%) were general discomfort. In the BNT162b2 group, 1883 (61.8%) were mild, 820 (26.9%) moderate, 334 (11%) severe and 9 (0.3%) emergency reactions of which 1 (0,03%) were nausea/vomiting, 2 (0,07%) were joint pain, 1 (0,03%) were muscle pain, 2 (0,07%) were fatigue, 1 (0,03%) were chills, 1 (0,03%) were headache, 1 (0,03%) were lymphadenopathy and 1 (0,03%) were general discomfort.
In both the mRNA-1273 and BNT162b2 groups, the most frequent systemic adverse reactions after the first and second dose were: general discomfort (mRNA-1273: 35.5% vs 79.1%; BNT162b2: 18.9% vs 55.1%), fatigue (mRNA-1273: 37.4% vs 75.3%; BNT162b2: 24.4% vs 55%), chills (mRNA-1273: 22.6% vs 70%; BNT162b2: 12.2% vs 42.4%), muscle pain (mRNA-1273: 29.7% vs 69.2%; BNT162b2: 16.4% vs 47.4%) and joint pain (mRNA-1273: 22.6% vs 70%; BNT162b2: 12.2% vs 42.4%) (Figure 2).
Figure 2.
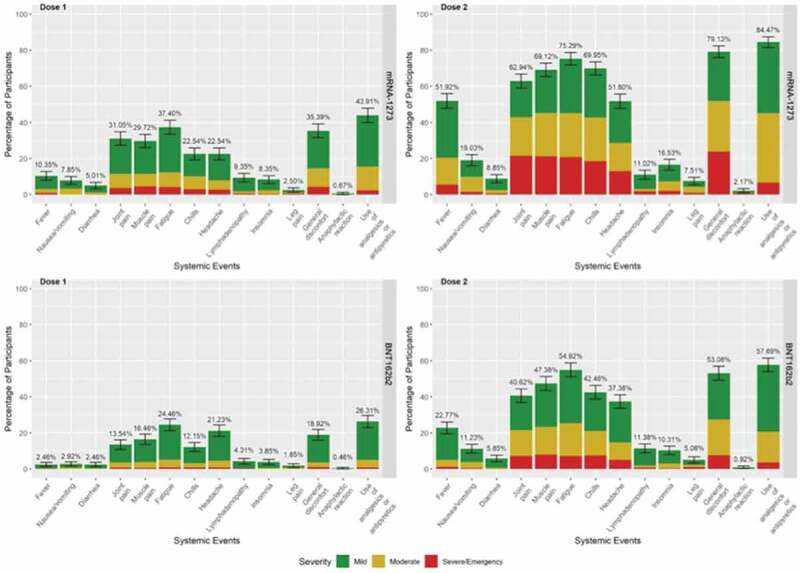
Systemic reactions reported within 7 days after injection of mRNA-1273 or BNT162b2 vaccines.
In contrast to localized adverse reactions, systemic reactions occurred in a higher percentage of individuals after administration of the second dose compared to the first dose for both vaccines. What remains the same is the higher percentage of affected individuals in the mRNA-1273 group compared to the BNT162b2 group and the statistical significance.
3.3. Severe and emergency reactions
Significant differences were observed between mRNA-1273 and BNT162b2 vaccines in individuals who experienced severe and emergent reactions with p-value<0.05. In total 601 (48.1%) respondents reported a severe-type reaction and 93 (7.4%) reported an emergent-type reaction. Specifically, 379 individuals (63.3%) in the mRNA-1273 group reported a severe reaction compared to 222 (34.2%) in the BNT162b2 group and 60 individuals (10%) in the mRNA-1273 group reported an emergent reaction compared to 33 (5.1%) in the BNT162b2 group.
Among the severe and emergent reactions reported by the respondents, 302 of the individuals (24.2%) reported a local reaction and 331 (26.5%) a systemic reaction. 203 individuals (33.9%) in the mRNA-1273 group confirmed a local reaction compared to 99 (15.2%) in the BNT162b2 group and 222 individuals (37.1%) in the mRNA-1273 group reported a systemic reaction compared to 109 (16.8%) in the BNT162b2 group.
3.4. Seropositive
For the different vaccines, there are no differences in the occurrence of local or systemic reactions between seropositive and seronegative patients. Within seropositive patients, there are no differences in the presence of local reactions between mRNA-1273 and BNT162b2 but the presence of systemic reactions showed significant differences when comparing by vaccine with p-value<0.001; where the proportion of patients with some type of these reactions was 100% for mRNA-1273 versus 79.7% for BNT162b2.
3.5. Age
Significant differences (with p-value<0.05) were also observed between mRNA-1273 and BNT162b2 vaccines in the different age groups. 18–30 year age group had a higher frequency of adverse events than the 30–55 and >55 age groups for the mRNA-1273 group: pain at the injection site (100% vs 95,9% vs 89,8%), arm mobility (78,4% vs 74,4% vs 62%), fever (73% vs 55,6% vs 47,3%), fatigue (89,2% vs 80,9% vs 75,6%) and lymphadenopathy (27% vs 14,7% vs 11,7%). Similarly, the frequency of adverse reactions is higher in the younger group for the BNT162b2 vaccine: pain at the injection site (95,5% vs 92,3% vs 84,6%), arm mobility (69,3% vs 55,9% vs 41,2%), fever (29,5% vs 25,4% vs 15,4%), muscle pain (61,4% vs 53,8% vs 35,3%), fatigue (70,5% vs 62,4% vs 47,8%) and general discomfort (71,6% vs 58% vs 47,8%), with some exceptions such as itching at the injection site (1,1% vs 8,7% vs 8,1%), chills (46,6% vs 49,8% vs 34,6%) and lymphadenopathy (8% vs 16,4% vs 3,7%).
3.6. Analgesic and antipyretic use
In the mRNA-1273 group, 263 (43.9%) respondents reported requiring analgesic or antipyretic use in the first week after administration of the first dose and 506 (84.4%) in the first week after administration of the second dose. In the BNT162b2 group, the number of individuals who used analgesics or antipyretics in the first week after administration of the first dose was 171 (26.4%) and 375 (57.7%) after the second dose. This difference between BNT162b2 and mRNA-1273 was statistically significant (p < 0.05).
3.7. Allergies and comorbidities
The presence of one or more previous allergies was associated with a higher frequency of nausea (28% vs 19.1%), redness (58.2% vs 45.8%), swelling (66.4% vs 53.7%) and itching (39.2% vs 28.6%) at the injection site for the mRNA-1273 group and general discomfort (64, 1% vs 54.3%), fatigue (66.4% vs 57.4%), headache (50.2% vs 40.5%), nausea (16.6% vs 9.6%), swelling at the injection site (43% vs 27.4%) and difficulty in arm mobility (63.7% vs 49.9%) for the BNT162b2 group.
Having any comorbidity was associated with a higher frequency of diarrhea (14.9% vs. 9.4%), redness (55.6% vs. 47%) and itching (38.7% vs. 28.5%) at the injection site and anaphylactic reactions (4% vs. 1.4%) for the mRNA-1273 group. For the BNT162b2 group only, it was associated with a higher frequency of insomnia (15.1% vs. 9.4%).
3.8. Absence from work
Significant differences were observed between mRNA-1273 and BNT162b2 in the time of absence from work with p-value<0.05; where the proportion of workers not absent from work was 64.6% mRNA-1273 vs 89.2% BNT162b2; the proportion of absence between 1 to 2 days was 31.9% mRNA-1273 vs 9.8% BNT162b2; while for more than 2 days of absence the proportions were 3.5% mRNA-1273 vs 0.9% BNT162b2.
4. Discussion
The serious global crisis caused by the appearance of SARS-COV-2 at the end of 2019 has made it possible to develop more than one vaccine against this infection in less than a year, making it the fastest development of a vaccine in history, as until now the record was held by the vaccine against mumps, which took 4 years from its inception to its commercialization [17].
The first vaccines to reach the Spanish market were BNT162b2 and mRNA-1273. Both vaccines had a very high degree of efficacy, 95% and 94.5%, respectively. However, this does not exclude the occurrence of a wide variety of adverse effects similar to those of other vaccines already on the market.
The short time that has elapsed since the vaccines were marketed means that there are very few studies available that compare the marketed vaccines, and even fewer studies that evaluate the use of these vaccines in real post-marketing conditions, hence the importance of our study.
The patients in our study are generally well balanced, as is the case with pivotal clinical trials of vaccines. However, it should be noted that our study has the limitation of the maximum age of personnel, in contrast to the lack of age limit in the pivotal trials, and although there are differences in the number of subjects receiving one or the other vaccine depending on the age group of 31–55, this is compensated by the following group of >55, so it is assumed that the distribution of subjects is correct. It should also be considered that in our study the majority are women.
Overall, the safety data for both vaccines are reassuring, with no unexpected adverse effects detected that could alter the vaccination pattern. The local adverse reactions described in our study are similar to those described in the pivotal clinical trials of both vaccines [19,20], mainly pain and tenderness at the injection site and mobility problems. These similarities are seen in both the type and the number of adverse events, as in the pivotal clinical trial with mRNA-123 [19], the number of local adverse events is 84.2% and 88.6% for the first and second dose, respectively. In the case of the pivotal BNT162b2 trial, these numbers are slightly lower, at 83% and 78% for those under 55 and 71% and 66% for those over 55.
As for systemic reactions, our study shows that in both vaccines these adverse effects were similar, although in all cases the data show a higher percentage of these events with the second dose of either vaccine, and the data are always higher with the mRNA-1273 vaccine. These results are consistent with data published in other comparative studies such as that of Meo et al [17]. in which the adverse effects described are also lower with the BNT162b2 vaccine than with the mRNA-1273 vaccine. The difference in reactions between the mRNA-1273 and BNT162b2 vaccines could be due to the higher dose of RNA present in the mRNA-1273 vaccine or to the different excipients present in the two vaccines. However, we have not found any studies to justify the reason for the difference in the occurrence of these adverse events.
These percentages described in the studies are much higher than those reported in the latest Pharmacovigilance report [21] (27/07/2021) of the Spanish Ministry of Health, in which, for example, with the BNT162b2 vaccine, the most common adverse effects described in the population were: pyrexia 37%, headache 27%, myalgia 20%, injection site pain 14%, malaise 12%; and with the mRNA-1273 vaccine they were: pyrexia 50%, headache 30%, myalgia 25%, injection site pain 19%, malaise 13%. The fact that these data are lower than those in our study could be due to the fact that the patient did not report the adverse event and not to the fact that they did not actually occur.
Our data are comparable to those of other studies in hospital workers. For example, a study on the adverse effects of the BNT162b2 vaccine has recently been published in professionals in the Czech Republic [22], which showed a higher percentage of systemic adverse effects 93.1% compared to our 81.5%. In addition, both studies agree that allergic patients had a higher number of cases of injection site redness, headache or fatigue. In another study of hospital workers in Germany [23] immunized with these vaccines, 78.3% of patients suffered a local adverse event and 61% a systemic adverse event, the latter being lower than in our study. These results are also similar to another study with the BNT162b2 vaccine [24], with a similar percentage of adverse events.
It is also important to highlight the fact that these adverse reactions were analyzed according to whether the patient had previous contact with the virus or not, where no differences were found between seropositive and seronegative patients, but there were differences between seropositive patients with one vaccine and those with the other, who were at greater risk of suffering systemic adverse events with mRNA-1273.
Our study has several limitations. One of the most important is that as this was an analytical cross-sectional survey-based study, the adverse events recorded are subjective and depend on the patients’ recollection. There was no medical control of them, so everything depends on the patients’ assessment at the time, and we cannot associate it with an objective scale, so we cannot be sure that the number of adverse effects could be even higher.
Another limitation of the study is that considering the data available at the time, most of the subjects (98.8%) were administered the full vaccination regimen, whether or not they had passed the Covid test, although it has subsequently been established that for those individuals who had been positive, a single dose of vaccine would be sufficient, so there have been patients who have experienced unnecessary adverse effects with the second dose of vaccine.
5. Conclusion
This study shows results that are in accordance with the data available in the clinical trials on which the authorization of both vaccines was based and in the few comparative studies that have been carried out to date, confirming that although the adverse effects that occur after the administration of both vaccines are not particularly serious, they do appear in a large majority of the people vaccinated and in greater numbers after the administration of the mRNA-1273 vaccine.
This study confirms what we already know; however, more extensive studies with a more varied population profile are needed in order to draw more general conclusions.
Supplementary Material
Acknowledgments
Data were partially presented at the 26th European Congress of Hospital Pharmacy.
Funding Statement
This paper was not funded.
Author contributions
Design of the work: MVR, MACH, MRLV, OSC, RCG; JCR, MIST; Data collection: OSC, RCG; Data analysis and interpretation: MVR, JCR, RCG, MIST; Drafting the article: RCG, MIST, MVR, OSC; Critical revision of the article: MVR, MIST JCR, MRLV; Final approval of the revision to be published: MACH, MVR, JCR, MRLV, MIST.
Declaration of Interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary material
Supplemental data for this article can be accessed here.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Coronavirus disease 2019 (COVID-19). Situation Report–51; Accessed 01 February 2021 (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf)
- 3.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020. Mar-Apr;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss P, Murdoch DR.. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(10229):1014–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article is of considerable interest to the reader regarding the importance of COVID-19
- 5.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020Jul1; 180(7)934–943.Erratum in: JAMA Intern Med. 2020 Jul 1;180(7):1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . Coronavirus disease 2019 (COVID-19). Weekly epidemiological update - 6-19 July 2021. Accessed 19 July 2021 (: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—6-july-2021)
- 7.European Centre for Disease Prevention and Control . Guidelines for the use of non-pharmaceutical measures to delay and mitigate the impact of 2019-nCoV. Accessed 22 February 2021 (https://www.ecdc.europa.eu/en/publications-data/guidelines-use-non-pharmaceutical-measures-delay-and-mitigate-impact-2019-ncov)
- 8.Anderson RM, Heesterbeek H, Klinkenberg D, et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395(10228):931–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teslya A, Pham TM, Godijk NG, et al. Impact of self-imposed prevention measures and short-term government-imposed social distancing on mitigating and delaying a COVID-19 epidemic: a modelling study. PLoS Med. 2020Jul21; 17(7)e1003166.Erratum in: PLoS Med. 2020 Dec 4;17(12):e1003499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roper RL, Rehm KE. SARS vaccines: where are we? Expert Rev Vaccines. 2009. Jul;8(7):887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nota informativa MUH AEMPS, 38/2020 La EMA recomienda la autorización de la primera vacuna frente a la COVID-19. 2020. Accesed 01 February 2021 (https://www.aemps.gob.es/informa/notasinformativas/laaemps/2020-laaemps/la-ema-recomienda-la-autorizacion-de-la-primera-vacuna-frente-a-la-covid-19/)
- 12.Nota informativa MUH AEMPS, 1/2021: la EMA recomienda la autorización de la segunda vacuna frente a la COVID-19. Accessed 01 February 2021. https://www.aemps.gob.es/informa/notasinformativas/laaemps/2021-2/la-ema-recomienda-la-autorizacion-de-la-segunda-vacuna-frente-a-la-covid-19/
- 13.European Medicines Agency . Spikevax (previously COVID-19 vaccine Moderna): summary of product characteristics. 2021. Accessed 02 February 2021 (https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf).; • This document is of interest to the reader in order to learn as much as possible about the vaccine.
- 14.European Medicines Agency . Comirnaty: summary of product characteristics. Accessed 02 February 2021 (https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf).; • This document is of interest to the reader in order to learn as much as possible about the vaccine.
- 15.Wise J. Covid-19: people who have had infection might only need one dose of mRNA vaccine. BMJ. 2021. Feb 2;372:n308. [DOI] [PubMed] [Google Scholar]
- 16.Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021. Apr 8;384(14):1372–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meo SA, Bukhari IA, Akram J, et al. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna vaccines. Eur Rev Med Pharmacol Sci. 2021. Feb;25(3):1663–1669. [DOI] [PubMed] [Google Scholar]; • This paper is of interest to the reader in understanding pharmacological aspects of both vaccines.
- 18.Guidance for Industry . Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. FDA, Sept 2007. https://www.fda.gov/media/73679/download; 2021. [cited 2021 Feb 7]. [DOI] [PubMed]
- 19.Baden LR, El Sahly HM, Essink B, et al., Efficacy and Safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 384(5): 403–416. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper is of considerable interest to the reader regarding that these results led to the licensing of the vaccine.
- 20.Polack FP, Thomas SJ, Kitchin N, et al., Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 383(27): 2603–2615. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper is of considerable interest to the reader regarding that these results led to the licensing of the vaccine.
- 21.7º Informe de Farmacovigilancia sobre Vacunas Covid-19. https://www.aemps.gob.es/informa/boletines-aemps/boletin-fv/2021-boletin-fv/7o-informe-de-farmacovigilancia-sobre-vacunas-covid-19/; 2021. [cited 2021 Jul 27].
- 22.Riad A, Pokorná A, Attia S, et al. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med. 2021;10(7):1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klugar M, Riad A, Mekhemar M, et al. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology (Basel). 2021;10(8):752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riad A, Hocková B, Kantorová L, et al. Side effects of mRNA-based COVID-19 vaccine: nationwide Phase IV study among healthcare workers in Slovakia. Pharmaceuticals. 2021;14(9):873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


