ABSTRACT
Introduction
Appearances of SARS-CoV-2 variants have created havoc and additional challenges for the ongoing vaccination drive against pandemic COVID-19. Interestingly, several vaccine platforms are showing great potential to produce successful vaccines against SARS-CoV-2 and its variants. Billions of COVID-19 vaccine doses have been administered worldwide. Mix-and-Match COVID-19 vaccines involving the mixing of the same platform vaccines and also two different vaccine platforms may provide greater protection against SARS-CoV-2 and its variants. COVID-19 vaccines have become one of the most important tools to mitigate the ongoing pandemic COVID-19.
Areas covered
We describe SARS-Cov-2 variants, their impact on the population, COVID-19 vaccines, diverse vaccine platforms, doses of vaccines, the efficacy of vaccines against SARS-CoV-2 and its variants, mitigation of the COVID-19 transmission- alternatives to vaccines.
Expert opinion
Diverse vaccine platforms may safeguard against ongoing, deadly SARS-CoV-2 and its infectious variants. The efficacies of COVID-19 vaccines are significantly high after the administration of the second dose. Further, it protects individuals including vulnerable patients with co-morbidities from SARS-CoV-2 and its variants. The hospitalizations and deaths of the individuals may be prevented by COVID-19 vaccines.
KEYWORDS: COVID-19, sars-cov-2, vaccines, diverse, variants, platforms
1. Introduction
COVID-19 is a highly infectious, airborne viral disease with a big threat for patients having respiratory, cardiovascular diseases, compromised immune systems, hypertension, diabetes and other diseases [1–3]. It was declared a global pandemic on 11 March 2020 by World Health Organization (WHO). The ongoing SARS-CoV-2 pandemic has challenged human life and the peace of the world. The virus SARS-CoV-2 uses its spike (S) glycoprotein to enter the human body by binding to its angiotensin-converting enzyme 2 (ACE2) receptors [4,5]. SARS-CoV-2 is a positive-sense single-stranded RNA virus and has a greater chance of mutation in its sequence for its survival. The complementary mutations might have evolved to preserve the structural integrity of the virus.
COVID-19 has infected more than billions of humans worldwide and has caused more than a hundred thousand deaths as reported by the World Health Organization (WHO) [6]. This disease has destabilized the health system and economy of the entire world. Notably, the number of infections and mortality rates due to COVID-19 are different worldwide. The global mortality rate is 2.08% [6]. Since the first case of SARS-CoV-2 infection reported in January 2020, its several variants have evolved with changes in the receptor-binding domain of the spike protein [7]. Meanwhile, the structural analysis of the variants has suggested that the mutations change the conformation of the sidechain to reduce the effectiveness of interactions with antibodies [8].
The global pandemic COVID-19 highlighted the importance of the development of safe and effective vaccines with high priority. Therefore, vaccination at a large scale is the need of the hour to control this ongoing pandemic. Vaccination is driven by recent technologies involving the growth of viruses in cell culture, synthetic biology, recombinant DNA, genomics and chemical conjugation [9]. The approval for vaccines is required to go through different stages such as pre-clinical in animals and phase I/II/III/IV in humans. The number of participants, age, doses of vaccines and their response including side effects are key points for the approval of vaccines [10]. Further, infant, neonatal, pregnancy and other severe/chronic diseases are also taken into consideration while approving the vaccines. In most of the clinical trials, the participants’ age is between 18 and 55 years while in certain cases it’s between 12 and 60 years and between 60 and 80 years. Generally, the number of participants varies from about 30 to 30,000 in number in diverse clinical studies [10,11].
Interestingly, a great achievement in the field of vaccinology was in the development of COVID-19 vaccines which involved design, testing and approval within a year in few countries (Table 1) [12,13]. The S protein of SARS-CoV-2 is the main target for the design of the COVID-19 vaccine as SARS-CoV-2 uses its S protein to bind to host ACE2 receptors [14]. Many vaccines are now available and many vaccine developments are under clinical trials (Tables 1 and 2). Notably, on 11 August 2020, Russia has approved and registered the first COVID-19 vaccine made by Gamaleya Research Institute and named it ‘Sputnik V’ and published the result of clinical trial phase 1/2 studies on 4 September 2020 (Clinical Trials No.: NCT04436471 and NCT04437875) [15]. However, vaccination of such a huge population is one of the challenging tasks [16]. The authorized COVID-19 vaccines have shown 65–95% efficacy against non-variant strain [17]. However, the efficacy of authorized COVID-19 vaccines may be affected by SARS-CoV-2 variants.
Table 1.
Diverse platform vaccines against COVID-19 are in emergency use listing (EUL)/ prequalification evaluation process at WHO
| Sl. No. | Vaccine Name | Platform/Type of Vaccine | Manufacturer Industry/Academy |
NRA of Record | EOI accepted at WHO | Status of assessment of WHO |
|---|---|---|---|---|---|---|
| 1 | BNT162b2/COMIRNATY Tozinameran (INN) | Nucleoside modified mNRA | Pfizer, BioNTech Manufacturing GmbH | EMA USFDA |
Yes | Finalized |
| 2 | AZD1222 Vaxzevria, Covishield (ChAdOx1_nCoV-19) |
Recombinant ChAdOx1 adenoviral vector encoding the Spike protein antigen of the SARS-CoV-2. | AstraZeneca, AB, University of Oxford |
EMA, MFDS KOREA, Japan MHLW/PMDA, Australia TGA, DCGI | Yes | Finalized |
| 3 | Ad26.COV2.S | Recombinant, replicationincompetent adenovirus type 26 (Ad26) vectored vaccine encoding the (SARS-CoV-2) Spike (S) protein | Janssen–Cilag International NV | EMA | Yes | Finalized |
| 4 | mRNA-1273 | mNRA-based vaccine encapsulated in lipid nanoparticle (LNP) | Moderna Biotech | EMA, USFDA | Yes | Finalized |
| 5 | SARS-CoV-2 Vaccine (Vero Cell), Inactivated (lnCoV) | Inactivated, produced in Vero cells | Sinopharm/Beijing Institute of Biological Products Co., Ltd. (BIBP) | NMPA | Yes | Finalized |
| 6 | COVID-19 Vaccine (Vero Cell), Inactivated/ CoronavacTM | Inactivated, produced in Vero cells | Sinovac Life Sciences Co., Ltd. | NMPA | Yes | Finalized |
| 7 | Sputnik V | Human Adenovirus Vector-based Covid-19 vaccine | The Gamaleya National Center | Russian NRA | Yes | Ongoing |
| 8 | SARS-CoV-2 Vaccine, Inactivated (Vero Cell)/ COVAXIN | Whole-Virion Inactivated Vero Cell | Bharat Biotech, India | DCGI | Yes | Ongoing |
| 9 | Inactivated SARS-CoV-2 Vaccine (Vero Cell) | Inactivated, produced in Vero cells | Sinopharm/WIBP | NMPA | Yes | Ongoing |
| 10 | Ad5-nCoV | Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) | CanSinoBio | NMPA | Yes | - |
| 11 | NVX-CoV2373/Covovax | Recombinant nanoparticle prefusion spike protein formulated with Matrix-M™ adjuvant | Novavax, Serum Institute of India |
EMA, DCGI |
Yes | - |
| 12 | CoV2 preS dTM-AS03 vaccine | Recombinant, adjuvanted | Sanofi | EMA | Yes | - |
| 13 | SCB-2019 | Novel recombinant SARS-CoV-2 Spike (S)-Trimer fusion protein | Clover Biopharmaceuticals | NMPA | Yes | - |
| 14 | Zorecimeran (INN) concentrate and solvent for dispersion for injection; Company code: CVnCoV/CV07050101 | mNRA-based vaccine encapsulated in lipid nanoparticle (LNP) | Curevac | EMA | Yes | - |
| 15 | EpiVacCorona | Peptide antigen | Vector State Research Center of Viralogy and Biotechnology | Russian NRA | Letter Received | - |
| 16 | Recombinant Novel Coronavirus Vaccine (CHO Cell) | Recombinant protein subunit | Zhifei Longcom, China | NMPA | in process | - |
| 17 | SARS-CoV-2 Vaccine, Inactivated (Vero Cell) | Inactivated | IMBCAMS, China | NMPA | in process | - |
| 18 | Soberana 01, Soberana 02 Soberana Plus Abdala | SARS-CoV-2 spike protein conjugated chemically to meningococcal B or tetanus toxoid or Aluminum | BioCubaFarma – Cuba | CECMED | in process | - |
Table 2.
Diverse platform vaccines are under clinical trial against COVID-19
| Sl. No. | Vaccine Platform | Type/Name of COVID-19 Vaccine | Manufacturer Industry/Academy |
2PhaseDoseDay of DoseRoute | References/Clinical Trial |
|---|---|---|---|---|---|
| Phase 4 | |||||
| 1 | Inactivated virus | CoronaVac; inactivated SARS-CoV-2 vaccine (vero cell) | Sinovac Research and Development Co., Ltd | Phase 4 |
NCT04756830 NCT04747821 NCT04775069 NCT04789356 NCT04754698 NCT04801888 NCT04894227 NCT04892459 NCT04911790 NCT04953325 NCT04962308 NCT04993365 |
| 2 Dose | |||||
| Day 0 + 14 | |||||
| IM | |||||
| 2 | Inactivated virus | Inactivated SARS-CoV-2 vaccine (Vero cell), vaccine name BBIBP-CorV | Sinopharm + China National Biotec Group Co + Beijing Institute of Biological Products | Phase 4 | NCT04863638 |
| 2 Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 3 | Viral vector (Non-replicating) | ChAdOx1-S – (AZD1222) (Covishield) (Vaxzevria) |
AstraZeneca + University of Oxford | Phase 4 |
NCT04760132 NCT04775069 EUCTR2021-002327-38-NL NCT04914832 ACTRN12621000661875 |
| 1–2Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 4 | Viral vector (Non-replicating) | Recombinant novel coronavirus vaccine (Adenovirus type 5 vector) Ad5-nCoV | CanSino Biological Inc./Beijing Institute of Biotechnology | Phase 3 | NCT04892459 |
| 1Dose | |||||
| Day 0 | |||||
| IM | |||||
| 5 | Viral vector (Non-replicating) | Ad26.COV2.S | Janssen Pharmaceutical, Johnson & Johnson |
Phase 4 | EUCTR2021-002327-38-NL |
| 1–2Dose | |||||
| Day 0 or Day 0 + 56 | |||||
| IM | |||||
| 6 | RNA based vaccine | mRNA −1273 | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | Phase 4 |
NCT04760132 NCT04792567 NCT04885907 EUCTR2021-002327-38-NL EUCTR2021-003388-90-NL NCT04952402 EUCTR2021-003618-37-NO NCT04969250 |
| 2Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 7 | RNA based vaccine | BNT162b2 (3 LNP-mRNAs), also known as ‘Comirnaty’ | Pfizer/BioNTech + Fosun Pharma | Phase 4 |
NCT04760132 ACTRN12621000661875 EUCTR2021-000412-28-BE EUCTR2021-002327-38-NL NCT04780659 NCT04961229 NCT04775069 EUCTR2021-000893-27-BE EUCTR2021-000930-32-BE NCT04852861 NCT04878211 EUCTR2021-003388-90-NL EUCTR2021-003618-37-NO NCT04955626 NCT04952766 NCT04969250 |
| 2Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 8 | RNA based vaccine | mRNA-1273.351. A lipid nanoparticle (LNP)-encapsulated mRNA-based vaccine that encodes for a full-length, prefusion stabilized S protein of the SARS-CoV-2 B.1.351 variant. |
Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | Phase 4 | EUCTR2021-000930-32 NCT04878211 NCT04869358 |
| 1 or 2 Dose | |||||
| Day 0 or Day 0 + 28 or 56 | |||||
| IM | |||||
| Phase 3 | |||||
| 9 | Inactivated virus | Inactivated SARS-CoV-2 vaccine (Vero cell) | Sinopharm + China National Biotec Group Co + Wuhan Institute of Biological Products | Phase 3 | ChiCTR2000034780 ChiCTR2000039000 NCT04510207 NCT04612972 |
| 2Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 10 | Viral vector (Non-replicating) | Gam-COVID-Vac Adeno-based (rAd26-S+ rAd5-S) Sputnik V COVID-19 vaccine |
Gamaleya Research Institute; Health Ministry of the Russian Federation | Phase 3 |
NCT04530396 NCT04564716 NCT04642339 NCT04656613 NCT04741061 |
| 2Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 11 | Protein subunit | SARS-CoV-2 rS/Matrix M1-Adjuvant (Full length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M) NVX-CoV2373 |
Novavax | Phase 3 |
NCT04611802 EUCTR2020-004123-16-GB NCT04583995 |
| 2Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 12 | Protein subunit | Recombinant SARS-CoV-2 vaccine (CHO Cell) | Anhui Zhifei Longcom Biopharmaceutical + Institute of Microbiology, Chinese Academy of Sciences | Phase 3 | NCT04646590 |
| 2–3Dose | |||||
| Day 0 + 28 or Day 0 + 28 + 56 | |||||
| IM | |||||
| 13 | RNA based vaccine | CVnCoV Vaccine | CureVac AG | Phase 3 |
NCT04674189 NCT04838847 NCT04838847 |
| 2 | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 14 | Inactivated virus | SARS-CoV-2 vaccine (vero cells) | Institute of Medical Biology + Chinese Academy of Medical Sciences | Phase 3 | NCT04659239 |
| 2Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 15 | Inactivated virus | QazCovid-in® – COVID-19 inactivated vaccine | Research Institute for Biological Safety Problems, Rep of Kazakhstan | Phase 3 | NCT04691908 |
| 2Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 16 | DNA based vaccine | nCov vaccine | Zydus Cadila | Phase 3 | CTRI/2020/07/026352 |
| 3Dose | |||||
| Day 0 + 28 + 56 | |||||
| IM | |||||
| 17 | Inactivated virus | Whole-Virion Inactivated SARS-CoV-2 Vaccine (BBV152) | Bharat Biotech International Limited | Phase 3 | NCT04641481; CTRI/2020/11/028976 |
| 2Dose | |||||
| Day 0 + 14 | |||||
| IM | |||||
| 18 | Protein subunit | VAT00002: SARS-CoV-2 S protein with adjuvant | Sanofi Pasteur + GSK | Phase 3 | PACTR202011523101903 NCT04904549 |
| 2Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 19 | Inactivated virus | Inactivated SARS-CoV-2 vaccine (Vero cell) | Shenzhen Kangtai Biological Products Co., Ltd. | Phase 3 | NCT04852705 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 20 | Protein subunit | COVAX-19® Recombinant spike protein + adjuvant | Vaxine Pty Ltd. | Phase 3 | IRCT20150303021315N24 |
| 2 Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 21 | Protein subunit | FINLAY-FR-2 anti-SARS-CoV-2 Vaccine (RBD chemically conjugated to tetanus toxoid plus adjuvant) | Instituto Finlay de Vacunas | Phase 3 | RPCEC00000354 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 22 | Protein subunit | EpiVacCorona (EpiVacCorona vaccine based on peptide antigens for the prevention of COVID-19) | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology ‘Vector’ | Phase 3 | NCT04780035 |
| 2 Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 23 | Protein subunit | RBD (baculovirus production expressed in Sf9 cells) Recombinant SARS-CoV-2 vaccine (Sf9 Cell) | West China Hospital + Sichuan University | Phase 3 | NCT04887207 NCT04904471 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 24 | RNA based vaccine | SARS-CoV-2 mRNA vaccine (ARCoV) | Academy of Military Science (AMS), Walvax Biotechnology and Suzhou Abogen Biosciences | Phase 3 | NCT04847102 |
| 2 Dose | |||||
| Day 0 + 14 Or Day 0 + 28 | |||||
| IM | |||||
| 25 | Protein subunit | CIGB-66 (RBD+aluminum hydroxide) | Center for Genetic Engineering and Biotechnology (CIGB) | Phase 3 | RPCEC00000359 |
| 3 Dose | |||||
| Day 0 + 14 + 28 or Day 0 + 28 + 56 | |||||
| IM | |||||
| 26 | Inactivated Virus | VLA2001 | Valneva, National Institute for Health Research, United Kingdom | Phase 3 |
NCT04864561 NCT04956224 |
| 2 Dose | |||||
| Day 0 + 21, | |||||
| IM | |||||
| 27 | Protein subunit | Recombinant Sars-CoV-2 Spike protein, Aluminum adjuvanted (Nanocovax) | Nanogen Pharmaceutical Biotechnology | Phase 3 | NCT04922788 |
| 2 Dose | |||||
| Day 0 + 21, | |||||
| IM | |||||
| 28 | Inactivated Virus | ERUCOV-VAC, inactivated virus | Erciyes University | Phase 3 | NCT04942405 |
| 2 Dose | |||||
| Day 0 + 21, | |||||
| IM | |||||
| Phase 2/3 | |||||
| 29 | DNA based vaccine | INO-4800+ electroporation | Inovio Pharmaceuticals + International Vaccine Institute + Advaccine (Suzhou) Biopharmaceutical Co., Ltd | Phase 2/3 | NCT04642638 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 30 | DNA based vaccine | AG0301-COVID19 | AnGes + Takara Bio + Osaka University | Phase 2/3 | NCT04655625 |
| 2 Dose | |||||
| Day 0 + 14 | |||||
| IM | |||||
| 31 | Viral vector (Non-replicating) | GRAd-COV2 (Replication defective Simian Adenovirus (GRAd) encoding S) | ReiThera + Leukocare + Univercells | Phase 2/3 | NCT04791423 |
| 1 Dose | |||||
| 0 | |||||
| IM | |||||
| 32 | Protein subunit | SCB-2019 + AS03 or CpG 1018 adjuvant plus Alum adjuvant (Native like Trimeric subunit Spike Protein vaccine) | Clover Biopharmaceuticals Inc./GSK/Dynavax | Phase 2/3 | NCT04672395 |
| 2 Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 33 | Protein subunit | UB-612 (Multitope peptide based S1-RBD-protein based vaccine) | Vaxxinity | Phase 2/3 | NCT04683224 |
| 2 Dose | |||||
| Day 0 + 28, | |||||
| IM | |||||
| 34 | Virus like particle | Coronavirus-Like Particle COVID-19 (CoVLP) | Medicago Inc. | Phase 2/3 | NCT04636697 |
| 2 Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 35 | Viral vector (Replicating) | rVSV-SARS-CoV-2-S Vaccine (IIBR-100) | Israel Institute for Biological Research | Phase 2/3 | NCT04990466 |
| 1 Dose | |||||
| Day 0 | |||||
| IM | |||||
| 36 | Inactivated Virus | COVID-19 inactivated vaccine | Shifa Pharmed Industrial Co | Phase 2/3 | IRCT20201202049567N3 |
| 2 Dose | |||||
| Day 0 + 14, | |||||
| IM | |||||
| 37 | RNA based vaccine | mRNA-1273.211. A multivalent booster candidate combining mRNA-1273 plus mRNA-1273.351. | ModernaTX, Inc. | Phase 2/3 | NCT04927065 |
| 1 Dose | |||||
| Day 0 | |||||
| IM | |||||
| 38 | Viral vector (Non-replicating) | AZD2816; adenoviral vector ChAdOx platform and based on the Beta (B.1.351) variant | AstraZeneca + University of Oxford | Phase 2/3 | NCT04973449 |
| 2 Dose | |||||
| Day 0, 28 | |||||
| IM | |||||
| Phase 2 | |||||
| 39 | RNA based vaccine | ARCT-021 | Arcturus Therapeutics | Phase 2 |
NCT04668339 NCT04728347 |
| ND | |||||
| ND | |||||
| IM | |||||
| 40 | Protein subunit Protein subunit |
MVC-COV1901 (S-2P protein + CpG 1018) | Medigen Vaccine Biologics + Dynavax + National Institute of Allergy and Infectious Diseases (NIAID) | Phase 2 |
NCT04695652 NCT04822025 NCT04951388 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 41 | Protein subunit | FINLAY-FR1 anti-SARS-CoV-2 Vaccine (RBD + adjuvant) | Instituto Finlay de Vacunas | Phase 2 | RPCEC00000366 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 42 | Viral vector (Replicating) | DelNS1–2019-nCoV-RBD-OPT1 (Intranasal flu-based-RBD) | University of Hong Kong, Xiamen University and Beijing Wantai Biological Pharmacy | Phase 2 | ChiCTR2000039715 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| IN | |||||
| 43 | Protein subunit | Razi Cov Pars, recombinant spike protein | Razi Vaccine and Serum Research Institute | Phase 2 | IRCT20201214049709N2 |
| 3 Dose | |||||
| Day 0 + 21 + 51 | |||||
| IM or IN | |||||
| 44 | RNA based vaccine | MRT5500, an mRNA vaccine candidate | Sanofi Pasteur and Translate Bio | Phase 2, | IRCT20210206050259N2 |
| 2 Dose | |||||
| Day 0 + 21, | |||||
| IM | |||||
| 45 | Virus like particle | SARS-CoV-2 VLP Vaccine Vaccine-Wuhan; Vaccine-Alpha variant; Vaccine-Wuhan+Alpha variant |
The Scientific and Technological Research Council of Turkey | Phase 2, | NCT04962893 |
| 2 Dose | |||||
| Day 0 and later, | |||||
| SC | |||||
| 46 | Protein subunit | Recombinant SARS-CoV-2 Fusion Protein Vaccine (V-01) | Guangdong Provincial Center for Disease Control and Prevention/Gaozhou Center for Disease Control and Prevention | Phase 2 | ChiCTR2100045107 |
| 2 Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 47 | Protein subunit | SCB-2020S, an adjuvanted recombinant SARS-CoV-2 trimeric S-protein (from B.1.351 variant) | Clover Biopharmaceuticals AUS Pty Ltd | Phase 2 | NCT04950751 |
| 2 Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| Phase1/2 | |||||
| 48 | DNA based vaccine | GX-19 N | Genexine Consortium | Phase 1/2 |
NCT04445389 NCT04715997 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 49 | Protein subunit | KBP-COVID-19 (RBD-based) | Kentucky Bioprocessing Inc. | Phase 1/2 | NCT04473690 |
| 2 Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 50 | Virus like particle | RBD SARS-CoV-2 HBsAg VLP vaccine | Serum Institute of India + Accelagen Pty + SpyBiotech | Phase 1/2 | ACTRN12620000817943 ACTRN12620001308987 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 51 | Protein subunit | IMP CoVac-1 (SARS-CoV-2 HLA-DR peptides) | University Hospital Tuebingen | Phase 1/2 | NCT04954469 |
| 1 Dose | |||||
| Day 0 | |||||
| SC | |||||
| 52 | Viral vector (Non-replicating) + APC | LV-SMENP-DC vaccine. Dendritic cells are modified with lentivirus vectors expressing Covid-19 minigene SMENP and immune modulatory genes. CTLs are activated by LV-DC presenting Covid-19 specific antigens. | Shenzhen Geno-Immune Medical Institute | Phase 1/2 | NCT04276896 |
| 1 Dose | |||||
| Day 0 | |||||
| SC & IV | |||||
| 53 | Viral vector (Non-replicating) | Human Adenovirus Type 5: hAd5 S + N vaccine (S-Fusion + N-ETSD). E2b- Deleted Adeno. | ImmunityBio, Inc | Phase 1/2 |
NCT04843722 NCT04845191 |
| 1–2 Dose | |||||
| Day 0 + 21 | |||||
| SC or Oral | |||||
| 54 | Viral vector (Replicating) + APC | Dendritic cell vaccine AV-COVID-19. A vaccine consisting of autologous dendritic cells loaded with antigens from SARS-CoV-2, with or without GM-CSF | Aivita Biomedical, Inc. National Institute of Health Research and Development, Ministry of Health Republic of Indonesia | Phase 1/2 | NCT04386252 |
| 1 Dose | |||||
| Day 0 | |||||
| IM | |||||
| 55 | Protein subunit | CIGB-669 (RBD+AgnHB) | Center for Genetic Engineering and Biotechnology (CIGB) | Phase 1/2 | RPCEC00000345 |
| 3 Dose | |||||
| Day 0 + 14 + 28 or Day 0 + 28 + 56 | |||||
| IN | |||||
| 56 | Protein subunit | BECOV2 | Biological E. Limited | Phase 1/2 | CTRI/2020/11/029032 |
| 2 Dose | |||||
| Day 0 + 28, | |||||
| IM | |||||
| 57 | Viral vector (Replicating) | AdCLD-CoV19 (adenovirus vector) | Cellid Co., Ltd. | Phase 1/2 | NCT04666012 |
| 1 Dose | |||||
| Day 0 | |||||
| IM | |||||
| 58 | DNA based vaccine | GLS-5310 | GeneOne Life Science, Inc. | Phase 1/2 | NCT04673149 |
| 2 Dose | |||||
| Day 0 + 56, Day 0 + 84, | |||||
| IM | |||||
| 59 | Protein subunit | Recombinant protein vaccine S-268019 (using Baculovirus expression vector system) | Shionogi | Phase 1/2 | jRCT2051200092 |
| 2 Dose | |||||
| Day 0 + 21, | |||||
| IM | |||||
| 60 | Protein subunit | SARS-CoV-2-RBD-Fc fusion protein | University Medical Center Groningen + Akston Biosciences Inc. | Phase 1/2 | NCT04681092 |
| SC or IM | |||||
| 61 | Protein subunit | COVAC-1 and COVAC-2 sub-unit vaccine (spike protein) + SWE adjuvant | University of Saskatchewan | Phase 1/2 | NCT04702178 |
| 2 Dose | |||||
| Day 0 + 28, | |||||
| IM | |||||
| 62 | Protein subunit | GBP510, a recombinant surface protein vaccine with adjuvant AS03 (aluminum hydroxide) | SK Bioscience Co., Ltd. and CEPI | Phase 1/2 |
NCT04742738 NCT04750343 |
| 2 Dose | |||||
| Day 0 + 28, | |||||
| IM | |||||
| 63 | DNA based vaccine | COVID-eVax, a candidate plasmid DNA vaccine of the Spike protein | Takis + Rottapharm Biotech | Phase 1/2 |
NCT04788459 EUCTR2020-003734-20-IT |
| IM or IM + electroporation | |||||
| 64 | Inactivated virus | Inactivated (NDV-based) chimeric vaccine with or without the adjuvant CpG 1018 | The Government Pharmaceutical Organization (GPO); PATH; Dynavax | Phase 1/2 | NCT04764422 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 65 | Virus like particle | VBI-2902a. An enveloped virus-like particle (eVLP) of SARS-CoV-2 spike (S) glycoprotein and aluminum phosphate adjuvant. | VBI Vaccines Inc. | Phase 1/2, | NCT04773665 |
| 2 Dose | |||||
| Day 0 + 28, | |||||
| IM | |||||
| 66 | Protein subunit | EuCorVac-19; A spike protein using the recombinant protein technology and with an adjuvant. | POP Biotechnologies and EuBiologics Co.,Ltd | Phase 1/2, | NCT04783311 |
| 2 Dose | |||||
| Day 0 + 21, | |||||
| IM | |||||
| 67 | RNA based vaccine | DS-5670a, mRNA vaccine | Daiichi Sankyo Co., Ltd. | Phase 1/2, | NCT04821674 |
| 2 Dose | |||||
| IM | |||||
| 68 | Viral vector (Non-replicating) | COVIVAC. Newcastle Disease Virus (NDV) expressing membrane-anchored pre-fusion-stabilized trimeric SARS-CoV-2 S protein ± adjuvant CpG 1018 | Institute of Vaccines and Medical Biologicals, Vietnam | Phase 1/2, | NCT04830800 |
| 2 Dose | |||||
| Day 0 + 28, | |||||
| IM | |||||
| 69 | Protein subunit | Recombinant SARS-CoV-2 Vaccine (CHO cell) | National Vaccine and Serum Institute, China | Phase 1/2, | NCT04869592 |
| 2 Dose | |||||
| Day 0, | |||||
| IM | |||||
| 70 | RNA based vaccine | EXG-5003; a temperature-sensitive self-replicating RNA vaccine expressing the receptor binding domain of the SARS-CoV-2 spike protein. | Elixirgen Therapeutics, Inc | Phase 1/2, | NCT04863131 |
| 1 Dose | |||||
| Day 0, | |||||
| ID | |||||
| 71 | Inactivated Virus | Inactivated COVID-19 vaccine | KM Biologics Co., Ltd. | Phase 1/2, | jRCT2071200106 |
| 2 Dose | |||||
| Day 0, 28 | |||||
| IM | |||||
| 72 | Viral vector (Non-replicating) | Modified Vaccinia Virus Ankara (MVA) vector expressing a stabilized SARS-CoV-2 spike protein | German Center for Infection Research | Phase 1/2, | NCT04895449 |
| 2 Dose | |||||
| Day 0, 28 | |||||
| IM | |||||
| 73 | Protein subunit | QazCoVac-P – COVID-19 Subunit Vaccine | Research Institute for Biological Safety Problems | Phase 1/2, | NCT04930003 |
| 2 Dose | |||||
| Day 0, 21 | |||||
| IM | |||||
| 74 | DNA based vaccine | AG0302-COVID19 | AnGes, Inc | Phase 1/2, | NCT04993586 |
| 2–3 Dose | |||||
| Day 0,14,28 | |||||
| IM | |||||
| 75 | Protein subunit | Recombinant protein RBD fusion dimer adjuvanted vaccine (COVID-19 Vaccine Hipra) | Laboratorios Hipra, S.A. | Phase 1/2, | NCT05007509 |
| 2 Dose | |||||
| Day 0, 21 | |||||
| IM | |||||
| Phase 1 | |||||
| 76 | Viral vector (Non-replicating) | ChAdOx1-S – (AZD1222) (Covishield) (Vaxzevria) |
University of Oxford | Phase 1 | NCT04816019 |
| 1–2 Dose | |||||
| Day 0 + 28 | |||||
| IN | |||||
| 77 | Viral vector (Non-replicating) | VXA-CoV2-1 Ad5 adjuvanted Oral Vaccine platform | Vaxart | Phase 1 | NCT04563702 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| Oral | |||||
| 78 | Viral vector (Non-replicating) | MVA-SARS-2-S | University of Munich (Ludwig-Maximilians) | Phase 1 | NCT04569383 |
| 2 Dose | |||||
| Day 0 + 28 | |||||
| IM | |||||
| 79 | RNA based vaccine | LNP-nCoVsaRNA | Imperial College London | Phase 1 | ISRCTN17072692 |
| 2 Dose | |||||
| ND | |||||
| IM | |||||
| 80 | Viral vector (Replicating) + APC | Covid-19/aAPC vaccine. The Covid-19/aAPC vaccine is prepared by applying lentivirus modification with immune modulatory genes and the viral minigenes to the artificial antigen presenting cells (aAPCs). | Shenzhen Geno-Immune Medical Institute | Phase 1 | NCT04299724 |
| 3 Dose | |||||
| Day 0 + 14 + 28 | |||||
| SC | |||||
| 81 | Protein subunit | AdimrSC-2 f (recombinant RBD ± Aluminum) | Adimmune Corporation | Phase 1 | NCT04522089 |
| ND | |||||
| ND | |||||
| ND | |||||
| 82 | DNA based vaccine | Covigenix VAX-001 – DNA vaccines + proteo-lipid vehicle (PLV) formulation | Entos Pharmaceuticals Inc. | Phase 1 | NCT04591184 |
| 2 Dose | |||||
| Day 0 + 14 | |||||
| IM | |||||
| 83 | DNA based vaccine | CORVax – Spike (S) Protein Plasmid DNA Vaccine | Providence Health & Services | Phase 1 | NCT04627675 |
| 2 Dose | |||||
| Day 0 + 14 | |||||
| ID | |||||
| 84 | RNA based vaccine | ChulaCov19 mRNA vaccine | Chulalongkorn University | Phase 1 | NCT04566276 |
| 2 Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 85 | DNA based vaccine | bacTRL-Spike oral DNA vaccine | Symvivo Corporation | Phase 1 | NCT04334980 |
| 1 Dose | |||||
| Day 0 | |||||
| Oral | |||||
| 86 | Viral vector (Non-replicating) | COH04S1 (MVA-SARS-2-S) – Modified vaccinia ankara (sMVA) platform + synthetic SARS-CoV-2 | City of Hope Medical Center + National Cancer Institute | Phase 1 | NCT04639466 |
| 1–2 Dose | |||||
| Day 0, + 28 | |||||
| IM | |||||
| 87 | Live attenuated virus | COVI-VAC | Codagenix/Serum Institute of India | Phase 1 | NCT04619628 |
| 1–2 Dose | |||||
| Day 0 or Day 0 + 28 | |||||
| IN | |||||
| 88 | Protein subunit | MF59 adjuvanted SARS-CoV-2 Sclamp vaccine | The University of Queensland | Phase 1 | NCT04495933 |
| 2 Dose | |||||
| Day 0 + 28, | |||||
| IM | |||||
| 89 | DNA based vaccine | COVIGEN | University of Sydney, Bionet Co., Ltd Technovalia | Phase 1 | NCT04742842 |
| 2 Dose | |||||
| Day 0 + 28, | |||||
| ID or IM | |||||
| 90 | Viral vector (Non-replicating) | BBV154, Adenoviral vector COVID-19 vaccine | Bharat Biotech International Limited | Phase 1 | NCT04751682 |
| 1 Dose | |||||
| Day 0, | |||||
| IN | |||||
| 91 | RNA based vaccine | PTX-COVID19-B, mRNA vaccine | Providence Therapeutics | Phase 1, | NCT04765436 |
| 2 Dose | |||||
| Day 0 + 28, | |||||
| IM | |||||
| 92 | RNA based vaccine | CoV2 SAM (LNP) vaccine. A self-amplifying mRNA (SAM) lipid nanoparticle (LNP) platform + Spike antigen | GlaxoSmithKline | Phase 1, | NCT04758962 |
| 2 Dose | |||||
| Day 0 + 28, | |||||
| IM | |||||
| 93 | Protein subunit | SK SARS-CoV-2 recombinant surface antigen protein subunit (NBP2001) + adjuvanted with alum. | SK Bioscience Co., Ltd. | Phase 1, | NCT04760743 |
| 2 Dose | |||||
| Day 0 + 28, | |||||
| IM | |||||
| 94 | Viral vector (Non-replicating) | Chimpanzee Adenovirus serotype 68 (ChAd) and self-amplifying mRNA (SAM) vectors expressing spike alone, or spike plus Gritstone Oncology additional SARS-CoV-2 T cell epitopes. |
Gritstone Oncology | Phase 1, | NCT04776317 |
| 3, Dose | |||||
| Day 0 + 14 + 28 or Day 0 + 28 + 56 or Day 0 + 112, | |||||
| IM | |||||
| 95 | Protein subunit | SpFN (spike ferritin nanoparticle) uses spike proteins with a liposomal formulation QS21 (ALFQ) adjuvant. | Walter Reed Army Institute of Research (WRAIR) | Phase 1, | NCT04784767 |
| 2–3 Dose | |||||
| Day 0 + 28 + 180, | |||||
| IM | |||||
| 96 | Inactivated virus | Inactivated SARS-CoV-2 vaccine FAKHRAVAC (MIVAC) | Organization of Defensive Innovation and Research | Phase 1, | IRCT20210206050259N1 |
| 2 Dose | |||||
| Day 0 + 14 ± 21, | |||||
| IM | |||||
| 97 | Live attenuated virus | MV-014-212, a live attenuated vaccine that expresses the spike (S) protein of SARS-CoV-2 | Meissa Vaccines, Inc. | Phase 1, | NCT04798001 |
| 3 Dose | |||||
| Day 0 ± 35, | |||||
| IN | |||||
| 98 | Protein subunit | ReCOV: Recombinant two-component spike and RBD protein COVID-19 vaccine (CHO cell). | Jiangsu Rec-Biotechnology | Phase 1, | NCT04818801 |
| 2 Dose | |||||
| Day 0 + 21, | |||||
| IM | |||||
| 99 | Inactivated Virus | Inactivated COVID-19 vaccine | Kocak Farma | Phase 1, | NCT04838080 |
| 2 Dose | |||||
| Day 0 + 21, | |||||
| IM | |||||
| 100 | Viral vector (Non-replicating) | SC-Ad6-1, Adneviral vector vaccine | Tetherex Pharmaceuticals Corporation | Phase 1, | NCT04839042 |
| 1–2 Dose | |||||
| Day 0 ±21, | |||||
| IM | |||||
| 101 | Virus like particle | ABNCoV2 capsid virus-like particle (cVLP) ± adjuvant MF59 | Radboud University | Phase 1, | NCT04839146 |
| 2 Dose | |||||
| Day 0, Day 28, | |||||
| IM | |||||
| 102 | RNA based vaccine | HDT-301: Self-replicating mRNA vaccine formulated as a lipid nanoparticle. | SENAI CIMATEC | Phase 1, | NCT04844268 |
| 2 Dose | |||||
| Day 0, Day 28, | |||||
| IM | |||||
| 103 | Inactivated Virus | Adjuvanted inactivated vaccine against SARS-CoV-2 | The Scientific and Technological Research Council of Turkey (TÜBITAK) | Phase 1, | NCT04866069 |
| 2 Dose | |||||
| Day 0, Day 21, | |||||
| SC | |||||
| 104 | RNA based vaccine | mRNA-1283 | ModernaTX, Inc. | Phase 1, | NCT04813796 |
| 2 Dose | |||||
| Day 0, Day 28, | |||||
| IM | |||||
| 105 | Inactivated Virus | Live recombinant Newcastle Disease Virus (rNDV) vector vaccine | Laboratorio Avi-Mex | Phase 1, | NCT04871737 |
| 2 Dose | |||||
| Day 0, Day 21, | |||||
| IM or IN | |||||
| 106 | mRNA vaccine | mRNA COVID-19 vaccine | Shanghai East Hospital and Stemirna Therapeutics | Phase 1, | ChiCTR2100045984 |
| 2 Dose | |||||
| TBD | |||||
| IM | |||||
| 107 | Protein subunit | CoVepiT vaccine: SARS-CoV-2 multi-target peptide vaccine (targeting Spike, M, N, and several non-structural proteins) | OSE Immunotherapeutics | Phase 1 | NCT04885361 |
| 1-2 | |||||
| Day 0 ± 21 | |||||
| SC | |||||
| 108 | Protein subunit | CoV2-OGEN1, protein-based vaccine | USSF/Vaxform | Phase 1 | NCT04893512 |
| 1–2 Dose | |||||
| Day 0 ± 14 | |||||
| Oral | |||||
| 109 | RNA based vaccine | LNP-nCOV saRNA-02 vaccine; Self-amplifying RNA (saRNA) encapsulated in lipid nanoparticles (LNP) | MRC/UVRI and LSHTM Uganda Research Unit | Phase 1 | NCT04934111 |
| 2 Dose | |||||
| Day 0, + 28, | |||||
| IM | |||||
| 110 | Protein subunit | RBD protein recombinant SARS-CoV-2 vaccine | Bagheiat-allah University of Medical Sciences | Phase 1 | IRCT20210620051639N1 |
| 3 Dose | |||||
| Day 0 + 21 + 35 | |||||
| IM | |||||
| 111 | Protein subunit | Baiya SARS-CoV-2 VAX1, a plant-based subunit vaccine (RBD-Fc + adjuvant) |
Baiya Phytopharm Co., Ltd. | Phase 1 | NCT04953078 |
| 2 Dose | |||||
| Day 0 + 21 | |||||
| IM | |||||
| 112 | Viral vector (Non-replicating) | PIV5 vector that encodes the SARS-CoV-2 spike protein | CyanVac LLC | Phase 1 | NCT04954287 |
| 1 Dose | |||||
| Day 0 | |||||
| IN | |||||
| 113 | Protein subunit | 202-CoV; SARS-CoV-2 spike trimer protein + adjuvant, CpG7909. | Shanghai Zerun Biotechnology + Walvax Biotechnology + CEPI | Phase 1 | NCT04982068 |
| 2 Dose | |||||
| Day 0, + 28, | |||||
| IM | |||||
2. SARS-CoV-2 variants
As SARS-COV-2 is widely circulating in the population and getting more opportunities to spread, there is a high probability of an increase in its mutation. Depending on the location of the mutation in the virus genetic material, the properties of the virus such as transmission or severity are affected [18]. Resurfacing of COVID-19 with new SARS-CoV-2 variants has created panic worldwide [19,20]. These variants can influence the transmission, severity of COVID-19, its diagnostics, therapeutics, and natural and vaccine-induced immunity [21]. The documented variants of SARS-CoV-2 are more than ten in number such as Alpha, Beta, Gamma, Delta, Delta Plus, Epsilon, Eta, Theta, Iota, Kappa, and Lambda and the list will increase by the emergence of new variants (Table 3) [8]. The variants bind more efficiently to ACE2 receptors and have more transmissible ability compared to the original SARS-CoV-2. Unfortunately, variants originated from South Africa and Brazil can easily enter the human lungs [22,23]. Alpha variant (B.1.1.7) was first identified in the UK and the major mutations are on the spike protein of SARS-CoV-2 [24–26].
Table 3.
SARS-CoV-2: Variants of concern and interest (classified according to WHO)
| Sl. No. | WHO Label | Pango lineages | Spike Protein Substitutions: | Earliest documented samples |
|---|---|---|---|---|
| Variants of concern | ||||
| 1 | Alpha | B.1.1.7 | 69del, 70del, 144del, E484K, S494P, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H K1191N | United Kingdom, Sep-2020 |
| 2 | Beta | B.1.351, B.1.351.2, B.1.351.3 | D80A, D215G, 241del, 242del, 243del, K417N, E484K, N501Y, D614G, A701V | South Africa, May 2020, |
| 3 | Gamma | P.1 P.1.1 P.1.2 P.1.4 P.1.6 P.1.7 | L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I | Brazil, Nov 2020 |
| 4 | Delta | B.1.617.2 AY.1 AY.2 AY.3 AY.3.1 |
T19R, V70F, T95I, G142D, E156-, F157-, R158G, A222V, W258L, K417N, L452R, T478K, D614G, P681R, D950N | India, Oct2020 |
| Variants of interest | ||||
| 5 | Eta | B.1.525 | A67V, 69del, 70del, 144del, E484K, D614G, Q677H, F888 | Multiple countries, Dec-2020 |
| 6 | Iota | B.1.526 | L5F, D80G, T95I, Y144-, F157S, D253G, L452R, S477N, E484K, D614G, A701V, T859N, D950H, Q957R | United States of America Nov 2020 |
| 7 | Kappa | B.1.617.1 | T95I, G142D, E154K, L452R, E484Q, D614G, P681R, Q1071H | India, Oct-2020 |
| 8 | Lambda | C.37 | G75V, T76I, RSYLTPGD246-253 N, L452Q, F490S, T859N | Peru, Dec 2020 |
Several mutations occur in spike proteins including D614G, mutation N501Y in RBD, deletion [69,70, 144] in NTD, mutation P681H near the furin cleavage site. Beta Variant (B.1.351, N501Y.V2) was identified in South Africa and major mutations are on the receptor-binding domain. It has also several mutations in spike proteins including D614G [27,28]. Further, three mutations K417N, E484K, N501Y are found in the RBD region. In addition, deletion (242–244) and mutation R246I are found in NTD, along with the mutation in A701V near the furin cleavage site [19].
Meanwhile, the third Gamma variant (P.1) which is a descendant of variant B.1.1.28 was first identified in Brazil and the major mutations are on the receptor-binding domain including K417T, E484K, N501Y, D614G [29,30]. The major mutant E484K helps the virus to hide and escape easily from monoclonal antibodies and causes hindrance in antibody and plasma-based therapeutics. Notably, D614G is related to an increase in infection due to COVID-19 [31]. Two lineages are identified in India which are the Kappa variant (B.1.617.1) and Delta variant (B.1.617.2). Unfortunately, the Delta variant is one of the reasons for the spreading of the second wave of COVID-19 in India. Delta virus contains mutations K417N, L452R, T478K, D614G (Table 3).
The Delta (B.1.617.2) variant was first identified in India in December 2020 and is related to high transmissibility, virulence, hospitalizations, and deaths [32,33]. Further, the Delta variant affected younger age groups and the risk of hospital admission with the Delta variant is about twice as compared to the Alpha variant [34]. Interestingly, the transmissibility of the Delta variant is 60% more than the Alpha variant and its basic reproduction rate is between 5 and 8 as reported by US Centers for Disease Control and Prevention (CDC) [33]. The Delta Plus variant (AY.1 or B.1.617.2.1) was first detected in India and has spread to the United States through England and Japan. It has ≥20% high-prevalence mutations than in the Delta variant with the exclusive presence of mutations K417N, V70F, and W258L in the Spike region along with mutation of about 58% prevalence in ORF1a (A1146T) [8].
In Japan, the most prevalent variants were 501Y.V1 which was 53% and 452 R.V1 which was 24% according to the collected data from January 2020 to February 2021. A high correlation was found between fatalities and population density (rs = 0.81) and more than 90% fatality was found in patients with an age of more than 60 years [35].
A compartmental mathematical model was constructed to study the impact of the variant VOC-202012/01 of lineage B.1.1.7 (Alpha variant) on the population and it was found that the high transmissibility ability of the variant can infect more people. Further, the studies show health care institutions should involve more non-pharmaceutical interventions and vaccine inoculation to prevent disastrous outcomes in the population due to the high transmissibility ability of the variant [36]. Interestingly, a high reproduction number of 43–90%, compared to the predecessor lineage was estimated by the statistical and dynamic modeling approaches for variant VOC-202012/01 of lineage B.1.1.7 (Alpha variant) in England [37]. The Alpha variant (B.1.1.7) and Gamma (P.1) variants depicted about 66.0% and 5.0% of SARS-CoV-2 infections in the U.S. at the end of April 2021 [21]. The infection with VOC-202012/1 in a population is associated with high mortality [38].
The infection by SARS-CoV-2 also depends upon genetic variation in ACE2 [39]. Another study has shown that the Asian population has a higher expression of ACE2 compared to European, North American, and African populations. The population with lower ACE2 expression is responsible for the selection of D614G mutation and is associated with an increased transmission efficiency of D614G mutation. The variations in human population genetics are responsible for viral evolution [40].
3. COVID-19 Vaccines
Already, there are about 18 vaccines against COVID-19 that are in emergency use listing (EUL)/ prequalification evaluation process at WHO (Table 1, Figures 1 – 2). According to WHO, more than 112 candidate vaccines are in clinical trial evaluation while more than 183 candidate vaccines are in pre-clinical evaluation [41]. Notably, eight vaccines are in phase 4 clinical trial including ChAdOx1-S from University of Oxford/AstraZeneca, UK; Inactivated from Sinovac, China; LNP-encapsulated mRNA from Moderna/NIAID, USA; and LNP-mRNAs from BioNTech/Fosun Pharma/Pfizer, Germany (Table 2). In addition, 20 vaccines are in phase 3 clinical trials and 10 vaccines are in phase 2/3 clinical trials. Further, 9 vaccines are in phase 2 clinical trials, 28 vaccines are in phase 1/2 clinical trial and 38 vaccines are in clinical trial phase 1 (Table 2) (Figure 3).
Figure 1.
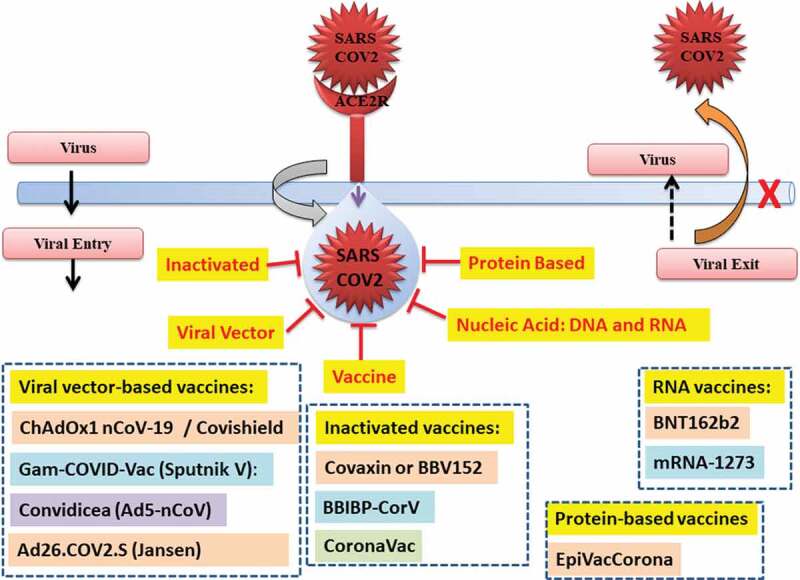
Vaccine against SARS-CoV-2.
Figure 2.
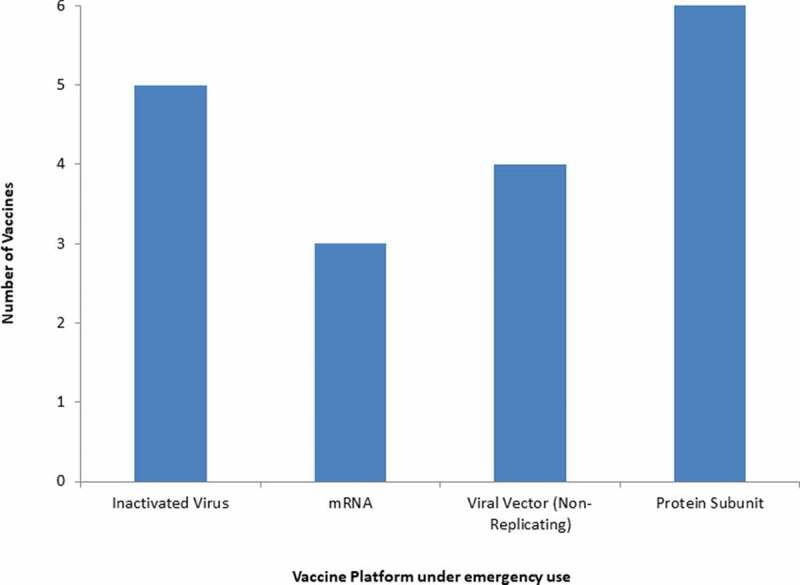
Vaccines for COVID-19 are in the emergency use listing (EUL)/ prequalification evaluation process at WHO.
Figure 3.
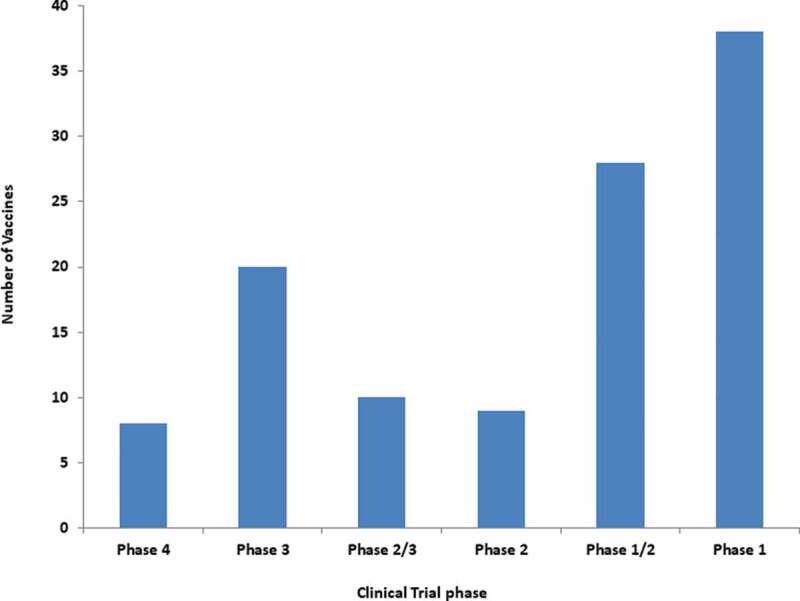
Vaccines for COVID-19 under the different phases of the clinical trial.
Most of the countries/ regulatory authorities have treated the vaccine development with topmost priority and also fast-tracked the vaccine development process and maybe this is the reason the world got some vaccines within a year. Further, most of the vaccines were approved for emergency use without completing all the phases of clinical trials except a few. Interestingly, a total of several billion COVID-19 vaccine doses have been administered worldwide mostly through intramuscular [6]. Notably, 86 candidates of vaccines will be delivered intramuscular (IM), 8 candidates of vaccines will be administered intranasal, 4 candidates of vaccines will be delivered intradermal (ID), 5 candidates of vaccines will be delivered subcutaneous and other 3 candidates of vaccines will be delivered orally (Figure 4) [41]. The success in nasal or oral vaccines will have great benefits as it will save syringes and plastic wastes and therefore will be environment friendly. The nasal or oral vaccines will be liked by young children and neonatal in the future.
Figure 4.
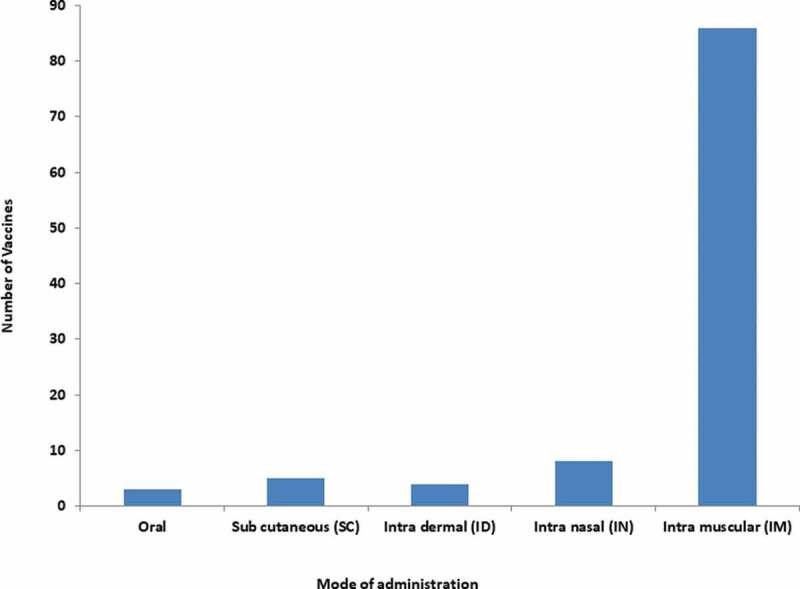
Mode of administration of COVID-19 vaccines.
4. Diverse vaccine platforms
The diverse vaccine platforms have been used for the development of COVID-19 vaccines. It can be divided into major platforms such as inactivated, viral vector, protein-based, nucleic acids (RNA and DNA) based platform, yeast-based vaccines, and conjugated vaccines with antimicrobial peptides. A total of about 18 vaccines for COVID-19 are in emergency use listing (EUL)/prequalification evaluation process at WHO with different platforms such as mRNA, inactivated virus vaccine, non-replicating viral vector and protein subunit-based vaccine (Table 1) (Figure 2).
According to WHO, more than 112 candidate vaccines are in a clinical trial including 16 Inactivated Virus, 17 Viral Vector Non-Replicating (VVnr), 38 Protein Subunit, 18 RNA, 11 DNA, 2 Viral Vector Replicating (VVr), 5 Virus-like particles, 2 VVr Antigen Presenting Cell, 2 Live Attenuated Virus and 1 VVnr Antigen Presenting Cell (Table 2, Figure 5) [41]. Notably, 183 potential candidate vaccines against SARS-CoV-2 are under pre-clinical evaluation using different vaccine platforms including 70 Protein Subunit, 21 Non-Replicating Viral Vector, 9 Inactivated, 24 RNA based vaccines, 16 DNA based vaccines, 19 Replicating Viral vectors, 2 Live attenuated Virus, 18 virus-like particles and 1 cellular-based vaccine (Figure 6).
Figure 5.
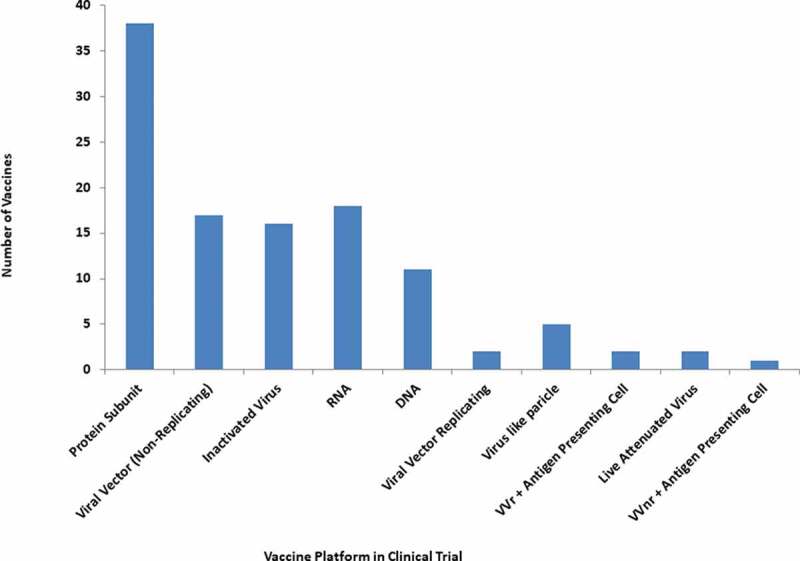
Diverse platform vaccines under clinical trial for COVID-19.
Figure 6.
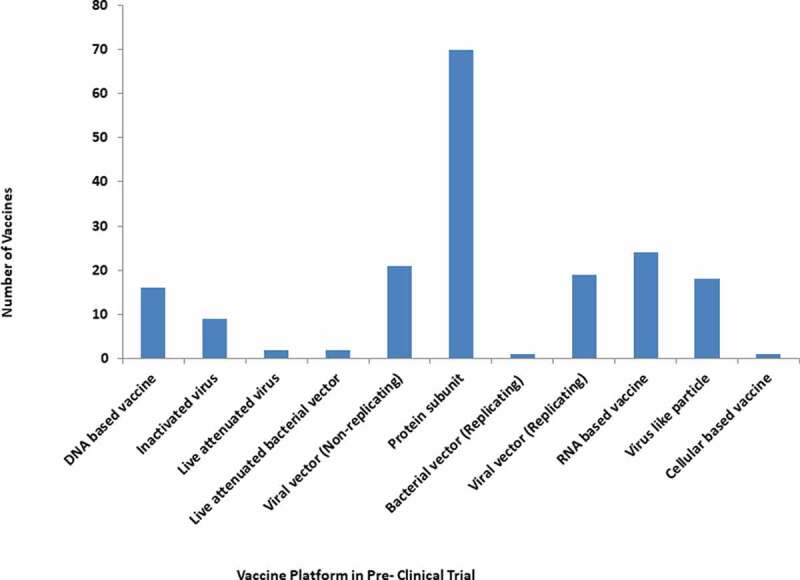
Diverse platform vaccines in Pre-clinical trial for COVID-19.
4.1. Viral vector-based vaccines: The harmless virus that is unable to cause disease was used as a platform to produce proteins coronavirus which successfully generated an immune response in the body. The viral-based vaccines belong to two categories such as non-replicating vaccines and replicating vaccines. The COVID-19 Vaccine-AstraZeneca (AZD1222) popularly known as Covishield [ChAdOx1-S-(AZD1222)] from Oxford/AstraZeneca [42–46], Sputnik V of Gamaleya Research Institute [15,47], Convidicea (Ad5-nCoV) from CanSino Biologics, recombinant vaccine (adenovirus type 5 vector) from China belongs to non-replicating viral vector-based vaccines [48,49].
4.1.1. ChAdOx1 nCoV-19/Covishield: Folegattiet et al from the University of Oxford/AstraZeneca, have selected 1077 healthy participants in the age group of 18 to 55 years and administered 5 × 1010 viral particles of chimpanzee adenovirus-vectored vaccine (ChAdOx1 nCoV-19) [42]. This vaccine expresses SARS-CoV-2 spike proteins in 543 participants. Further, the meningococcal conjugate vaccine (MenACWY) was used as a control in 534 participants. Notably, there were no adverse side effects, while common symptoms have been minimized by paracetamol. Significantly, spike-specific T-cell responses peaked on day 14 and Anti-spike IgG responses rose by day 28 and further the booster dose was given. Neutralizing antibody response was detected in more than 90% of participants after the first dose while it was detected in 100% of participants after the second dose [42]. The vaccine did not have any adverse side effects. Furthermore, this study may be extended to patients with chronic diseases, old age, neonatal and pediatrics thereby giving protection from SARS-CoV-2 infection and recovered SARS-CoV-2 individuals from re-infection. Notably, the efficacy of this vaccine should be tested against different recent circulating strains of SARS-CoV-2. Moreover, further studies are required to unearth the long duration of immunity caused by this vaccine and the requirement for booster doses in the future. The need to extend the suitability of this vaccine to different ethnic groups and persons with rare diseases such as blood clotting complications might be helpful.
Notably, Voysey et al have reported that ChAdOx1 nCoV-19 is efficacious for symptomatic COVID-19 and safe [43]. Further, Ramasamy et al have reported ChAdOx1 nCoV-19 (AZD1222) a chimpanzee adenovirus-vectored vaccine is well tolerated in older people above 70 years and has good immunogenicity after the booster dose [44]. Ewer et al have reported that the ChAdOx1 nCoV-19 vaccine was inducing a favorable immune profile in age groups of 18–55 years [45]. The booster dose of ChAdOx1 nCoV-19 vaccines induced stronger antibody responses and is safe and well-tolerated. Further, this vaccine substantially enhances anti-spike neutralizing antibody titers, Fc-mediated functional antibody responses, antibody-dependent neutrophil/monocyte phagocytosis, natural killer cell activation and complement activation [46].
4.1.2. Gam-COVID-Vac (Sputnik V): Sputnik V of Gamaleya Research Institute is a recombinant adenovirus (rAd26 and rAd5). Logunov et al [15] have reported a heterologous vaccine against SARS-CoV-2 using recombinant adenovirus platform containing rAd26 vector and rAd5 vector that has successfully induced humoral and cellular response with safety profile [15]. They have established that both frozen and lyophilized formulations of vaccines are safe and immunogenic during non-randomized clinical trial phases 1 and 2 against COVID-19. The studies involved 76 participants between 18 to 60 age groups including both genders in both phase 1 and 2 studies and measured successfully their antigen-specific humoral immunity, antigen-specific cellular immunity, and changes in neutralizing antibodies. In Phase 1, they have administered one dose either rAd26-S or rAd5-S in participants on day 0, while in Phase 2, rAd26-S was administered on day 0 and rAd5-S was administered on day 21 through intramuscularly as prime-boost vaccination [15]. Phase 3 trial of sputnik V reported immunogenicity, high efficacy as 91.6% against SARS-CoV-2 and well-tolerated in large clinical trial studies in 18 years and above [47].
4.1.3. Convidicea (Ad5-nCoV): Zhu et al from CanSino Biological Inc with Beijing Institute of Biotechnology have studied 108 participants during Phase 1 clinical trial and reported that the administration of AD5 vectored COVID-19 vaccine in healthy participants have been tolerable and immunogenic after 28 days of vaccination while the rapid specific T-cell response was observed after 14 days of vaccination [48]. Thereafter, Zhu et al. have studied 508 participants [1 × 1011 viral particles n = 253; 5 × 1010 viral particles n = 129; placebo n = 126) in Phase 2 clinical trial [49]. They reported AD5 vectored COVID-19 vaccine was safe and single immunization had successfully induced immune response in the majority of participants at the dose of 5 × 1010 viral particles. However, 1 × 1011 viral particle dose had shown adverse reactions in 24 (9%) participants while 5 × 1010 viral particle dose had shown adverse effect only in 1 (1%) participants. Notably, 1 × 1011 viral particle dose showed solicited adverse reactions in 183 (72%) of 253 participants while 5 × 1010 viral particle dose had shown solicited adverse reactions in 96 (74%) of 129 participants.
4.2. Inactivated or weakened virus vaccines: Notably, 16 vaccines in clinical trials of COVID-19 belonged to inactivated or weakened virus vaccines. The desired strain of the virus can be inactivated by using heat or chemicals including formalin/formaldehyde/Beta propiolactone (BPL). Hence the virus loses the ability to replicate and cannot cause the related disease. Thus, after injecting the inactivated virus into the human body, it generates an immune response. An Inactivated-based vaccine is safe as there is no live virus in the vaccine. Several vaccines have been made earlier using an inactivated platform such as Influenza, Hepatitis A, Polio, and Rabies.
Notably, there are three well-known inactivated virus-based vaccines for COVID-19. One such inactivated virus-based vaccine is Covaxin that was produced by Bharat Biotech International Limited and ICMR-India [50,51]. The other is BBIBP-CorV produced by Sinopharm with China National Biotec Group Co. and Beijing Institute of Biological Products [52,53]. Another is CoronaVac produced by Sinovac Research and Development Co Ltd respectively [54].
4.2.1 Covaxin: Covaxin or BBV152 is made in India as the SARS-CoV-2 vaccine. It has inactivated whole-virion and toll-like receptor 7/8 agonist molecule adsorbed to alum (Algel-IMDG). Ella et al [50,51] have reported that BBV152 is safe, well tolerable with enhanced immune responses, inducing both cell-mediated and humoral neutralizing responses based on Phase 1 clinical trial. Notably, both the doses of 3-μg and 6-μg Algel-IMDG vaccines were successfully able to induce T-cell responses and it was biased to T-helper-1 cells [50]. In the phase 2 trial, BBV152 has shown better reactogenicity and immune response in the expected line of phase 1 trial and suggested 6 μg with Algel-IMDG formulation for further clinical studies [51]. Further, the observation of humoral and cell-mediated response and neutralizing of antibody response was reported in different age groups and gender without any serious adverse events. Two doses of vaccine were needed to be administered with the first dose on day 0 and the second dose on day 28. Notably, the vaccine is immunogenic that persists for three months and preferable the storage temperature for the vaccine is 2–8°C.
4.2.2. BBIBP-CorV: It is an inactivated vaccine with the potential to prevent COVID-19. Wang et al have reported BBIBP-CorV was successfully able to induce significant neutralizing antibodies titers in several animals including mice, guinea pigs, rabbits and monkeys. The 2 mg/dose of BBIBP-CorV has shown better results in rhesus macaques with two-dose immunizations [52]. Further, Xia et al have reported that BBIBP-CorV is safe in humans and suggested that the two doses of immunization are better as compared to a single dose against COVID-19. Notably, 4 μg dose of BBIBP-CorV with double dose has shown that immunization on day 0 and day 21 or day 0 and day 28 both gave better results with significant neutralizing antibody titer as compared to a single dose of the different amount [53].
4.2.3. CoronaVac: Zhang et al have reported that inactivated vaccine with two doses of CoronaVac is safe and immunogenic. They recommended 3ug doses for phase 3 clinical trial based on phase 1/2 clinical trial study in 18–59 age groups [54]. Further Wu et al recommended CoronaVac for older people above the age of 60 years. They further found that in the phase 3 clinical trial, the 3 µg dose of the vaccine was successfully able to induce neutralizing antibodies [55].
4.3. Nucleic acid-RNA and DNA-based platform vaccines: The genetically engineered nucleic acids such as RNA and DNA were used to generate immune responses safely. Comirnaty (BNT162b2) of Pfizer-BioNTech [56–61] and COVID 19 Vaccine mRNA-1273 of Moderna/NIAID belongs to mRNA-based vaccines [62–68].
4.3.1. BNT162b2: A lipid nanoparticle-formulated nucleoside-modified mRNA vaccine, BNT162b1 has significant potential to protect from SARS-CoV-2 from Pfizer-BioNTech. It encodes the receptor-binding domain (RBD) of the spike protein1 of SARS-CoV-2 [61]. BNT162b2 can be used as a COVID-19 vaccine for 16 years and older. It is administered in two doses and provides remarkably 95% protection in COVID-19. Clinical trials have been done in a large population of 43,448 individuals that included 21,720 people receiving BNT162b2 injection while 21,728 people receiving placebo [56]. Monin et al have reported that BNT162b2 is safe and beneficial also in cancer patients especially when the second dose was given on 21 days after the first dose increased immunogenicity significantly, while single-dose alone yielded poor efficacy [59]. Dagan et al have reported mass vaccination by BNT162b2 that is effective in COVID-19 and protects from most of the COVID-19 outcomes including severe conditions [58]. Notably, BNT162b2 is also very effective after 14 days of the second dose against the Covid-19 and its variants Alpha (B.1.1.7) and Beta (B.1.351). It has been reported it is effective in the case of 89.5% infection from Alpha variant (B.1.1.7) and 75% infection from Beta variant (B.1.351), however, it is still effective in cases of severe and critical conditions [60].
4.3.2. mRNA-1273: The mRNA-1273 vaccine has successfully induced anti-SARS-CoV-2 immune response in all participants and notably no trial limiting safety issues had been observed during phase 1 human clinical trial in 45 healthy adults between the ages of 18 to 55 years without any adverse side effects [62]. This vaccine is successfully able to stabilize spike protein S-2P (prefusion spike trimer). Notably, structural rearrangement of fusion (S2) subunit can be prevented by substitution of two prolines on top of heptad repeat 1 that results in stabilization of coronavirus spike protein [62]. These studies have shown that the majority of the participants are from one ethnic group and place, therefore it would be helpful to expand these studies to the wider range of human populations including different ethnic groups and geographical areas. It is necessary to extend the clinical trials to different age groups, especially those below the age of 18 and above 55 years, and to the patients having preexisting chronic diseases and ethnicity to make this vaccine universal.
Anderson et al reported that 100-μg dose of mRNA-1273 vaccine on day 0 and day 28 had performed better as compared to a 25-μg dose based on neutralizing-antibody titers. Notably, the mRNA-1273 vaccine is safe and adverse events were either mainly mild or moderate in older adult people [66]. Baden et al have reported the phase 3 clinical trial of mRNA-1273 vaccine suggesting 94.1% efficacy in preventing Covid-19 related complications based on 30,420 volunteers. This lipid nanoparticle–encapsulated mRNA vaccine is safe and no safety concerns were observed, while it has only transient local and systemic reactions. Further, it suggests that mRNA-1273 vaccines are safe for a person with chronic diseases [65].
Widge et al have reported that administration of two doses of 100-μg dose of mRNA-1273 vaccine induces a high level of binding and neutralizing antibodies and remains elevated for 90 days after a booster dose of vaccination [64]. Doria‑Rose et al have reported that 100-μg dose of mRNA-1273 vaccine induces antibody activity and remarkably it remains high at 180 days after the second dose of vaccination in all the age groups [68]. Corbett et al have reported mRNA-1273 to induce significant neutralizing activity, quick protection in upper and lower airways and does not cause pathological changes [63]. Notably, mRNA-1273 induces potent neutralizing antibody responses to D614G mutant SARS-CoV-2 and wild type D614 along with CD8 + T cell responses and protects against SARS-CoV-2 infection in the upper respiratory tract (nose) and lower respiratory tract (lungs) [67].
4.4. Protein-based vaccines: The harmless fragments of proteins or their shells that mimic the COVID-19 disease virus have been used to generate an immune response. Interestingly, EpiVacCorona from Federal Budgetary Research Institution State Research Center of Virology and Biotechnology, Russia is a protein subunit vaccine [69].
4.5. Yeast-based vaccines: The availability of yeast expression technology provides significant benefits for the manufacture of inexpensive yeast-based SARS-CoV-2 vaccines [70]. The yeast-based vaccine such as the yeast Pichia pastoris expressed SARS-CoV-2 receptor-binding domain (RBD) combined with 3 M-052-alum adjuvants provided immunogenicity and protective efficacy in rhesus macaques suggesting promising SARS-CoV-2 vaccine candidate eligible for human trials as it is cost-effective, thermostable and scalable [71]. Another finding of yeast-based vaccine which yielded significant immune response in mice was by using oral administration of yeast S. cerevisiae-based SARS-CoV-2 vaccine EBY100/pYD1-RBD without any adjuvants [72]. Further, Zang J et al. found that the immunized mice with yeast (Pichia pastoris) derived RBD (either monomeric or dimeric) based recombinant SARS-CoV-2 vaccines effectively protects and neutralize SARS-COV-2 variants Alpha (B.1.1.7) and Beta (B.1.351) [73].
4.6. Conjugated vaccines with antimicrobial peptides: The mice immunized with the conjugates resulting from the synthesized peptide epitopes from the spike protein of SARS-CoV-2 attached covalently to cross-reactive material (CRM197) neutralized SARS-CoV-2 pseudovirus suggesting these conjugates as a potential COVID-19 vaccine candidate [74]. A Study by Outlaw et al., found that SARS-CoV-2 HRC-derived cholesterol conjugate inhibits Coronavirus Entry in vitro and ex vivo, therefore acting like a potential candidate for the COVID-19 vaccine [75].
5. Doses of vaccines
The majority of approved vaccines are working in two doses and 64 vaccines are under clinical trial for two doses (Figure 7). FDA has approved the Janssen COVID-19 vaccine that is working in a single dose against SARS-CoV-2. Earlier approved Pfizer-BioNTech COVID-19 vaccine and Moderna COVID-19 vaccine was working in two doses. Further, the AstraZeneca vaccine produced by the Serum Institute of India and Covaxin from Bharat Biotech is working in two doses. Furthermore, Sputnik V with two doses has been approved for emergency use in India (Table 2).
Figure 7.
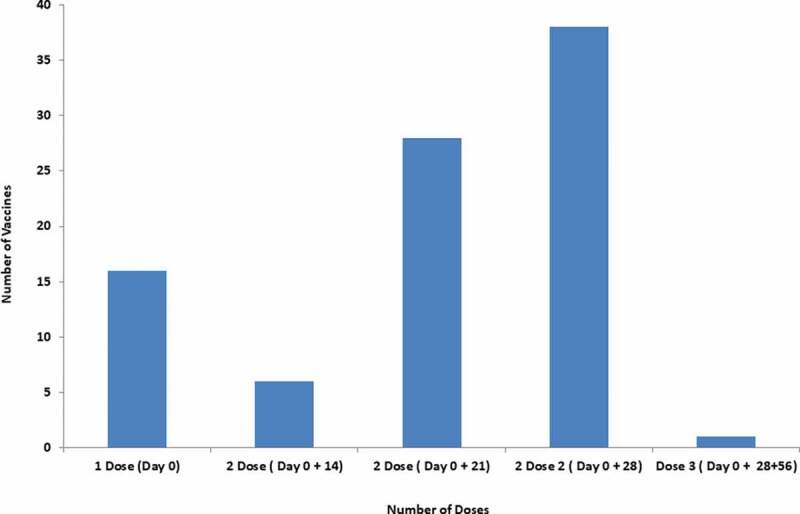
Doses of COVID-19 vaccines for immunization against SARS-CoV-2.
The benefits of the single-dose vaccine are it does not require follow-up and hence saves extra doses. The majority of vaccines have two doses. Notably, the time required for the second dose differs from vaccine to vaccine. Few vaccines need to be administered in 3 doses with the first dose on day 0, the second dose on 28 days, the third dose on 180 days for longer immunity. This is the longest time duration for three doses while some vaccine that requires shortest time duration for three doses involves the first dose on day 0, the second dose on 14 days and the third dose on 28 days (Figure 7).
Mix-and-Match or heterologous prime and boost of COVID-19 vaccine approaches are showing a bright future. Recently, the Spanish CombivacS trial enrolled with more than 600 people have presented promising trial results. The first dose administered was of Oxford–AstraZeneca vaccine with harmless chimpanzee adenovirus platform while the booster dose was of mRNA-based Pfizer-BioNTech. This combination produces a significantly higher level of antibodies compared to without booster dose and with the booster dose having the same adenovirus vaccine. Further, no severe adverse effects were observed [76]. Notably, Oxford–AstraZeneca vaccine induces significant T-cell responses and the Pfizer-BioNTech vaccine induce significantly high levels of antibodies [77]. Several studies are going on about the Mix-and-Match COVID-19 vaccine including mixing of same platform vaccine and also two different platform vaccines [78–80]. The Mix-and-Match approach of COVID-19 vaccines may provide significantly higher protection against SARS-CoV-2 and its variants.
6. Efficacy of vaccines against SARS-CoV-2 and variants
The reports about the effectiveness of different vaccines on the variants of SARS-CoV-2 are developing quickly. Different vaccines have different efficacies against SARS-CoV-2 such as Pfizer-BioNTech has 95% efficacy, Moderna has 94% efficacy, J&J has 72% efficacy, Astrazeneca has 62 to 90% efficacy and Sinovac has 50% efficacy. As SARS-CoV-2 has a high tendency of mutation, therefore it is difficult to predict, which vaccine will work for which variants. Notably, all these vaccines have some efficacy against Alpha variant (B.1.1.7, first detected in UK strain). Unfortunately, these vaccines have fewer efficacies against Beta Variant (B.1.351) (first detected in South Africa). Pfizer-BioNTech and Astrazeneca have the same efficacy against the Gamma variant (P.1, first detected in Brazil). Other vaccines have also reported different efficacies against SARS-CoV-2 such as sputnik V has 92% efficacy, Novavax has 96% efficacy, Sinopharm has 79 to 86% efficacy, Covaxin has 80% efficacy and CanSinoBIO has 66% efficacy [81–83].
The D614G substitution helps in virus replication in airway tissue and epithelial lung cells and causes higher infectivity and stability of the virus. Therefore, D614G mutation increases the transmission and viral load. Notably, D614G produces more infectious titer in the upper respiratory tract (Nasal, trachea) compare to the lower tract (lung) [81]. This indicates that further mutation in SARS-CoV-2 especially in spike protein will make it more infectious and lethal.
The ChAdOx1 nCoV-19 showed neutralization activity in the Alpha (B.1.1.7) variant, while it has shown better neutralization activity in non-B.1.1.7 (Alpha) variant. Efficacy for B.1.1.7 lineage was 70 · 4% compared to 81 · 5% for non-B.1.1.7 lineages [83]. The two doses of the ChAdOx1 nCoV-19 vaccine are unable to protect against the Beta (B.1.351) variant in mild to moderate COVID-19 [84]. Notably, the single dose of non-replicating adenovirus type 26 vaccine (Ad26.COV2.S from Janssen) has shown efficacy against Beta (B.1.351) variant such as 89% against severe COVID-19 and 57% against moderate to severe COVID-19 [84]. A single dose of Ad26.COV2.S has great efficacy against variants originated in South Africa and Brazil [85]. The NVX-CoV2373 vaccine has significant efficacy and cross-protection against the Beta (B.1.351) variant that originated in South Africa. Further, it has shown higher efficacy in HIV-negative groups [86]. Beta Variant (B.1.351) is more resistant compare to wild-type SARS-CoV-2. Mutation E484K in spike protein has made several variants more transmissible, tough and lethal including Alpha (B.1.1.7) and B.1.351 [19]. E484K mutation in B.1.1.7 makes the BNT162b2 vaccine less effective compared to wild type [87]. The mRNA-1273 vaccine may protect against COVID-19 from the Beta (B.1.351) variant as humoral immunity can be retained, despite the reduction in the efficacy of mRNA-1273 against the Beta (B.1.351) variant [88]. BBV152/COVAXIN significantly neutralizes the Alpha (B.1.1.7) variant that originated in the UK and sera of BBV152 is also protective against Delta (B.1.617) variant that originated in India [89,90].
SARS-CoV-2 dynamic multiple mutation ability has made the recent variants Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Kappa (B.1.617.1) and Delta (B.1.617.2) indicating SARS-CoV-2 antigenic drift has helped these to escape current prophylactics including some vaccines and therapeutics. Interestingly, all the individuals responded to the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Epsilon (B.1.429), Iota (B.1.526), and Delta (B.1.617.2) on the second dose of mRNA-1273 vaccine. However, Beta (B.1.351) had the lowest recognition by the antibody [91]. The Pfizer–BioNTech vaccine appeared to be more effective than Oxford–AstraZeneca vaccine in preventing Delta variant infection [34].
Two doses of Pfizer or the AstraZeneca vaccine have been found to produce a neutralizing response in 95% of individuals with three to five times lower effect against the Delta variant as compared to the Alpha variant while one dose of this vaccine had an even less inhibitory effect on the Delta variant. The Delta variant spreadability is related to getting away from antibodies that target receptor-binding domain (RBD) and non-RBD of the SARS-CoV-2 spike protein [92]. The two-dose vaccination scheme among the Guangzhou participants in the age group of 40–59 years with inactivated SARS-CoV-2 vaccine has the vaccine efficacy of 72.5% against the Delta variant infection and has been found to be more effective in females compared to the males [32].
In South Korea, the vaccination program using AstraZeneca-Oxford and Pfizer COVID-19 vaccines brought down the virus attack rate from 6.9% (without vaccination) to 3.9% over 150 days with a 50% reduction observed among 50–59 years individuals during the fourth wave of the COVID-19 using an age-structured, two-strain model of SARS-CoV-2 transmission and vaccination. Further, the vaccination has been thought to decrease the attack rate from 26.9% to 11.9% for the Delta variant [7].
7. Mitigation of the COVID-19 transmission- alternatives to vaccines
Mitigation of COVID-19 transmission can be achieved by personal, administrative and engineering controls. Personal controls include masking, physical distancing, and ensuring proper ventilation, while administrative and engineering controls include proper guidance, educational information, access to clean water and policies for implementing masking and physical distances in dense population and market areas [93]. Further, preventive measures such as appropriate mask-wearing, hand hygiene, physical distancing, promoting respiratory etiquette and frequent cleaning of high touch surfaces in crowded areas can help to mitigate the transmission of COVID-19. Notably, reducing the crowd at public places following occupational safety/health measures, avoiding unnecessary visits, possible remote work, proper ventilation and maximum open-air stay/circulation can help to minimize the exposure and spread of COVID-19. Routine screening, monitoring of the symptom, early diagnosis and quick proper treatments are important to mitigate the COVID-19 [93, 94].
8. Conclusions
Systematic clinical trial studies of COVID-19 vaccines have provided trust and confidence among clinicians and the general public. Several COVID-19 vaccines have been globally accepted due to their efficacy and safety that has helped to overcome vaccine hesitancy. Notably, COVID-19 vaccines protect vulnerable patients with co-morbidities. It can also be administered in pregnant ladies for the safety of the mother and the newborn.
Emerging of SARS-CoV-2 variants has challenged the ongoing vaccine drive against pandemic COVID-19. The diverse vaccine platforms have opened new avenues for quick and cost-effective production of COVID-19 vaccines to attain immunization globally. The vaccine effectiveness was more pronounced after the receipt of second dose. The vaccination will help in preventing hospitalizations and deaths. Further, if the vaccinated individuals get infected with COVID-19, they will recover without serious illness.
There is a potential requirement for additional boost vaccinations as the SARS-CoV-2 variants decrease the vaccine-induced protective immune response over time especially in individuals with medical co-morbidities. Mix-and-Match COVID-19 vaccines including mixing of same vaccine platform and also two different vaccines platform may provide higher protection against SARS-CoV-2 and its variants. The vaccines for the COVID-19 pandemic will help in preserving health care infrastructure, economy and may eventually end this pandemic.
9. Expert Opinion
The world is going through the havoc of an airborne coronavirus disease (COVID-19) pandemic caused by SARS-CoV-2 infection. The passage of the corona virus into the human body is through the upper respiratory tract (nose, mouth) to the lower respiratory tract (lung). This virus has infected more than billions of humans worldwide and has caused several thousand deaths. The multiple COVID-19 infection waves have created an increased demand for medical oxygen, hospitalization, ventilator and the usage of emergency drugs. The subsequent waves of COVID-19 have taught the value for continued usage of masks, avoidance of public gatherings and application of vaccines. This may be continued for months to years, to overcome the damage from the future wave of the COVID-19 pandemic as we know that SARS-CoV-2 has a high ability to mutate and form dangerous variants. Interestingly, the vaccination of the human population may help to overcome the infections from the emergence of new mutant strains of SARS-CoV-2.
To vaccinate billion of the population worldwide, there is an urgent requirement for resources and funds. Ongoing vaccination drive has administered several billion vaccine doses as reported by the World Health Organization (WHO). The worldwide vaccination, if completed simultaneously may lead to herd immunity and help in the eradication of COVID-19. If some groups of human populations are left unvaccinated, again there are chances for the emergence of new mutant strains of SARS-CoV-2 that may again start a new chain of infections.
Though intramuscular vaccines protect the lungs including protection from the severity of COVID-19 condition with the help of IgG1, however, they are unable to provide complete protection from SARS-CoV-2 infection and its transmission including breaking the chain of COVID-19 infection. Notably, intranasal or oral vaccines can protect the upper respiratory tract with the help of IgA1 thus preventing SARS-CoV-2 transmission. Therefore in this extraordinary pandemic situation, both intranasal and intramuscular COVID-19 vaccines provide better protection from infection, transmission and severe condition of COVID-19. Notably, the majority of vaccines that are under clinical trial are intramuscular while some as intranasal, and a few are oral. Besides, nasal and oral vaccines are environmentally friendly and may be a preferable candidate for vaccination to young children and neonatal in the future.
Interestingly, industry and academia have been the torchbearer in this pandemic and have provided several vaccines worldwide for adults and notably, the studies on vaccines for children are in process. In the ongoing pandemic situation, vaccines from diverse platforms may help to deal with the infection from SARS-CoV-2 and its variants. In the future, it may be possible to use diverse platforms to produce several vaccine doses to protect humans worldwide. The administration of two vaccine doses belonging to different platforms may play an important role in the handling of the ongoing SARS-CoV-2 and its variants. There is a potential need for additional boost vaccinations especially in individuals with medical co-morbidities. Additionally, more studies are required to be done for the mixing of vaccine doses belonging to the same and different platforms. These may provide better immunity against SARS-CoV-2 and its variants. Further, this may cope with the vaccine scarcity in low and middle-income nations and also in nations having large populations. However, patients’ side effects must be studied. The vaccination will help in preventing hospitalizations and deaths to the individuals.
Acknowledgments
BC has received the fellowship from Department of Science and Technology (DST) in area of Women Scientist Scheme A (WOS-A).
Funding Statement
This paper was not funded.
Article highlights
Resurfacing COVID-19 with new SARS-CoV-2 variants has created panic worldwide. These variants can influence several parameters including transmission, severity, diagnostics, therapeutics, and natural and vaccine-induced immunity.
COVID-19 vaccines came within a year as the vaccine development program was fast-tracked by several countries/ regulatory authorities. Several COVID-19 vaccines have been globally accepted due to their efficacy and safety thereby overcoming vaccine hesitancy.
The development of COVID-19 vaccines involved diverse vaccine platforms and is divided into major platforms such as inactivated, viral vector, protein-based, nucleic acids (RNA and DNA) based platform, yeast-based vaccines, and conjugated vaccines with antimicrobial peptides.
Studies about the effectiveness of different vaccines on the variants of SARS-CoV-2 are developing quickly including several studies about the Mix-and-Match COVID-19 vaccine as it may provide significantly higher protection against SARS-CoV-2 and its variants.
The vaccine effectiveness was more pronounced after the receipt of the second dose.
The vaccination will help in preventing hospitalizations and deaths.
Mitigation of COVID-19 transmission can be achieved by personal, administrative and engineering controls.
Author contributions
B Chatterjee and SS Thakur have conceived the idea and wrote the manuscript.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Thakur SS. Proteomics and Its Application in Pandemic Diseases. J Proteome Res. 2020;19(11):4215–4218. [DOI] [PubMed] [Google Scholar]
- 2.Krittanawong C, Kumar A, Hahn J.. Cardiovascular risk and complications associated with COVID-19. Am J Cardiovasc Dis. 2020;10(4):479–489. [PMC free article] [PubMed] [Google Scholar]
- 3.Apicella M, Campopiano MC, Mantuano MCOVID . 19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee. B, Thakur SS. ACE2 as potential therapeutic target for pandemic COVID-19. RSC Adv. 2020;10(65):39808–39813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Abajo FJ, Rodríguez-Martín S, Lerma V, et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395(10238):1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://covid19.who.int/
- 7.Shim E. Projecting the Impact of SARS-CoV-2 Variants and the Vaccination Program on the Fourth Wave of the COVID-19 Pandemic in South Korea. Int J Environ Res Public Health. 2021;18(14):7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannan SR, Spratt AN, Cohen AR, et al. Evolutionary analysis of the Delta and Delta Plus variants of the SARS-CoV-2 viruses. Journal of Autoimmunity. 2021;124:102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rappuoli R . Timeline: Vaccines. . Cell. 2020;183(2):552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.cdc.gov/vaccines/basics/test-approve.html
- 11.https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
- 12.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. [DOI] [PubMed] [Google Scholar]
- 13.Grigoryan. L, Pulendran B. The immunology of SARS-CoV-2 infections and vaccines. Semin Immunol.2020;50:101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bubar KM, Reinholt K, Kissler SM, et al. 19 vaccine prioritization strategies by age and serostatus. Science 2021;371(6532):916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christie. A, Mbaeyi, SA, Walensky RP. CDC Interim Recommendations for Fully Vaccinated People: an Important First Step. JAMA J Am Med Assoc. 2021;325::1501–1502. [DOI] [PubMed] [Google Scholar]
- 18.https://www.who.int/news-room/feature-stories/detail/the-effects-of-virus-variants-on-covid-19-vaccines
- 19.Wang P, Nair MS, Liu L, et al. Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7. Nature. 2021 Mar 8. DOI: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Jackson CB, Mou H, et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat Commun. 2020;11(1):6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul P, France AM, Aoki Y, et al. Genomic Surveillance for SARS-CoV-2 Variants Circulating in the United States, December 2020-May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(23):846–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore. JP, Offit, Offit PA PA.. SARS-CoV-2 Vaccines and the Growing Threat of Viral Variants. JAMA. 2021;325(9):821–822. [DOI] [PubMed] [Google Scholar]
- 23.Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burki T. Understanding variants of SARS-CoV-2. Lancet. 2021;397(10273):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frampton D, Rampling T, Cross A, et al. Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. Lancet Infect Dis. 2021;21(9):1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies NG, Jarvis CI, Edmunds WJ, et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021 Mar 15;593(7858):270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tegally H, Wilkinson E, Lessells RJ, et al. Sixteen novel lineages of SARS-CoV-2 in South Africa. Nat Med. 2021;27(3):440–446. [DOI] [PubMed] [Google Scholar]
- 28.Tegally H, Wilkinson E, Giovanetti M, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021;592(7854):438–443. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann M, Arora P, Groß R, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184(9):2384–2393.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plante JA, Mitchell BM, Plante KS, et al. The variant gambit: COVID-19’s next move. Cell Host Microbe. 2021;29(4):508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korber B, Fischer WM, Gnanakaran S, et al. Tracking Changes in SARS-CoV-2 Spike: evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020;182(4):812–827.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XN, Huang Y, Wang W, et al. Efficacy of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study. Emerg Microbes Infect. 2021; 10(1):1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Rio C, Malani PN, Omer SB.. Confronting the Delta Variant of SARS-CoV-2, Summer 2021. JAMA 2021 Aug 18;326(11):1001. [DOI] [PubMed] [Google Scholar]
- 34.Sheikh A, McMenamin J, Taylor B, et al. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharif N, Ahmed SN, Opu RR, et al. Impact of meteorological parameters and population density on variants of SARS-CoV-2 and outcome of COVID-19 pandemic in Japan. Epidemiol Infect. 2021;149:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Parra G, Martínez-Rodríguez D, Villanueva-Micó RJ.. Impact of a New SARS-CoV-2 Variant on the Population: a Mathematical Modeling Approach. Mathematical Comput Appl. 2021;26(2):25. [Google Scholar]
- 37.Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538):eabg 3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Challen R, Brooks-Pollock E, Read JM, et al. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021 Mar 9;372:n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo X, Chen Z, Xia Y, et al. Investigation of the genetic variation in ACE2 on the structural recognition by the novel coronavirus (SARS-CoV-2). J Transl Med. 2020 Aug 24;18(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang SW, Miller SO, Yen CH, et al. Impact of Genetic Variability in ACE2 Expression on the Evolutionary Dynamics of SARS-CoV-2 Spike D614G Mutation. Genes (Basel). 2020;12(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 42.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020;396(10249):467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ewer KJ, Barrett JR, Belij-Rammerstorfer S, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27(2):270–278. [DOI] [PubMed] [Google Scholar]
- 46.Barrett JR, Belij-Rammerstorfer S, Dold C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27(2):279–288. [DOI] [PubMed] [Google Scholar]
- 47.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ella R, Vadrevu KM, Jogdand H.. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21(5):637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021; 21(7):950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Zhang Y, Huang B, et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell. 2020;182(3):713–721.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine. 2020;383(27):2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walsh EE, RW Frenck Jr, Falsey AR.. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383(25):2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dagan N, Barda N, Kepten E.. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384(15):1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monin L, Laing AG, Muñoz-Ruiz M.. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abu-Raddad LJ, Chemaitelly H, Butt AA.. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021 May 5;385(2):187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sahin U, Muik A, Derhovanessian ECOVID. 19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature. 2020;586(7830):594–599. [DOI] [PubMed] [Google Scholar]
- 62.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS-CoV-2 — preliminary Report. N Engl J Med. 2020;383:1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corbett KS, Flynn B, Foulds KE.. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med. 2020;383(16):1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Widge AT, Rouphael NG, Jackson LA.. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med. 2021;384(1):80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baden LR, El Sahly HM, Essink B.. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine. 2021 Feb 4;384(5):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson EJ, Rouphael NG, Widge AT.. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383(25):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corbett KS, Edwards DK, Leist SR.. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doria-Rose N, Suthar MS, Makowski M.. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021 Apr 6;384(23):2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doroftei B, Ciobica A, Ilie O-D, et al. Mini- Review Discussing the Reliability and Efficiency of COVID-19 Vaccines. Diagnostics. 2021;11:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hotez PJ, Bottazzi ME.. Developing a low-cost and accessible COVID-19 vaccine for global health. PLoS Negl Trop Dis. 2020;14(7):e0008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pino M, Abid T, Pereira Ribeiro S, et al. A yeast expressed RBD-based SARS-CoV-2 vaccine formulated with 3M-052-alum adjuvant promotes protective efficacy in non-human primates. Sci Immunol. 2021;6(61):eabh3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao T, Ren Y, Li S, et al. Immune response induced by oral administration with a Saccharomyces cerevisiae-based SARS-CoV-2 vaccine in mice. Microb Cell Fact. 2021;20(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zang J, Zhu Y, Zhou Y, et al. Yeast-produced RBD-based recombinant protein vaccines elicit broadly neutralizing antibodies and durable protective immunity against SARS-CoV-2 infection. Cell Discov. 2021;7(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Q, Gao Y, Xiao M, et al. Synthesis and immunological evaluation of synthetic peptide based anti-SARS-CoV-2 vaccine candidates. Chem Commun. 2021;57(12):1474–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Outlaw VK, Bovier FT, Mears MC, et al. Inhibition of Coronavirus Entry In Vitro and Ex Vivo by a Lipid-Conjugated Peptide Derived from the SARS-CoV-2 Spike Glycoprotein HRC Domain. mBio. 2020;11(5):e01935–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Callaway E. Mix-and-match COVID vaccines trigger potent immune response. Nature. 2021;593(7860):491. [DOI] [PubMed] [Google Scholar]
- 77.Lewis D. Mix-and-match COVID vaccines: the case is growing, but questions remain. Nature. 2021;595(7867):344–345. [DOI] [PubMed] [Google Scholar]
- 78.Kunal S, Sakthivel P, Gupta N, et al. Mix and match COVID-19 vaccines: potential benefit and perspective from India. Postgrad Med J. 2021:2021–140648. [DOI] [PubMed] [Google Scholar]
- 79.Deming ME, Lyke KE.. A ‘mix and match’ approach to SARS-CoV-2 vaccination. Nat Med. 2021 Jul 26;27(9):1510–1511. [DOI] [PubMed] [Google Scholar]
- 80.Shaw RH, Stuart A, Greenland M, et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397(10289):2043–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plante JA, Liu Y, Liu J.. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592(7852):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luchsinger LL, Hillyer CD, Sills J.. Vaccine efficacy probable against COVID-19 variants. Science. 2021;371(6534):1116. [DOI] [PubMed] [Google Scholar]
- 83.Emary KRW, Golubchik T, Aley PK.. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397(10282):1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021; 384(20):1885–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021 Apr 21;384(23):2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021 May 5;384(20):1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Collier DA, De Marco A, Ferreira IATM, et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature. 2021;593(7857):136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edara VV, Norwood C, Floyd K, et al. Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe. 2021;29(4):516–521.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sapkal GN, Yadav PD, Ella R, et al. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B 1.1.7 variant of SARS-CoV-2. J Travel Med. 2021;28:taab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yadav PD, Sapkal GN, Abraham P, et al. Neutralization of variant under investigation B.1.617 with sera of BBV152 vaccinees. Clin Infect Dis 2021. ciab411 10.1093/cid/ciab411. [DOI] [PubMed] [Google Scholar]
- 91.Pegu A, O’Connell S, Schmidt SD, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021;eabj4176. 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. [DOI] [PubMed] [Google Scholar]
- 93.https://www.cdc.gov/coronavirus/2019-ncov/global-covid-19/global-urban-areas.html
- 94.Xiao Y, Torok ME.. Taking the right measures to control COVID-19. Lancet Infect Dis. 2020;20(5):523–524. [DOI] [PMC free article] [PubMed] [Google Scholar]


